Abstract
Background
The Dutch Working Party on Antibiotic Policy (SWAB) in collaboration with relevant professional societies, has updated their evidence-based guidelines on empiric antibacterial therapy of sepsis in adults.
Methods
Our multidisciplinary guideline committee generated ten population, intervention, comparison, and outcome (PICO) questions relevant for adult patients with sepsis. For each question, a literature search was performed to obtain the best available evidence and assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. The quality of evidence for clinically relevant outcomes was graded from high to very low. In structured consensus meetings, the committee formulated recommendations as strong or weak. When evidence could not be obtained, recommendations were provided based on expert opinion and experience (good practice statements).
Results
Fifty-five recommendations on the antibacterial therapy of sepsis were generated. Recommendations on empiric antibacterial therapy choices were differentiated for sepsis according to the source of infection, the potential causative pathogen and its resistance pattern. One important revision was the distinction between low, increased and high risk of infection with Enterobacterales resistant to third generation cephalosporins (3GRC-E) to guide the choice of empirical therapy. Other new topics included empirical antibacterial therapy in patients with a reported penicillin allergy and the role of pharmacokinetics and pharmacodynamics to guide dosing in sepsis. We also established recommendations on timing and duration of antibacterial treatment.
Conclusions
Our multidisciplinary committee formulated evidence-based recommendations for the empiric antibacterial therapy of adults with sepsis in The Netherlands.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-022-07653-3.
Keywords: Antibacterial therapy, Duration of antibiotic therapy, Sepsis, Guidelines, Empirical therapy, Pharmacokinetics, Pharmacodynamics, Enterobacterales, Pseudomonas aeruginosa, Antimicrobial resistance, Penicillin allergy
Background
Sepsis is currently defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1–4]. Sepsis and septic shock are common reasons for intensive care unit (ICU) admission and associated with high mortality rates, even at long-term follow-up [5–12]. Worldwide in 2017, nearly 50 million cases of sepsis were recorded resulting in 11 million sepsis-related deaths [13]. In the Netherlands the estimated annual number of admissions for severe sepsis in Dutch ICU’s was 7700–9500 in 2004 [14]. The incidence of sepsis may have risen in recent decennia, possibly due to ageing and increasing numbers of immunocompromised patients [6, 8, 15]. Antibacterial treatment is an essential part of effective sepsis management. Inappropriate or delayed antibacterial treatment in patients with sepsis and septic shock have been associated with increased morbidity and mortality [16–21].
The Dutch Working Party on Antibiotic Policy (SWAB), initiated by the Dutch Association of Internal Medicine, the Dutch Society for Medical Microbiology and the Dutch Association of Hospital Pharmacists, coordinates activities in the Netherlands intending to optimize antibiotic use, to contain the development of antimicrobial resistance, and to limit the costs of antibiotic use. The general objective of the SWAB sepsis guidelines is to guide medical professionals in the empirical antibacterial treatment for adults with sepsis and septic shock in hospitals. The current guidelines on empirical antibacterial therapy of sepsis in the Netherlands is an update of the SWAB sepsis guidelines published in 2010 [22].
Providing evidence-based recommendations on empirical antibacterial therapy in sepsis is challenging. There is considerable heterogeneity among sepsis studies as to included patients (comorbidities, disease severity, source of infection), microbiological characteristics (availability of culture results, pathogens involved, local antimicrobial resistance), interventions (drug dosing, source control, timing of treatment) as well as to the outcome parameters assessed. In particular, antimicrobial resistance is much lower in the Netherlands than in other countries [23–25]. Another important consideration is that most trials and meta-analyses on antibacterial therapy are not powered to assess relevant outcomes such as adverse events and the development of antimicrobial resistance [26–28].
In this publication, we summarize the most important literature and changes in recommendations for the antibacterial treatment of adults with sepsis.
Methods
For a complete description of the methodology of the guideline, we refer to the original document. In short, the guideline was written according to the Appraisal of Guidelines for Research and Evaluation (AGREE II) instrument [29].
A multidisciplinary guideline committee consisting of experts delegated from relevant professional societies followed a guideline development process comparable to that of the Infectious Diseases Society of America (IDSA), which includes a systematic method of grading both the quality of evidence (very low, low, moderate, and high) and the strength of the recommendation (weak or strong) [30]. We aimed to provide an overview of the quality of available evidence and give evidence-based recommendations for antibacterial treatment of sepsis in adults (≥ 18 years old). We restricted the guideline to the most important causes of sepsis, i.e., pneumonia, abdominal infections, urinary tract infections, complicated skin and soft tissue infections, as well as to sepsis in general or of (yet) unknown origin. Neutropenic sepsis, sepsis due to viral or fungal infections, sepsis in patients with prosthetic material or long term central venous catheters, sepsis due to osteomyelitis, meningitis, mediastinitis and endocarditis and children were outside the scope of the guideline.
The committee generated ten population, intervention, comparison, and outcomes (PICO) questions relevant for adult patients with sepsis in the Netherlands (Table 1). For each question we reviewed existing national and international guidelines and performed additional pragmatic literature searches. For evidence on drug resistance in the Netherlands, the guideline committee used surveillance data from 2017 in the NethMap annual report 2018 [23]. Reports of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guided the interpretation of susceptibility test results [31].
Table 1.
Key questions SWAB guideline for empirical antibacterial therapy of sepsis in adults
| I Causative bacterial pathogens in sepsis |
| 1 Which bacteria are most frequently isolated from patients with sepsis in the Netherlands? |
| 2 What are the resistance patterns of the most frequently isolated bacteria in patients with sepsis in the Netherlands? |
| 3 Which patients are at risk for sepsis due to third-generation cephalosporin-resistant Enterobacterales (3GCR-E) or P. aeruginosa in the Netherlands? |
| II Empirical antibacterial therapy of sepsis |
| 4 What is the importance of appropriate empirical therapy in patients with sepsis? |
| 5 What is the effect of double active empirical antibacterial therapy compared to monotherapy in patients with sepsis? |
| 6 What is the optimal choice of empirical therapy in patients with sepsis in the Netherlands? |
| 7 What is the optimal empirical antibacterial therapy of sepsis in patients with a penicillin allergy? |
| III Timing and duration of antibacterial therapy in sepsis |
| 8 What is the optimal timing of empirical antibacterial therapy in patients with sepsis? |
| 9 What is the optimal duration of antibacterial treatment for sepsis? |
| IV Pharmacokinetic and pharmacodynamic considerations in sepsis |
| 10 In patients with sepsis, should we recommend pharmacokinetic/pharmacodynamic dosing optimization for empirical antibacterial therapy? |
Included guidelines and studies were assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. We graded the quality of evidence for clinically relevant outcomes from high to very low. In structured consensus meetings, the committee formulated recommendations as strong or weak. When evidence could not be obtained, recommendations could be provided based on opinions and experiences (good practice statements).
The draft guideline was submitted to the members of relevant professional societies for external review. The guideline working group adjusted the guideline according to comments in the external review through group discussion. Both comments and responses of the committee are available at www.swab.nl. The final version received formal approval from the SWAB executive board.
Results
Causative bacterial pathogens in sepsis and their antibiotic susceptibility
Which bacteria are most frequently isolated from patients with sepsis in The Netherlands?
In the Netherlands, the most commonly cultured pathogens in blood cultures are coagulase-negative staphylococci (CNS) (34%), Escherichia coli (23%), Staphylococcus aureus (10%), Klebsiella pneumonia (4%) and Enterococcus faecalis/faecium (5%) [23]. In patients with sepsis and ICU admission, gram-negative pathogens were more likely to be involved [32, 33]. Of note, Acinetobacter baumannii was not an important cause of sepsis due to hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP) as it was hardly isolated in respiratory cultures of hospitalized patients in Dutch surveillance data [23]. Reported pathogens in sepsis due to intra-abdominal infections were E. coli, enteric anaerobes, other Enterobacterales, Enterococcus spp. and Streptococcus spp. [34]. In central line-associated bloodstream infections (CLABSI), the most reported pathogens were CNS, gram-negative bacteria (fermenters and non-fermenters), S. aureus, Enterococcus spp. and Candida albicans [35, 36].
What are the resistance patterns of the most frequently isolated bacteria in patients with sepsis in The Netherlands?
A Dutch study among 648 intensive care unit (ICU) patients with non-pneumonia derived sepsis reported microbiological culture results of (surveillance) samples obtained two days before until two days after ICU admission. Resistance percentages of pathogenic bacteria in these patients were 10% for 3rd generation cephalosporins, 8% for ciprofloxacin, 6% for gentamicin, 2% for piperacillin-tazobactam, and 0.5% for meropenem [37]. Dutch surveillance data showed that the rate of extended-spectrum beta-lactamase (ESBL)-producing bacteria in blood cultures has increased over the past years. In 2017, 6% of E. coli and 10% of K. pneumoniae blood isolates were resistant to 3rd generation cephalosporins (Table 2) [23]. The prevalence of carbapenem resistance in all E. coli and K. pneumoniae isolates was stable over five years and low at 0.03% and 0.42%. The risk of methicillin-resistant S. aureus (MRSA) bacteraemia has remained stable over the last ten years and low at 1% of all S. aureus bacteraemias [23].
Table 2.
Percentage of growth and resistance of most frequent pathogens in blood cultures of patients in unselected departments in the Netherlands in 2017 [23]
| In blood culture | Amoxicillin-clavulanic acid | Cefuroxime | Ceftriaxone | Gentamicin | Ciprofloxacin | Piperacillin-tazobactam | Amoxi-clav + gentamicin | Amoxi-clav + ciprofloxacin | Cefuroxime + gentamicin | Cefuroxime + ciprofloxacin | Ceftriaxone + gentamicin | Ceftriaxone + ciprofloxacin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | 23% | 37% | 12% | 6% | 4% | 14% | 5% | 3% | 9% | 2% | 6% | 1% | 4% |
| K. pneumoniae | 4% | 17% | 14% | 10% | 5% | 14% | 7% | 4% | 9% | 4% | 9% | 4% | 7% |
| P. mirabilis | 1% | 8% | 1% | 1% | 5% | 11% | 1% | 2% | 2% | 0% | 0% | 0% | 0% |
| E. cloacae | 1% | 3% | 5% | ||||||||||
| Other Enterobacterales | 5% | ||||||||||||
| P. aeruginosa | 2% | 2% | 9% | 5% | |||||||||
| S. aureus | 10% | 1% | 1% | 0% | 6% | 1% | |||||||
| Other Gram-positives | 12% |
Which patients are at risk for sepsis due to third-generation cephalosporin-resistant Enterobacterales (3GCR-E) or Pseudomonas aeruginosa in the Netherlands?
One systematic review summarized colonization and risk of subsequent bacteraemia with ESBL-producing Enterobacterales in patients with solid and haematological malignancies [38]. Patients with known colonization with an ESBL-producing Enterobacterales as detected by surveillance cultures (mostly at admission) were 13 times more likely to develop a bacteraemia with these pathogens compared to patients not that were not colonized. Two specific risk factors for sepsis due to 3GCR-E have been externally validated by in a Dutch retrospective study of 9442 episodes in which blood cultures were drawn and iv antibacterial therapy was started [39]. Positive predictive values (PPV) of prior (90 days and 1 year) colonization with 3GCR-E were 7.4% and 6.1% for predicting bacteraemia and 34.4% and 28.2% for predicting any culture-positive infection with 3GCR-E. PPVs of prior (30 days) treatment with cephalosporins or fluoroquinolones were 1.3% for predicting bacteraemia and 6.9% for predicting any culture-positive infection with 3GCR-E. No other studies were found that externally validated predictors for sepsis due to 3GCR-E or P. aeruginosa.
Based on currently available evidence, we concluded that prior (1 year) infection or colonization is the strongest and most common risk factor predicting subsequent infection with 3GCR-E [38, 40–42]. It was challenging to provide general recommendations on other risk factors that should be taken into account to guide the decision to start empirical antibiotic therapy in sepsis directed against 3GCR-E or P. aeruginosa. Until high-quality and externally validated prediction rules are available, the guideline committee recommends that the following factors should be taken into account to decide if empirical antibacterial therapy against 3GCR-E in patients with sepsis is appropriate: local prevalence of 3GCR-E [43], whether the sepsis is hospital-acquired [40, 44, 45], and to a lesser extent healthcare-associated, versus community-acquired, whether the patient received prior (2 months) antibiotic treatment and whether or not the patient receives selective decontamination of the digestive tract (SDD) [40, 45, 46]. It is essential to realize the limitations of using risk factors for the decision to treat for 3GCR-E, to weigh potential risk factors against the associated risk of overtreatment and to ensure antibiotic de-escalation if possible.
In addition, the committee regarded the high colonization rate with highly resistant micro-organisms (HRMO) in travellers from highly endemic countries such as the Indian subcontinent as another risk factor to consider in the choice of empirical antibiotic therapy in patients with sepsis. As many travellers will not be colonized anymore after several months, we suggest including three months prior travel to highly endemic countries in the individual risk assessment (https://resistancemap.cddep.org). The committee felt that the risk of 3GCR-E involvement to be high in patients with sepsis recently hospitalized abroad for > 24 h. There is no strong evidence to support this statement, but it is in accordance to national infection prevention guidelines on which patients to screen for HRMO [47]. We therefore included this as a separate suggestion.
Regarding P. aeruginosa, the committee suggests to empirically start targeted treatment in patients with sepsis when prior (1-year) cultures showed P. aeruginosa. In addition, we suggest covering P. aeruginosa in patients with sepsis due to HAP/VAP or suspected infected central venous catheter (CVC) infection.
Empirical antibacterial therapy in sepsis
What is the importance of appropriate empirical therapy in patients with sepsis?
The importance of appropriate empirical antibacterial therapy in patients with sepsis has been supported by systematic reviews of observational studies only [21, 48, 49]. The reported effect has been consistent and includes reduced mortality, costs and length of hospital stay, although with considerable heterogeneity between studies [21, 48, 49]. Very low quality evidence showed a trend towards improved outcomes of appropriate empirical therapy in patients with sepsis due to HRMO and anaerobic pathogens [43, 50–52]. For Enterococcus spp, empirical treatment strategies in community-acquired intra-abdominal infections showed no difference in clinical outcomes comparing antibiotic regimens with and without activity against Enterococci [52]. There is no clear evidence to support or refute empirical treatment of enterococci in hospital-acquired intra-abdominal infections, patients that have no adequate source control, immunocompromised patients and patients with sepsis [52].
Based on the available evidence, the committee strongly recommends empirical broad-spectrum antibacterial therapy for patients presenting with sepsis to cover all pathogenic bacteria that are likely to be involved. Prior (< 1 year), relevant cultures and local distribution of pathogens associated with sepsis and their antimicrobial susceptibilities should guide the ultimate choice. Although there is a lack of strong evidence, the committee suggests to empirically cover HRMO when these are likely to be involved and to cover anaerobic bacteria in patients presenting with abdominal sepsis or necrotizing soft tissue infections. We suggest against the routine empirical treatment of anaerobic bacteria in sepsis due to aspiration pneumonia, unless empyema or a lung abscess is suspected. Similarly we recommend against the routine empirical treatment of enterococci, but to consider treatment in individual patients with sepsis, such as those who have a high likelihood of enterococcal involvement based on recent relevant cultures and those with recent complicated intra-abdominal surgery or a suspected CVC infection and substantial exposure to broad spectrum antibiotics.
What is the effect of double active empirical antibacterial therapy compared to monotherapy in patients with sepsis?
We defined double active antibacterial therapy as treatment with two classes of antibiotics, both targeting the known or suspected causing pathogen(s) (e.g., ceftriaxone and an aminoglycoside to target gram-negative pathogens) and with the specific purpose to accelerate pathogen clearance rather than to broaden antimicrobial coverage.
Pooled data in a meta-analysis showed no additional effect on all-cause mortality and clinical failure of beta-lactam plus aminoglycoside double active therapy compared to the same or a different beta-lactam when given as monotherapy in patients with sepsis [53]. An increased risk of clinical failure and nephrotoxicity for beta-lactam plus aminoglycoside double active therapy compared to a different beta-lactam given as monotherapy was reported [53]. Other meta-analyses and randomized trials also showed no additional effect of empirical double active antibacterial therapy compared to empirical monotherapy on clinical outcomes in patients with sepsis and septic shock [54], patients with S. aureus bacteraemia [55], patients with severe P. aeruginosa infections [53, 56, 57], and patients with VAP [58, 59].
Based on these data the committee recommends against the use of double active antibacterial therapy in patients with sepsis and septic shock, provided that the chosen single antibacterial agent is active against the most likely pathogens involved. In line, we suggest against double active antibacterial therapy in patients with sepsis due to P. aeruginosa and S. aureus.
What is the optimal choice of empirical therapy in patients with sepsis in The Netherlands
Most trials in patients with severe infections compared cephalosporins, carbapenems, piperacillin-tazobactam and some fluoroquinolones. Clinical outcomes did not consistently support that one of these classes of antibiotics is considerably more effective than others in patients with sepsis. Aminoglycoside-based regimens for sepsis due to HAP or VAP were associated with lower clinical response rates [59]. For sepsis due to intra-abdominal infections, aminoglycoside monotherapy was less effective compared to beta-lactam treatment [60, 61]. One large randomized multicentre trial (MERINO) compared definitive therapy with piperacillin-tazobactam to meropenem in patients with bloodstream infections caused by ceftriaxone-resistant, piperacillin-tazobactam and meropenem sensitive E. coli and K. pneumonia [62]. The 30-day all-cause mortality was 12.3% in patients treated with piperacillin-tazobactam and 3.7% in patients treated with meropenem. There were no trials available on optimal antibiotic treatment of sepsis and high likelihood of S. aureus involvement.
The guideline committee concluded that based on the current data about efficacy and safety of beta-lactams, the experience with beta-lactams and the large number of trials using a beta-lactam, beta-lactams are most appropriate as empirical and definite therapy in the majority of patients with sepsis. Based on the available literature, fluoroquinolones are acceptable alternatives when the risk of fluoroquinolone resistance is considered low. However, clinicians should be aware that the use of fluoroquinolones has significant disadvantages regarding toxicity and the development of resistance [63–66]. Regarding aminoglycosides, the committee expresses their concerns on potential lower efficacy and higher toxicity risk, but settled that current (lack of) evidence still supports short-term (i.e., maximum of 2 days) aminoglycoside treatment added to a beta-lactam agent in patients with sepsis with the only purpose of increasing the empirical antibacterial spectrum of activity until susceptibility results are available. This strategy is therefore mainly applicable to gram-negative bacteria such as 3GCR-E or P. aeruginosa. Although questions remain, the committee found the evidence on the difference in mortality in the MERINO trial convincing enough to currently recommend against the use of BL/BI and specifically piperacillin-tazobactam for the treatment of sepsis in patients at risk of or with proven involvement of 3GCR Enterobacterales [62].
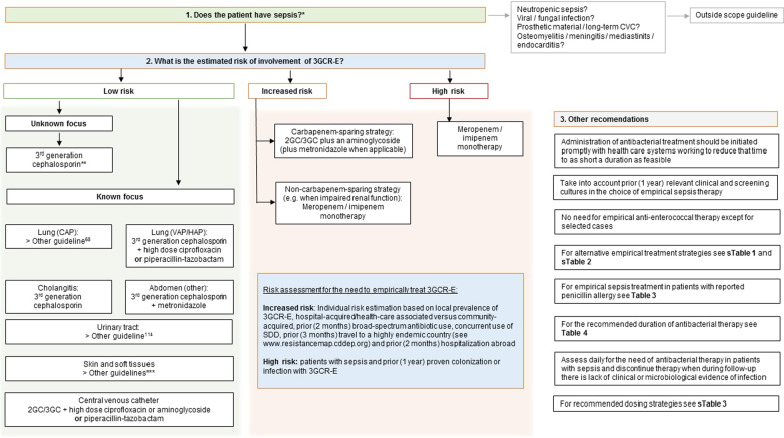
The choice of empirical sepsis therapy is primarily based on the source of infection. Empirical treatment strategies should be further dictated by the likelihood of involvement of a resistant causative pathogen, by the desire to prevent overuse of reserve antibiotics from a stewardship perspective and by risks of toxicity and other potential adverse events for the patient. The committee therefore provided pragmatic suggestions and alternative strategies for patients with low risk of 3GCR-E involvement and patients at increased or high risk of involvement of 3GCR-E (Additional file 1: Tables S1 and S2). Recommendations are also summarized in Fig. 1. If a definite diagnosis is established one should be referred to other guidelines for empiric antibiotic therapy, e.g., current community-acquired pneumonia (CAP) guidelines do apply in the case of pneumonia-derived sepsis [67].
Fig. 1.
Flow chart of guideline recommendations on empirical antibiotic treatment of sepsis. *For the diagnosis and non-antibiotic treatment of sepsis we refer to the Dutch guideline ‘Sepsis fase 1’ [4]. **For this guideline, 3GC includes ceftriaxone and cefotaxime and does not include the anti-pseudomonal cephalosporin ceftazidime. ***Guidelines on skin and soft tissue infections [109, 110]. 3GCR-E: 3rd generation cephalosporin-resistant Enterobacterales; 2GC: second generation cephalosporin; 3GC: 3rd generation cephalosporin; SDD: selective decontamination of the digestive tract. CVC: central venous catheter; CAP: community-acquired pneumonia; VAP: ventilator-associated pneumonia; HAP: hospital-acquired pneumonia
What is the optimal empirical antibacterial therapy of sepsis in patients with a penicillin allergy?
Accumulating data from observational cohort studies indicate that true penicillin allergy in patients with a reported penicillin allergy is relatively rare and that avoiding beta-lactams negatively affects treatment outcome [68, 69]. The committee set up a pragmatic approach based on available observational studies including a strong recommendation to obtain information (i.e., medical history and skin test results) about the presumed allergy if possible (Table 3).
Table 3.
Empirical antibacterial therapy of sepsis in patients with a penicillin allergy label
| Available allergy label data for penicillins (e.g., amoxicillin, amoxicillin-clavulanic acid, flucloxacillin, penicillin G) | Administration of a penicillin during sepsis | Administration of a cephalosporin or carbapenem during sepsis |
|---|---|---|
| Immediate type or delayed typea reaction very unlikely | Yes | Yes |
| Possible immediate type reaction occurred > 10 years ago AND symptoms were mild to moderate | Nob | Yes |
| Possible immediate type reaction occurred < 10 years ago AND/OR reaction was severe (i.e., anaphylactic shock, airway oedema etc.) | Nob | Yesc |
| Allergy testing previously confirmed immediate type penicillin allergy | No | Yesc |
| Information about the allergy label is not available | Nob | Yes |
aIn case of delayed type reactions e.g., Stevens-Johnson syndrome (SJS), toxic epidermic necrolysis (TEN), tubulointerstitial nephritis (TIN), on a beta-lactam antibiotic, avoid the respective penicillin and choose alternative treatment or consult expert; bAfter the patient has recovered from sepsis, skin testing and/or controlled challenge with a beta-lactam may be considered to confirm or rule out allergy to beta-lactams; cRisk of a severe immediate type cross allergic reaction is still estimated to be < 1%; Exposure may be avoided until skin-tests or controlled challenge is possible
Timing and duration of antibacterial therapy in sepsis
What is the optimal timing of empirical antibacterial therapy in patients with sepsis?
In the previous edition of the SWAB sepsis guidelines, it was recommended to start antibacterial therapy in adult patients with severe sepsis and septic shock as soon as possible, preferably within the first hour of presentation [22]. The recommendation was mainly based on the results of one study showing that each hour delay in antibiotic therapy resulted in an average decrease in survival of 7.6% [19], an observation that was underlined by other retrospective observational studies [70–73]. However, a more recent meta-analysis, which included the aforementioned observational studies, did not show a significant mortality benefit of administering antibiotics within 3 h of ER triage or within 1 h of shock recognition in sepsis [74]. In line, a randomized trial on this topic could not demonstrate an effect of faster (prehospital) antibiotic administration for sepsis on outcome in a Dutch setting [32]. This study however only included a small number of patients with septic shock.
Based on available evidence, the committee deemed it reasonable to state that in patients with septic shock, antibiotics should be administered as soon as possible [71, 72]. On the other hand, in sepsis patients without shock, rapid antibiotic administration should be weighed against the negative impact of potentially unjustified antibiotic use when the patient turns out not to suffer from sepsis [75–77].
The guidelines committee therefore agreed not to recommend a specific timeframe in which antibiotics should be administered in patients with sepsis and septic shock. In line with a Dutch trial on the impact of emergency department staff training on time to antibiotic administration and with an earlier Infectious Disease Society of America (IDSA) position statement, the committee recommends that the administration of antibacterial treatment in patients with sepsis or septic shock should be initiated promptly with health care systems working to reduce that time to as short as feasible [32, 75].
What is the optimal duration of antibacterial treatment for sepsis?
Several meta-analyses [59, 78, 79], an RCT [80] and a large propensity-adjusted observational study [81] consistently showed that shorter duration of treatment is as effective and safe as the traditional, longer duration of treatment, in patient with sepsis. Similar results have been found in patients with mild to moderate-severe CAP [67], pyelonephritis [82], uncomplicated cellulitis [83], and bacteraemia [84]. In line, indirect evidence from the studies on PCT-guided discontinuation of antibacterial treatment in patients with sepsis in the ICU setting also suggests that shorter antibacterial treatment duration is safe without a negative effect on mortality [85–88]. These data, together with the potential adverse effects of antibiotic overuse, strengthened the committee to generally suggest durations of antibiotic therapy in most patients with sepsis that are shorter than historical treatment durations. Table 4 shows recommended treatment durations based on source of infection. Based on available evidence [85, 89–91], the committee suggests using PCT levels to support shortening the duration of antibacterial therapy in patients with sepsis if the optimal duration of antibiotic therapy is unclear.
Table 4.
Suggested antibacterial therapy duration in patients with sepsis
| Focus of sepsis | Suggested antibacterial treatment duration |
|---|---|
| Intra-abdominal infections, following effective source control and with favourable clinical response | Four days [111–114] |
| Cholangitis, following adequate drainage of the biliary tree | Up to 3 days [115] |
| VAP | Seven days [59] |
| HAP | Seven days |
| CVC infection with gram-negative pathogen, following removal of the CVC and with favourable clinical response | Up to 7 days [80] |
| CVC infection with CNS or enterococci, following removal of the CVC and with favourable clinical response | Zero to 7 days |
| No clear focus | Seven days [80] |
Studies showed conflicting findings on the efficacy and safety of antibiotic de-escalation (ADE) [92–95]. Within the SWAB sepsis guideline committee there was consensus that ADE is appropriate in many clinical situations. In line with other relevant guidelines the committee recommends to consider ADE in all patients who are on sepsis treatment after 48 h of treatment [88, 96]. We also suggest this would include patients in whom only limited or indirect cultures show no causative pathogen. In contrast, with current conflicting evidence, including the negative outcomes of ADE in one trial on ICU length of stay the committee felt it is defendable not to perform ADE in selected individual patients [95].
Pharmacokinetic and pharmacodynamic considerations in sepsis
In patients with sepsis, should we recommend pharmacokinetic/pharmacodynamic dosing optimization for empirical antibacterial therapy?
Many pathophysiological changes typical for sepsis patients can alter the pharmacokinetic properties of antibiotics and can lead to inadequate antibiotic concentrations when using standard antibiotic dosing schedules [97–102]. These pathophysiologic changes include kidney dysfunction, augmented renal clearing (the enhanced renal function sometimes seen in critically ill patients), hypoalbuminemia and increased third space due to fluid therapy [96, 98]. Drug concentrations of hydrophilic antibacterial agents (such as beta-lactams, aminoglycosides and vancomycin) are generally more sensitive to pharmacokinetic changes in patients with sepsis than lipophilic antibacterial agents (such as fluoroquinolones). In addition, patients with sepsis may generally be at higher risk to be infected with bacteria with higher MICs in comparison to other patients [98].
Pooled RCT data in patients with sepsis showed that extended or continuous infusion of beta-lactams in general was associated with decreased all-cause mortality, increased clinical cure with no effect on adverse events and development of resistance compared to intermittent infusion. Evidence was particularly strong for extended infusion of piperacillin-tazobactam and meropenem [103–105]. There was lack of evidence for the effect of pharmacokinetic/pharmacodynamic (PK/PD)-based dosing on clinical outcomes of aminoglycosides, vancomycin and ciprofloxacin and in obese patients. The committee felt that the available evidence supports a recommendation of PK/PD-based dosing [96, 98, 106–108]. Since EUCAST recommendations on breakpoints are generally accepted and based on PK/PD principles, we followed the EUCAST dosing recommendations for specific pathogens (Additional file 1: Table S3) [31]. We recommended therapeutic drug monitoring (TDM) for all patients on aminoglycoside and vancomycin treatment.
For a complete list of guidelines recommendations, see Table 5. A flow chart is provided in Fig. 1, which summarizes the given recommendations on the empirical antibacterial treatment of sepsis. See Text box 1 for a summary of all the new recommendations compared with the 2010 guideline. The full guidelines text, literature review and rebuttal of the received commentaries are available at www.swab.nl.
Table 5.
Recommendations of the SWAB sepsis guideline 2021
| Recommendation | Strength | Quality of evidence |
|---|---|---|
| I Causative bacterial pathogens in sepsis | ||
| Which patients are at risk for sepsis due to third-generation cephalosporin-resistant Enterobacterales or P. aeruginosa in the Netherlands? | ||
| 1. We recommend empirical therapy against 3GCR-E in patients with sepsis and prior (1 year) proven infection or colonization with 3GCR-E | Strong | Very low |
|
2. We suggest that clinicians take into account the risk of 3GCR-E involvement in sepsis on an individual patient basis to decide if empirical antibacterial therapy against 3GCR-E is appropriate Factors to guide this decision include local prevalence of 3GCR-E, if the infection is hospital-acquired/health-care associated versus community-acquired, prior (2 months) broad-spectrum antibiotic use, concurrent use of SDD, prior (3 months) travel to a highly endemic country (see https://resistancemap.cddep.org/) and prior (2 months) hospitalization abroad |
Weak | Very low |
| 3. We recommend empirical therapy against P. aeruginosa in patients with sepsis and prior (1 year) infection or colonization with P. aeruginosa | Strong | Very low |
| II Empirical antibacterial therapy in sepsis | ||
| What is the importance of appropriate empirical therapy in patients with sepsis? | ||
| 4. We recommend empirical broad-spectrum antibacterial therapy for patients presenting with sepsis to cover all pathogenic bacteria that are likely to be involved | Strong | Moderate |
| 5. We recommend to take into account prior (1 year) resistance in relevant clinical and screenings cultures in the choice of empirical sepsis therapy | Strong | Very low |
| 6. We recommend that empirical antibacterial therapy is guided by the local distribution of pathogens associated with sepsis and their antimicrobial susceptibilities | Strong | Very low |
| 7. We suggest empirical antibacterial therapy for patients presenting with sepsis to cover HRMO when these are likely to be involved | Weak | Very low |
| 8. We suggest empirical antibacterial therapy covering anaerobic bacteria for patients presenting with sepsis and intra-abdominal infections of the lower intestinal tract or necrotizing soft tissue infections | Weak | Very low |
| 9. We generally suggest against routine empirical treatment of anaerobic bacteria in patients presenting with sepsis due to aspiration pneumonia, unless empyema or a lung abscess is suspected | Weak | Very low |
| 10. We generally recommend against routine empirical treatment of enterococci in patients presenting with sepsis | Strong | Moderate |
| 11. We suggest that anti-enterococcal therapy could be considered in individual patients with sepsis, e.g., those who have a high likelihood of enterococcal involvement based on recent relevant cultures and those with recent complicated intra-abdominal surgery or a suspected CVC infection and substantial exposure to broad spectrum antibiotics | Weak | Very low |
| What is the effect of double active empirical antibacterial therapy compared to monotherapy in patients with sepsis? | ||
| 12. We recommend against routine double active empirical antibacterial therapya for patients with sepsis or septic shock | Strong | Moderate |
| 13. We suggest that double active therapy is not routinely used as definite therapy for patients with sepsis due to P. aeruginosa infection | Weak | Very low |
| 14. We suggest that double active therapy is not routinely used as definite therapy for patients with sepsis due to S. aureus infection not associated to prosthetic material | Weak | Moderate |
| What is the optimal choice of empirical therapy in patients with sepsis in the Netherlands? | ||
| Antibacterial therapy in patients with sepsis in general | ||
| 15. In patients with sepsis, we generally recommend using a beta-lactam antibiotic covering the most likely involved pathogens | Strong | Moderate |
| 16. In patients with sepsis in general / with no obvious source of infection, we suggest a 3rd generation cephalosporin (3GC). Alternative empirical treatment strategies are listed in Additional file 1: Table S1 | Weak | Low |
| 17. In patients with sepsis due to HAP or VAP, we suggest that there are equivalent empirical treatment strategies, listed in Additional file 1: Table S1 | Weak | Low |
| 18. In patients with sepsis due to cholangitis, we suggest a 3GC. Alternative empirical treatment strategies are listed in Additional file 1: Table S1 | Weak | Low |
|
19. In patients with sepsis due to intra-abdominal infection, we suggest a combination of a 3GC with metronidazole Alternative empirical treatment strategies are listed in Additional file 1: Table S1 |
Weak | Low |
| 20. In patients with sepsis and a suspected CVC infectionb, we recommend prompt removal of the line | Strong | GPS |
|
21. In patients with sepsis and suspected CVC infection, we suggest empirical treatment with a 3GCc with gentamicin or high dose ciprofloxacin Alternative treatment strategies are listed in Additional file 1: Table S1 |
Weak | GPS |
| 22. For the empirical treatment of sepsis due to UTI, CAP and SSSI’s, we refer to other guidelines [67, 109, 110, 116] | ||
| Antibacterial therapy in patients with sepsis and increased risk of involvement of 3GCR-E | ||
|
23. In patients with sepsis and high risk of involvement of 3GCR-E based on prior (1 year) infection/colonization, we recommend meropenem or imipenem as empirical antibacterial therapy Alternative strategies are listed in Additional file 1: Table S2 |
Strong | Moderate |
| 24. In patients with sepsis and increased risk of involvement of 3GCR-E but no prior (1 year) infection/colonization, we suggest that a carbapenem-sparing strategy Additional file 1: Table S2 is acceptable | Weak | Very low |
| 25. We cannot provide a recommendation for or against empirical or definite treatment with piperacillin-tazobactam in patients with sepsis due to chromosomal AmpC-producing Enterobacterales (such as Enterobacter, Serratia, Citrobacter, Providencia and Morganella spp) | – | – |
| 26. In patients with sepsis due to ESBL-producing Enterobacterales, we recommend against piperacillin-tazobactam as definite antibacterial therapy regardless of the in vitro susceptibility | Strong | Moderate |
| Antibacterial therapy in patients with sepsis and increased risk of involvement of Staphylococcus aureus | ||
| 27. There is insufficient evidence to recommend against empirical use of other beta-lactam antibiotics than flucloxacillin or cefazolin in patients with sepsis in which S. aureus is a likely pathogen | - | - |
| 28. For definite therapy of patients with sepsis due to S. aureus, we refer to the Dutch guideline on S. aureus bacteraemia [117] | ||
| What is the optimal empirical antibacterial therapy of sepsis in patients with a penicillin allergy? | ||
| 29. In patients with sepsis and a reported penicillin allergy, we recommend to obtain information (i.e., medical history and skin test results) about the presumed allergy if possible | Strong | GPS |
| 30. In patients with sepsis and a reported penicillin allergy but in whom the allergy is very unlikely, we suggest that penicillins can be used if needed (see Table 3) | Weak | Very low |
| 31. In patients with sepsis and a reported penicillin allergy that was proven, possible or unspecified, we suggest to avoid penicillins during the primary sepsis treatment and to choose alternative beta-lactams (cephalosporins, carbapenems) | Weak | Very low |
| 32. In patients with sepsis and an unspecified or possible immediate type penicillin allergy, we suggest to plan penicillin allergy testing and/or a controlled penicillin challenge when the patient has recovered from sepsis | Weak | Very low |
| III Timing and duration of antibacterial therapy in sepsis | ||
| What is the optimal timing of empirical antibacterial therapy in patients with sepsis? | ||
| 33. In patients with sepsis or septic shock, we recommend that the administration of antibacterial treatment should be initiated promptly with health care systems working to reduce that time to as short a duration as feasible | Strong | Low |
| What is the optimal duration of antibacterial treatment for sepsis? | ||
| 34. For treatment duration of sepsis due to CAP, UTI, SSSI and of sepsis due to S. aureus infection, we refer to other guidelines [67, 109, 110, 116–118] | ||
| 35. We recommend source control interventions when possible to support antibacterial treatment in patients with sepsis | Strong | Low |
| 36. We recommend that a 4-day course of antibacterial treatment is appropriate for patients with sepsis due to intra-abdominal infections following effective source control and with favourable clinical response | Strong | Moderate |
| 37. We suggest that shorter courses of antibacterial treatment (up to 3 days) are appropriate in patients with sepsis and cholangitis following adequate drainage of the biliary tree | Weak | Very low |
| 38. We recommend that an antibacterial treatment duration of 7 days is adequate for most patients with sepsis due to VAP | Strong | Moderate |
| 39. We suggest that an antibacterial treatment duration of 7 days is adequate for most patients with sepsis due to HAP | Weak | Very low |
| 40. We suggest that an antibacterial treatment duration of 7 days at maximum is adequate for most patients with sepsis due to suspected CVC infection with gram-negative pathogens following removal of the CVC and with favourable clinical response | Weak | Very low |
| 41. We suggest that an antibacterial treatment duration of 0 to 7 days is adequate for most patients with sepsis due to suspected CVC infection with CNS or enterococci following removal of the CVC and with favourable clinical response | Weak | GPS |
| 42. We suggest that an antibacterial treatment duration of 7 days is adequate for sepsis and septic shock without a clear focus in most patients with favourable clinical response | Weak | Low |
| 43. We recommend daily assessment for the need of antibacterial therapy in patients with sepsis and to discontinue therapy when during follow-up there is lack of clinical or microbiological evidence of infection | Strong | GPS |
| 44. We suggest that procalcitonin levels are used to support shortening the duration of antibacterial therapy in patients with sepsis if optimal duration of antibiotic therapy is unclear | Weak | Moderate |
| 45. We recommend to consider antibiotic de-escalation (resulting in smaller spectrum antibiotics) in all patients on antibiotics for sepsis on a daily basis and based on pathogen identification, sensitivities and risk of adverse events | Strong | Very low |
| 46. We recommend to stop empirical aminoglycoside therapy within a maximum of two days | Strong | Low |
| 47. We recommend to switch systemic antibiotic therapy from intravenous to oral antibiotic therapy after 48–72 h on the basis of the clinical condition and when oral treatment is feasible | Strong | Very low |
| IV Pharmacokinetic and pharmacodynamic considerations in sepsis | ||
| In patients with sepsis, should we recommend pharmacokinetic/pharmacodynamic dosing optimization for empirical antibacterial therapy? | ||
| 48. In patients with sepsis, we suggest that dosing strategies of antibacterial therapy be optimized based on accepted pharmacokinetic / pharmacodynamic principles and specific drug properties (Additional file 1: Table S3) | Weak | Low |
| 49. In patients with sepsis we recommend prolonged or continuousd infusion of piperacillin-tazobactam and carbapenems | Strong | High |
| 50. In patients with sepsis we suggest prolonged or continuousd infusion of other beta-lactam antibiotics than piperacillin-tazobactam and carbapenems | Weak | Low |
| 51. In patients with sepsis, we recommend direct therapeutic drug monitoring (including either mid-dosing or both peak and through levels) during aminoglycoside treatment in patients with sepsis and septic shock | Strong | GPS |
| 52. In patients with sepsis, we recommend therapeutic drug monitoring during vancomycin treatment in patients with sepsis and septic shock | Strong | GPS |
| 53. In patients with sepsis, we suggest therapeutic drug monitoring when there are concerns on target attainment of other antibacterial drugs than aminoglycoside and vancomycin (e.g., extreme body weight, augmented or decreased renal clearance, hypoalbuminemia) | Weak | GPS |
| 54. In patients with sepsis, we suggest continuousd infusion of vancomycin | Weak | GPS |
| 55. In patients with sepsis in whom ciprofloxacin is indicated, we suggest empirical ciprofloxacin three times daily 400 mg iv | Weak | GPS |
aWe defined double active antibacterial therapy as treatment with two classes of antibiotics, both targeting the known or suspected causing pathogen(s) (e.g., ceftriaxone and an aminoglycoside to target gram-negative pathogens) and with the specific purpose to accelerate pathogen clearance rather than to broaden antimicrobial coverage. Also frequently referred to as combination antibiotic therapy. Of note, the use of two antibiotics for the increased likelihood of covering the causing agent (broadening the spectrum), or for covering multiple causing agents (e.g., aerobic and anaerobic bacteria) was not included in the definition of double active therapy
bRecommendations for sepsis due to suspected long-term CVC’s were not included in this guideline
c3GC may be given in high dose for more optimal PK/PD for S. aureus infections in accordance to EUCAST
dContinuous infusion includes one intermittent dose as a loading dose
Textbox 1: What is new since the Dutch 2010 SWAB guidelines were published?
| • One important revision is the distinction between low, increased and high risk of infection with Enterobacterales resistant to third generation cephalosporins (3GRC-E) to guide the choice of empirical therapy. 3GCR-E is often used as a proxy for ESBL-production. The committee recommends covering 3GCR-E in patients if prior (1-year) culture revealed 3GCR-E. In patients without prior (1-year) cultures showing 3GCR-E the decision to empirically cover 3GCR-E should be made on an individual patient basis, taking into account multiple risk factors |
| • The choice of empirical antibacterial treatment of sepsis is dictated by the likelihood of involvement of a resistant causative pathogen, by the desire to prevent overuse of reserve antibiotics from a stewardship perspective and by risks of toxicity and other potential adverse events for the patient. Strong recommendations on the best empirical treatment in sepsis based on the currently available literature cannot be given since only subtle differences between strategies on clinical outcomes are found in studies that were also frequently not generalizable to the Dutch clinical setting. Every strategy has advantages and disadvantages depending on the mentioned perspectives (resistance epidemiology, pharmacokinetic/pharmacodynamic (PK/PD) properties, antibiotic stewardship, adverse events). Consequently, the committee provided pragmatic suggestions for empirical treatment choices in patients with sepsis based on current evidence, reported resistance rates nationally, the antibiotic stewardship perspective and risk of adverse events |
| • In patients with sepsis, we generally recommend using a beta-lactam antibiotic covering the most likely involved pathogens. Also, we recommend covering pathogens in prior (1-year) relevant cultures in general. We added suggestions on empirical therapy in case Pseudomonas aeruginosa, Staphylococcus aureus and Enterococcus spp are considered |
| • We provided new suggestions for empirical therapy in patients with sepsis and a reported penicillin allergy |
| • Regarding the duration of therapy, we generally recommended shorter treatment durations of patients with sepsis than the previous guidelines. The committee also underscores the responsibility of clinicians to de-escalate antibacterial therapy in patients with sepsis. Due to toxicity concerns, we strongly recommend stopping empirical aminoglycoside treatment after 2 days |
| • Among recommendations on PK/PD considerations in patients with sepsis, the committee strongly recommends continuous or prolonged infusion of piperacillin-tazobactam and meropenem based on high quality evidence. Therapeutic drug monitoring is recommended for all patients on aminoglycoside and vancomycin treatment |
Conclusions
We described the most important findings and recommendations of our multidisciplinary guideline committee for the 2020 SWAB sepsis guidelines. We formulated 55 recommendations on the antibacterial management of sepsis in adults in total. One crucial revision is the distinction between low, increased and high risk of infection with Enterobacterales resistant to third generation cephalosporins (3GRC-E) to guide the choice of empirical therapy. Other new topics included empirical antibacterial therapy in patients with a reported penicillin allergy and the role of pharmacokinetics and pharmacodynamics to guide dosing in sepsis.
Supplementary Information
Additional file 1. sTable 1. Alternative empirical treatment strategies in sepsis and low estimated risk of involvement of 3GCR-E. sTable 2. Alternative empirical treatment strategies in sepsis and increased or high estimated risk of involvement of 3GCR-E. sTable 3. Recommended iv doses of empirical antibacterial treatment for sepsis.
Acknowledgements
The Guidelines Committee would like to thank all individuals and societies who contributed to the development of these guidelines.
Author contributions
All authors contributed to the project conception and design. Data collection and first analysis were performed by ES, HIB, JJH, MGJB, MK, JS and WJW. The first draft of the manuscript was written by ES, HIB, MGJB, MK, PDL, DCM, JAS, JMP, WJW and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This projected was funded by the Dutch Working Party on Antibiotic Policy (SWAB), Leiden, the Netherlands.
Availability of data and materials
Literature searches are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Members of the preparatory committee reported the following potential conflicts of interest: ES: None; HIB: None; JJH: None; MGJB: Chair Stichting Werkgroep Antibioticabeleid, Chair COIG Infectieziekten en Immuniteit, NIV; MB: Institutional payment for consultancy / advisory board for Johnson&Johnson, Bard, Acelity, Gore, Institutional grants Johnson&Johnson, Mylan, Bard, Acelity, LifeCell/Allergan, New Compliance; MJMB: Consultant for Janssen Vaccines, (paid to UMCU), Research funding Janssen Vaccines, Immunexpress, Vedanta, (paid to UMCU), research funding Innovative Medicines Initiative (paid to UMCU); DD: None; RGW: Board member EAACI (European Academy of Allergy and Clinical Imunology), president UEMS Section and Board of Allergology, member of several national and international guideline committees; NPJ: Research funding ZonMW, Sanquin, Horizon2020, CSL Behring (not related to the current guideline); MK: None; PDL: Treasurer Stichting Werkgroep Antibioticabeleid; DCM: None; PP: Consultancy board memberships (not related to the current guideline), chair SepsisNet Nederland Foundation (unpaid); JAS: Member Wetenschappelijke Adviesraad (paid), Radboud Center Infectious Diseases, ESGAP (ESCMID) secretary, SWAB member, unrestricted educational Grant MSD; JRR: Curriculum-, en implementatiecommissie NVSHA, visitatiecommissie NVSHA, member of local sepsis guideline committee; ARHZ: Head of ICU & Research, Ziekenhuis Gelderse Valei, Ede, chair SKMS guideline Sepsis, research grants Adrenoss, Nutricia, Beacon, Cardinal Health, lectures (paid) not related to antibacterial therapy of Abbott, Baxter, BBraun, Fresenius-Kabi, Lyric, Nutricia, Nestle. The SKMS Sepsis guideline has been developed parallel to the SWAB sepsis guideline. JMP: editor in chief digital guideline antimicrobial therapy “SWAB ID”, (paid to AMC), member Wetenschappelijke Adviesraad Zorginstituut Nederland, CG (paid), member Board Stichting de Merel (non-profit, paid to AMC); WJW: Research funding from NWO, ZonMW and Horizon2020 (not related to the current guideline).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Zanten AR, Sankatsing SU, de Regt MJ, Derde LP, Klijn A, Schellaars R, Wilting RM, et al. Guideline Sepsis fase 1 (2020). Available at: https://richtlijnendatabase.nl/richtlijn/sepsis_fase_1/vroege_herkenning_van_dreigende_sepsis.html.
- 5.Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 6.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med. 2004;30(4):589–596. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 8.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318(13):1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- 10.Davis JS, He V, Anstey NM, Condon JR. Long term outcomes following hospital admission for sepsis using relative survival analysis: a prospective cohort study of 1,092 patients with 5 year follow up. PLoS ONE. 2014;9(12):e112224. doi: 10.1371/journal.pone.0112224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreiher J, Almog Y, Sprung CL, Codish S, Klein M, Einav S, Bar-Lavie Y, Singer PP, Nimrod A, Sachs J, et al. Temporal trends in patient characteristics and survival of intensive care admissions with sepsis: a multicenter analysis*. Crit Care Med. 2012;40(3):855–860. doi: 10.1097/CCM.0b013e318236f7b8. [DOI] [PubMed] [Google Scholar]
- 12.Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States-an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46(12):1889–1897. doi: 10.1097/CCM.0000000000003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Gestel A, Bakker J, Veraart CP, van Hout BA. Prevalence and incidence of severe sepsis in Dutch intensive care units. Crit Care. 2004;8(4):R153–162. doi: 10.1186/cc2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadri SS, Rhee C, Strich JR, Morales MK, Hohmann S, Menchaca J, Suffredini AF, Danner RL, Klompas M. Estimating ten-year trends in septic shock incidence and mortality in united states academic medical centers using clinical data. Chest. 2017;151(2):278–285. doi: 10.1016/j.chest.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barie PS, Hydo LJ, Shou J, Larone DH, Eachempati SR. Influence of antibiotic therapy on mortality of critical surgical illness caused or complicated by infection. Surg Infect (Larchmt) 2005;6(1):41–54. doi: 10.1089/sur.2005.6.41. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118(1):146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 20.Vazquez-Guillamet C, Scolari M, Zilberberg MD, Shorr AF, Micek ST, Kollef M. Using the number needed to treat to assess appropriate antimicrobial therapy as a determinant of outcome in severe sepsis and septic shock. Crit Care Med. 2014;42(11):2342–2349. doi: 10.1097/CCM.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 21.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010;54(11):4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SWAB guidelines for Antibacterial therapy of adult patients with Sepsis (2010). Available at: http://www.swab.nl/swab/cms3.nsf/uploads/65FB380648516FF2C125780F002C39E2/$FILE/swab_sepsis_guideline_december_2010.pdf.
- 23.NethMap 2018—Consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands in 2017. Availabl at: https://www.swab.nl/swab/cms3.nsf/uploads/AF0C15331EF7438AC12582BF00389A16/$FILE/NethMap-Maran%202018.pdf.
- 24.van der Steen M, Leenstra T, Kluytmans JA, van der Bij AK. Trends in expanded-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae among Dutch Clinical Isolates, from 2008 to 2012. PLoS ONE. 2015;10(9):e0138088. doi: 10.1371/journal.pone.0138088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlek AL, Frentz D, Haenen A, Bootsma HJ, Notermans DW, Frakking FN, de Greeff SC, Leenstra T. Detection and epidemiology of carbapenemase producing Enterobacteriaceae in the Netherlands in 2013–2014. Eur J Clin Microbiol Infect Dis. 2016;35(7):1089–1096. doi: 10.1007/s10096-016-2636-6. [DOI] [PubMed] [Google Scholar]
- 26.Bakhit M, Jones M, Baker J, Nair R, Yan K, Del Mar C, Scott AM. Reporting of adverse events, conflict of interest and funding in randomised controlled trials of antibiotics: a secondary analysis. BMJ Open. 2021;11(7):e045406. doi: 10.1136/bmjopen-2020-045406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips R, Hazell L, Sauzet O, Cornelius V. Analysis and reporting of adverse events in randomised controlled trials: a review. BMJ Open. 2019;9(2):e024537. doi: 10.1136/bmjopen-2018-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tacconelli E, Cataldo MA, Paul M, Leibovici L, Kluytmans J, Schröder W, Foschi F, De Angelis G, De Waure C, Cadeddu C, et al. STROBE-AMS: recommendations to optimise reporting of epidemiological studies on antimicrobial resistance and informing improvement in antimicrobial stewardship. BMJ Open. 2016;6(2):e010134. doi: 10.1136/bmjopen-2015-010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. J Clin Epidemiol. 2010;63(12):1308–1311. doi: 10.1016/j.jclinepi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62(4):e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 8.0 2018. Available at: http://www.eucast.org/clinical_breakpoints/.
- 32.Alam N, Oskam E, Stassen PM, Exter PV, van de Ven PM, Haak HR, Holleman F, Zanten AV, Leeuwen-Nguyen HV, Bon V, et al. Prehospital antibiotics in the ambulance for sepsis: a multicentre, open label, randomised trial. Lancet Respir Med. 2018;6(1):40–50. doi: 10.1016/S2213-2600(17)30469-1. [DOI] [PubMed] [Google Scholar]
- 33.Klein Klouwenberg PM, Cremer OL, van Vught LA, Ong DS, Frencken JF, Schultz MJ, Bonten MJ, van der Poll T. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care. 2015;19:319. doi: 10.1186/s13054-015-1035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sartelli M, Catena F, Ansaloni L, Leppaniemi A, Taviloglu K, van Goor H, Viale P, Lazzareschi DV, Coccolini F, Corbella D, et al. Complicated intra-abdominal infections in Europe: a comprehensive review of the CIAO study. World J Emerg Surg. 2012;7(1):36. doi: 10.1186/1749-7922-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altorf-van der Kuil W, Schoffelen AF, de Greeff SC, Thijsen SF, Alblas HJ, Notermans DW, Vlek AL, van der Sande MA, Leenstra T. National laboratory-based surveillance system for antimicrobial resistance: a successful tool to support the control of antimicrobial resistance in the Netherlands. Euro Surveill. 2017; 22(46). [DOI] [PMC free article] [PubMed]
- 36.PREZIES - Referentiecijfers 2012 t/m 2016: lijnsepsis. Available at: https://www.rivm.nl/sites/default/files/2021-06/Referentiecijfers%202016.pdf.
- 37.Ong DSY, Frencken JF, Klein Klouwenberg PMC, Juffermans N, van der Poll T, Bonten MJM, Cremer OL. Short-course adjunctive gentamicin as empirical therapy in patients with severe sepsis and septic shock: a prospective observational cohort study. Clin Infect Dis. 2017;64(12):1731–1736. doi: 10.1093/cid/cix186. [DOI] [PubMed] [Google Scholar]
- 38.Alevizakos M, Karanika S, Detsis M, Mylonakis E. Colonisation with extended-spectrum beta-lactamase-producing Enterobacteriaceae and risk for infection among patients with solid or haematological malignancy: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;48(6):647–654. doi: 10.1016/j.ijantimicag.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Rottier WC, Bamberg YR, Dorigo-Zetsma JW, van der Linden PD, Ammerlaan HS, Bonten MJ. Predictive value of prior colonization and antibiotic use for third-generation cephalosporin-resistant Enterobacteriaceae bacteremia in patients with sepsis. Clin Infect Dis. 2015;60(11):1622–1630. doi: 10.1093/cid/civ121. [DOI] [PubMed] [Google Scholar]
- 40.Rottier WC, van Werkhoven CH, Bamberg YRP, Dorigo-Zetsma JW, van de Garde EM, van Hees BC, Kluytmans J, Kuck EM, van der Linden PD, Prins JM et al. Development of diagnostic prediction tools for bacteraemia caused by third-generation cephalosporin-resistant enterobacteria in suspected bacterial infections: a nested case-control study. Clin Microbiol Infect 2018. [DOI] [PubMed]
- 41.Detsis M, Karanika S, Mylonakis E. ICU acquisition rate, risk factors, and clinical significance of digestive tract colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae: a systematic review and meta-analysis. Crit Care Med. 2017;45(4):705–714. doi: 10.1097/CCM.0000000000002253. [DOI] [PubMed] [Google Scholar]
- 42.Bruyere R, Vigneron C, Bador J, Aho S, Toitot A, Quenot JP, Prin S, Charles PE. Significance of prior digestive colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae in patients with ventilator-associated pneumonia. Crit Care Med. 2016;44(4):699–706. doi: 10.1097/CCM.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 43.Carrara E, Pfeffer I, Zusman O, Leibovici L, Paul M. Determinants of inappropriate empirical antibiotic treatment: systematic review and meta-analysis. Int J Antimicrob Agents. 2018;51(4):548–553. doi: 10.1016/j.ijantimicag.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Cardoso T, Almeida M, Carratala J, Aragao I, Costa-Pereira A, Sarmento AE, Azevedo L. Microbiology of healthcare-associated infections and the definition accuracy to predict infection by potentially drug resistant pathogens: a systematic review. BMC Infect Dis. 2015;15:565. doi: 10.1186/s12879-015-1304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson SW, Anderson DJ, May DB, Drew RH. Utility of a clinical risk factor scoring model in predicting infection with extended-spectrum beta-lactamase-producing enterobacteriaceae on hospital admission. Infect Control Hosp Epidemiol. 2013;34(4):385–392. doi: 10.1086/669858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Smet AM, Kluytmans JA, Blok HE, Mascini EM, Benus RF, Bernards AT, Kuijper EJ, Leverstein-van Hall MA, Jansz AR, de Jongh BM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis. 2011;11(5):372–380. doi: 10.1016/S1473-3099(11)70035-4. [DOI] [PubMed] [Google Scholar]
- 47.Dutch Working party on Infection Prevention (WIP)—highly resistant micro-organisms (HRMO) in hospitals(2017). Available at: https://www.rivm.nl/sites/default/files/2018-11/130424%20BRMO.pdf.
- 48.Marquet K, Liesenborgs A, Bergs J, Vleugels A, Claes N. Incidence and outcome of inappropriate in-hospital empiric antibiotics for severe infection: a systematic review and meta-analysis. Crit Care. 2015;19:63. doi: 10.1186/s13054-015-0795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raman G, Avendano E, Berger S, Menon V. Appropriate initial antibiotic therapy in hospitalized patients with gram-negative infections: systematic review and meta-analysis. BMC Infect Dis. 2015;15:395. doi: 10.1186/s12879-015-1123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(12):2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 51.Rottier WC, Ammerlaan HS, Bonten MJ. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother. 2012;67(6):1311–1320. doi: 10.1093/jac/dks065. [DOI] [PubMed] [Google Scholar]
- 52.Mazuski JE, Tessier JM, May AK, Sawyer RG, Nadler EP, Rosengart MR, Chang PK, O'Neill PJ, Mollen KP, Huston JM, et al. The surgical infection society revised guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt) 2017;18(1):1–76. doi: 10.1089/sur.2016.261. [DOI] [PubMed] [Google Scholar]
- 53.Paul M, Lador A, Grozinsky-Glasberg S, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2014;1:CD003344. doi: 10.1002/14651858.CD003344.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunkhorst FM, Oppert M, Marx G, Bloos F, Ludewig K, Putensen C, Nierhaus A, Jaschinski U, Meier-Hellmann A, Weyland A, et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307(22):2390–2399. doi: 10.1001/jama.2012.5833. [DOI] [PubMed] [Google Scholar]
- 55.Thwaites GE, Scarborough M, Szubert A, Nsutebu E, Tilley R, Greig J, Wyllie SA, Wilson P, Auckland C, Cairns J, et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10121):668–678. doi: 10.1016/S0140-6736(17)32456-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vardakas KZ, Tansarli GS, Bliziotis IA, Falagas ME. beta-Lactam plus aminoglycoside or fluoroquinolone combination versus beta-lactam monotherapy for Pseudomonas aeruginosa infections: a meta-analysis. Int J Antimicrob Agents. 2013;41(4):301–310. doi: 10.1016/j.ijantimicag.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Hu Y, Li L, Li W, Xu H, He P, Yan X, Dai H. Combination antibiotic therapy versus monotherapy for Pseudomonas aeruginosa bacteraemia: a meta-analysis of retrospective and prospective studies. Int J Antimicrob Agents. 2013;42(6):492–496. doi: 10.1016/j.ijantimicag.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Arthur LE, Kizor RS, Selim AG, van Driel ML, Seoane L. Antibiotics for ventilator-associated pneumonia. Cochrane Database Syst Rev. 2016;10:CD004267. doi: 10.1002/14651858.CD004267.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratala J, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bailey JA, Virgo KS, DiPiro JT, Nathens AB, Sawyer RG, Mazuski JE. Aminoglycosides for intra-abdominal infection: equal to the challenge? Surg Infect (Larchmt) 2002;3(4):315–335. doi: 10.1089/109629602762539544. [DOI] [PubMed] [Google Scholar]
- 61.Falagas ME, Matthaiou DK, Karveli EA, Peppas G. Meta-analysis: randomized controlled trials of clindamycin/aminoglycoside vs. beta-lactam monotherapy for the treatment of intra-abdominal infections. Aliment Pharmacol Ther. 2007;25(5):537–556. doi: 10.1111/j.1365-2036.2006.03240.x. [DOI] [PubMed] [Google Scholar]
- 62.Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018;320(10):984–994. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.EMA - Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics (2018). Available at: https://www.ema.europa.eu/en/documents/press-release/disabling-potentially-permanent-side-effects-lead-suspension-restrictions-quinolone-fluoroquinolone_en.pdf.
- 64.Etminan M, Sodhi M, Ganjizadeh-Zavareh S, Carleton B, Kezouh A, Brophy JM. Oral fluoroquinolones and risk of mitral and aortic regurgitation. J Am Coll Cardiol. 2019;74(11):1444–1450. doi: 10.1016/j.jacc.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 65.Lee CC, Lee MG, Hsieh R, Porta L, Lee WC, Lee SH, Chang SS. Oral fluoroquinolone and the risk of aortic dissection. J Am Coll Cardiol. 2018;72(12):1369–1378. doi: 10.1016/j.jacc.2018.06.067. [DOI] [PubMed] [Google Scholar]
- 66.Yu X, Jiang DS, Wang J, Wang R, Chen T, Wang K, Cao S, Wei X. Fluoroquinolone use and the risk of collagen-associated adverse events: a systematic review and meta-analysis. Drug Saf. 2019;42(9):1025–1033. doi: 10.1007/s40264-019-00828-z. [DOI] [PubMed] [Google Scholar]
- 67.Wiersinga WJ, Bonten MJ, Boersma WG, Jonkers RE, Aleva RM, Kullberg BJ, Schouten JA, Degener JE, van de Garde EMW, Verheij TJ, et al. Management of community-acquired pneumonia in adults: 2016 guideline update from the Dutch Working Party on Antibiotic Policy (SWAB) and Dutch Association of Chest Physicians (NVALT) Neth J Med. 2018;76(1):4–13. [PubMed] [Google Scholar]
- 68.Blumenthal KG, Parker RA, Shenoy ES, Walensky RP. Improving clinical outcomes in patients with methicillin-sensitive Staphylococcus aureus bacteremia and reported penicillin allergy. Clin Infect Dis. 2015;61(5):741–749. doi: 10.1093/cid/civ394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacFadden DR, LaDelfa A, Leen J, Gold WL, Daneman N, Weber E, Al-Busaidi I, Petrescu D, Saltzman I, Devlin M, et al. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis. 2016;63(7):904–910. doi: 10.1093/cid/ciw462. [DOI] [PubMed] [Google Scholar]
- 70.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C, Levy MM. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 71.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, Escobar GJ. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997–1000. doi: 10.1097/CCM.0000000000003119. [DOI] [PubMed] [Google Scholar]
- 74.Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta-analysis. Crit Care Med. 2015;43(9):1907–1915. doi: 10.1097/CCM.0000000000001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalil AC, Gilbert DN, Winslow DL, Masur H, Klompas M. Infectious Diseases Society of America (IDSA) POSITION STATEMENT: why IDSA did not endorse the surviving sepsis campaign guidelines. Clin Infect Dis. 2018;66(10):1631–1635. doi: 10.1093/cid/cix997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klompas M, Calandra T, Singer M. Antibiotics for sepsis-finding the equilibrium. JAMA. 2018;320(14):1433–1434. doi: 10.1001/jama.2018.12179. [DOI] [PubMed] [Google Scholar]
- 77.Singer M. Antibiotics for sepsis: does each hour really count, or is it incestuous amplification? Am J Respir Crit Care Med. 2017;196(7):800–802. doi: 10.1164/rccm.201703-0621ED. [DOI] [PubMed] [Google Scholar]
- 78.Dimopoulos G, Poulakou G, Pneumatikos IA, Armaganidis A, Kollef MH, Matthaiou DK. Short- vs long-duration antibiotic regimens for ventilator-associated pneumonia: a systematic review and meta-analysis. Chest. 2013;144(6):1759–1767. doi: 10.1378/chest.13-0076. [DOI] [PubMed] [Google Scholar]
- 79.Pugh R, Grant C, Cooke RP, Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 2015(8):CD007577. [DOI] [PMC free article] [PubMed]
- 80.Yahav D, Franceschini E, Koppel F, Turjeman A, Babich T, Bitterman R, Neuberger A, Ghanem-Zoubi N, Santoro A, Eliakim-Raz N, et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: a non-inferiority randomized controlled trial. Clin Infect Dis. 2018;69:1091. doi: 10.1093/cid/ciy1054. [DOI] [PubMed] [Google Scholar]
- 81.Chotiprasitsakul D, Han JH, Cosgrove SE, Harris AD, Lautenbach E, Conley AT, Tolomeo P, Wise J, Tamma PD. Comparing the outcomes of adults with enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis. 2018;66(2):172–177. doi: 10.1093/cid/cix767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection– 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183–2191. doi: 10.1093/jac/dkt177. [DOI] [PubMed] [Google Scholar]
- 83.Hepburn MJ, Dooley DP, Skidmore PJ, Ellis MW, Starnes WF, Hasewinkle WC. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Intern Med. 2004;164(15):1669–1674. doi: 10.1001/archinte.164.15.1669. [DOI] [PubMed] [Google Scholar]
- 84.Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15(6):R267. doi: 10.1186/cc10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wirz Y, Meier MA, Bouadma L, Luyt CE, Wolff M, Chastre J, Tubach F, Schroeder S, Nobre V, Annane D, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care (London, England) 2018;22(1):191. doi: 10.1186/s13054-018-2125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iankova I, Thompson-Leduc P, Kirson NY, Rice B, Hey J, Krause A, Schonfeld SA, DeBrase CR, Bozzette S, Schuetz P. Efficacy and safety of procalcitonin guidance in patients with suspected or confirmed sepsis: a systematic review and meta-analysis. Crit Care Med. 2018;46(5):691–698. doi: 10.1097/CCM.0000000000002928. [DOI] [PubMed] [Google Scholar]
- 87.de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, Loef BG, Dormans T, van Melsen GC, Kluiters YC, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819–827. doi: 10.1016/S1473-3099(16)00053-0. [DOI] [PubMed] [Google Scholar]
- 88.Schuts EC, Hulscher ME, Mouton JW, Verduin CM, Cohen Stuart JW, Overdiek JW, van der Linden PD. SWAB Guidelines for Antimicrobial Stewardship 2016. Available at: https://swab.nl/en/exec/file/download/81. [DOI] [PubMed]
- 89.Lam SW, Bauer SR, Fowler R, Duggal A. Systematic review and meta-analysis of procalcitonin-guidance versus usual care for antimicrobial management in critically ill patients: focus on subgroups based on antibiotic initiation, cessation, or mixed strategies. Crit Care Med. 2018;46(5):684–690. doi: 10.1097/CCM.0000000000002953. [DOI] [PubMed] [Google Scholar]
- 90.Meier MA, Branche A, Neeser OL, Wirz Y, Haubitz S, Bouadma L, Wolff M, Luyt CE, Chastre J, Tubach F, et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: a patient-level meta-analysis of randomized trials. Clin Infect Dis. 2018;69:388. doi: 10.1093/cid/ciy917. [DOI] [PubMed] [Google Scholar]
- 91.Pepper DJ, Sun J, Rhee C, Welsh J, Powers JH, 3rd, Danner RL, Kadri SS. Procalcitonin-guided antibiotic discontinuation and mortality in critically ill adults: a systematic review and meta-analysis. Chest. 2019;155(6):1109–1118. doi: 10.1016/j.chest.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paul M, Dickstein Y, Raz-Pasteur A. Antibiotic de-escalation for bloodstream infections and pneumonia: systematic review and meta-analysis. Clin Microbiol Infect. 2016;22(12):960–967. doi: 10.1016/j.cmi.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 93.Tabah A, Cotta MO, Garnacho-Montero J, Schouten J, Roberts JA, Lipman J, Tacey M, Timsit JF, Leone M, Zahar JR, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009–1017. doi: 10.1093/cid/civ1199. [DOI] [PubMed] [Google Scholar]
- 94.Guo Y, Gao W, Yang H, Ma C, Sui S. De-escalation of empiric antibiotics in patients with severe sepsis or septic shock: a meta-analysis. Heart Lung. 2016;45(5):454–459. doi: 10.1016/j.hrtlng.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 95.Leone M, Bechis C, Baumstarck K, Lefrant JY, Albanese J, Jaber S, Lepape A, Constantin JM, Papazian L, Bruder N, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial. Intensive Care Med. 2014;40(10):1399–1408. doi: 10.1007/s00134-014-3411-8. [DOI] [PubMed] [Google Scholar]
- 96.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 97.Varghese JM, Roberts JA, Lipman J. Antimicrobial pharmacokinetic and pharmacodynamic issues in the critically ill with severe sepsis and septic shock. Crit Care Clin. 2011;27(1):19–34. doi: 10.1016/j.ccc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med. 2009;37(6):2071–2078. doi: 10.1097/CCM.0b013e3181a0054d. [DOI] [PubMed] [Google Scholar]
- 100.Goncalves-Pereira J, Povoa P. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of beta-lactams. Crit Care. 2011;15(5):R206. doi: 10.1186/cc10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bos JC, van Hest RM, Misticio MC, Nunguiane G, Lang CN, Beirao JC, Mathot RAA, Prins JM. Pharmacokinetics and pharmacodynamic target attainment of benzylpenicillin in an adult severely ill sub-Saharan African patient population. Clin Infect Dis. 2018;66(8):1261–1269. doi: 10.1093/cid/cix961. [DOI] [PubMed] [Google Scholar]
- 102.Bos JC, Prins JM, Misticio MC, Nunguiane G, Lang CN, Beirao JC, Mathot RAA, van Hest RM. Pharmacokinetics and pharmacodynamic target attainment of ceftriaxone in adult severely ill sub-Saharan African patients: a population pharmacokinetic modelling study. J Antimicrob Chemother. 2018;73(6):1620–1629. doi: 10.1093/jac/dky071. [DOI] [PubMed] [Google Scholar]
- 103.Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal beta-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18(1):108–120. doi: 10.1016/S1473-3099(17)30615-1. [DOI] [PubMed] [Google Scholar]
- 104.Roberts JA, Abdul-Aziz MH, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, Bellomo R, Lipman J. Continuous versus intermittent beta-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. 2016;194(6):681–691. doi: 10.1164/rccm.201601-0024OC. [DOI] [PubMed] [Google Scholar]
- 105.Rhodes NJ, Liu J, O'Donnell JN, Dulhunty JM, Abdul-Aziz MH, Berko PY, Nadler B, Lipman J, Roberts JA. Prolonged infusion piperacillin-tazobactam decreases mortality and improves outcomes in severely Ill patients: results of a systematic review and meta-analysis. Crit Care Med. 2018;46(2):236–243. doi: 10.1097/CCM.0000000000002836. [DOI] [PubMed] [Google Scholar]
- 106.Blot S, Koulenti D, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Martin C, Montravers P, Rello J, et al. Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI study. Crit Care. 2014;18(3):R99. doi: 10.1186/cc13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bakke V, Sporsem H, Von der Lippe E, Nordoy I, Lao Y, Nyrerod HC, Sandvik L, Harvig KR, Bugge JF, Helset E. Vancomycin levels are frequently subtherapeutic in critically ill patients: a prospective observational study. Acta Anaesthesiol Scand. 2017;61(6):627–635. doi: 10.1111/aas.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, De Backer D, Layeux B, Wallemacq P, Vincent JL, et al. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14(4):R126. doi: 10.1186/cc9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Necrotiserende wekedeleninfecties 2015. Available at: https://richtlijnendatabase.nl/richtlijn/necrotiserende_wekedeleninfecties/startpagina_-_nwdi.html.
- 110.Lavrijsen AP, Damstra RJ, van Dissel JT, Draijer LW, van Everdingen JJ, Go PM, Kwa D, Komen DJ. Richtlijn cellulitis en erysipelas van de onderste extremiteiten 2013.
- 111.Sawyer RG, Claridge JA, Nathens AB, Rotstein OD, Duane TM, Evans HL, Cook CH, O'Neill PJ, Mazuski JE, Askari R, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372(21):1996–2005. doi: 10.1056/NEJMoa1411162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hassinger TE, Guidry CA, Rotstein OD, Duane TM, Evans HL, Cook CH, O'Neill PJ, Mazuski JE, Askari R, Napolitano LM, et al. Longer-duration antimicrobial therapy does not prevent treatment failure in high-risk patients with complicated intra-abdominal infections. Surg Infect (Larchmt) 2017;18(6):659–663. doi: 10.1089/sur.2017.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rattan R, Allen CJ, Sawyer RG, Askari R, Banton KL, Claridge JA, Cocanour CS, Coimbra R, Cook CH, Cuschieri J, et al. Patients with complicated intra-abdominal infection presenting with sepsis do not require longer duration of antimicrobial therapy. J Am Coll Surg. 2016;222(4):440–446. doi: 10.1016/j.jamcollsurg.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 114.Montravers P, Tubach F, Lescot T, Veber B, Esposito-Farese M, Seguin P, Paugam C, Lepape A, Meistelman C, Cousson J, et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: the DURAPOP randomised clinical trial. Intensive Care Med. 2018;44(3):300–310. doi: 10.1007/s00134-018-5088-x. [DOI] [PubMed] [Google Scholar]
- 115.van Lent AU, Bartelsman JF, Tytgat GN, Speelman P, Prins JM. Duration of antibiotic therapy for cholangitis after successful endoscopic drainage of the biliary tract. Gastrointest Endosc. 2002;55(4):518–522. doi: 10.1067/mge.2002.122334. [DOI] [PubMed] [Google Scholar]
- 116.SWAB Guidelines for Antimicrobial Therapy of Complicated Urinary Tract Infections in Adults 2013. Available at: https://www.swab.nl/swab/cms3.nsf/uploads/41949F6BD9ED10EDC1257B7F00212560/$FILE/revised%20uti%20guideline%20FINAL%20010413.pdf.
- 117.Verduin K, Ammerlaan H, Blaauw G, Bleeker-Rovers CP, van Drie-Pierik RJ, Ekkelenkamp M, Glaudemans AW, van Hout D. Richtlijn Staphylococcus aureus bacteriëmie 2019 (NVMM). Available at: https://richtlijnendatabase.nl/richtlijn/staphylococcus_aureus_bacteriemie/startpagina.html.
- 118.SWAB Guidelines on Antibacterial Therapy of Patients with Bacterial Central Nervous System Infections 2012. Available at: https://www.swab.nl/swab/cms3.nsf/uploads/FE54A057082AA54CC1257A2B00293B1D/$FILE/SWAB_CNSguideline_%20June12.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. sTable 1. Alternative empirical treatment strategies in sepsis and low estimated risk of involvement of 3GCR-E. sTable 2. Alternative empirical treatment strategies in sepsis and increased or high estimated risk of involvement of 3GCR-E. sTable 3. Recommended iv doses of empirical antibacterial treatment for sepsis.
Data Availability Statement
Literature searches are available from the corresponding author upon request.