Abstract
Background
Sepsis is one of the leading causes of death in intensive care units. Dexmedetomidine is a sedative agent with anti-inflammatory properties. This study is designed to differentiate the impact of two different doses of dexmedetomidine on lung injury induced by sepsis.
Methods
Adult male Wistar rats were randomly divided into four groups: sham (n = 6), control (n = 12), 5DEX (n = 12), and 10DEX (n = 12). Cecal ligation puncture (CLP) was applied for sepsis initiation. The 5DEX group received 5 μg.kg-1.h-1 and the 10DEX group received 10 μg.kg-1.h-1 dexmedetomidine intravenous infusions for a 1-hour period. Six hours after CLP, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and intercellular adhesion molecule-1 (ICAM-1) levels were analyzed in blood samples. Twenty-four hours after CLP, lung samples from the remaining rats were collected for the measurement of myeloperoxidase (MPO) activity, histological examination, and TdT- (terminal deoxynucleotidyl transferase) mediated fluorescent-dUTP labeling staining for apoptosis detection.
Results
Serum cytokine release, MPO activity, and apoptosis in the lung were significantly increased in the CLP group compared with the sham and dexmedetomidine groups (p < 0.05). TNF-α, ICAM-1, and MPO were significantly lower in the 10DEX group compared with both 5DEX and control groups, while IL-1β, total injury score, and apoptotic cell count had significantly lower values in both 10DEX and 5DEX groups compared with the control group (p < 0.05).
Conclusion
Dexmedetomidine administration played a protective role against CLP-induced lung injury. High-dose dexmedetomidine was needed for suppressing the leukocyte-mediated lung injury and apoptosis of lung tissue.
Keywords: Sepsis, Lung injury, Dexmedetomidine
Introduction
Sepsis is a life-threatening clinical syndrome that disrupts the host response to infectious pathogens.1 Sepsis syndrome is related to major clinical problems, including hemodynamic instability, coagulation problems, and multiple organ dysfunctions. The most vulnerable organ to sepsis is the lung; acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are the leading causes of mortality in sepsis.2 ALI/ARDS is a multifactorial clinical condition associated with multiple organ failure contributing to increased mortality and morbidity.3 The excessive release of inflammatory cytokines, the upregulation of adhesion molecules, neutrophil accumulation, the loss of vascular integrity, and apoptosis of alveolar epithelial and pulmonary vascular endothelial cells are major pathological changes in sepsis-related lung injury.4, 5, 6
Intercellular adhesion molecule 1 (ICAM-1) is found in leukocyte and endothelial cell membranes, and regulates the interaction between leukocytes and the endothelium.4 Therefore, ICAM-1 plays an important role in leukocyte-mediated organ injury and has been even shown to be an early marker of lung injury diagnosis.7, 8 Neutrophil infiltration into the lung plays an important role in the initiation of ALI by releasing neutrophil proteases, causing alveolar damage.9 Apoptosis of lung epithelial cells may promote alveolar destruction and lung fibrosis, contributing to the initiation and progression of ALI.10, 11
The selective α2 agonist dexmedetomidine has sedative, anxiolytic, and analgesic properties and is commonly administered to patients in intensive care units to provide sedation.12 In addition, potential anti-inflammatory and antiapoptotic effects of dexmedetomidine have been reported.13 Moreover, clinical reports of patients with severe sepsis have shown that dexmedetomidine improves the survival rate via its anti-inflammatory effects.14 However, the protective impact of dexmedetomidine on sepsis-related lung injury and the effective dose that reduces inflammation and alveolar apoptosis are not clear.
The primary objective of this study was to differentiate the effects of two different doses of dexmedetomidine on lung injury induced by sepsis, and the secondary objective was to demonstrate whether the effects of dexmedetomidine on lung injury are related to systemic ICAM-1 release, neutrophil accumulation, and/or the apoptosis of alveolar epithelial cells. Based on previous findings, we hypothesized that dexmedetomidine medication could attenuate sepsis induced lung injury.
Materials and methods
Ethics statement
The experimental procedures and the care of animals were approved by the Instructional Animal Care and Use Committee of Gazi University (Approval No. GÜET-16.002). All animals were maintained in accordance with the recommendations of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Animal groups, randomization, and experimental design
Healthy adult male pathogen-free Wistar rats (n = 42) weighing 250–300 g (GÜDAM – Laboratory Animals Raising and Experimental Research Center) were included in this study. All animals were housed at 21 °C to 23 °C, 30% to 40% humidity, and on a 12-hour light-dark cycle with ad libitum access to rat chow and water. The block randomization scheme was generated by a computer-generated random assignment sequence prepared in advance. An independent statistician who was not directly participant in the conduct of the trial generated the randomization sequence with computer. Anesthesia was induced and maintained by intramuscular injection of 50 mg.kg-1 ketamine hydrochloride, 5 mg.kg-1 xylazine, and intravenous 2 μg.kg-1.h-1 fentanyl infusion in all rats. Anesthesia depth was determined by the toe pinch response method. Hydration and drug infusions were provided through a tail vein catheter. Hydration was maintained by infusion of isotonic sodium chloride solution at a rate of 0.5 mL.h-1. During the experiment, rats were allowed to breathe spontaneously while monitoring peripheral oxygen saturation. Rats were randomly divided into four groups according to a computer-generated list: sham (n = 6), control (n = 12), 5DEX (n = 12), and 10DEX (n = 12) groups. There are several murine sepsis models used in experimental studies. We decided to use the cecal ligation and puncture (CLP) model since it is polymicrobial and creates conditions similar to sepsis and septic shock seen in humans, such as perforated appendicitis, diverticulitis or colon perforation.15
Cecal ligation puncture was applied to rats in the control, 5DEX, and 10DEX groups, while in the sham group the abdominal cavity was exposed without CLP by a midline incision. After CLP surgery and the sham procedure, the rats in the 5DEX group received 5 μg.kg-1.h-1 dexmedetomidine; rats in the 10DEX group received 10 μg.kg-1.h-1 dexmedetomidine intravenous infusions for a 1-hour period; rats in the sham and control groups received equal volumes of saline. Six hours after CLP, 3 rats from the sham group and 6 rats each from the control, 5DEX, and 10DEX groups were anesthetized with ketamine/xylazine combination, and blood samples were withdrawn for the measurements of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and ICAM-1 analysis. Twenty-four hours after CLP, the remaining rats from every group were euthanized via intravenous injection of 200 mg.kg-1 pentobarbital, and lung samples were collected for the analysis of myeloperoxidase (MPO) concentration, histological examination, and TdT- (terminal deoxynucleotidyl transferase) mediated fluorescent-dUTP labeling (TUNEL) staining for apoptosis detection.
Cecal ligation and puncture procedure
Sepsis was initiated by cecal ligation and puncture as previously described.16 Briefly, intraperitoneal ketamine and xylazine were used to maintain anesthesia, and aseptic conditions were provided. Laparotomy was performed via a 3-cm midline incision to explore, ligate, and perforate the cecum. The cecum was ligated with 3-0 silk and perforated twice with an 18G needle, gently squeezed, and a small amount of feces was extruded. Then, the cecum was repositioned in the abdominal cavity, and the laparotomy incision was closed. After the CLP procedure, the rats were returned to their individual cages with free access to food and water. Rats in the sham group underwent all surgical procedures except cecal ligation and puncture.
Cytokine and ICAM-1 measurements
Blood samples were centrifuged, and plasma was separated and stored at -80 °C. Plasma TNF-α and IL-1β levels were measured in duplicate by an enzyme-linked immunosorbent assay (ELISA) kit (Elabscience Biotechnology Co., Ltd.) according to the manufacturer’s guidelines, and the results are expressed as picograms per milliliter (pg.mL-1). Plasma ICAM-1 levels were determined in duplicate with the Quantikine ICAM-1 ELISA kit (Elabscience Biotechnology Co., Ltd.), according to the manufacturer’s instructions. Serum cytokine levels were expressed in pg.mL-1 of analyzed serum.
Lung tissue MPO activity assay
Lung tissue samples were homogenized by sonication in potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide. After centrifugation, the supernatant was diluted in reaction solution containing o-dianisidinedihydrochloride and H2O2. The rate of change in optical density (OD) for 1 minute was measured at 460 nm to calculate the MPO activity. MPO levels were expressed as units of MPO per gram of protein.
Histology
The lungs were fixed in a mixture of 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4), dehydrated with a graded alcohol series, and embedded in paraffin at 52 °C. The sections were prepared and stained with hematoxylin and eosin (H & E) for histological evaluation. A pathologist blinded to the study groups scored each lung section for lung injury. The alveolar capillary congestion, hemorrhage, infiltration, or aggregation of neutrophils in the vessel wall, and thickness of the alveolar wall were assessed. Each item was graded according to the following scale: 0 = minimal damage; 1 = mild damage; 2 = moderate damage; 3 = severe damage; and 4 = maximal damage.17
In situ apoptosis in lung tissue
In situ DNA fragmentation in lung tissue was determined by the terminal deoxynucleotidyl transferase (TdT)-mediated fluorescent-dUTP labeling (TUNEL) method. TUNEL was performed using the ApopTag Peroxidase in situ apoptosis detection kit (S7110, Chemicon International, Inc., Temecula, CA, USA) according to the manufacturer's instructions. The peroxidase substrate 3.3-diaminobenzidine was used to visualize apoptotic cells. Methyl green (0.5%) was used for nuclear staining. TUNEL-positive cells were visualized under a light microscope (Olympus, Bx51, Japan). After counting TUNEL-positive cells in 5 different large growth fields outside the areas of lymphoid follicle formation in the lungs, the averages were calculated.
Statistics
Statistical evaluation was performed using SPSS (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp; 2016). The suitability of univariate data for normal distribution was evaluated with the Kolmogorov-Smirnov test and Shapiro-Wilk francia test. In comparing more than two groups with respect to quantitative data, using the Kruskal-Wallis H Test, Monte Carlo Simulation results, Dunn’s Test was used for Post Hoc analysis. Quantitative variables were expressed as Median (minimum/maximum) in the figures. Given that the lung injury score was a categorical variable, Fisher’s correction was applied. In all statistical analyses, p < 0.05 was regarded as the significance limit.
Results
Influence of dexmedetomidine treatment on serum cytokine and ICAM-1 release
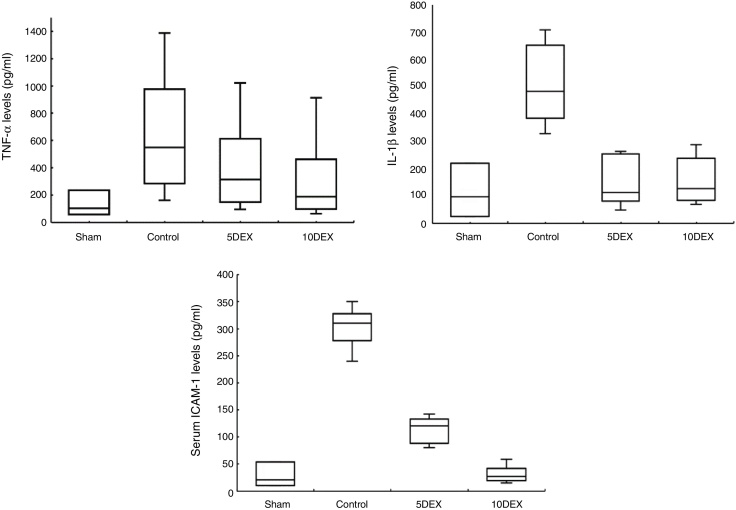
The data obtained from the comparison of serum cytokine and ICAM-1 values between groups are presented in Figure 1. TNF-α levels were significantly lower in the 10DEX group than in both the 5DEX and control groups (p < 0.05), while IL-1β values were significantly lower in both the 10DEX and 5DEX groups than in the control group (p < 0.05). Serum ICAM-1 levels were significantly lower in the 10DEX group than in the 5DEX and control groups (p < 0.05).
Figure 1.
Serum TNF-α, serum IL-1β, and serum ICAM-1 concentrations in rats six hours after the CLP (cecal ligation puncture) and sham procedures. Each bar represents the Median (minimum/maximum). 5DEX, 5 μg.kg-1.h-1 dexmedetomidine + CLP; 10DEX, 10 μg.kg-1.h-1 dexmedetomidine + CLP; Control, saline + CLP.
Influence of dexmedetomidine treatment on neutrophil infiltration in lung tissue
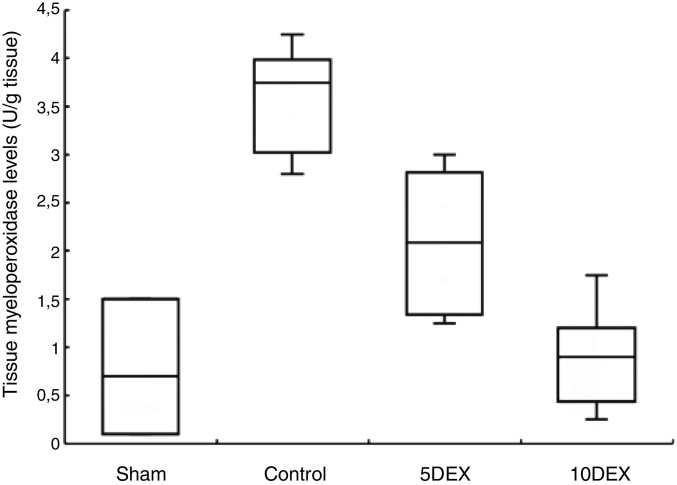
The effects of low and high doses of dexmedetomidine treatment on MPO formation in the lung are presented in Figure 2. The activity of MPO in the lungs significantly increased in the control group 24 hours after CLP compared to the sham and study groups. MPO activity was reduced significantly in lung tissue samples obtained from rats in the 10DEX group compared to the 5DEX and control groups (p < 0.05).
Figure 2.
Lung tissue MPO levels of the rats 24 hours after the CLP and sham procedures. Each bar represents the Median (minimum/maximum). 5DEX, 5 μg.kg-1.h-1 dexmedetomidine + CLP; 10DEX, 10 μg.kg-1.h-1 dexmedetomidine + CLP; Control, saline + CLP.
Influence of dexmedetomidine treatment on lung histopathology and apoptosis
Table 1 presents the results of H&E and TUNEL staining of lung tissue specimens. The total lung injury score was lower in both the 5DEX and 10DEX groups than in the control group (p < 0.05). Moreover, the apoptotic cell count was found to be lower in the 10DEX and 5DEX groups than in the control group (p < 0.05). Examination of H&E staining showed normal alveolar structure without congestion, hemorrhage, or infiltration in the sham group. Lung tissues from the control group showed thickening of the alveolar wall and intense neutrophil aggregation. In the dexmedetomidine infusion groups, alveolar capillary congestion, alveolar wall thickness, and neutrophil aggregation were alleviated compared with the control group. Representative images of different injury categories and apoptotic cells from each experimental group detected 24 hours after CLP are shown in Figure 3, Figure 4.
Table 1.
Injury scores and apoptotic changes in lung tissues of the rats twenty-four hours after CLP (cecal ligation puncture) and sham procedures.
| Groups | Apoptosis (mean ± SD) | Lung Injury Scores (mean ± SD) |
|---|---|---|
| Sham (n = 3) | 0.03 ± 0.001β | 1.33 ± 0.002β |
| Control (n = 6) | 6.40 ± 0.55 | 9.60 ± 0.89 |
| 5DEX (n = 6) | 4.17 ± 3.06α | 4.33 ± 1.21α |
| 10DEX (n = 6) | 2.13 ± 1.97α | 3.67 ± 1.63α |
Values are expressed as mean ± standard deviation; 5DEX, 5 μg.kg-1.h-1 dexmedetomidine + CLP; 10DEX, 10 μg.kg-1.h-1 dexmedetomidine + CLP; Control, saline + CLP; αp < 0.05 compared with the control group; βp < 0.05 compared with the control, 5DEX, 10DEX groups.
Figure 3.
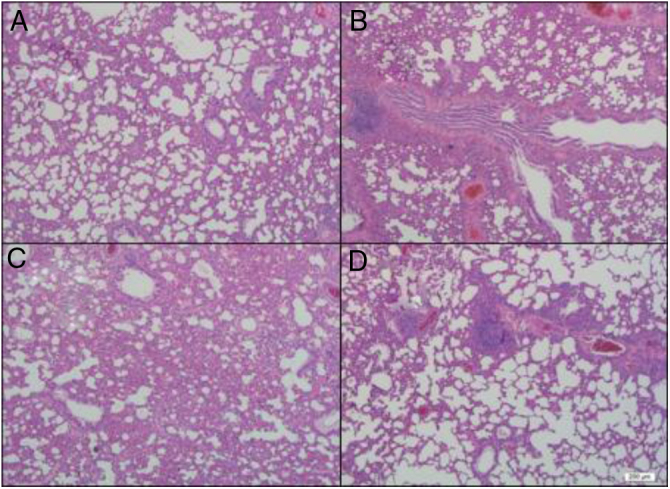
Representative images of lung sections obtained 24 hours after the CLP and sham procedures. A: normal structures and clear alveoli in the sham group. B: intense neutrophil aggregation and thickening of the alveolar wall in the control group. C: moderate neutrophil aggregation and thickening of the alveolar wall in the 5 μg.kg-1.h-1 dexmedetomidine infusion group. D: few neutrophil aggregations and thickening of the alveolar wall in the 10 μg.kg-1.h-1 dexmedetomidine infusion group (hematoxylin and eosin, Mag ×400).
Figure 4.
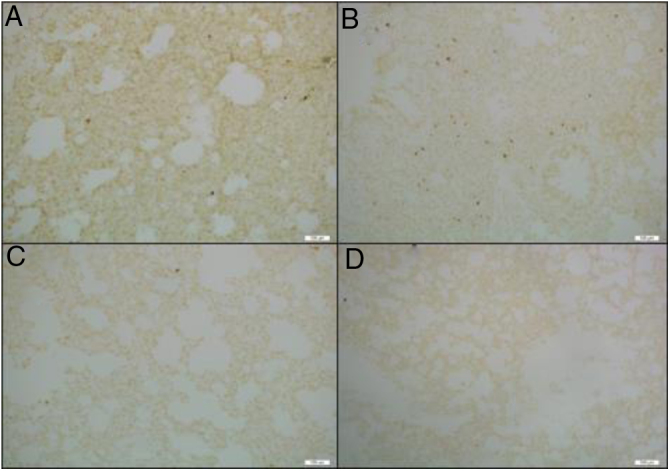
Images of lung sections ApopTag (+) cells with TUNEL staining obtained 24 hours after the CLP and sham procedure. ApopTag (+) cell nuclei were stained dark brown. Tissue samples obtained from A: sham group; B: saline + CLP (control) group; C: 5 μg.kg-1.h-1 dexmedetomidine + CLP group; D: 10 μg.kg-1.h-1 dexmedetomidine + CLP group (Mag × 100).
During the experiment none of the rats needed vasopressor drugs or experienced a decrease in peripheral oxygen saturation.
Discussion
In the present study, we investigated the effects of low and high doses of dexmedetomidine on sepsis-related lung injury. Our results showed that high-dose dexmedetomidine administration provided protection against CLP-induced lung injury, as evidenced by decreases in ICAM-1, MPO and apoptotic cell counts. Low-dose dexmedetomidine effectively suppressed the systemic cytokine response but did not improve leukocyte adhesion, neutrophil accumulation, or apoptosis in lung tissue.
Lung injury is a serious complication of sepsis and is associated with high mortality rates.2 Alveolar capillary barrier damage, oxidative stress, inflammatory cascade stimulation, increased cytokine response, upregulation of adhesion molecules, neutrophil infiltration, and apoptosis of alveolar epithelium and endothelial cells are pathological processes associated with lung injury.3, 18, 19
Patients with sepsis generally require sedation to maintain effective mechanical ventilation, to ensure patient compliance with this therapy, and to reduce anxiety. Dexmedetomidine is a potent, highly selective α2-adrenergic receptor agonist, and it is preferred for sedation in intensive care units due to its analgesic and sedative effects without respiratory depression. Dexmedetomidine has been demonstrated to have anti-inflammatory and antiapoptotic effects apart from sedative, anxiolytic, analgesic sparing, and sympatholytic effects.20 Liu Z et al.21 showed that intraperitoneal administration of dexmedetomidine inhibited the inflammatory response in the lung tissue of mice with sepsis, and this effect was partially mediated by the cholinergic anti-inflammatory pathway.
Although the anti-inflammatory and immunomodulatory effects of dexmedetomidine have been shown in many studies, the effective dose range that provided these reported effects was not defined clearly. Ma Y et al.22 studied the effects of low, medium, and high doses of dexmedetomidine infusion on immunomodulation and mortality; their results revealed that dexmedetomidine had immunomodulatory effects that were initiated within 5 hours, and dexmedetomidine infusion improved mortality rates dose-dependently 24 hours after CLP. Their results showed that both medium and high doses of dexmedetomidine infusion suppressed systemic cytokine release and decreased the mortality rate, while immune regulation was maintained more effectively with medium doses of dexmedetomidine, as evidenced by HLA-DR levels. However, they did not study the effects of dexmedetomidine on specific organ functions. In the present study, we evaluated the effects of two different doses of dexmedetomidine on lung injury in CLP-induced sepsis in rats. Our results showed that high-dose dexmedetomidine was needed for the effective control of systemic inflammation and neutrophil accumulation in lung tissue.
Enhanced levels of serum cytokines and ICAM-1 have been shown to be associated with endothelial damage in experimental studies. Moreover, clinical data has shown that sepsis survivors have decreased cytokine and ICAM-1 levels compared to nonsurvivors.23
Jing W et al.24 showed that the release of inflammatory cascade mediators, such as TNF-α, IL-6, IL-1β, and ICAM-1, plays an essential role in lung parenchyma injury. IL-1β is an important modulator in the acute phase of inflammation and is known to participate in alveolar epithelial repair.25 TNF-α activates human bronchial epithelial cells and facilitates the generation of other inflammatory mediators.26 Moreover, these proinflammatory cytokines are involved in the recruitment of neutrophils and promotion of MPO activity.27 ICAM-1 stimulates the inflammatory cascade and contributes to the migration, accumulation and activation of neutrophils to lung tissue, resulting in ALI.4 In the present study, 6 hours after CLP, high-dose dexmedetomidine decreased the activity of TNF-α and ICAM-1. Pulmonary neutrophil accumulation is a major marker of inflammation and tissue damage, and is related to ALI initiation.28 Pulmonary MPO activity has been reported to be a reliable marker of pulmonary neutrophil infiltration.29 Consequently, we determined the MPO activity in the lung tissue of septic rats treated with different doses of dexmedetomidine 24 hours after sepsis initiation. Our results showed that dexmedetomidine provided dose-related protection against sepsis-induced neutrophil accumulation in lung tissue, as evidenced by decreased MPO levels. But in this experiment, we did not investigate the underlining mechanism of this effect and this needs further studies.
Initiation of the systemic inflammatory cascade and neutrophil accumulation in lung tissue may promote apoptosis, resulting in lung dysfunction and increased mortality during sepsis. Alveolar apoptosis has been shown to disrupt epithelial barrier function, initiating ALI.30 In the present study, apoptotic cells were detected to identify the effects of dexmedetomidine on lung injury. Our results showed that both low- and high-dose dexmedetomidine were effective for controlling sepsis-related lung apoptosis.
There are some limitations to our study. First, the sample size was relatively small. Second, conducting continuous invasive monitoring and comparing hemodynamic parameters might have strengthened the study since the diagnosis of sepsis is made clinically, and patients with sepsis are usually treated in intensive care units with invasive monitoring. Last, a survival study might have been included. Therefore, further studies with larger sample sizes, which include clinical parameters and survival analysis, are needed, and these findings should be supported with randomized clinical studies.
Conclusions
Our study demonstrated that dexmedetomidine infusion at high doses effectively suppressed the leukocyte-mediated lung injury and apoptosis of lung tissue.
Funding
This study was supported by the Gazi University Scientific Research Projects Coordination Department (Project Number: 01/2016-04).
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Singer M., Deutschman C.S., Seymour C.W., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenfeld G.D., Caldwell E., Peabody E., et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X.D., Hou J.F., Qin X.J., et al. Pentoxifylline inhibits intercellular adhesion molecule-1 (ICAM-1) and lung injury in experimental phosgene-exposure rats. Inhal Toxicol. 2010;22:889–895. doi: 10.3109/08958378.2010.493900. [DOI] [PubMed] [Google Scholar]
- 5.Gong Y., Lan H., Yu Z., et al. Blockage of glycolysis by targeting PFKFB3 alleviates sepsis-related acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem Biophys Res Commun. 2017;491:522–529. doi: 10.1016/j.bbrc.2017.05.173. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y., Wang L., Kang Q., et al. Heat Shock Protein A12B Protects Vascular Endothelial Cells Against Sepsis-Induced Acute Lung Injury in Mice. Cell Physiol Biochem. 2017;42:156–168. doi: 10.1159/000477308. [DOI] [PubMed] [Google Scholar]
- 7.Hill J., Lindsay T., Valeri C.R., et al. A CD18 antibody prevents lung injury but not hypotension after intestinal ischemia-reperfusion. J Appl Physiol. 1993;74:659–664. doi: 10.1152/jappl.1993.74.2.659. [DOI] [PubMed] [Google Scholar]
- 8.Marcus B.C., Hynes K.L., Gewertz B.L. Loss of endothelial barrier function requires neutrophil adhesion. Surgery. 1997;122:420–427. doi: 10.1016/s0039-6060(97)90035-0. [DOI] [PubMed] [Google Scholar]
- 9.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–9. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 10.Ma X., Xu D., Ai Y., et al. Fas inhibition attenuates lipopolysaccharide-induced apoptosis and cytokine release of rat type II alveolar epithelial cells. Mol Biol Rep. 2010;37:3051–3056. doi: 10.1007/s11033-009-9876-9. [DOI] [PubMed] [Google Scholar]
- 11.Chuang C.Y., Chen T.L., Cherng Y.G., et al. Lipopolysaccharide induces apoptotic insults to human alveolar epithelial A549 cells through reactive oxygen species-mediated activation of an intrinsic mitochondrion-dependent pathway. Arch Toxicol. 2011;85:209–218. doi: 10.1007/s00204-010-0585-x. [DOI] [PubMed] [Google Scholar]
- 12.Hsu Y.W., Cortinez L.I., Robertson K.M., et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–1076. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Ran K., Zhang S.B., et al. Dexmedetomidine may upregulate the expression of caveolin-1 in lung tissues of rats with sepsis and improve the short-term outcome. Mol Med Rep. 2017;15:635–642. doi: 10.3892/mmr.2016.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandharipande P.P., Sanders R.D., Girard T.D., et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poli-de-Figueiredo L.F., Garrido A.G., Nakagawa N., et al. Experimental models of sepsis and their clinical relevance. Shock. 2008;30:53–59. doi: 10.1097/SHK.0b013e318181a343. [DOI] [PubMed] [Google Scholar]
- 16.Ebong S., Call D., Nemzek J., et al. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun. 1999;67:6603–6610. doi: 10.1128/iai.67.12.6603-6610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torre D., Minoja G., Maraggia D., et al. Effect of recombinant IL-1 beta and recombinant gamma interferon on septic acute lung injury in mice. Chest. 1994;105:1241–1245. doi: 10.1378/chest.105.4.1241. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y., Liu Y., Huang H., et al. Dexmedetomidine inhibits inflammatory reaction in lung tissues of septic rats by suppressing TLR4/NF-kappaB pathway. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/562154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma C., Zhu L., Wang J., et al. Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. J Ethnopharmacol. 2015;168:349–355. doi: 10.1016/j.jep.2015.03.068. [DOI] [PubMed] [Google Scholar]
- 20.Wang X., Zhao B., Li X. Dexmedetomidine attenuates isoflurane-induced cognitive impairment through antioxidant, anti-inflammatory and anti-apoptosis in aging rat. Int J Clin Exp Med. 2015;8:17281–17288. [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z., Wang Y., Wang Y., et al. Dexmedetomidine attenuates inflammatory reaction in the lung tissues of septic mice by activating cholinergic anti-inflammatory pathway. Int Immunopharmacol. 2016;35:210–216. doi: 10.1016/j.intimp.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y., Yu X.Y., Wang Y. Dose-related effects of dexmedetomidine on immunomodulation and mortality to septic shock in rats. World J Emerg Med. 2018;9:56–63. doi: 10.5847/wjem.j.1920-8642.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hein O.V., Misterek K., Tessmann J.P., et al. Time course of endothelial damage in septic shock: prediction of outcome. Crit Care. 2005;9:R323. doi: 10.1186/cc3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing W., Chunhua M., Shumin W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-kappaB pathway in vivo and in vitro. Toxicol Appl Pharmacol. 2015;285:128–135. doi: 10.1016/j.taap.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Chen T., Mou Y., Tan J., et al. The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2015;25:55–64. doi: 10.1016/j.intimp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Chen T., Wang R., Jiang W., et al. Protective Effect of Astragaloside IV Against Paraquat-Induced Lung Injury in Mice by Suppressing Rho Signaling. Inflammation. 2016;39:483–492. doi: 10.1007/s10753-015-0272-4. [DOI] [PubMed] [Google Scholar]
- 27.Huang X., Liu Y., Lu Y., et al. Anti-inflammatory effects of eugenol on lipopolysaccharide-induced inflammatory reaction in acute lung injury via regulating inflammation and redox status. Int Immunopharmacol. 2015;26:265–271. doi: 10.1016/j.intimp.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X., Dai Q., Huang X. Neutrophils in acute lung injury. Front Biosci. 2012;17:2278–2283. doi: 10.2741/4051. [DOI] [PubMed] [Google Scholar]
- 29.McCabe A.J., Dowhy M., Holm B.A., et al. Myeloperoxidase activity as a lung injury marker in the lamb model of congenital diaphragmatic hernia. J Pediatr Surg. 2001;36:334–337. doi: 10.1053/jpsu.2001.20709. [DOI] [PubMed] [Google Scholar]
- 30.Bardales R.H., Xie S.S., Schaefer R.F., et al. Apoptosis is a major pathway responsible for the resolution of type II pneumocytes in acute lung injury. Am J Pathol. 1996;149:845–852. [PMC free article] [PubMed] [Google Scholar]