Abstract
The purpose of the Brazilian Society of Anesthesiology (SBA)’s Regional Anesthesia Safety Recommendations Update is to provide new guidelines based on the current relevant clinical aspects related to safety in regional anesthesia and analgesia. The goal of the present article is to provide a broad overview of the current knowledge regarding pre-procedure asepsis and antisepsis, risk factors, diagnosis and treatment of infectious complications resulting from anesthetic techniques. It also aims to shed light on the use of reprocessed materials in regional anesthesia practice to establish the effects of aseptic handling of vials and ampoules, and to show cost-effectiveness in the preparation of solutions to be administered continuously in regional blockades. Electronic databases were searched between January 2011 (final date of the literature search for the past SBA recommendations for safety in regional anesthesia) and September 2019. A total of 712 publications were found, 201 of which were included for further analysis, and 82 new publications were added into the review. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system was used to assess the quality of each study and to classify the strength of evidence. The present review was prepared by members of the SBA Technical Standards Committee.
Keywords: Regional anesthesia, Infection, Patient safety
Resumo
O propósito desta atualização das Recomendações da Sociedade Brasileira de Anestesiologia para Segurança em Anestesia Regional foi apresentar novas diretrizes com base na relevância e atualidade clínica nos aspectos de segurança relacionados a analgesia e anestesia regional. Este artigo visa prover uma visão ampla sobre o conhecimento atual no tocante a assepsia e antissepsia pré-procedimento, fatores de risco, diagnóstico e tratamento das complicações infecciosas decorrentes das técnicas anestésicas. Também visa esclarecer sobre o uso de materiais reprocessados na prática da anestesia regional, estabelecer as implicações no manejo asséptico de frascos e ampolas e elucidar sobre a relação custo-efetividade no preparo de soluções a serem administradas continuamente em bloqueios regionais. As bases de dados eletrônicas foram pesquisadas entre Janeiro de 2011 (final da pesquisa de literatura das diretrizes anteriores da SBA sobre segurança em anestesia regional) e Setembro de 2019. Um total de 712 artigos foram encontrados, dos quais 201 foram incluídos para análise posterior e 82 novos estudos foram acrescentados nesta revisão. O sistema de Avaliação, Desenvolvimento e Avaliação da Classificação das Recomendações (GRADE) foi utilizado para avaliar a qualidade do estudo individual e classificar a força da evidência. Esta revisão foi elaborada por membros da Comissão de Normas Técnicas da SBA.
PALAVRAS-CHAVE: Anestesia regional, Infecção, Segurança do paciente
Description of method for collecting evidence
The present article updates the 2011 Recommendations of the Brazilian Society of Anesthesiology for Safety in Regional Anesthesia1 and takes into account studies published between January 1, 2011 and September 31, 2019, in addition to articles published between 1965 and 2011, already considered in the previous review.1 A review protocol was used to identify, retrieve and assess evidence in the following databases: PubMed, Cochrane Library, and LILACS. Cross-references with the collected material were also used to identify articles with better methodological designs. The search was later limited to studies performed in humans and published in English, French, German, Portuguese, or Spanish.
The search strategies used for this update were identical to the 2011 Recommendations:
-
1
“regional anaesthesia” OR “anesthesia, conduction” [MeSH Terms] AND “infection” [MeSH Terms] AND “prevention and control” [Subheading] OR “prevention” AND “control” OR “prevention and control” [MeSH Terms]
-
2
“regional anaesthesia” OR “anesthesia, conduction” [MeSH Terms] AND “infection” [MeSH Terms]
-
3
“regional anaesthesia” OR “anesthesia, conduction” [MeSH Terms] AND “infection” [MeSH Terms] AND “etiology” [Subheading] OR “etiology” OR “causality” [MeSH Terms]
-
4
“regional anaesthesia” OR “anesthesia, conduction” [MeSH Terms] AND “immunocompromised host” OR “immunocompromised patient” [MeSH Terms]
-
5
“regional anaesthesia” OR “anesthesia, conduction” [MeSH Terms] AND “meningitis” [MeSH Terms]
-
6
“regional anaesthesia” OR “anesthesia, conduction” [MeSH Terms] AND “epidural abscess” [MeSH Terms]
-
7
“anesthesia, epidural” [MeSH Terms] AND “catheters” [MeSH Terms] AND “colonization” AND “infection” [MeSH Terms]
-
8
“single-use” AND “equipment and supplies” [MeSH Terms] AND “devices” OR “medical devices” AND “reprocessing”
-
9
“single-use” AND “equipment and supplies” [MeSH Terms] AND “devices” OR “medical devices” AND “reprocessing” AND “anaesthesia” [MeSH Terms]
-
10
“Medication Errors” [Mesh Terms] AND "Anesthesia, Conduction" [Mesh Terms]
-
11
“cost-effective” AND “pharmaceutical solutions” [MeSH Terms] AND “regional anaesthesia” OR “anesthesia, conduction” [MeSH Terms]
-
12
“drug contamination” [MeSH Terms] AND “ampoules”
After searches, a critical analysis of content was performed followed by classification according to the strength of evidence. The list of selected articles was decided by peer review. In case of disagreement, the article was included for reading and a later decision as to its inclusion.
In a second stage, two new searches were performed (this time in the period between January 1, 1965 and September 31, 2019) for the inclusion of articles dealing with the new topics included in this review: techniques for surgical antisepsis of hands and the use of ultrasound devices for anesthetic blockades, focusing on its cleaning, or as a factor for increasing infection in regional anesthesia. The search was also limited to human studies and English, French, German, Portuguese, or Spanish publications. The following search strategies were used:
"antisepsis" [Mesh] AND "hand disinfection" [Mesh] AND "surgical wound infection" [Mesh]
"anesthesia, conduction" [Mesh] AND "ultrasonography" [Mesh] AND "infection" [Mesh]
"anesthesia, conduction" [Mesh] AND "ultrasonography" [Mesh] AND "disinfection" [Mesh]
"anesthesia, conduction" [Mesh] AND "ultrasonography" [Mesh] AND "safety" [Mesh]
Degrees of recommendation and strength of evidence
-
A)Experimental or observational studies of better consistency.
-
B)Experimental or observational studies of lower consistency.
-
C)Case reports or case series (uncontrolled studies).
-
D)Opinion devoid of critical assessment, based on consensus, expert opinions, physiological studies or animal models.
-
A)
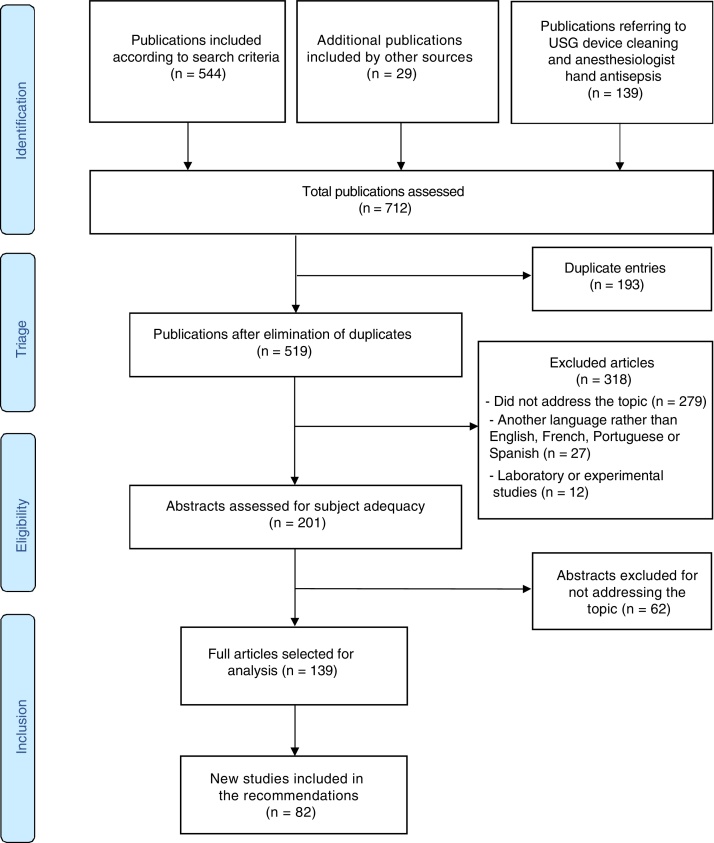
Fig. 1 describes the sequence of information at the different steps of this systematic review, with the number of records included and excluded, and with the reasons for exclusions.
Figure 1.
Flowchart of publication search and selection process for systematic reviews and meta-analyses using PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysis).
Introduction
During the regional anesthesia procedure, there are aspects that may cause or worsen infectious processes. Certain aspects are related to patient characteristics (sepsis or local infection, diabetes, immunodeficiency, use of immunosuppressive drugs) or to supplies (reuse, contamination or poor sterilization). Now and then, however, anesthesiologists can contribute to increase the incidence of infection by not complying with aseptic techniques or by contaminating the material to be used.2
Infectious complications related to regional anesthesia, although rare, are associated with increased hospital stay, costs, acute and chronic pain, morbidity and mortality.2 Infectious complications associated with neuraxial anesthesia can be devastating, such as meningitis, paralysis and death. The estimated risk of major infectious complications is 1:40,000, according to data from the 3rd National Audit Project of the Royal College of Anaesthesiologists in the United Kingdom, in which 15 epidural abscesses and three cases of meningitis were reported in 700,000 neuraxial anesthesia cases. According to the audit, epidural anesthesia or analgesia was associated with a higher chance of complications when compared to spinal anesthesia.3
The frequency of infections associated with peripheral nerve blockades is even more unclear. Risk factors that seem to be associated with a greater likelihood of infectious complications include the absence of antibiotic prophylaxis, use of a catheter for more than 48 hours, use of a catheter in the axillary and femoral sites, frequency of changing the dressing to protect the catheter, in addition to patient requiring ICU admission.4
A catheter used for neuraxial blockade, even if inserted under aseptic technique, can be colonized with patient skin flora, and favors epidural or subarachnoid infection.5
The importance of the aseptic technique
Does hand washing by the anesthesiologist reduce the incidence of infectious complications in regional anesthesia?
In 1847, Hungarian doctor Ignaz Philipp Semmelweis (1818‒1865) established that hospital infections could be transmitted by the hands of health professionals, and that simple washing with chlorine solutions could result in significant risk reduction. From Semmelweis’ study onwards, hands of health professionals have been linked to the transmission of microorganisms in the hospital environment.6
Basic hand washing is a simple and formidable component of the aseptic technique, and it has been stimulated and emphasized worldwide by the World Health Organization (WHO) message “clean care is safe care”. The WHO message emphasizes that proper hand washing needs to be considered as the most important technique in preventing cross-contamination between health professionals and their patients.7
Multi-resistant bacteria and fungi can be part of the components of the transient microbiota of hands. Hand contamination of health professionals can occur by direct contact during handling of patients, and by indirect contact with other objects and equipment (bed, stethoscope, anesthesia machine and other operating room materials). Studies show the association of contaminated hands with infection outbreaks in health facilities.8, 9, 10
The hands of the anesthesiologist can act as a source of contamination in the procedures performed in the operating room, and correct hand hygiene is vital to prevent infectious complications.11 Simple actions are essential anesthesia safety measures, including hand hygiene between cases.12 Thus, it is important to observe the five moments for hand hygiene recommended by WHO: 1) Before touching a patient; 2) Before a clean or aseptic procedure, such as providing anesthesia; 3) After risk of exposure to body fluids, such as following glove removal after performing anesthesia; 4) After touching a patient; 5) After touching patient surroundings, which constitutes a variant of moment 4 (it occurs after hand exposure to any surface in the patient's area and before subsequent exposure of the hand to any surface in the care area, but without touching the patient. This normally encompasses objects contaminated by patient flora that are to be decontaminated or discarded).
Washing hands with soap and water removes bacteria from the surface of the skin, but it is not efficient to kill microorganisms. The use of alcohol (e.g., isopropyl) or alcohol-based solutions will provide improved disinfection when compared to alcohol-free antiseptic solutions (e.g., iodopovidone11 or chlorhexidine 4%13). When these antiseptic solutions are combined with an alcohol compound (e.g., chlorhexidine gluconate in ethyl alcohol), bacterial growth is reduced to very low levels. Generally, the risk of intraoperative bacterial transmission and infections associated with healthcare professionals can be significantly reduced by improving compliance with hand hygiene.14 Proper hygiene can also reduce medical costs related to the treatment of blood-borne infections caused by methicillin-resistant S. aureus.15
Therefore, correct hand hygiene means the combination of the five WHO moments with the correct aseptic technique, and it is one of the most important components in performing anesthetic procedures.14
Adequate asepsis should always be used in the preparation of regional anesthesia, both for single shot and catheter insertion techniques. Still, a study assessing the members of the Pediatric Anesthesiology Society of Australia and New Zealand showed that 3.6% of anesthesiologists did not wash their hands or did not use sterile gloves to perform caudal epidural blockade.16
Watches and rings are risk factors for infectious complications. Studies show greater potential for contamination when adornments are not removed, as they preclude correct hand hygiene.17 Long nails and with cracked polish also hamper proper hygiene. Although there is controversy on the subject, adornment removal is recommended as a prophylactic measure against infections.18 Avoiding wearing artificial nails, and nail glues or gems is also recommended.19
Measures can be implemented to spread the importance of hand washing among health professionals, such as educational material and the availability of sinks and alcohol gel dispensers in easily accessible places. Alcohol-based products used for hand hygiene in healthcare services are available in solution (liquid), gel and foam forms. Thanks to their formulations, gel-based products have a more comfortable feeling, with less alcohol smell and tend to have greater acceptance, although they do not have superior antimicrobial efficacy to other formulations.7
Recommendations
-
1
Hand washing prior to procedures11 is recommended as an important step in the aseptic technique when performing regional anesthesia, both for single shot puncture and techniques with catheter insertion.14
-
2
Adornments, such as watches, rings and artificial nails, should be removed as a prophylactic measure to improve the technique.17, 18, 19
Does the use of surgical garments by anesthesiologists reduce the risk of infectious complications when performing continuous epidural blocks?
Sterile gloves should be considered as a supplement, not a replacement for hand washing.20 Accessories such as rings, watches or bracelets should be removed before hand washing.6, 17 Sterile gloves protect not only patients, but also health professionals themselves from contamination.5
As for glove perforation, it has been well established that this type of incident occurs more often with vinyl gloves than with latex gloves, causing contamination of the hands of health professionals.5 To date, no study has assessed the risk of microbial contamination or perforation of sterile latex or neoprene gloves. Sterile single-use or disposable gloves should never be washed, re-sterilized, or disinfected, and a new pair must be used for each new procedure.21
Surgical gowns are used as a strategy to prevent cross-contamination between patients, preventing infectious material from coming into contact with the healthcare professional's clothes. However, investigations have shown that wearing a gown did not reduce the rates of colonization, infection, or mortality in neonatal intensive care units.22 Another study showed that patients undergoing labor analgesia under continuous epidural anesthesia did not show significant differences in rates of colonization of the catheter tip between groups with or without gown (9.2% vs. 7.6%, respectively), with coagulase-negative Staphylococcus being the most commonly found microorganism.23
So far, the evidence is insufficient to make definitive recommendations regarding the use of a routine gown within the operating room environment during regional blockade, both for simple punctures and for placement of a short-term neuraxial catheter.5, 24
Reports have drawn an analogy between the installation of a central venous line and neuraxial blockade, suggesting the use of surgical garments. However, some aspects are questioned, such as the increase in time to perform the procedure and the increased associated costs.25 It is argued that, if surgical gowning is indicated for insertion of a central venous line, then it should also be indicated for neuraxial blockade.26, 27 In central venous line placement techniques, full barrier precautions (sterile gloves, surgical gown, mask, cap and large sterile drapes) reduce the incidence of infection associated with central venous catheters, when compared to standard precautions (sterile gloves and small drapes). The incidence of infection is 2.3% and 7.2% when complete barrier precautions and standard precautions are used, respectively. The extrapolated infection rates were 227:10,000 and 718:10,000 with the use of complete barrier devices and standard barrier devices, respectively.25
However, the incidence of infection associated with neuraxial anesthesia is in the order of 1/718 of the infection incidence associated with central venous access (considering 1:10,000 with standard precautions).25 So far, there are no consistent data to recommend using surgical gowns for single-shot or for catheter insertion neuraxial procedures.
The surgical mask, initially considered a protective barrier mechanism for health professionals against patient secretions and blood,21 is now considered mandatory due to cases of post-spinal puncture meningitis described as the result of contamination of the epidural space or subarachnoid with pathogens of the anesthesiologist's oropharyngeal mucosa.28, 29, 30 In Schneeberger et al.,29 four cases of iatrogenic meningitis after spinal anesthesia were described over a period of 4 years, all involving the same anesthesiologist, who had a history of recurrent pharyngitis, did not wear a mask, and frequently spoke during the procedure. Its use seems to be critically important for protecting patients against physicians who have sore throats, suffer from pharyngitis or recurrent tonsillitis, or who are chronic carriers of Staphylococcus aureus in the nasal region.28, 29, 30
The routine use of surgical masks while performing regional anesthesia techniques has been recommended since 2006 by the American Society of Regional Anesthesia and Pain Medicine in its advisory on infectious complications associated with regional anesthesia. It recommends wearing masks correctly, covering the entire nose and mouth. This recommendation starts with the exposure of sterile materials during the anesthetic-surgical procedure.26, 27 The replacement of the surgical mask should be considered between cases, if it is soiled or has moistness.26
Recommendations
-
1
Sterile gloves should always be used and are considered as a supplement, not a replacement of hand washing.5, 20, 26
-
2
So far, there are no consistent data to recommend the use of surgical gowns to perform single shot or catheter insertion procedures.24
-
3
The use of the face mask is recommended, being strongly advised for anesthesiologists who present signs and symptoms of upper airway infections,28, 29, 30 and should always cover mouth and nose.26
What is the best antiseptic technique for preparation of the patient’s skin before performing the regional blockade?
Disinfection is the process of destroying the vegetative form of microorganisms, pathogenic or not, present in inanimate objects. Antisepsis is the set of measures used to destroy or inhibit the growth of existing microorganisms in the superficial (transient microbiota) and deep (resident microbiota) layers of skin and mucous membranes. Such measures involve the application of germicidal agents called antiseptics.24 Such products must have immediate antimicrobial action, persistent residual effect and must not be toxic, allergenic or irritating. It is recommended that they should be mild and cost-effective.31, 32 The antiseptic activity of alcohol occurs by denaturing proteins and removing lipids, including the envelopes of some viruses. To achieve maximum germicidal activity, alcohol must be diluted with water, which enables protein denaturation. The recommended concentration to achieve greater microbicide speed is 70%. However, its volatility, flammability, rapid evaporation at room temperature, and low or no residual activity on surfaces limit its use as a standard antiseptic. In addition, the presence of high concentrations of organic matter can decrease the microbicide activity of alcohol. Alcohol-based preparations are not appropriate when the skin is visibly soiled or contaminated with protein material. The emergence of antibiotic- and antiseptic-resistant bacteria has underlined the need for more effective, well-tolerated and easy-to-handle antiseptic formulations. Most studies on alcohol have evaluated its individualized effect at different concentrations, or emphasized its combination with solutions containing limited amounts of hexachlorophene, quaternary ammonium compounds, polyvinylpyrrolidone iodine (PVP-I), triclosan or chlorhexidine gluconate.33
Using products containing triclosan has been questioned, as they do not seem to be as effective, given triclosan is mainly bacteriostatic, inactive against Pseudomonas aeruginosa, and has been associated with water pollution in lakes.34 In regional anesthesia, the most frequently used alcohol-based preparations are those with chlorhexidine or iodophors (PVP-I). When they are compared in terms of prevention of epidural catheter colonization in children, using chlorhexidine showed superior bactericidal effect, faster action and greater residual effect.35
Thus, despite initial controversies on the best antiseptic solution for skin preparation before regional anesthesia, there is consensus that the antiseptic that most satisfies requirements for application in living tissues is alcohol diluted in water in combination with chlorhexidine gluconate solution. Chlorhexidine is a potent germicide and, when added to alcohol, accelerates this effect.35 Chlorhexidine gluconate adheres to the skin corneum stratum, resulting in prolonged action.33
A meta-analysis study presented by Cochrane in 2015 evaluated antiseptic solutions for preventing surgical wound infection, and described evidence that preoperative skin preparation with 0.5% chlorhexidine in denatured alcohol was associated with lower infection rates after clean surgeries when compared with PVP-I. However, the study leaves professionals free to choose other alternatives, based on costs or side effects.36
A systematic review evaluating the primary outcome of the incidence of surgical wound infection and secondary skin bacterial contamination revealed that in all studies that compared chlorhexidine with iodophor, chlorhexidine showed lower incidences of both outcomes (wound infection surgical - Risk Ratio [RR = 0.70]; 95% Confidence Interval [95% CI 0.52‒0.92] and bacterial skin colonization [RR = 0.45; 95% CI 0.36‒0.55]). The conclusion was that there is moderate quality evidence that supports chlorhexidine use for preoperative skin antisepsis and high-quality evidence indicating that chlorhexidine is associated with fewer positive skin cultures.37
Recommendation
For providing safe and effective patient skin antisepsis before performing regional anesthesia, the recommendation is to remove any organic or inorganic material from unclean skin by cleaning it with water, soap and rinsing it;24 chlorhexidine can be used in back and forth movements for 30 seconds, waiting for it to dry completely.35, 36, 37
What concentration of antiseptic solution should be used and how, and what are its associated risks?
Adhesive arachnoiditis is a rare, but well recognized cause of neurological deficit after neuraxial anesthesia. In the cases described in the literature, there is an evident time relationship, but the mechanisms proposed are still speculative and uncertain, including the inflammatory response to the presence of blood in the subarachnoid space, the local anesthetic intrinsic action (previously implicated as the major cause), or the accidental contamination of the injectate by neurotoxic substances.36, 38
In 2008, alcoholic chlorhexidine was implicated as the causative agent of the disease in a court case in England and, since then, it has been considered the most consistent mechanism for the development of adhesive arachnoiditis.39 Injections above 0.1 mL (about 2 drops) are defined as enough to trigger adhesive arachnoiditis.38 Chlorhexidine in higher concentrations (2%) has also been implicated as a cause of adhesive arachnoiditis.40
The solution commonly used in Brazil is chlorhexidine gluconate 0.5% in 70% isopropyl alcohol. Solutions of 2% chlorhexidine gluconate in 70% alcohol are authorized by ANVISA (trade name BD Chloraprep®, registration 10033430733), but the company does not recommend using the antisepsis product in neuraxial anesthesia procedures.
Chlorhexidine in spray is used in regional blockades in some countries. Its proponents argue that the solution is kept in a closed bottle, which is used away from the equipment tray and can be applied by an assistant who will not take direct part in the procedure.39 However, spraying the antiseptic solution from a distance increases the possibility of droplets going astray. When inhaled, released aerosols can irritate the airways, exacerbating asthma or causing occupational asthma. There is also a risk of permanent eye damage and ototoxicity after contact with the tympanic membrane.41
Regarding the number of applications required, despite the lack of evidence to support the practice, it is common to perform two applications of chlorhexidine for greater efficiency of skin antisepsis before regional anesthesia. A study comparing bacterial growth after one or two applications showed the absence of a number of colony-forming units after the first use of the solution, and that, therefore, a new application was devoid of advantage, because it could add risks of neurotoxicity of the solution.42
Antiseptic solution of 0.5% chlorhexidine in 70% alcohol significantly reduces the probability of colonization of the catheter and the puncture site, and maximizes the speed and power of bactericidal activity when compared to other solutions. Therefore, it should be considered the antiseptic of choice before regional anesthesia techniques.24
After antisepsis, some recommend waiting at least 2 minutes before performing regional anesthesia,3 but it seems reasonable to start the procedure only after visible drying of the skin and, therefore the excess that may still be present in the liquid form should not be removed.
Recommendation
A meticulous technique is recommended when 0.5% chlorhexidine in 70% alcohol is used for skin antisepsis. Every effort must be made to avoid dripping or splashing and to avoid contamination of the injectate, including the removal of any sources of chlorhexidine[39,40 by using the appropriate amount of antiseptic, avoiding removing excess liquid and waiting for its evaporation, in order to guarantee actual effectiveness of the solution.3, 24, 35
What is the best technique for antisepsis for the anesthesiologist before regional anesthesia?
Despite the apparent need for surgical antisepsis before anesthetic-surgical procedures, it has never been proven through a randomized controlled clinical trial, most likely because such a study would never be accepted by an ethics committee.43 Thus, antisepsis of hands has been recommended since the 19th century as a measure to reduce surgical-related infection through indirect evidence. When correctly performed, it is associated with a significant reduction in morbidity and impacts positively on patient safety and health resource expenses.6, 44 Historically, surgical preparation of hands consists of washing them with water and antimicrobial soap, often with brushes. Brushing time varies considerably among institutions.43
Currently, almost all studies discourage the use of brushes, which are no longer recommended for preoperative hand preparation, given using a disposable sponge or brush/sponge combination reduces the bacterial count on hands as effectively as rubbing with a brush.45, 56 The World Health Organization (WHO) does not recommend the use of brushes for this purpose due to their abrasive effect.47
Several studies have evaluated techniques for pre-surgical hand antisepsis. When comparing the use of sponges (with soap) with hand rubbing (with alcohol-based formulations), it was found that both methods are suitable for prevention of surgical wound infection. However, although medicinal soaps are still used worldwide by surgical teams, it is important to note that the antibacterial efficacy of products containing high concentrations of alcohol far exceeds that of any medicinal soap.48 A randomized clinical trial comparing hand rubbing with alcohol-based product versus hands brushed with degerming chlorhexidine demonstrated similarity in the incidence of surgical site infection, despite the significantly better in vitro activity of the alcohol-based formulation to rub hands.49 A study comparing sponge with chlorhexidine gluconate at 4% (degerming) with hand rubbing with a solution containing 57% ethanol and 22.5% n-propanol showed greater, statistically significant (p < 0.001), reduction in microbial count with the use of alcohol-based solution, with approximately -0.63 colony forming units. In this study, the alcohol-based solution reduced by 23.4% the microbial count by cm-2 when compared to 4% chlorhexidine.50
The initial reduction in the cutaneous resident flora is so fast and effective that bacterial regrowth to the baseline in gloved hand takes more than 6 hours,51 which makes the requirement of sustained effect products superfluous. Thus, there is a strong reason to prefer alcohol-based products. Fast antimicrobial action, broader spectrum of activity, fewer side effects and no risk of hand contamination by washing water clearly favor the use of alcohol-based products, especially in resource-limited countries where water supply is scarce or of suspicious quality.43
Other benefits of hand rubbing with alcohol-based products include time saving and no risk of recontamination by rinsing hands with water.51 Finally, there is the advantage of an ecological perspective, by reducing water consumption and generating less waste for disposal, without the use of sponges.52
However, some surgeons consider the time required for surgical hand washing to be a ritual for preparing an intervention. The potential policy change in an institution must be carefully prepared and understood by all members of the process.53
The protocol for hand hygiene practice in health services, published by the Ministry of Health/ANVISA/Fiocruz in 2013, establishes practices for hand hygiene of health professionals.54 The Anvisa website also provides a tool for planning and calculating costs of alcohol-based preparations for hand hygiene to help health facility managers to check the feasibility of implementing accessibility of health professionals to alcohol-based preparations for hand hygiene.55
But which product would be the most suitable? Ideally, the surgical antiseptic should enable complete elimination from hands of transient microbiota, significant reduction in resident flora at the beginning of the procedure and inhibit its growth in gloved hands until the end of the surgery.56 Alcohol Preparations (AP) have been used in Europe for surgical hand antisepsis for roughly 30 years.57 To be marketed, they must be approved by the EN 1500 (antiseptic hand rubbing) and EN 12791 (surgical hand antisepsis) standards of the Comité Européen de Normalisation (CEN). When this solution (45% alcohol and 18% n-propanol) was applied for 90 seconds, compared to the mixture 61% alcohol and 1% chlorhexidine applied for 180 seconds, it was found to achieve significantly greater microbial reduction in both measured time points (p ≤ 0.025 immediately after application and p ≤ 0.01 maintenance after 6 hours under surgical gloves, according to the protocol EN 12791).58 Among the different types of alcohol (ethanol, isopropanol and n-propanol) commonly used for hand and skin antisepsis, n-propanol has the most potent general microbicidal activity at relatively low concentrations.59 In Brazil, a solution tested by EN 1500, EN 12054/13727, EN 1275/13624 and EN 12791 which contain <55% ethanol and <25% propanol-1-ol is available. The solution has been approved by Anvisa (MS nº 2.0151.0002) since 2010, for the purpose of hand antisepsis and surgical hand antisepsis.
For surgical hand antisepsis with alcohol-based products, Anvisa recommends a duration of 60 seconds, subsequent to hand washing with liquid soap and water upon arrival at the operating room. The sequence must be repeated two to three times, as recommended by the manufacturer. It is essential to wait for hands to dry completely. Between surgical procedures, if there is any residue of powder/talc or body fluids when removing gloves, hands should be washed with liquid soap and water.59, 60
In a cost-effectiveness study, Graf ME et al found advantages in the use of alcohol-based solutions for hand antisepsis in pre-surgical preparations, among them, significant reduction in microbial count, improvement in professionals' adherence due to shorter preparation time with the use of alcohol-based solutions (1 minute vs. 3 minutes for cleaning with chlorhexidine), and less irritating effect on the skin, in addition to greater water savings and reduced waste material. The economic model demonstrated a 46% cost reduction when compared to chlorhexidine degermation.61 Findings were consistent with those found in a previous review.62
A systematic review on the outcome of microbial hand count, or rates of surgical site infections, showed that alcohol-based preparations presented an equal and/or greater microbial reduction compared to traditional products in 17 studies, and lower in 4, while surgical site infection rates were similar. The authors concluded that there is evidence to support the safety of alcohol-based preparations for surgical hand antisepsis.63 However, a meta-analysis study presented by Cochrane in 2016 found no evidence of superiority associated with one specific type of hand antisepsis regarding reduction in surgical site infection. Hand rubbing with chlorhexidine gluconate can reduce the number of Colony-Forming Units (CFU) compared to rubbing with povidone-iodine; however, the clinical relevance of this outcome is unclear. Rubbing with alcohol with additional antiseptic ingredients can reduce the number of colonies compared to rubbing with water. There is no evidence that nail brushes have an impact on the number of CFUs remaining on hands.64
Thus, it is concluded that there have been major changes in the past decades regarding surgical hand antisepsis, favoring the use of alcohol-based solutions without using water and brushes, and generating cost-effectiveness and ecological sustainability when compared to traditional procedures such as surgical hand degerming using chlorhexidine gluconate or polyvinylpyrrolidone iodine. To incorporate best practices based on scientific evidence, a programmatic approach must be adopted. Policies that govern the processes and products used must be implemented, in addition to monitoring compliance.65
Studies on the preparation of the hands of the anesthesiologist before performing regional anesthesia are scarce, but they confirm the findings regarding the preoperative preparation of surgeons.66 Thus, it can be inferred that the same procedures should be followed.24
Recommendations
Brushes for brushing are not recommended for the anesthesiologist’s hand hygiene before performing regional anesthesia.45, 46, 47
Although hand washing with sponges with chlorhexidine soap and water is still used, current evidence favors hand rubbing with products containing alcohol and n-propanol, especially in places where the quality of rinsing water cannot be guaranteed. The procedure should take 60 seconds and follow the steps recommended by ANVISA.48, 49, 50, 60, 61, 62, 63, 64
Factors associated with infectious complications in regional anesthesia
What are the risk factors related to infection in regional anesthesia with or without catheter insertion?
Severe CNS infections, such as arachnoiditis, meningitis, and abscesses are rare complications of neuraxial anesthesia. However, literature data show an increasingly frequent occurrence of these events, perhaps because there are more publications on the topic or more frequent use of long-term catheters.67, 68, 69
Epidural abscess occurs more frequently in immunocompromised patients, with prolonged epidural catheterization. Staphylococcal species are the organisms most commonly found in epidural infection, related or not to the use of catheters. Only 15% are caused by other bacteria.70
Reports show that patients who developed meningitis after neuraxial anesthesia were healthy and underwent spinal anesthesia.71, 72, 73 Case reports and literature reviews alert to the occurrence of cases of meningitis caused by Streptococcus salivarius, a bacterial species prevalent in normal oral flora in humans, whose source of infection was upper airway droplets of health professionals (confirmed by genotyping techniques).74 Anesthesia professionals who perform neuraxial procedures without wearing a surgical mask, expose patients to devastating risks and infectious complications, including death.75
Epidural catheter-related infections can occur by direct dissemination of cutaneous flora that migrate along the catheter, contamination of the infused solution or blood-borne dissemination from a distant source.76 Direct propagation with bacterial growth along the catheter is the most common cause, which may result in superficial or deep infection. Tunneled catheters reduce the likelihood of infection of the epidural space by increasing the distance to the site.76 Contamination of the solution can occur by breaking a closed system, by accidental disconnection of the catheter, or during bag solution change. Bacterial filters can potentially reduce the risk of epidural contamination.76 The main agent described in this case is Serratia marcecens, an opportunistic gram-negative bacterium that colonizes the human gastrointestinal tract.77, 78 Disconnection of the epidural catheter and infusion system exposes the catheter internal lumen to the environment, which can lead to subsequent epidural infusion of contaminated solution. A study with catheter contaminated by Staphylococcus epidermidis concluded that cutting the proximal end of the catheter exposed to the environment, with disinfection of the contaminated site and subsequent infusion of the local anesthetic, can prevent contamination of the infusate.69
Studies have shown that 0.5% bupivacaine and 2% lidocaine inhibit the growth of microorganisms (e.g. S. aureus and coagulase negative Staphylococcus) in culture media. However, the bactericidal effect decreases significantly at low concentrations of local anesthetics, which are typically used to promote analgesia. Further studies are needed to investigate the bactericidal effect in vivo of local anesthetic solutions at low concentrations.79 Opioid solutions do not exhibit any ability to inhibit bacterial growth.80 The number of disconnections, duration, portion of catheter to be removed and the decision on whether to proceed with the infusion are still controversial parameters, and studies must be carried out to elucidate such questions.70, 81 Thus, disconnections and the length of catheter stay should be limited.26, 27, 82, 83, 84
Delays in diagnosing patients with epidural/spinal abscess are common, often causing irreversible neurological deficits. Risk factor assessment is more sensitive than using the classic diagnostic triad (fever, spinal pain and neurological deficits) to screen patients.85 ESR can be a useful screening test before MRI in selected populations, such as immunocompromised patients.86 Hematogenous spread of infection from another site to the epidural space is very rare. Intravenous drug users, patients with dental abscesses and patients with long-term central venous catheters constitute a higher risk population.76 Additional risk factors are the presence of infection in adjacent anatomical structures and the colonization of central venous devices.75
Dressings for the epidural catheter must be transparent and cover the catheter entry site in order to maintain sterility during the infusion. Dressings impregnated with chlorhexidine were assessed by culture at the catheter entry site, observing 3.4% of positive bacterial culture compared to 40.1% for dressings without chlorhexidine. In addition, patients should be instructed to keep the area clean and dry during therapy to avoid compromising the dressing.76
The incidence of epidural catheter-associated infections can be minimized by the use of catheters implanted with subcutaneous injection ports or externalized silicone catheters with subcutaneous Dacron cuffs (e.g., Du Pen®; Bard Access Systems®).76
Currently, two main types of bacterial filters being used are the particulates (5 µg pores) and the antimicrobials (0.2 µg pores). They are used to decrease the risk of bacterial contamination while changing solution medication bags. There is a recommendation by the American Society of Anesthesiologists Task Force on Infectious Complications Associated with Neuraxial Techniques to routinely use the antimicrobial filter in situations of long-term continuous infusion.76
The epidural catheter insertion site influences the occurrence of colonization and potential infection of the puncture site.87, 88, 89 Continuous caudal epidural catheters are more frequently colonized than continuous lumbar epidural catheters.88, 89 Short-term catheters (up to 120 hours) show direct correlation between bacterial skin colonization around the insertion site and bacterial growth from the subcutaneous segment to the catheter tip.90
Some patients are at higher risk for infection. Diabetes is identified as a risk factor in several studies, with a higher incidence of spontaneous epidural abscesses. One study demonstrated that obesity is also an important risk factor for infections; however, it was not related to a higher risk for neuraxial catheterizations. Immunocompromised patients (on treatment with chronic steroids or immunosuppressants, and those with autoimmune disorders, cancer, human immunodeficiency virus/AIDS, chronic kidney disease and liver cirrhosis) are also at high risk.76 Data suggest that performing epidural or spinal anesthesia during an episode of bacteremia is a risk factor for neuraxial infection.67, 69, 91, 92 However, studies showed that is safe to insert and keep a catheter in patients with infection at a distant site.87, 93 In an observational, prospective and multicenter study, performing epidural analgesia for ICU patients was observed to be safe and with benefits in improving pain control, and reducing pain impact on cardiovascular and immune functions. However, several precautions for early diagnosis and infection control must be carefully observed when performing epidural analgesia in ICU patients.94
Adverse events that occur in the ward, such as catheter occlusion, damage or replacement of transparent dressings, partial displacement of catheters, disconnections, blood transfusion and positive skin culture close to the insertion site are important risk factors for bacterial colonization of the epidural catheter. It is argued that maintaining sterile skin around the insertion site can reduce tip colonization.90, 95
As for peripheral regional anesthesia techniques, the frequency, diagnosis and prognosis of infectious complications remain uncertain. Several series involving continuous peripheral blockade technique have reported erythema at the insertion site and high incidence of colonization (20%‒60%),96, 97 Specifically observing the risk of infection with continuous peripheral nerve blockade, bacterial colonization is present in 29% of catheters, the most common agent being Staphylococcus epidermidis. The incidence of local inflammation is present in 3% of patients. There is no correlation between inflammation and the presence of fever. Risk factors for local infection/inflammation are ICU admission, male, catheter kept for more than 48 hours and absence of antibiotic prophylaxis.96 The incidence of infectious complications in continuous femoral catheters occurs in most catheters examined 48 hours later, with S. epiderminidis being the main agent (71%).97
Bomberg H et al. demonstrated that antibiotic prophylaxis in a single dose is related to reducing the risk of catheter infection for peripheral and epidural catheters, with no difference whether the antibiotic was administered before or shortly after catheter insertion.98
Recommendations
-
1
For epidural techniques, assess the patient for conditions that add risk to developing infections, such as diabetes, obesity, dental abscess, IV drug abuse, bacteremia and presence of a long-term central venous catheter. The period of time the catheter remains in-place in these patients should be as short as possible, and the anesthesiologist must comply with the correct technique of catheter insertion and maintenance. Using a bacterial filter also adds to security.99
-
2
Handle catheters carefully to avoid contamination, reduce breakage of closed systems, and use and exchange bacterial filters.100
-
3
Professionals assisting the anesthesiologist in regional anesthesia procedures should routinely wear surgical masks.75
-
4
Prophylactic single-dose antibiotics should be administered before or shortly after the insertion of epidural catheters.98
What is the risk of infectious complications in regional anesthesia in the febrile or infected patient?
There are specific recommendations regarding regional anesthesia in febrile or infected patients.101, 102 Severe neuraxial infections, such as arachnoiditis, meningitis and abscess related to epidural or spinal anesthesia are rare. The decision to perform a regional anesthetic technique must be individualized, considering anesthetic alternatives, benefits of regional anesthesia and the risk of CNS infection, which in theory can occur in patients with bacteremia. In patients with orthopedic prosthesis infection, neuraxial anesthesia has been considered safe.100, 103
The placement of an epidural or subarachnoid catheter in this group of patients remains controversial. The epidural catheter must be removed in the presence of erythema and/or local discharge, and there is no convincing data suggesting that concomitant infections in remote sites or the absence of antibiotic therapy are risk factors for infection. Even a few hours of delay in the diagnosis and treatment of major CNS infections can significantly worsen neurological outcomes.102
Recommendation
Patients with evidence of systemic infection can be submitted to spinal anesthesia as long as, prior to the puncture, antibiotic therapy has been initiated and some treatment response demonstrated, such as reduction of fever.99, 100, 103
What is the risk of infectious complications in regional anesthesia in immunocompromised patients?
Patients with impaired immune function (e.g., cancer, diabetes, infected with Acquired Immunodeficiency Virus (HIV) or Herpes Simplex Virus (HSV), drug and alcohol abuse, on glucocorticoid therapy, on immunosuppressive treatment and/or chemotherapy for inflammatory bowel diseases, autoimmune diseases and transplant patients) have been increasingly submitted to regional anesthesia.86, 101 These patients are more susceptible to infection by opportunistic germs. Antimicrobial therapy is less effective in these cases, resulting in greater morbidity and mortality when compared to patients with preserved immune function. Immune system suppression increases both frequency and severity of infection, in addition to diminishing its characteristic signs and symptoms. In these individuals, the extent and duration of granulocytopenia (< 500 granulocytes.mL-1) is a well-known infection risk factor. When granulocytopenia persists for 6 to 10 days, the risk is higher (30% risk of infection with leukopenia < 1000 m L-1 or 50% with granulocytes < 100 m L-1). If granulocytopenia lasts more than 10 days, patient is classified as high-risk (70% risk of bacterial infections).86
The number of pathogenic microorganisms (atypical and/or opportunistic pathogens) is higher in the immunocompromised host than in the general population. The delay in diagnosis and treatment of CNS infections worsens neurological outcome and increases mortality. For these patients the risk of epidural abscess increases proportionally to the period the epidural catheter remains in situ. Neuraxial anesthesia has been shown to be safe in patients with recurrent HSV virus infection, although there are reports of exacerbation of HSV-1 infection associated with the use of epidural or intrathecal opioids.101
Some scenarios in immunocompromised patients deserve special attention:
-
a)Chronic glucocorticoid therapy: The degree of hypercortisolism correlates with the risk of opportunistic bacterial diseases and infections. Even after a single dose of glucocorticoids, lymphopenia, monocytopenia and eosinopenia last for 4‒6 hours. Cell count is reduced and cell function is impaired. The risk of infection in chronic glucocorticoid therapy depends on the type, dose, administration route and duration of treatment. In patients with rheumatoid arthritis, the risk of severe bacterial infection has been shown to double when glucocorticoids are used when compared to methotrexate, with a dose-effect relationship when using doses of prednisone above 5 mg per day. Prednisone has also been shown to be a risk factor for serious infections in patients with Crohn's disease.86
-
b)Chemotherapeutic agents: The main side effect is on hematopoiesis, resulting in anemia, leukopenia/neutropenia and thrombocytopenia. Neutropenia is the most frequent effect and its degree and duration are directly correlated with the incidence of infections.86 In a study carried out with children and young patients from an oncology clinic and undergoing epidural analgesia for a lengthier time period than usually recommended showed low incidence of infection. The results suggest that the pain control method is safe, with limited risk for infection.104
-
c)Immunosuppression after organ transplantation: Intra and postoperative neuraxial techniques are increasingly used in organ transplant centers to improve perfusion during solid organ transplantation. However, only a few studies have been published addressing the potential risk of complications in individuals undergoing transplants combined with epidural anesthesia. Among them, Trzebicki et al. reported no complications associated with thoracic epidural anesthesia in patients undergoing liver transplantation. There are, however, two reports describing spontaneous epidural abscess during immunosuppressive treatment without any anesthesia procedure.86
-
d)HIV infection: It has been suggested that approximately 20%‒25% of HIV-positive patients may require surgery during the period of their illness. Pregnant patients who are HIV positive with high viral loads are referred for elective cesarean surgery in order to reduce transmission rate to the child. In the study by Gronwald et al, regional anesthesia (epidural and spinal) was performed without complications. However, the authors themselves emphasize safety criteria such as CDC stages A2 or B2 (CDC stages: A, asymptomatic HIV infection; B, symptoms and diseases associated with HIV, but no AIDS-defining disease; C, AIDS-defining diseases; 11/4 CD4 cell count > 350 cells.mL-1, 21/4 CD4 cell count < 50 cells.mL-1, 31/4 CD4 cell count < 200 cells.mL-1.86 Some data suggest that, in HIV-infected patients, peripheral and neuraxial blockades are feasible, including blood patch. Pre-existing neurological diseases are common in these patients and should be considered when performing neuraxial techniques.95
-
a)
Recommendations
-
1
Limit epidural catheterization time to up to 72 hours in immunosuppressed patients. In patients who will receive immunosuppressive therapy, it may be useful to measure absolute and differential leukocyte counts preoperatively and perform follow-up measurements.86
-
2
Change dressing at the insertion site with aseptic technique. Daily inspections and early treatment in case of suspected complications should be routine.86
-
3
In case of suspected neuraxial infection, it is recommended to request consultation from the infectious disease service to help early and effective initiation of antibiotic therapy.101
Diagnosis and treatment of regional anesthesia-related infections
How are meningitis and epidural abscess diagnosed and treated?
A high level of suspicion is vital for diagnosing an infectious complication. The incidence of catheter-related epidural abscess is low, and cases of meningitis and osteomyelitis are rarer.76, 78 Delaying diagnosis and treatment of major CNS infections, even for a few hours, significantly worsens neurological outcome. Bacterial meningitis is a medical emergency. The mortality rate ranges from 10% to 30%. Sequelae, such as nerve damage and hearing loss, occur in 5% to 40% of patients.104, 105, 106
Meningitis presents more often with fever, headache, altered level of consciousness and meningism. The diagnosis is confirmed by lumbar puncture. Usually clinical manifestations start 48 hours after performing spinal anesthesia puncture. Antibiotic therapy can delay the onset of symptoms and laboratory evaluation is a useful complement to confirm the diagnosis. Tests may include a combination of laboratory tests, wound and cerebrospinal fluid cultures, white blood cell count, Erythrocyte Sedimentation Rate (ESR) and C Reactive Protein (CRP) analysis.107, 108 CRP may be a more sensitive indicator of infection than other biochemical markers as it has more predictable kinetics and is more responsive in the postoperative period, being regarded as the best diagnostic test. Repeated CRP tests and assessment of CRP trends are valuable measures for establishing diagnosis of infection.76, 85, 109
Cerebrospinal fluid analysis shows leukocytosis with increase in polymorphonuclear cells, low glucose (< 30 mg.dL-1), high proteins (> 150 mg.dL-1). Gram-stain reveals bacterial presence and culture is positive for bacteria.73 Level of lactate in CSF (greater than 35 mg.dL-1) is recommended to differentiate bacterial from aseptic meningitis, since the use of previous antibiotics can reduce clinical accuracy.110, 111, 112
Lumbar puncture should not be performed if an epidural abscess is suspected, as it can also cause contamination of the subarachnoid space. Abscess formation after epidural or spinal anesthesia can be superficial, requiring limited surgical drainage and intravenous antibiotic administration, rarely leading to neurological problems, unless they are not treated. The epidural abscess usually occurs from days to weeks after neuraxial blockade and when the patient has already been discharged.113, 114, 115, 116 The time of onset of symptoms may suggest the etiologic agent.74 It presents as a progressive disease, starting with localized back pain (most common initial symptom), local hypersensitivity, and fever and chills in the first stage; nerve root irritation and headache occur in the second stage; neurological deficits, such as muscle weakness, sensory deficits, bladder and bowel dysfunction occur in the third stage, followed by paralysis in the fourth stage.76, 87, 108 Initial back pain and nerve root symptoms can remain stable for hours to weeks. However, after the onset of muscle weakness, the condition progresses quickly to complete paralysis within 24 hours.117, 118 The radiological image of an epidural mass associated with variable neurological deficit are diagnostic. Magnetic resonance imaging with gadolinium administration is recommended because it is the most sensitive test for assessing spinal cord, when an infectious process is suspected,119 as it defines the extension of the lesion and helps differential diagnosis.108
The combination of antibiotic therapy and surgical approach (drainage and/or debridement) is the treatment of choice.107, 119, 120 Neurological recovery depends on several factors, and almost half of survivors are left with neurological deficits, 15% of them with complete paresis or paralysis, with reports of mortality rates ranging from 5% to 16%.107, 119, 120, 121
Recommendations
-
1
Meningitis should be suspected if a patient presents fever, headache, altered level of consciousness and meningism within 48 hours after spinal puncture. Sequelae will depend on early diagnosis and timely treatment.104, 105, 106
-
2
Epidural abscess should be suspected in patients presenting localized back pain, local hypersensitivity and fever, chills, root irritation, headache and neurological deficit in days or weeks after neuraxial block, usually after the patient has been discharged.76, 84, 108 Magnetic resonance imaging is the diagnostic test of choice.119
Antibiotic prophylaxis and regional anesthesia
Should the patient undergoing continuous regional analgesia receive antibiotic prophylaxis?
Systemic infection, or local abscess due to a regional analgesia catheter is rare, although colonization of the catheter is more frequent.122 Depending on the catheter insertion site, the incidence of infection ranges from 0% to 7% for peripheral catheters, and from 0.8% to 4.2% for epidural catheters.98 Tunneling of a short-term catheter (mean of 48 hours) seems to decrease bacterial colonization of the catheter tip. The incidence of colonization is 6.2%, and is higher in trauma patients. Keeping epidural catheters in situ for an average of 56 hours without tunneling have shown 28% of positive cultures, with no correlation with the type of preoperative antibiotic administered.123 According to a study involving 40,362 cases of peripheral and epidural catheters for continuous use, patients who received single-dose antibiotic prophylaxis had significant lower levels of peripheral catheter-related infections (1.1%) compared to those without prophylaxis (2.4%, p < 0.001, nnT = 76). In patients with an epidural catheter, single-dose antibiotic prophylaxis reduced the incidence of infections (3.1%) in comparison to those without prophylaxis (5.2%, p < 0.001, nnT = 49). Administration of systemic antibiotics within 24 hours postoperatively significantly decreases the risk of catheter colonization.98, 123
Recommendations
-
1
Single-dose antibiotic prophylaxis is associated with a reduced risk of catheter-related infections for peripheral and epidural catheters, and should therefore be performed.98
-
2
Tunneling of long-term catheters is recommended in order to reduce colonization of catheter tips.123
-
3
Handling short-term catheters should take place under aseptic technique with minimal handling of the catheter after insertion, use of transparent dressings, and surveillance of the puncture site two to three times a day, and the day following removal.
-
4
In case of inflammatory signs at the site, catheter removal, catheter culture request and antibiotic treatment (ceftriaxone ‒ 2 g each 12 hours associated with vancomycin ‒ 1 g each 12 hours) are mandatory. Magnetic resonance imaging should be ordered to guide future decisions.124
Reuse of materials in regional anesthesia
Are there materials that can be reprocessed for regional anesthesia practice (glass syringes, needles)?
Recycling or reuse of hospital supplies is one of the most controversial issues discussed by health care systems worldwide. Many industries are against reprocessing, claiming possible dangers of reuse. Many health services are favorable to reprocessing, considering the economic and ecological impacts. Several types of materials intended for health services are produced and labeled by manufacturers as for single use, ensuring safety both for product operation and sterilization, and avoiding any possibility of cross infection. The items used for providing regional anesthesia are considered critical,125 and the current literature does not provide sufficient evidence for practicing reprocessing,126 given the risk of infection and other complications do not justify the adoption of this measure.125, 127 Reprocessing can mechanically, thermally and chemically affect products, compromising their effective performance. Besides, the reprocessed item must have the safety equivalent to the one provided by the manufacturer, so that the patient is not exposed to any kind of risk.128, 129
Thus, using reprocessed products offers a potential risk related to improper cleaning, disinfection and/or sterilization, which can result in chemical or microbiological contamination.130, 131 There is evidence that using reprocessed products is related to transmission of diseases caused by viruses or by unconventional agents (Creutzfeldt-Jakob disease).131 After studying different reprocessed items, 11% of them had some type of malfunction, which compromised their safety use.132 Sterilization of reusable materials is usually performed using ethylene oxide, mixed with steam and formaldehyde, oxidizing gas (hydrogen peroxide), ozone or peracetic acid. Waste gases from the sterilization process can remain, compromising item safety and efficiency, especially if the item is reprocessed several times, and thus loses its biocompatibility.133 The presence of chemical residues that may remain after cleaning or by absorption of the re-sterilized material is a latent and important danger to be considered.134 The analysis from a questionnaire that assessed doctor and patient perceptions regarding reprocessing of materials reported that more than 90% of respondents considered that hospitals have the responsibility to inform patients about the practice as part of their care.135
Regarding the matter, the ANVISA RDC 156 defines clear criteria for reprocessing materials, with clear norms for reusing items that can be reused, including prohibiting their commercialization.133
Recommendation
Materials for use in regional anesthesia, such as needles, glass syringes or catheters should not be reprocessed.133
Safety in the administration of drugs
How to improve safety in the administration of drugs in regional anesthesia?
A wide range of drugs is used during regional anesthesia techniques,136 and currently, medication administration errors are considered a worldwide epidemic, resulting in thousands of deaths annually. The incidence of the event has increased over the years, generating human fatalities and significant financial losses.137
The medication error is characterized, according to ANVISA’s definition, as any preventable event that can, actually or potentially, lead to incorrect administration of medication. The event may be related to professional practice and to healthcare products, procedures, communication issues (prescription, labeling, packaging, naming), preparation, dispensing, distribution, administration, education, monitoring and use of medications.138
Following, we describe some measures for reducing errors in medication administration. The measures have strong evidence for recommendation, such as carefully reading the label of every drug, ampoule or syringe before use, double checked by a second person;139, 140, 141, 142 using ampoules and syringes that have a clear identification label and follow standards defined by the pertaining agency;142, 143, 144, 145, 146, 147, 148, 149 routine identification of syringes;141 systematic organization of drugs routinely used during anesthesia;139, 142, 143 review of drug administration error incidents during anesthesia, registered at the organization;145 drug handling technique focused on minimizing the possibility of administration error;142, 143, 144, 145 avoid handling drugs that have similar presentations;139 and using drug color identification based on drug class, complying with national recommendations or international standards.139, 142
There are essential and necessary elements to improve safety and avoid drug administration errors,146 such as the development of a safety culture among team members; logistical support to the team, encouraging the report of adverse events; integration between areas (anesthesiology, pharmacy, organization risk management); encouraging comprehensive report of the facts by professionals involved; and sharing safety lessons among team members.5
The Brazilian norms on this matter are described in ANVISA Resolutions, which establish criteria for labels and tags for Small Volume Parenteral Solutions (SPPV). The norms were established by collegiate resolutions RDC nº 9, of January 2, 2001. In 2009, a new resolution, RDC nº 71, on Drug Labeling was published. Among the innovations incorporated by this RDC, one in particular was very well received by companies that own the brands of reference products. According to article 17, item V, of this resolution, “using labels with a layout (packaging) similar to that of a pharmaceutical with the same active ingredient, pharmaceutical form and concentration, previously registered by another company, is prohibited”. An improvement in the identification of pharmaceuticals and, consequently increase in safety is expected.138, 139, 140, 141, 142, 143, 144, 145, 146, 147
Recommendation
Adopt a safety routine (preferably with the development of a culture of institutional safety) to avoid accidents during a regional anesthesia procedure, such as detailed reading of any medication label before administration; regular review of label legibility on packaging or ampoules of drugs; identification of syringes filled with drugs; formal organization of drugs routinely used; medication double checking by a second person; and, if possible, use of drugs dispensed in pre-filled and pre-labeled syringes139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149
Does using solutions in vials or ampoules in sterile packaging for regional anesthesia seem to be safer?
To test the likelihood of contamination of the local anesthetic dispensed in ampoules, a swab with S. epidermidis was rubbed on the neck of 16 1% lidocaine ampoules; the necks of half of these ampoules (eight) were subsequently cleaned with prepackaged alcohol pads, and all ampoules were opened in the supine position with sterile gauze. The results described that none of the alcohol-cleaned ampoules showed bacterial growth, while three of eight lidocaine ampoules not cleaned with alcohol showed strong bacterial growth.149 A survey performed in the UK, reported that only 21% of anesthesiologists claimed that they cleaned the neck of non-sterile ampoules with alcohol.34 It is argued that the risk of contamination of solutions stored in ampoules during handling and opening is small, but should not be overlooked. Possible answers for the contamination of solutions while opening ampoules should include changing medication packaging into different formats, such as single-use ampoules sealed with a rubber septum; cleaning the neck of ampoules with alcohol before opening; or sterilizing the outside of glass ampoules and subsequent sterile packaging, as is already the case of some ampoules for spinal and epidural anesthesia.149
Regarding bacterial contamination, no differences were observed between withdrawing fentanyl solution after cleaning the ampoule neck with isopropyl alcohol, or when the solution was withdrawn aspirated with a needle with an antibacterial filter.150
When sterility and microbial (bacteria and fungi) load on the outside of ampoules and vials of hyperbaric bupivacaine from conventional or sterile packaging were compared, the use of sterile packaging reduced the microbial load, and decreased the possibility of potential anesthetic solution contamination.151
Recommendations
-
1
Cleaning glass ampoule necks with alcohol before opening or withdrawing drugs using antibacterial filter needles should be part of the anesthesiologist`s routine.149, 150
-
2
Sterile packaging is recommended as a way of increasing safety and reducing bacterial contamination of the solutions used in regional anesthesia.149, 151
Is there cost-effectiveness in handling and preparation of sterile solutions for patient-controlled analgesia in the out-of-hospital environment?
Although not widely reported, contamination of solutions can cause serious infectious complications in regional anesthesia. The adoption of handling practices that minimize contamination should be a priority for anesthesiologists, especially when such solutions are infused in unmonitored patients in settings outside the hospital environment. Because continuous infusion during several days is considered of medium risk, solutions must be purchased as pre-made sterile products, or handled according to USP‒797 guidelines.152 It is recommended that all sterile solutions be prepared in a laminar flow environment, with particle counts between 0 and 46,262 per cubic meter (class ISSO 0‒6). Therefore, they must be prepared outside operating rooms and by qualified pharmacy personnel.153
A study assessing cost-effectiveness of solutions (local anesthetic or opioids) for epidural administration via catheter, all prepared in the pharmacy department using aseptic technique and horizontal laminar airflow, found bacterial growth in five of the 115 samples prepared, and it was associated with handling contamination during sample collection, since most of the identified microorganisms supported this assumption. Based on these findings, it is recommended that for solution mixtures prepared with opioid, local anesthetic/opioid or only local anesthetic, both the infusion system replacement and solution handling should not be more frequent than every 72 hours. This is a clear pharmacoeconomic-oriented recommendation, especially for services that routinely adopt continuous epidural analgesia for postoperative acute pain treatment.154
However, as replacement of the infusion solution bag requires disconnection of the catheter, it is preferable to use larger volume bags, to minimize contamination risk.76
Evaluating the sterile viability of local anesthetic and opioid solutions to be used in continuous infusion for chronic pain home treatment, solutions were stored in a regular home refrigerator and no bacterial growth in the solutions was observed after seven months from preparation. It is recommended, in selected patients undertaking out-of-hospital basis treatment, and being followed up at home and with a long-term epidural catheter in situ, to use solutions prepared with a sterile technique, stored in a regular home refrigerator, for a period not exceeding 14 days.155
Regarding the compatibility between different solutions, ropivacaine associated with morphine, sufentanil, fentanyl or clonidine was studied in plastic bag commercially available solutions of 0.2% ropivacaine with 214 mL. The bags were diluted once again with 0.9% saline using controlled aseptic technique to result in a 1 mg.mL-1 solution. The new dilution was later associated with different concentrations of opioids and clonidine.125 Solutions were stored for 30 days, at 30 °C and relative humidity of 40%. The mixtures of 1‒2 mg.mL-1 of ropivacaine, with 20‒100 μg.mL-1 of morphine sulfate, 0.4‒4 μg.mL-1 of sufentanil, 1‒10 μg.mL-1 of fentanyl or 5‒50 μg.mL-1 of clonidine were observed to be chemically and physically compatible and stable for 30 days after preparation when stored in plastic bags kept at 30 °C.156 A levobupivacaine solution mixed with sufentanil and sodium chloride to produce a concentration of 1 μg.mL-1 of sufentanil and 1 mg.mL-1 of levobupivacaine, was stored in polypropylene syringes, protected from light for 30 days. The results of the microbiology and chemical stability analysis showed that the solution can be stored at 4 °C or 21 °C. It should not be stored at 36 °C due to the potential bacterial growth.157 The stability in PVC infusion bags of the mixture of sufentanil citrate (500 μg) with levobupivacaine hydrochloride (625 mg) in 500 mL 0.9% sodium chloride solution, enables the solution to be prepared in advance by a specialized service, in sterile conditions, and stored for 58 days at a temperature of 4 °C, without changes in the concentration of the product.158 The stability of pre-manufactured solutions of 0.1% bupivacaine in PVC infusion bags containing 2 μg.mL-1 of fentanyl citrate and epinephrine (1 mg) allows us to conclude that this epidural infusion solution is stable when stored at temperatures of 4 °C and 22 °C for 184 days, with refrigeration as the preferred storage condition.159
Several recommendations can also be made regarding the duration of regional anesthetic infusions. Evidence suggests that when the local anesthetic or the mixture of local anesthetic with opioids is prepared under sterile conditions, microbiological stability is maintained for 72 hours.153
Recommendations
-
1
It is recommended to prepare the solution in a sterile environment.152 The solution can be stored at low temperatures (4 °C or 21 °C) for several days, as it retains its physical-chemical characteristics without bacterial contamination.157, 158, 159
-
2
It is recommended to replace the analgesic solution after 72 hour infusion.154
Care while using ultrasound-guided regional anesthesia technique
Precautions regarding ultrasound-guided regional anesthesia
Ultrasound has revolutionized the way we approach regional anesthesia. Nonetheless, consistent evidence supporting the effectiveness of ultrasound-guided regional anesthesia in reducing the incidence in local anesthetic systemic toxicity, vascular injury, hemi diaphragmatic paralysis and pneumothorax, and its impact on patient safety remains under study.160, 161 There is no solid evidence that ultrasound guidance significantly affects the incidence of peripheral nerve injury associated with regional anesthesia.162 It is important to note that safety does not depend on a single technology. During the performance of regional anesthesia, safety is related to the level of training of the professional, and to the technique chosen, patient anatomy, and equipment.160, 162
Regional anesthesia is also becoming increasingly frequent in pediatric anesthesiology. In this scenario, different studies have shown that regional anesthesia, when properly performed, has low morbidity in infants and children, presenting itself as a promising tool to increase regional anesthesia safety.163
The occurrence of infection related to ultrasound-guided blocks has already been reported, with serious associated complications.164
Given regional anesthesia requires a sterile technique and that the USG device and probe (or transducer) are Reusable Medical Devices (RMD), every precaution must be taken to avoid patient cross-infection transmission via blood or other body fluids. Although studies often mention that ultrasound-guided blockades should be carried out under aseptic conditions, the way they should be performed (with both protective cover and conductive gel sterile) has been neglected. Due to the lack of specific studies on use during regional anesthesia, general recommendations for intraoperative ultrasound use can be adopted. Thus, the general recommendations used for disinfecting RMD are usually adopted to USG device care. 165
According to the use and risk of infection transmission, and based on the Spaulding classification, RMD are divided into:166
Critical: Devices that penetrate tissue, sterile cavity or vascular system (high risk of infection). Invasive surgical devices require sterility or high-level disinfection. Example: Ultrasound probe used by the surgeon during the surgery.
Semi-critical: Devices in contact with intact mucosa or non-intact skin (medium risk of infection). Disinfection must be of intermediate level and is achieved by immersion in disinfectant solution after cleaning. Example: endovaginal and transesophageal probes.
Non-critical: Devices that do not have direct contact with the patient or come into contact with intact skin (low risk of infection). They require low level disinfection. Transcutaneous ultrasonic probes fall into this category.
The USG probe is usually placed on healthy, prepped, disinfected skin and does not penetrate the tissue. Thus, it could be considered a non-critical device according to the Spaulding classification,166 and, therefore, subject to low-level disinfection.
The guideline of the Center for Disease Control and Prevention (CDC) for disinfection and sterilization of healthcare facilities defines cleaning as removal of visible grime (for example, organic and inorganic material) from the transducer surface, and it is usually done manually or mechanically using water with detergents or enzyme products. Disinfection is described as the process that eliminates many or all pathogenic microorganisms, except bacterial spores. Cleaning should always be carried out before disinfection, as the inorganic and organic materials that may remain on the surface of devices can interfere with the effectiveness of the process.167
To provide appropriate USG probe care after regional blockades one should know if the device was contaminated by body fluids, if it was kept in a sterile environment, and if device components allow sterilization or disinfection.168 Therefore, care requirements for the USG probe for regional anesthesia match those of the camera used in video-assisted surgeries. Disinfection of the camera is performed by cleaning it with a disinfectant detergent solution with the goal of minimizing the number of microorganisms present on its surface. A sterile cover is subsequently applied before using the camera in the surgical field.169
A recent recommendation by the American College of Emergency Physicians established some principles for cleaning transducers, which include the disinfection based on the pathogens possibly found in each patient and on the emphasis on initial cleaning (including carefully removing gel) and performing the correct level of disinfection. The levels of disinfection range from low (destroys most bacteria, some viruses and some fungi by using soap and water and quaternary ammonia sprays) to high (removes all microorganisms, except bacterial spores by using chemical/germicidal sterilizers or by physical sterilization). According to the American College of Emergency Physicians,170 the following precautions should be followed:
-
a)Transducers (linear or curved) placed on clean and intact skin (for example, when scanning structures without performing puncture) are considered non-critical devices and require low level cleaning after each use.
-
b)Transducers that are used during percutaneous procedures (vascular access, lumbar puncture, regional anesthesia and other procedures) should be covered with a sterile single-use probe cover during the procedure, and then cleaned with low level disinfection between uses.
-
c)Internal transducers (probes for intraoral procedures and transesophageal probes) are semi-critical devices that must be covered with a single-use cover and subjected to high-level disinfection between cases. When removing the probe cover, care must be taken to avoid probe contamination with patient fluids. Upon completion of the examination, the operator must perform adequate hand hygiene. Operators should be aware of hospital procedures for high-level disinfection and workflow that may include communicating with supply technicians, adopting equipment covers or probe tracking systems.
-
a)
Therefore, during the procedure, probes used during regional anesthesia must be covered with a sterile single-use probe cover, and then cleaned with low-level disinfection between cases.170
There is only one study that deals with the direct relationship between ultrasound, and regional anesthesia and anesthetic injections. Alakkad et al. examined 7,476 medical charts of patients who underwent peripheral nerve blockade with a single injection guided by ultrasound. A low-level disinfection technique was used in combination with a transparent sterile barrier protection glove to cover the ultrasound transducer. Results showed no evidence of infection related to the blockade. Thus, the authors suggested that the low-level disinfection technique associated with a sterile transparent film barrier dressing to cover the ultrasound transducer result in an extremely low infection rate.171
For single shot or continuous peripheral nerve blocks, sterile gel and a sterile probe cover are required. Ideally, a telescope plastic cover should be used, secured in place by a sterile rubber band instead of an adhesive tape, which poses a greater risk of tearing during removal and, therefore, offering a real possibility of soiling probes. Between two patients, probes must at least undergo low-level disinfection (it is a non-critical device). Using sterile gel for ultrasound is recommended, as non-sterile gel has already been linked to nosocomial infection outbreaks. If non-sterile gel is used, care should be taken to dispose multiple-dose recipients when empty (that is, avoid replenishment) and avoid direct contact between gel container/tube and surface of the transducer or the skin. Once opened, gel containers must be discarded after 28 days. Gel used on a patient should not be reused. Sterile single-use gel packs should be used for invasive procedures that involve skin puncture, for examinations performed on non-intact skin or near sites with surgical scars.170
The protective barriers used during venipuncture and regional anesthesia are medical gloves and probe covers. Covers with pore sizes < 30 nm are recommended because they block most viruses, including HPV, which is 50 nm in size. Sterile adhesive film dressings (e.g., Tegaderm®, OPSITE®) are considered effective barriers against microorganisms larger than 27 nm in size. One must judge on a case-by-case basis and observe the manufacturer's recommendations when contact with non-intact skin is necessary.170 Even if the cover is intact after removal, the probe must be cleaned with detergent, rinsed, dried and maintained in a clean environment. 167
In case of damage to the protective cover, the transducer must undergo higher level disinfection. Most manufacturers do not offer probes and cables that can be completely submerged in disinfectant products. The material of the transducer can be previously disinfected with detergent, but not always with a disinfectant, such as peracetic acid. The manufacturer must provide information on alternative methods for the disinfection process, which may be automated rapid disinfection devices, such as ultraviolet C light (physical disinfection) or hydrogen peroxide (chemical disinfection). The pre-cleaning process is important to assure effective disinfection.172
Due to the chance of protective cover perforation of around 10%, some protocols recommend that high-level disinfection should be routinely performed.167 As USG probes are not resistant to high temperatures, it is not possible to autoclave them. An alternative to obtain high-level disinfection is Ultraviolet C (UV-C) light, currently available in Brazil. One study evaluated the effectiveness of UV-C disinfection after previous probe infection (48 hours before) with gel contaminated with Staphylococcus aureus, Escherichia coli and Enterococcus faecalis. As the UV-C disinfection protocol requires performing a primary cleaning step before disinfection, according to the study protocol, transducers were first cleaned with dry paper to remove gel, and then with paper impregnated with disinfectant. The results found showed that, after 5 to 7 minutes of exposure to the inoculated gel, all probes were infected (> 150 CFU ‒ Colony Forming Units). All were considered sterile (< 10 CFU) after the disinfection protocol. Ultraviolet C light appears to offer a triple advantage over conventional techniques. First, it provides high-level disinfection. Second, it allows the use of the ultrasound probe without a protective cover, which can simplify US-guided techniques and improve echogenicity. In addition, it is a quick process (cycle lasts 90 seconds).173
After using the transducer cover, it must be removed and discarded with common waste. If it contains body fluids, the cover must be discarded as biological waste. Gel residues should be removed (e.g., wipe with a lint-free cloth moistened with running water), and probe and cable should be inspected and disconnected from the device to clean. After cleaning, probes must be stored dry and protected. It is recommended that they be stored in a closed and ventilated space outside the procedure room. The entire unit (keyboards and carts) should be cleaned regularly.167, 170
Protocols for cleaning and disinfection of the materials involved must be developed and monitored. Nevertheless, recently, a study that evaluated the cleaning of ultrasound equipment in five institutions in Australia with defined protocols,174 concluded that for more than 90% of the devices, cleaning standards could be significantly improved.175
Anesthesiologists have varying levels of interpretation in relation to care after use. In research evaluating the risk of infection in regional anesthesia, cross-transmission was considered low, moderate or high by 43%, 48% and 9% of anesthesiologists, respectively. Regarding the use of probe gel, sterile single-dose packets were reported in 83% of cases, while a non-sterile, multi-dose plastic bottle, used for more than 24 hours after opening, was used by 13% of those surveyed. There was no protocol for skin disinfection in 73% of cases. Regarding probe protection, 68% of respondents reported using nonspecific protective covers, 52% reported using semi-permeable transparent occlusive dressing (e.g., OPSITE®) and 5% did not use any type of covering. Still, only 27% believed they were using appropriate protective covers. Probe decontamination between two patients was reported by 86% of professionals. The multiplicity of precaution measures taken reflects the imperative need for studies and the establishment of specific recommendations by specialty societies,176 because, although the use of sterile and self-adhesive ultrasound films is useful in daily clinical practice, there is still no scientific and economic evidence regarding its efficacy in regional anesthesia.177
Recommendations
It is recommended that aseptic measures be taken for the ultrasound probe used in regional blockades. For simple or continuous peripheral nerve blocks, sterile gel and a sterile transducer covers are required. Ideally, a telescopic cover should be used and secured in place by sterile rubber band. Between two patients, the transducer must undergo low-level disinfection (non-critical device). The entire unit should be cleaned regularly.162, 164, 167, 172, 173
-
1
Ultrasound probes used for external use on intact skin for diagnosis only (without punctures for injections) and without contamination of blood or body fluids, should be cleaned with low-level disinfection.167, 170, 172
-
2
Ultrasound probes used externally for percutaneous procedures must be covered with single-use protective covers and sterile gel applied. Subsequently, they should be cleaned with low-level disinfection.167, 170, 172
-
3
Ultrasound probes used internally in mucous membranes and internal orifices must be covered with a single-use sterile cover for each examination, followed by high-level disinfection between each use.167, 170, 172
-
4
If available, ultraviolet C light seems to offer an advantage over conventional techniques, as it provides high-level disinfection, it is a quick process (90 second cycle) and allows US probe use without a protective cover (which improves echogenicity).173
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Paper supervised by the Technical Norms Committee of the Brazilian Society of Anesthesiology (SBA).
References
- 1.Fernandes C.R., Fonseca N.M., Rosa D.M., et al. Recomendações da Sociedade Brasileira de Anestesiologia para Segurança em Anestesia Regional. Rev Bras Anestesiol. 2011;61:668–694. doi: 10.1016/S0034-7094(11)70077-0. [DOI] [PubMed] [Google Scholar]
- 2.Hebl J.R., Niesen A.D. Infectious complications of regional anesthesia. Curr Opin Anaesthesiol. 2011;24:573–580. doi: 10.1097/ACO.0b013e32834a9252. [DOI] [PubMed] [Google Scholar]
- 3.Cook T.M., Counsell D., Wildsmith J.A.W. Major complications of central neuraxial block: Report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102:179–190. doi: 10.1093/bja/aen360. [DOI] [PubMed] [Google Scholar]
- 4.Neuburger M., Büttner J., Blumenthal S., et al. Inflammation and infection complications of 2285 perineural catheters: a prospective study. Acta Anaesthesiol Scand. 2007;51:108–114. doi: 10.1111/j.1399-6576.2006.01173.x. [DOI] [PubMed] [Google Scholar]
- 5.Hebl J.R., Neal J.M. Infectious complications: a new practice advisory. Reg Anesth Pain Med. 2006;31:289–290. doi: 10.1016/j.rapm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Trampuz A., Widmer Fa. Hand hygiene: a frequently missed lifesaving opportunity during patient care. Mayo Clinic Proc. 2004;79:109–116. doi: 10.4065/79.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Guidelines for Hand Hygiene in Health Care (Advanced Draft) World Health Organization; Geneva: 2006. [Google Scholar]
- 8.Samore M.H., Venkataraman L., DeGirolami P.C., et al. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med. 1996;100:32–40. doi: 10.1016/s0002-9343(96)90008-x. [DOI] [PubMed] [Google Scholar]
- 9.Levin A.S., Costa S.F., Mussi N.S., et al. Candida parapsilosis fungemia associated with implantable and semiimplantable central venous catheters and the hands of healthcare workers. Diagn Microbiol Infect Dis. 1998;30:243–249. doi: 10.1016/s0732-8893(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 10.Cassettari V.C., Silveira I.R., Balsamo A.C., et al. Surto em berçário por Klebsiella pneumoniae produtora de beta-lactamase de espectro estendido atribuído à colonização de profissional de saúde portador de onicomicose. J Pediatr. 2006;82:313–316. doi: 10.2223/JPED.1519. [DOI] [PubMed] [Google Scholar]
- 11.Loftus R.W., Muffly M.K., Brown J.R., et al. Hand contamination of anesthesia providers is an important risk factor for intraoperative bacterial transmission. Anesth Analg. 2011;112:98–105. doi: 10.1213/ANE.0b013e3181e7ce18. [DOI] [PubMed] [Google Scholar]
- 12.Raymond C.R., Sorin J.B., John H.E. Surgical site infections and the anesthesia professionals’ microbiome: we’ve all been slimed! Now what are we going to do about it? Anesth Analg. 2011;112:4–7. doi: 10.1213/ANE.0b013e3181fe4942. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings. MMWR Morb Mortal Wkly Rep. 2002;51:1–48. [Google Scholar]
- 14.Koff M.D., Loftus R.W., Burchman C.C., et al. Reduction in intraoperative bacterial contamination of peripheral intravenous tubing through use of a novel device. Anesthesiology. 2009;110:978–985. doi: 10.1097/ALN.0b013e3181a06ec3. [DOI] [PubMed] [Google Scholar]
- 15.Cummings K.L., Deverick D.J., Kaye K.S. Hand hygiene noncompliance and the cost of hospital- acquired methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2010;31:357–364. doi: 10.1086/651096. [DOI] [PubMed] [Google Scholar]
- 16.Fahy C.J., Costi D.A., Cyna A.M. A survey of aseptic precautions and needle type for paediatric caudal block in Australia and New Zealand. Anaesth Intensive Care. 2013;41:102–107. doi: 10.1177/0310057X1304100117. [DOI] [PubMed] [Google Scholar]
- 17.William E.T., Michael O.V., Robert A.H., et al. Impact of ring wearing on hand contamination and comparison of hand hygiene agents in a hospital. Clin Infect Dis. 2003;36:1383–1390. doi: 10.1086/374852. [DOI] [PubMed] [Google Scholar]
- 18.Jeans A.R., Moore J., Nicol C., et al. Wristwatch use and hospital-acquired infection. J Hosp Infect. 2010;74:16–21. doi: 10.1016/j.jhin.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A., Della-Latta P., Todd B. Outbreak of extended-spectrum beta-lactamase producing Klebsiella pneumoniae in a neonatal intensive care unit linked to artificial nails. Infect Control Hosp Epidemiol. 2004;25:210–215. doi: 10.1086/502380. [DOI] [PubMed] [Google Scholar]
- 20.Olsen R.J., Lynch P., Coyle M.B., et al. Examination gloves are barriers to hand contamination in clinical practice. JAMA. 1993;270:350–353. [PubMed] [Google Scholar]
- 21.Horlocker T.T., Wedel D.J. Infectious complications of regional anesthesia. Best Pract Res Clin Anaesthesiol. 2008;22:451–475. doi: 10.1016/j.bpa.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Pelke S., Ching D., Easa D., et al. Gowning does not affect colonization or infection rates in a neonatal intensive care unit. Arch Pediatr Adolesc Med. 1994;148:1016–1020. doi: 10.1001/archpedi.1994.02170100014004. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui N.T., Davies S., McGeer A., et al. The effect of gowning on labor epidural catheter colonization rate - a randomized controlled trial. Reg Anesth Pain Med. 2014;39:520–524. doi: 10.1097/AAP.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 24.Hebl J.R. The importance and implications of aseptic techniques during regional anesthesia. Reg Anesth Pain Med. 2006;31:311–323. doi: 10.1016/j.rapm.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Raad I.I., Hohn D.C., Gilbreath B., et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15:227–230. [PubMed] [Google Scholar]
- 26.Practice advisory for the prevention, diagnosis, and management of infectious complications associated with neuraxial techniques – report by the American Society of Anesthesiologists Task Force on infectious complications associated with neuraxial techniques. Anesthesiology. 2010;112:530–545. doi: 10.1097/ALN.0b013e3181c4c7d8. [DOI] [PubMed] [Google Scholar]
- 27.Lambert D.H. Gloved and masked: will gowns be next? Let the data (not logic) decide this issue. Anesthesiology. 2007;106:877–888. doi: 10.1097/01.anes.0000264758.26050.c7. [DOI] [PubMed] [Google Scholar]
- 28.Couzigou C., Vuong T.K., Botherel A.H., et al. Iatrogenic Streptococcus salivarius meningitis after spinal anaesthesia: need for strict application of standard precautions. J Hosp Infect. 2003;53:313–314. doi: 10.1053/jhin.2002.1366. [DOI] [PubMed] [Google Scholar]
- 29.Schneeberger P.M., Janssen M., Voss A. Alpha-hemolytic streptococci: a major pathogen of iatrogenic meningitis following lumbar puncture. Case reports and a review of the literature. Infection. 1996;24:29–35. doi: 10.1007/BF01780647. [DOI] [PubMed] [Google Scholar]
- 30.Trautmann M., Lepper P.M., Schmitz F.J. Three cases of bacterial meningitis after spinal and epidural anesthesia. Eur J Clin Microbiol Infect Dis. 2002;21:43–45. doi: 10.1007/s10096-001-0643-7. [DOI] [PubMed] [Google Scholar]
- 31.Wickett R.R., Visscher M.O. Structure and function of the epidermal barrier. Am J Infect Control. 2006;34:S98–110. [Google Scholar]
- 32.Kaiser E.N., Newman J.L. Formulation technology as a key component in improving hand hygiene practices. Am J Infect Control. 2006;34:S82–97. [Google Scholar]
- 33.Kampf G., Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev. 2004;17:863–893. doi: 10.1128/CMR.17.4.863-893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balmer M.E., Poiger T., Droz C., et al. Occurrence of methyl triclosan, a transformation product of the bactericide triclosan, in fish from various lakes in Switzerland. Environ Sci Technol. 2004;38:390–395. doi: 10.1021/es030068p. [DOI] [PubMed] [Google Scholar]
- 35.Kinirons B., Mimoz O., Lafendi L., et al. Chlorhexidine versus povidone iodine in preventing colonization of continuous epidural catheters in children: a randomized, controlled trial. Anesthesiology. 2001;94:239–244. doi: 10.1097/00000542-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Dumville J.C., McFarlane E., Edwards P., et al. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database of Systematic Reviews. 2015 doi: 10.1002/14651858.CD003949.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Privitera G.P., Costa A.L., Brusaferro S., et al. Skin antisepsis with chlorhexidine versus iodine for the prevention of surgical site infection: A systematic review and meta-analysis. Am J Infect Control. 2017;45:180–189. doi: 10.1016/j.ajic.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Angelique Sutcliffe vs Aintree Hospitals NHS Trust. EWCA Civ 179. UK Court of Appeal. 2008. [Google Scholar]
- 39.Bogod D. The sting in the tail: antiseptics and the neuraxis revisited. Anaesthesia. 2014;67:1305–1320. doi: 10.1111/anae.12060. [DOI] [PubMed] [Google Scholar]
- 40.Killeen T., Kamat A., Walsh D., et al. Severe adhesive arachnoiditis resulting in progressive paraplegia following obstetric spinal anaesthesia: a case report and review. Anaesthesia. 2012;67:1386–1394. doi: 10.1111/anae.12017. [DOI] [PubMed] [Google Scholar]
- 41.Girgirah K., MacNab W.R. Arachnoiditis: is chlorhexidine spray a safe option? Anaesthesia. 2013;68:425. doi: 10.1111/anae.12197. [DOI] [PubMed] [Google Scholar]
- 42.Malhotra S., Dharmadasa A., Yentis S.M. One vs two applications of chlorhexidine⁄ ethanol for disinfecting the skin: implications for regional anaesthesia. Anaesthesia. 2011;66:574–578. doi: 10.1111/j.1365-2044.2011.06706.x. [DOI] [PubMed] [Google Scholar]
- 43.Widmer A.F., Rotter M., Voss A., et al. Surgical hand preparation: state-of-the-art. Journal of Hospital Infection. 2010;74:112–122. doi: 10.1016/j.jhin.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 44.Loeb M.B., Wilcox L., Smaill F., et al. A randomized trial of surgical scrubbing with a brush compared to antiseptic soap alone. Am J Infect Control. 1997;25:11–15. doi: 10.1016/s0196-6553(97)90047-x. [DOI] [PubMed] [Google Scholar]
- 45.Liu L.Q., Mehigan S. The effects of surgical hand scrubbing protocols on skin integrity and surgical site infection rates: a systematic review. AORN J. 2016;103:468–482. doi: 10.1016/j.aorn.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization (WHO) 2009. WHO guidelines on hand hygiene in health care. First global patient safety challenge. Clean care is safe care. http://apps.who.int/iris/bitstream/handle/10665/44102/9789241597906_eng.pdf?sequence=1& TSPD_101_R0=af301d35619af62b8dbbe612ccbd7e27p6K0000000000000002134e63b1ffff000000 00000000000000000000005bafeea900275b3a1f. [PubMed] [Google Scholar]
- 47.Kampf G., Ostermeyer C. Efficacy of alcohol-based gels compared with simple hand wash and hygienic hand disinfection. J Hosp Infect. 2004;56:13–15. doi: 10.1016/j.jhin.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 48.Parienti J.J., Thibon P., Heller R., et al. Hand-rubbing with an aqueous alcoholic solution vs. traditional surgical hand- scrubbing and 30-day surgical site infection rates. J Am Med Assoc. 2002;288:722–727. doi: 10.1001/jama.288.6.722. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh H.F., Chiu H.H., Lee F.P. Surgical hand scrubs in relation to microbial counts: systematic literature review. J Adv Nurs. 2006;55:68–78. doi: 10.1111/j.1365-2648.2006.03876.x. [DOI] [PubMed] [Google Scholar]
- 50.Rotter M.L., Kampf G., Suchomel M., et al. Population kinetics of the skin flora on gloved hands following surgical hand disinfection with 3 propanol-based hand rubs: a prospective, randomized, double-blind trial. Infect Control Hosp Epidemiol. 2007;28:346–350. doi: 10.1086/510865. [DOI] [PubMed] [Google Scholar]
- 51.Jehle K., Jarrett N., Matthews S. Clean and green: saving water in the operating theatre. Annals of the Royal College of Surgeons of England. 2008;90:22–24. doi: 10.1308/003588408X242277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greer R.B., 3rd The ritual at the scrub sink. Orthop Ver. 1994;23(97) [PubMed] [Google Scholar]
- 53.Protocolo para a prática de higiene das mãos em serviços de saúde. Ministério da Saúde/Anvisa/Fiocruz. 07/09/2013. https://www20.anvisa.gov.br/segurancadopaciente/index.php/publicacoes/item/higiene-das- maos. [Acessado em 29 de setembro de 2018].
- 54.Como fazer a fricção antisséptica das mãos com preparação alcoólica. Ministério da Saúde/Anvisa/Fiocruz. 06/04/2018. https://www20.anvisa.gov.br/segurancadopaciente/index.php/publicacoes/category/higieniz acao-das-maos/. [Acessado em 11 de outubro de 2018].
- 55.Boyce J.M., Pittet D. Healthcare Infection Control Practices Advisory Committee. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/ SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm Rep. 2002;51:1–45. [PubMed] [Google Scholar]
- 56.European Standards . Brussels; 2005. CSN EN 12791. Chemical disinfectants and antiseptics – Surgical hand disinfection – Test method and requirements (phase2/step2) [Google Scholar]
- 57.Hennig T., Werner S., Naujox K., et al. Chlorhexidine is not an essential component in alcohol- based surgical hand preparation: a comparative study of two handrubs based on a modified EN 12791 test protocol. Antimicrobial Resistance and Infection Control. 2017;6:96–101. doi: 10.1186/s13756-017-0258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kampf G., Ostermeyer C. Efficacy of two distinct ethanol-based hand rubs for surgical hand disinfection – a controlled trial according to prEN 12791. BMC Infect Dis. 2005;5:17–20. doi: 10.1186/1471-2334-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Técnica para antissepsia cirúrgica das mãos com produto à base de álcool. Ministério da Saúde/Anvisa/ Fiocruz. Data de publicação: 20/03/2017. https://www20.anvisa.gov.br/segurancadopaciente/index.php/publicacoes/item/tecnica-para- antissepsia-cirurgica-das-maos-com-produto-a-base-de-alcool. [Acessado em 30 de dezembro de 2018].
- 60.Graf M.E., Machado A., Mensor L.L., et al. Antissepsia cirúrgica das mãos com preparações alcóolicas: custo-efetividade, adesão de profissionais e benefícios ecológicos no cenário de saúde. J Bras Econ Saúde. 2014;6:71–80. [Google Scholar]
- 61.Tavolacci M.P., Pitrou I., Merle V., et al. Surgical hand rubbing compared with surgical hand scrubbing: comparison of efficacy and costs. J Hosp Infect. 2006;6:55–59. doi: 10.1016/j.jhin.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 62.Gonçalves K.J., Graziano K.U., Kawagoe J.Y. Revisão sistemática sobre antissepsia cirúrgica das mãos com preparação alcoólica em comparação aos produtos tradicionais. Rev Esc Enferm USP. 2012;46:1484–1493. doi: 10.1590/s0080-62342012000600028. [DOI] [PubMed] [Google Scholar]
- 63.Tanner J., Dumville J.C., Norman G., et al. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD004288.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawagoe J.Y. Tendências e desafios do preparo cirúrgico das mãos. Rev SOBECC. 2016;21:217–222. [Google Scholar]
- 65.Jochum D., Iohom G., Bouaziz H. Asepsis in regional anesthesia. Int Anesthesiol Clin. 2010;48:35–44. doi: 10.1097/AIA.0b013e3181f89b21. [DOI] [PubMed] [Google Scholar]
- 66.Siddiqui N., Friedman Z., McGeer A., et al. Optimal had washing technique to minimize bacterial contamination before neuraxial anesthesia: a randomized control trial. Int J Obstet Anesth. 2017;29:39–44. doi: 10.1016/j.ijoa.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Von Peltz C., Bennet A., Patil V. Central neurological complications following obstetric neuraxial blockade. Curr Opin Anaesthesiol. 2019;32:315–324. doi: 10.1097/ACO.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 68.Wang L.P., Hauerberg J., Schmidt J.F. Incidence of spinal epidural abscess after epidural analgesia: a national 1-year survey. Anesthesiology. 1999;91:1928–1936. doi: 10.1097/00000542-199912000-00046. [DOI] [PubMed] [Google Scholar]
- 69.Scholle D., Kipp F., Reich A., et al. Influence of protective measures after epidural catheter disconnection on catheter lumen colonization: an in vitro study. J Hosp Infect. 2014;86:133–137. doi: 10.1016/j.jhin.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Baer E.T. Post-dural puncture bacterial meningitis. Anesthesiology. 2006;105:381–393. doi: 10.1097/00000542-200608000-00022. [DOI] [PubMed] [Google Scholar]
- 71.Videira R.L., Ruiz-Neto P.P., Brandão Neto M. Post spinal meningitis and asepsis. Acta Anaesthesiol Scand. 2002;46:639–646. doi: 10.1034/j.1399-6576.2002.460602.x. [DOI] [PubMed] [Google Scholar]
- 72.Rubin L., Sprecher H., Kabaha A., et al. Meningitis following spinal anesthesia: 6 cases in 5 years. Infect Control Hosp Epidemiol. 2007;28:1187–1190. doi: 10.1086/520748. [DOI] [PubMed] [Google Scholar]
- 73.Ganem E.M., Castiglia Y.M.M., Vianna P.T.G. Complicações neurológicas determinadas pela anestesia subaracnóidea. Rev Bras Anestesiol. 2002;52:471–480. [PubMed] [Google Scholar]
- 74.Suy F., Verhoeven P.O., Lucht F., et al. Nosocomial meningitis due to Streptoccus salivarius linked to the oral flora of an anesthesiologist. Infect Control Hosp Epidemiol. 2013;34:331–332. doi: 10.1086/669517. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Z., Xu X., Ni H. Disseminated Staphylococcus aureus infection following spinal anesthesia: a case report. J Clin Anesth. 2016;33:438–441. doi: 10.1016/j.jclinane.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 76.Hayek S.M., Goomber R. A review of epidural catheter-related infections. Hosp Pract (1995) 2012;(40):176–185. doi: 10.3810/hp.2012.04.983. [DOI] [PubMed] [Google Scholar]
- 77.Ersoz G., Uguz M., Aslan G., et al. Outbreak of meningitis due to Serratia marcescens after spinal anaesthesia. J Hosp Infect. 2014;87:122–125. doi: 10.1016/j.jhin.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Hadzic A., Koluder-Cimic N., Hadzovic-Cengic M., et al. Serratia marcescens meningitis following spinal anaesthesia and arthroscopy. Med Arch. 2012;66:54–55. [PubMed] [Google Scholar]
- 79.De Leon-Casasola O.A., Parker B., Lema M., et al. Postoperative epidural bupivacaine-morphine therapy: experience with 4,227 surgical cancer patients. Anesthesiology. 1994;81:368–375. doi: 10.1097/00000542-199408000-00015. [DOI] [PubMed] [Google Scholar]
- 80.Feldman J.M., Chapin-Robertson K., Turner J. Do agents used for epidural analgesia have antimicrobial properties? Reg Anesth. 1994;19:43–47. [PubMed] [Google Scholar]
- 81.McKenzie A.G., Darragh K. A national survey of prevention of infection in obstetric central neuraxial blockade in the UK. Anaesthesia. 2011;66:497–502. doi: 10.1111/j.1365-2044.2011.06705.x. [DOI] [PubMed] [Google Scholar]
- 82.Smitt P.S., Tsafka A., Teng-van Z.F., et al. Outcome and complications of epidural analgesia in patients with chronic cancer pain. Cancer. 1998;83:2015–2022. [PubMed] [Google Scholar]
- 83.De Jong, Kansen P.J. A comparison of epidural catheters with or without subcutaneous injections ports for treatment of cancer pain. Anesth Analg. 1994;78:94–100. [PubMed] [Google Scholar]
- 84.Holt H.M., Andersen S.S., Andersen O., et al. Infections following epidural catheterization. J Hosp Infect. 1995;30:253–260. doi: 10.1016/0195-6701(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 85.Davis D.P., Wold R.M., Patel R.J., et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26:285–291. doi: 10.1016/j.jemermed.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 86.Gronwald C., Vowinkel T., Hahnenkamp K. Regional anesthetic procedures in immunosuppressed patients: risk of infection. Curr Opin Anaesthesiol. 2011;24:698–704. doi: 10.1097/ACO.0b013e32834cd2f0. [DOI] [PubMed] [Google Scholar]
- 87.Lai T.T., Jaeger L., Jones B.L., et al. Continuous peripheral nerve block catheter infections in combat- related injuries: a case report of five soldiers from Operation Enduring Freedom/Operation Iraqi Freedom. Pain Med. 2011;12:1676–1681. doi: 10.1111/j.1526-4637.2011.01251.x. [DOI] [PubMed] [Google Scholar]
- 88.McNeely J.K., Trentadue N.C., Rusy L.M., et al. Culture of bacteria from lumbar and caudal epidural catheters used for postoperative analgesia in children. Reg Anesth. 1997;22:428–431. doi: 10.1016/s1098-7339(97)80028-4. [DOI] [PubMed] [Google Scholar]
- 89.Kost-Byerly S., Tobin J.R., Greenberg R.S., et al. Bacterial colonization and infection rate of continuous epidural catheters in children. Anesth Analg. 1998;86:712–716. doi: 10.1097/00000539-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 90.Yuan H.B., Zuo Z., Yu K.W., et al. Bacterial colonization of epidural catheters used for short-term postoperative analgesia: microbiological examination and risk factor analysis. Anesthesiology. 2008;108:130–137. doi: 10.1097/01.anes.0000296066.79547.f3. [DOI] [PubMed] [Google Scholar]
- 91.Dahlgren N., Tornebrandt K. Neurological complications after anaesthesia. A follow-up of 18,000 spinal and epidural anaesthetics performed over three years. Acta Anaesth Scand. 1995;39:872–880. doi: 10.1111/j.1399-6576.1995.tb04190.x. [DOI] [PubMed] [Google Scholar]
- 92.Kane R.E. Neurologic deficits following epidural or spinal anesthesia. Anesth Analg. 1981;60:150–161. [PubMed] [Google Scholar]
- 93.Darchy B., Forceville X., Bavoux E., et al. Clinical and bacteriologic survey of epidural analgesia in patients in the intensive care unit. Anesthesiology. 1996;85:988–998. doi: 10.1097/00000542-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 94.Jabaudon M., Chabanne R., Sossou A., et al. Epidural analgesia in the intensive care unit: An observational series of 121 patients. Anaesth Crit Care Pain Med. 2015;34:217–223. doi: 10.1016/j.accpm.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Du Pen S.L., Peterson D.G., Williams A., et al. Infection during chronic epidural catheterization: diagnosis and treatment. Anesthesiology. 1990;73:905–909. doi: 10.1097/00000542-199011000-00018. [DOI] [PubMed] [Google Scholar]
- 96.Capdevila X., Pirat P., Bringuier S., et al. Continuous peripheral nerve blocks in hospital wards after orthopedic surgery. Anesthesiology. 2005;103:1035–1045. doi: 10.1097/00000542-200511000-00018. [DOI] [PubMed] [Google Scholar]
- 97.Cuvillon P., Ripart J., Lalourcey L., et al. The continuous femoral nerve block catheter for postoperative analgesia: bacterial colonization, infectious rate and adverse effects. Anesth Analg. 2001;93:1045–1049. doi: 10.1097/00000539-200110000-00050. [DOI] [PubMed] [Google Scholar]
- 98.Bomberg H., Krotten D., Kubulus C., et al. Single-dose antibiotic prophylaxis in regional anesthesia: A retrospective registry analysis. Anesthesiology. 2016;125:505–515. doi: 10.1097/ALN.0000000000001218. [DOI] [PubMed] [Google Scholar]
- 99.De Cicco M., Matovic M., Castellani G.T., et al. Time-dependent efficacy of bacterial filters and infection risk in long-term epidural catheterization. Anesthesiology. 1995;82:765–771. doi: 10.1097/00000542-199503000-00019. [DOI] [PubMed] [Google Scholar]
- 100.Rasouli M.R., Cavanaugh P.K., Restrepo C., et al. Is neuraxial anesthesia safe in patients undergoing surgery for treatment of periprosthetic joint infection? Clin Orthop Relat Res. 2015;473:1472–1477. doi: 10.1007/s11999-015-4175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horlocker T.T., Wedel D.J. Regional anesthesia in the immunocompromised patient. Reg Anesth Pain Med. 2006;31:334–345. doi: 10.1016/j.rapm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 102.Wedel D.J., Horlocker T.T. Regional anesthesia in the febrile or infected patient. Reg Anesth Pain Med. 2006;31:324–333. doi: 10.1016/j.rapm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 103.Gritsenko K., Marcello D., Liguori G.A., et al. Meningitis or epidural abscesses after neuraxial block for removal of infected hip or knee prostheses. Br J Anaesth. 2012;108:485–490. doi: 10.1093/bja/aer416. [DOI] [PubMed] [Google Scholar]
- 104.Do corticosteroids improve outcome in meningitis? Drug Ther Bull. 2010;48:116–120. doi: 10.1136/dtb.2010.10.0050. [DOI] [PubMed] [Google Scholar]
- 105.Baer E.T. Post-dural puncture bacterial meningitis. Anesthesiology. 2006;105:381–393. doi: 10.1097/00000542-200608000-00022. [DOI] [PubMed] [Google Scholar]
- 106.Laguna del E.P., Castañeda P.A., López-Cano G.M. Bacterial meningitis secondary to spinal analgesia and anaesthesia. Neurologia. 2010;25:552–556. doi: 10.1016/j.nrl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 107.Shioya N., Ishibe Y., Kan S., et al. Sternoclavicular joint septic arthritis following paraspinal muscle abscess and septic lumbar spondylodiscitis with epidural abscess in a patient with diabetes: a case report. BMC Emerg Med. 2012;12:7–8. doi: 10.1186/1471-227X-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bomberg H., Albert N., Schmitt K., et al. Obesity in regional anesthesia a risk factor for peripheral catheter-related infections. Acta Anaesthesiol Scand. 2015;59:1038–1048. doi: 10.1111/aas.12548. [DOI] [PubMed] [Google Scholar]
- 109.Sendi P., Bregenzer T., Zimmerli W. Spinal epidural abscess in clinical practice. QJM. 2008;101:1–12. doi: 10.1093/qjmed/hcm100. [DOI] [PubMed] [Google Scholar]
- 110.Sakushima K., Hayashino Y., Kawaguchi T. Diagnostic accuracy of cerebrospinal fluid lactate for differentiating bacterial meningitis from aseptic meningitis: a meta-analysis. J Infect. 2011;62:255–262. doi: 10.1016/j.jinf.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 111.Huy N.T., Thao N.T., Diep D.T., et al. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: a systemic review and meta-analysis. Crit Care. 2010;14:R240. doi: 10.1186/cc9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prasad K., Sahu J.K. Cerebrospinal fluid lactate: Is it a reliable and valid marker to distinguish between acute bacterial meningitis and aseptic meningitis? Crit Care. 2011;15:104. doi: 10.1186/cc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Phillips J.M.G., Stedeford J.C., Hartsilver E., et al. Epidural abscess complicating insertion of epidural catheters. Br J Anaesth. 2002;89:778–782. [PubMed] [Google Scholar]
- 114.Gosavi C., Bland D., Poddar R., et al. Epidural abscess complicating insertion of epidural catheters. Br J Anaesth. 2004;89:294–298. doi: 10.1093/bja/aeh517. [DOI] [PubMed] [Google Scholar]
- 115.Pondé J.M., Valente E., Lemos J. Abscesso após anestesia peridural: Relato de caso. Rev Bras Anestesiol. 1996;46:427–430. [Google Scholar]
- 116.Abreu M.P., Deda R.G., Cangiani L.H. Abscesso peridural após analgesia controlada pelo paciente por via peridural: relato de caso. Rev Bras Anestesiol. 2004;54:78. [Google Scholar]
- 117.Danner R.L., Hartman B.J. Update of spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis. 1987;9:265–274. doi: 10.1093/clinids/9.2.265. [DOI] [PubMed] [Google Scholar]
- 118.Reynolds F. Neurological infections after neuraxial anesthesia. Anesthesiol Clin. 2008;26:23–52. doi: 10.1016/j.anclin.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Reihsaus E., Waldbaur H., Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Ver. 2000;23:175–204. doi: 10.1007/pl00011954. [DOI] [PubMed] [Google Scholar]
- 120.Bluman E.M., Palumbo M.A., Lucas P.R. Spinal epidural abscess in adults. J Am Acad Orthop Surg. 2004;12:155–163. doi: 10.5435/00124635-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 121.Van Rappard J.R., Tolenaar J.L., Smits A.B., et al. Spinal epidural abscess and meningitis following short-term epidural catheterisation for postoperative analgaesia. BMJ Case Rep. 2015;20:1–4. doi: 10.1136/bcr-2015-210867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grewal S., Hocking G., Wildsmith J.A.W. Epidural abscesses. Br J Anaesth. 2006;96:292–302. doi: 10.1093/bja/ael006. [DOI] [PubMed] [Google Scholar]
- 123.Gasparini J.R., Mello S.S., Marques R.S., et al. Analgesia pós-operatória plexular contínua. Estudo dos efeitos colaterais e do risco de infecção dos cateteres. Rev Bras Anestesiol. 2008;58 6:602-6. [Google Scholar]
- 124.Jeffreys A., Horton R., Evans B. Epidural abscesses. Br J Anaesth. 2006;97:115–120. doi: 10.1093/bja/ael123. [DOI] [PubMed] [Google Scholar]
- 125.Hailey D., Jacobs P.D., Ries N.M., et al. Reuse of single use medical devices in Canada: clinical and economic outcomes, legal and ethical issues, and current hospital practice. Int J Technol Assess Health Car. 2008;24:430–436. doi: 10.1017/S0266462308080562. [DOI] [PubMed] [Google Scholar]
- 126.Jacobs P., Polisena J., Hailey D., et al. Economic analysis of reprocessing single-use medical devices: a systematic literature review. Infect Control Hosp Epidemiol. 2008;29:297–301. doi: 10.1086/529587. [DOI] [PubMed] [Google Scholar]
- 127.Polisena J., Hailey D., Moulton K., et al. Reprocessing and reuse of single-use medical devices: a national survey of Canadian acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:437–439. doi: 10.1086/587648. [DOI] [PubMed] [Google Scholar]
- 128.Kramer A., Assadian O. Ethical and hygiene aspects of the reprocessing of medical devices in Germany. GMS Krankenhhyg Interdiszip. 2008;3:25. [PMC free article] [PubMed] [Google Scholar]
- 129.Kraft M. Framework conditions and requirements to ensure the technical functional safety of reprocessed medical devices. GMS Krankenhhyg Interdiszip. 2008;3:23. [PMC free article] [PubMed] [Google Scholar]
- 130.Adams S.B., Moore G.E., Elrashidy M., et al. Effect of needle size and type, reuse of needles, insertion speed, and removal of hair on contamination of joints with tissue debris and hair after arthrocentesis. Vet Surg. 2010;39:667–673. doi: 10.1111/j.1532-950X.2010.00649.x. [DOI] [PubMed] [Google Scholar]
- 131.Armitage W.J., Tullo A.B., Ironside J.W. Risk of Creutzfeldt-Jakob disease transmission by ocular surgery and tissue transplantation. Eye. 2009;23:1926–1930. doi: 10.1038/eye.2008.381. [DOI] [PubMed] [Google Scholar]
- 132.Roth K., Heeg P., Reichl R. Specific hygiene issues relating to reprocessing and reuse of single- use devices for laparoscopic surgery. Surg Endosc. 2002;16:1091–1097. doi: 10.1007/s00464-001-9190-7. [DOI] [PubMed] [Google Scholar]
- 133.RDC nº 156, de 11 de agosto de 2006. Dispõe sobre o registro, rotulagem e reprocessamento de produtos médicos, e dá outras providências. https://www20.anvisa.gov.br/segurancadopaciente/index.php/legislacao/category/outras-4. [Acessado em agosto de 2019].
- 134.Magetsari R., Van der Houwen E.B., Bakker M.T., et al. Biomechanical and surface physico- chemical analyses of used osteosynthesis plates and screws – Potential for reuse in developing countries? J Biomed Mater Res B, Appl Biomater. 2006;79:236–244. doi: 10.1002/jbm.b.30534. [DOI] [PubMed] [Google Scholar]
- 135.Grantcharov P., Ahmed S., Wac K., et al. Reprocessing and reuse of single-use medical devices: perceptions and concerns of relevant stakeholders toward current practices. Int J Evid Based Healthc. 2019;17:53–57. doi: 10.1097/XEB.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 136.Bailard N.S., Ortiz J., Flores R.A. Additives to local anesthetics for peripheral nerve blocks: Evidence, limitations, and recommendations. Am J Health Syst Pharm. 2014;71:373–385. doi: 10.2146/ajhp130336. [DOI] [PubMed] [Google Scholar]
- 137.Phillips D.P., Christenfeld N., Glynn L.M. Increase in US medication error deaths between 1983 and 1993. Lancet. 1998;351:643–644. doi: 10.1016/S0140-6736(98)24009-8. [DOI] [PubMed] [Google Scholar]
- 138.Formulário de Erro de Medicação. Ministério da Saúde. http://portal.anvisa.gov.br/resultado-de-busca?p_p_id=101&p_p_lifecycle=0&p_p_state=maximized&p_p_mode=view&p_p_col_id=column-1&p_p_col_count=1&_101_struts_action=%2Fasset_publisher%2Fview_content&_101_assetEntryId=402388&_101_type=content&_101_groupId=33868&_101_urlTitle=informe-snvs-anvisa-gfarm-n-2-de-16-de-abril-de-2007&redirect=http%3A%2F%2Fportal.anvisa.gov.br%2Fresultado-de-busca%3Fp_p_id=3%26p_p_lifecycle=0%26p_p_state=normal%26p_p_mode=view%26p_p_col_id=column-1%26p_p_col_count=1%26_3_groupId=0%26_3_keywords=Formul%25C3%25A1rio+de+Erro+de+Medica%25C3%25A7%25C3%25A3o%26_3_cur=1%26_3_struts_action=%252Fsearch%252Fsearch%26_3_format=%26_3_formDate=1441824476958&inheritRedirect=true. [Acessado em 22 de agosto de 2018].
- 139.Merry A.F., Webster C.S., Weller J., et al. Evaluation in an anaesthetic simulator of a prototype of a new drug administration system designed to reduce error. Anaesthesia. 2002;57:256–263. doi: 10.1046/j.0003-2409.2001.02397.x. [DOI] [PubMed] [Google Scholar]
- 140.Bergman I.J., Kluger M.T., Short T.G. Awareness during general anaesthesia: a review of 81 cases from the Anaesthetic Incident Monitoring Study. Anaesthesia. 2002;57:549–556. doi: 10.1046/j.1365-2044.2002.02565.x. [DOI] [PubMed] [Google Scholar]
- 141.Sinclair M., Simmons S., Cyna A. Incidents in obstetric anaesthesia and analgesia – an analysis of 5000 AIMS reports. Anaesth Intensive Care. 1999;27:275–281. doi: 10.1177/0310057X9902700309. [DOI] [PubMed] [Google Scholar]
- 142.Currie M., Mackay P., Morgan C., et al. The Australian Incident Monitoring Study. The ‘wrong drug’ problem in anaesthesia: an analysis of 2,000 incident reports. Anaesth Intensive Care. 1993;21:596–601. doi: 10.1177/0310057X9302100517. [DOI] [PubMed] [Google Scholar]
- 143.Short T.G., O’Regan A., Lew J., et al. Critical incident reporting in an anaesthetic department quality assurance programme. Anaesthesia. 1993;48:3–7. doi: 10.1111/j.1365-2044.1993.tb06781.x. [DOI] [PubMed] [Google Scholar]
- 144.Fasting S., Gisvold S.E. Adverse drug errors in anesthesia, and the impact of coloured syringe labels. Can J Anesth. 2000;47:1060–1067. doi: 10.1007/BF03027956. [DOI] [PubMed] [Google Scholar]
- 145.Jensen L.S., Merry A.F., Webster C.S., et al. Evidence-based strategies for preventing drug administration errors during anaesthesia. Anaesthesia. 2004;59:493–504. doi: 10.1111/j.1365-2044.2004.03670.x. [DOI] [PubMed] [Google Scholar]
- 146.Glavin R.J. Drug errors: consequences, mechanisms, and avoidance. Br J Anaesth. 2010;105:76–82. doi: 10.1093/bja/aeq131. [DOI] [PubMed] [Google Scholar]
- 147.RDC nº 71, de 22 de dezembro de 2009. Estabelece regras para a rotulagem de medicamentos. http://e-legis.bvs.br/leisref/. [Acessado em agosto de 2018].
- 148.Merry A.F., Webster C.S., Connell H. A new infusion syringe label system designed to reduce task complexity during drug preparation. Anaesthesia. 2007;62:486–491. doi: 10.1111/j.1365-2044.2007.04993.x. [DOI] [PubMed] [Google Scholar]
- 149.Hemingway C.J., Malhotra S., Almeida M., et al. The effect of alcohol swabs and filter straws on reducing contamination of glass ampoules used for neuroaxial injections. Anaesthesia. 2007;62:286–288. doi: 10.1111/j.1365-2044.2007.04977.x. [DOI] [PubMed] [Google Scholar]
- 150.Merriman S., Paech M.J., Keil A.D. Bacterial contamination in solution aspirated from non-sterile packaged fentanyl ampoules. Anaesth Intensive Care. 2009;37:608–612. doi: 10.1177/0310057X0903700413. [DOI] [PubMed] [Google Scholar]
- 151.Freitas R.R., Tardelli M.A. Comparative analysis of ampoules and vials in sterile and conventional packaging as to microbial load and sterility test. Einstein. 2016;14:226–230. doi: 10.1590/S1679-45082016AO3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.USP Chapter 797with proposed revisions. 2006. http://www.usp. org/pdf/EN/USPNF/PF797redline.pdf. [Acessado em 20 de setembro de 2018].
- 153.Head S., Enneking F.K. Infusate contamination in regional anesthesia: What every anesthesiologist should know. Anesth Analg. 2008;107:1412–1418. doi: 10.1213/01.ane.0000286228.57455.91. [DOI] [PubMed] [Google Scholar]
- 154.Sevarino F.B., Pizarro C.W., Sinatra R. Sterility of epidural solutions – recommendations for cost- effective use. Reg Anesth Pain Med. 2000;25:368–371. doi: 10.1053/rapm.2000.4148. [DOI] [PubMed] [Google Scholar]
- 155.McIntosh D., Spaven J., Hagen N.A. How long do prepared epidural solutions remain sterile? J Pain Symptom Manage. 1999;18:137–139. doi: 10.1016/s0885-3924(99)00052-4. [DOI] [PubMed] [Google Scholar]
- 156.Svedberg O.K., Chem E., McKenzie J., et al. Compatibility of ropivacaine with morphine, sufentanil, fentanyl, or clonidine. J Clin Pharmacy Therap. 2002;27:39–45. doi: 10.1046/j.1365-2710.2002.00386.x. [DOI] [PubMed] [Google Scholar]
- 157.Jäppinen A., Turpeinen M., Kokki H., et al. Stability of sufentanil and levobupivacaine solutions and a mixture in a 0.9% sodium chloride infusion stored in polypropylene syringes. Eur J Pharm Sci. 2003;19:31–36. doi: 10.1016/s0928-0987(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 158.Boitquin L., Hecq J.D., Evrard J.M., et al. Long-term stability of sufentanil citrate with levobupivacaine hydrochloride in 0.9% sodium chloride infusion PVC bags at 4 degrees C. J Pain Symptom Manage. 2004;28:4–6. doi: 10.1016/j.jpainsymman.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 159.Priston M.J., Hughes J.M., Santillo M., et al. Stability of an epidural analgesic admixture containing epinephrine, fentanyl and bupivacaine. Anaesthesia. 2004;59:979–983. doi: 10.1111/j.1365-2044.2004.03803.x. [DOI] [PubMed] [Google Scholar]
- 160.Neal Jm. Ultrasound-Guided Regional Anesthesia and Patient Safety: Update of an Evidence- Based Analysis. Reg Anesth Pain Med. 2016;41:195–204. doi: 10.1097/AAP.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 161.Barrington M.J., Uda Y. Did ultrasound fulfill the promise of safety in regional anesthesia? Curr Opin Anaesthesiol. 2018;31:649–655. doi: 10.1097/ACO.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 162.Bouaziz H., Aubrun F., Belbachir A., et al. Ultrasound-guided regional anesthesia. Ann Fr Anesth Reanim. 2013;32:119–120. doi: 10.1016/j.annfar.2013.07.790. [DOI] [PubMed] [Google Scholar]
- 163.Ecoffey C. Safety in pediatric regional anesthesia. Paediatr Anaesth. 2012;22:25–30. doi: 10.1111/j.1460-9592.2011.03705.x. [DOI] [PubMed] [Google Scholar]
- 164.Lalueza A., López-Medrano F., del Palacio A., et al. Cladosporium macrocarpum brain abscess after endoscopic ultrasound-guided celiac plexus block. Endoscopy. 2011;43:9–10. doi: 10.1055/s-0030-1255804. [DOI] [PubMed] [Google Scholar]
- 165.Nyhsen C.M., Humphreys H., Koerner R.J., et al. Infection prevention and control in ultrasound - best practice recommendations from the European Society of Radiology Ultrasound Working Group. Insights Imaging. 2017;8:523–535. doi: 10.1007/s13244-017-0580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Spaulding E.H. Principles and application of chemical disinfection. AORN Journal. 1963;1:36–46. [Google Scholar]
- 167.Healthcare Infection Control Practices Advisor Committee . 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities. Updated February 2017. Centers for Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/. [Acessado em 12 de janeiro de 2019]. [Google Scholar]
- 168.Bloc S., Garnier T., Bounhiol C., et al. Ultrasound guided regional anaesthesia: an effective method for cleaning the probes [in French] Ann Fr Anesth Reanim. 2008;27:994–998. doi: 10.1016/j.annfar.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 169.Jochum D. Hygiène relative à l’utilisation des ultrassons. Ann Fr Anesth Reanim. 2012;31:e219–21. doi: 10.1016/j.annfar.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 170.Policy Statement of American College Emergency Physicians . 2018. Guideline for Ultrasound Transducer Cleaning and Disinfection. https://www.acep.org/globalassets/new-pdfs/policy-statements/guideline-for-ultrasound- transducer-cleaning-and-disinfection.pdf. [Acessado em 20 de janeiro de 2019]. [Google Scholar]
- 171.Alakkad H., Naeeni A., Chan V.W., et al. Infection related to ultrasound-guided single-injection peripheral nerve blockade: a decade of experience at Toronto Western Hospital. Reg Anesth Pain Med. 2015;40:82–84. doi: 10.1097/AAP.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 172.College of Physicians and Surgeons of British Columbia. Steps for Cleaning and Disinfection of Ultrasound Probes. https://www.cpsbc.ca/files/pdf/Steps-Cleaning-Disinfecting- Ultrasound-Probes.pdf. [Acessado em 28 de dezembro de 2018].
- 173.Bloc S., Mercadal L., Garnier T., et al. Evaluation of a new disinfection method for ultrasound probes used for regional anesthesia: Ultraviolet C light. J Ultrasound Med. 2011;30:785–788. doi: 10.7863/jum.2011.30.6.785. [DOI] [PubMed] [Google Scholar]
- 174.Basseal J.M., Westerway S.C., Juraja M., et al. Guidelines for reprocessing ultrasound transducers. Australas J Ultrasound Med. 2017;20:30–40. doi: 10.1002/ajum.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Whiteley G.S., Glasbey T.O., Westerway S.C., et al. A new sampling algorithm demonstrates that ultrasound equipment cleanliness can be improved. Am J Infect Control. 2018;46:887–892. doi: 10.1016/j.ajic.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 176.Bouaziz H., Aubrun F., Belbachir A.A., et al. Échographie en anesthésie locorégionale Locoregional anaesthesia and echography. Ann Fr Anesth Reanim. 2011;30:33–35. doi: 10.1016/j.annfar.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 177.Marhofer P., Schebesta K., Marhofer D. Hygieneaspekte in der ultraschallgestützten Regionalanästhesie. Anaesthesist. 2016;65:492–498. doi: 10.1007/s00101-016-0168-1. [DOI] [PubMed] [Google Scholar]