Abstract
The temperate bacteriophage K139 is highly associated with pathogenic O1 Vibrio cholerae strains. The nucleotide sequence of the major control region of K139 was determined. The sequences of four (cox, cII, cI, and int) of the six deduced open reading frames and their gene order indicated that K139 is related to the P2 bacteriophage family. Two genes of the lysogenic transcript from the mapped promoter PL encode homologs to the proteins CI and Int, with deduced functions in prophage formation and maintenance. Between the cI and int genes, two additional genes were identified: orf2, which has no significant similarity to any other gene, and the formerly characterized gene glo. Further analysis revealed that Orf2 is involved in preventing superinfection. In a previous report, we described that mutations in glo cause an attenuation effect in the cholera mouse model (J. Reidl and J. J. Mekalanos, Mol. Microbiol. 18:685–701, 1995). In this report, we present strong evidence that Glo participates in phage exclusion. Glo was characterized to encode a 13.6-kDa periplasmic protein which inhibits phage infection at an early step, hence preventing reinfection of vibriophage K139 into K139 lysogenic cells. Immediately downstream of gene int, the attP site was identified. Upon analysis of the corresponding attB site within the V. cholerae chromosome, it became evident that phage K139 is integrated between the flagellin genes flaA and flaC of O1 El Tor and O139 V. cholerae lysogenic strains.
In 1992 to 1993, a new Vibrio cholerae serogroup, designated O139, was found to be responsible for a cholera epidemic in southern and eastern parts of India and Bangladesh (2, 45, 51). It was also reported that this new serogroup carries temperate bacteriophages related to the kappa family of O1 V. cholerae bacteriophages (23). Kappa phages appear to be widely distributed in O1 V. cholerae El Tor and are probably synonymous with the alpha phage described by Nicolle et al. (44), typing phage 3 (9), phage 32 (33), and probably also other phages, such as VcA-2 (21), K139 (47), and V86 (13a). Kappa phages have thus far been classified as vibriophages with a Bradley group A morphology and a host range restriction to V. cholerae biotype El Tor, serogroup O1 (1, 12, 57, 58).
Mainly due to a lack of further characterization, it was not possible to define the kappa phage more precisely, e.g., based on molecular characteristics. For example, nothing is known about the regulation of the two developmental states, lytic development and lysogeny. For temperate integrating Escherichia coli phages, entry into these phases is controlled by a lysis-lysogeny transcriptional regulation system and its coordination with an integration-excision, site-specific recombinational switch. Maintenance of the stable lysogenic state is regulated, for example, by the CI repressor in phage λ (25) and P186 (32). The CI repressor and often additional regulators (e.g., the tripartite immunity system of phages P1 and P7 [22]) are expressed during lysogeny, which in consequence will also prevent the propagation of a superinfecting phage. This property of lysogens is called immunity. Beside this mechanism, other proteins which also act to prevent superinfection of the lysogenic cells by other complete mechanisms, e.g., prevention of adsorption or phage exclusion systems, are often expressed during lysogeny (56). For example, Salmonella typhimurium phage 22 encodes three different systems represented by proteins A1, SieA, and SieB (54) which prevent superinfection by the same, related, or lytic phages.
In a recent report (47), we described K139 as a temperate phage derived from a clinical isolate of V. cholerae serogroup O139. Based on morphology analysis, we concluded that phage K139 belongs to the kappa phage family and showed that this phage or highly related K139 phages were widely distributed throughout different serogroups (O1 and O139) and biotypes (El Tor and classical) of V. cholerae. In addition, evidence was provided that the phage encoded an exported gene product, named Glo. This protein was expressed during the lysogenic phase, and evidence was provided that it may encode a hypothetical virulence determinant of lysogenic V. cholerae strains.
This study was initiated to elucidate Glo’s function, leading to the preliminary result that Glo participates in phage biology. We present data indicating that the phage-encoded glo gene product is involved in phage exclusion. We also present the DNA sequence of the putative lysis-lysogeny switch region of the phage. It is further shown that phage integration occurs at a distinct locus on the V. cholerae chromosome, linked to the flagellin genes flaA and flaC in O1 El Tor and O139 V. cholerae strains.
MATERIALS AND METHODS
Bacterial strains, phages, plasmids, and media.
E. coli LE392 (F− supF supE hsdR galK trpR metB lacY tonA) (53) was used as the recipient strain for construction of plasmids and overexpression of proteins. V. cholerae M799, M804, M807, M817 (27), AI1838, and MO10 (64) were all clinical isolates, whereas O395 and MAK757 (37) are defined derivatives of laboratory-adapted stocks. Cultures were grown in Luria broth (LB), LB agar, or TB top-agar medium (38). Unless noted otherwise, antibiotics were used at the following concentrations for E. coli and V. cholerae, respectively: ampicillin, 100 and 50 μg/ml; kanamycin, 50 and 30 μg/ml; chloramphenicol, 1.5 μg/ml; and tetracycline, 12 and 1 μg/ml. Plasmids and phages used in this study are listed in Table 1.
TABLE 1.
Plasmids and bacteriophages used in this study
| Plasmid or phage | Description | Reference |
|---|---|---|
| Plasmid | ||
| pBR322 | Apr Tcr | 65 |
| pACYC184 | Tcr Cmr | 49 |
| pACYC177 | Apr Kmr | 48 |
| pAKcat | Tn10d-cat Kmr Cmr | 30 |
| pTrc99A | Expression plasmid, Apr | 5 |
| pTrcglo | pTrc99A-glo Apr | This study |
| pTrcglokan | pTrcglo Aps Kmr | This study |
| pTrckan | pTrc99A Aps Kmr | This study |
| pJBcII-int | pBR322 ′cII cox cI orf2 glo int′ Tcr Aps | This study |
| pJBcII-glo | pBR322 ′cII cox cI orf2 glo′ Tcr Aps | This study |
| pJBcII-orf2 | pBR322 ′cII cox cI orf2′ Tcr Aps | This study |
| Phage | ||
| K139 | wt phage | 47 |
| K139.A | K139 glo::Tn10d-bla Apr | 47 |
| K139.cm2 | K139 orf2::Tn10d-cat Cmr | This study |
| K139.cm9 | K139 orf2::Tn10d-cat Cmr | This study |
Oligonucleotides.
The primers seq 1 to 18 (Fig. 1) were used for sequencing of the phage control region. The primers used for construction, cloning, and sequencing of the Glo expression system and amplification of the flaA and flaC regions were GloNcoI (5′-AACATGCCATGGTGCGATTACTACCTCTA-3′), GloPstI (5′-AACTGCAGATCTTTAAGGTTACGGACGG-3′), JN-int (5′-CGATGGGCGTTCTGTTTCTA-3′), JN-flaA (5′-GCTCAGACGTGGGTATGTAAT-3′), and JN-flaC (5′-GTCTCGGAAAACCAAGCAGTT-3′). The synthetic oligonucleotide AR6, described elsewhere (47), was used to determine the integration sites of Tn10d-cat in the K139 phage genome.
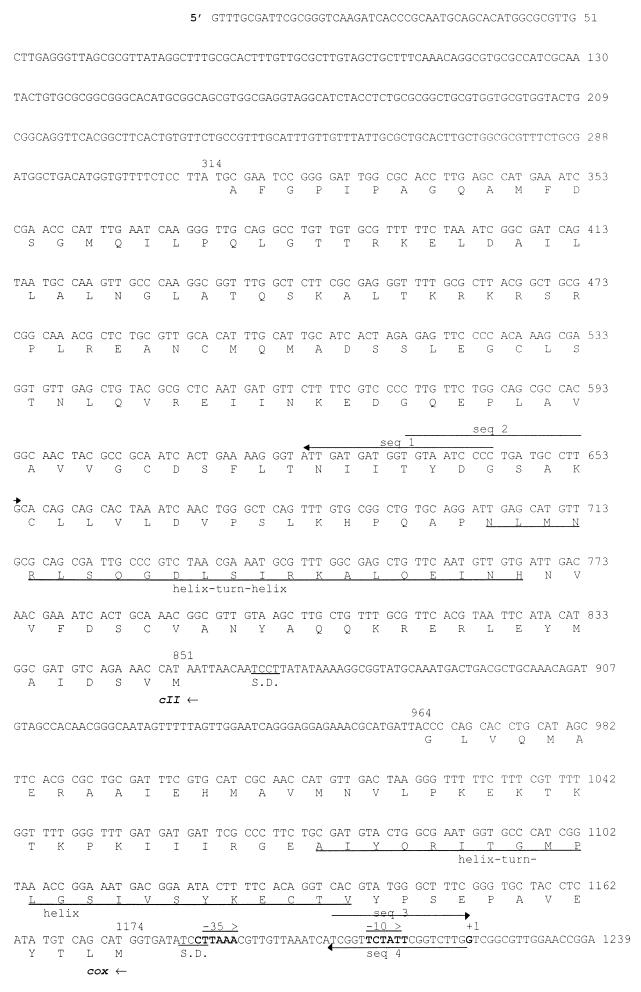
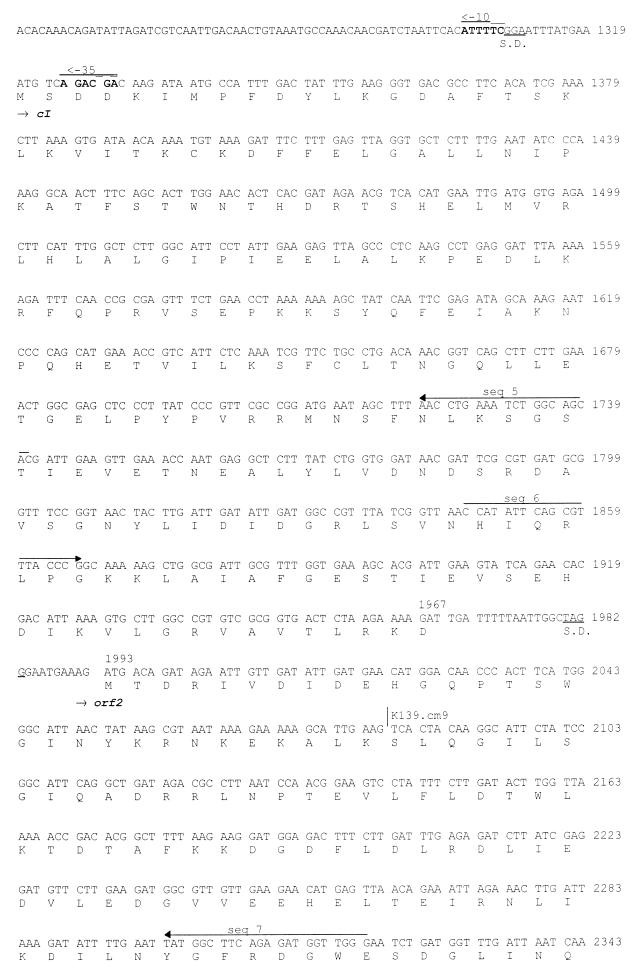
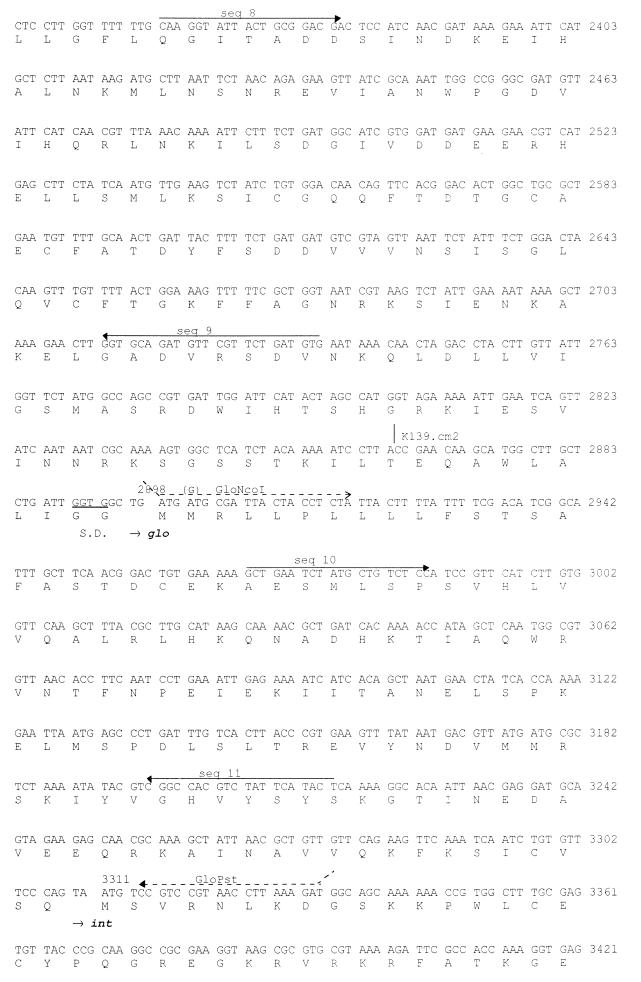
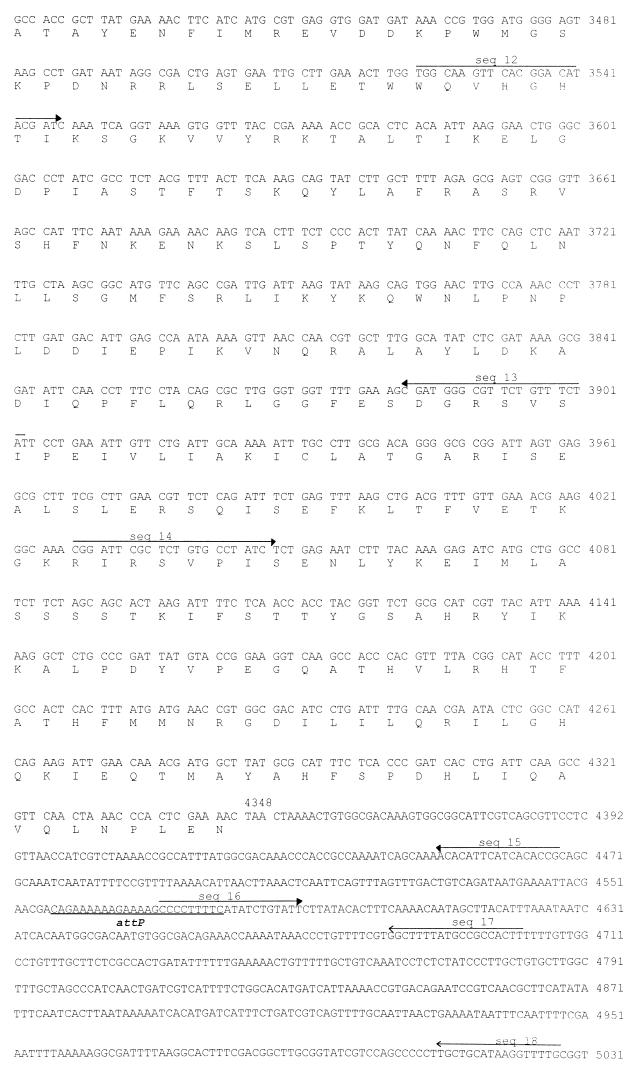
FIG. 1.
DNA sequence of the phage K139 control region. The deduced amino acid sequences are shown below the DNA sequence. Oligonucleotides used for sequencing (seq 1 to 18) are indicated by solid-line arrows, and those used for the construction of the Glo expression system are shown as dashed-line arrows. Transcriptional and translational relevant sites are indicated; ribosomal binding sites are marked as S.D., and putative promoters (PL and PR) are marked by arrows. The attP site is underlined. Tn10d-cat insertions are indicated at bp 2031 and 2863. The mapped start site of transcription is indicated at position 1222. Putative helix-turn-helix motifs are marked by underlining of the amino acids comprising CII and Cox.
Plasmid constructions.
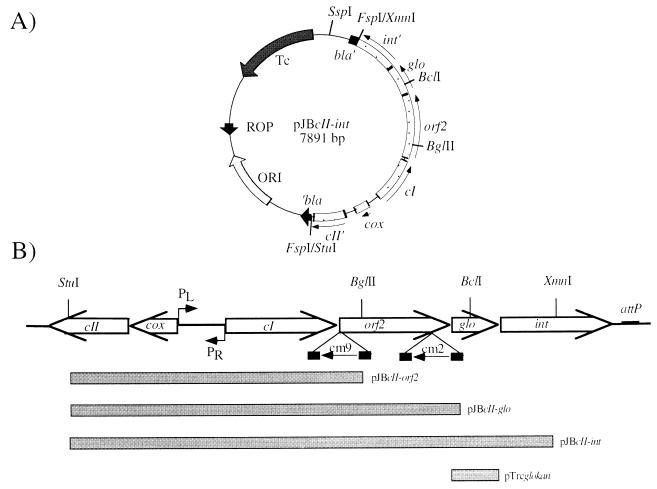
Plasmid pJBcII-int is a pBR322 derivative containing a 3.53-kb StuI-XmnI phage DNA fragment subcloned into the FspI site of pBR322 (Fig. 2A). The orientation of the insert was verified by sequencing with primer seq 1. Plasmid pJBcII-glo was generated by deletion of a 1.2-kb BclI-SspI (located downstream on pBR322) fragment from pJBcII-int. Deletion of a 2-kb BglII-SspI fragment from plasmid pJBcII-int resulted in pJBcII-orf2 (Fig. 2B). Plasmid pTrcglo, a pTrc99A (5) derivative, was constructed by subcloning glo as an NcoI-PstI PCR product, generated with primers GloNcoI and GloPstI (Fig. 1). To obtain a functional Glo-expressing system, the second codon (Met) of the signal sequence was modified (by primer GloNcoI) to a Val codon. The resulting construct was verified by DNA sequencing with primers seq 10 and seq 11. pTrcglo was further modified to obtain pTrcglokan by subcloning of a kanamycin resistance (Kmr) gene (StuI fragment originated from plasmid pACYC177 [48]) into the FspI site of the bla gene. This modification was necessary to provide a β-lactamase-negative Glo expression system for use in the lysogenic assay. As a control plasmid, pTrckan was constructed accordingly.
FIG. 2.
Construction of the control region encoding plasmid pJBcII-int and its derivatives. (A) Orientation of the subcloned StuI-XmnI phage fragment in the FspI site of pBR322. (B) Deduced ORFs of the 5,031-bp DNA phage fragment along with the putative intergenic region and promoters PL and PR. Filled bars indicate the corresponding DNA fragments, subcloned on pBR322 (pJBcII-orf2, pJBcII-glo, and pJBcII-int) and pTrckan (pTrcglokan). cm9 and cm2 indicate the locations and direction of Tn10d-cat insertions.
DNA analytical methods, PCRs, DNA sequencing, and computer analysis.
Chromosomal DNA of V. cholerae was prepared as described elsewhere (37), and phage DNA preparation was carried out according to the Qiagen lambda kit protocol. Restriction enzyme digestion and analysis were carried out as described by Maniatis et al. (36). PCRs were performed as described by Mullis and Faloona (40). DNA sequencing was performed by the dideoxynucleotide chain termination method of Sanger et al. (50), with cycling reaction as specified by Amersham Life Sciences. DNA separation and data collection were performed with the automatic sequencing LiCor system (MWG Biotech GmbH). Both DNA strands were sequenced from position 690 to the end; one single strand was sequenced from positions 1 to 690. The glo-containing region (bp 2848 to 3308) has been published previously (GenBank accession no. U24280 [47]) and was resequenced. Sequence analysis was performed with Basic Blast Search 2.0 (4) at the National Center for Biological Information, the Genetics Computer Group Wisconsin Package, and the promoter algorithm of Mulligan et al. (39).
Nonradioactive primer extension analysis.
The transcriptional start site of the PL promoter was determined by using total RNA of lysogenic V. cholerae MAK757 K139 cells. RNA was isolated from cells by the hot-acidic phenol method (61). Nonradioactive primer extension analysis was performed as described previously (3) with the cI-specific oligonucleotide seq 5 (Fig. 1).
Enrichment of the Glo protein and generation of Glo-specific antiserum.
Glo-overexpressing plasmid pTrcglo was transformed into E. coli LE392 or V. cholerae strains. Transformants were grown to an optical density at 600 nm (OD600) of 0.5 in LB-ampicillin (100 μg/ml) and induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h. Cells were harvested by centrifugation and washed once with phosphate-buffered saline (pH 7.4) at 4°C. To obtain periplasmic extracts (24), the pellet was resuspended in 30 ml of phosphate-buffered saline containing 2 mg of polymyxin B per ml and incubated for 10 min on ice. Cells were then centrifuged (20,000 × g, 4°C, 10 min), the supernatant was recovered, and proteins were precipitated with 80% ammonium sulfate. The pellet was resuspended in 2 ml of 20 mM Tris-HCl (pH 7.5) and subsequently dialyzed for 12 h against the same buffer. This extract was loaded on an equilibrated MonoQ column (Pharmacia). Highly enriched Glo fraction was obtained by isocratic elution (washing the column with 20 mM Tris-HCl [pH 7.5]). Glo samples were concentrated by centrifugation (Centriprep 3; Amicon) and stored in protein sample buffer (20 mM Tris-HCl [pH 7.5], 10% glycerol, 1 mM dithiothreitol, 1 mM EDTA) at −80°C. The enriched Glo protein fraction from E. coli was used to generate a polyclonal rabbit antiserum (BioTrend).
Fractionation of V. cholerae cells.
Early-stationary-phase cells of 150-ml LB cultures were harvested by centrifugation, washed, and resuspended in 10 ml of cold phosphate buffer (10 mM, pH 7). The cells were then lysed in a French press at 1,000 lb/in2. Cell debris was removed by centrifugation (10,000 × g, 10 min, 4°C), and the recovered supernatant was further fractionated by centrifugation (100,000 × g, 45 min, 4°C). The remaining supernatant was referred to as the cytosolic extract, and the pellet (envelope fraction) was fractionated further in inner and outer membrane preparations as described by Aono et al. (6). Periplasmic extract (24) was prepared from 2 ml of the same LB culture.
Protein analysis.
Protein concentrations were determined as described by Bradford (11), using a commercial reagent (Bio-Rad). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out as described by Laemmli (31). After SDS-PAGE, proteins were detected either by staining with Coomassie brilliant blue or by Western blot analysis (59) using the Amersham ECL reagent. Protein masses were determined by ion spray mass spectrometry. The samples were desalted over 0.1 μl of POROS R2 reversed-phase matrix (Perseptive Biosystems, Framingham, Mass.) and eluted in 1 μl of 60% methanol in 5% formic acid. Electrospray mass spectra were acquired on an API 365 triple-quadrupole mass spectrometer (PE-Sciex, Toronto, Ontario, Canada). The N-terminal sequencing analysis was performed on an Applied Biosystems 494 protein sequencer.
Transposon mutagenesis of phage K139.
First, minitransposon Tn10d-cat, encoded by pAKcat (30), was transformed into competent MO10 cells. A single transformant was then isolated, inoculated under transposase-inducing conditions (1 mM IPTG) in 5 ml of LB medium, and grown for 5 to 6 h. Then, the supernatant was collected, filtered (0.2-μm-pore-size filter; Nalgene), and used for phage transduction with reference strain MAK757. Phage transductants were then plated onto LB agar plates supplemented with chloramphenicol (1.5 μg/ml). Chloramphenicol-resistant (Cmr) MAK757 colonies were obtained and further characterized as K139 lysogenic cells. From these lysogens, phage lysates were obtained and used to isolate phage DNA. The location of the Tn10d-cat element on the phage genome was determined by sequencing using oligonucleotide AR6 (47).
Bacteriophage techniques.
Isolation of K139 wild-type (wt) or mutant phages, determination of the phage titer, and identification of lysogenic cells were performed as described previously (47). Plaque assay analysis was done by phage titer determination. One hundred microliters of MAK757 overnight culture and 100 μl of appropriate dilutions of phage lysates were mixed, added to 8 ml of TB top agar containing 10 mM CaCl2, and poured onto LB agar plates; then phage titer (PFU per milliliter) was determined.
Phage K139 is not inducible by UV or chemical mutagens; therefore, the titer of spontaneously produced phage lysate of phage K139 was determined. As phage donors, K139 lysogenic cells harboring plasmids pTrcglokan, pTrckan, pBR322, and pJBcII-orf2, -cII-glo, and -cII-int were used. Cells were grown overnight or to late stationary growth phase. Cells were then harvested by centrifugation, and the supernatant was filtered (0.2-μm-pore-size filter; Nalgene). The filtrate was referred as K139 lysate. Phage titers were determined as described above.
Phage lysogeny assays were performed with a K139.A (glo::Tn10d-bla) or K139.cm2 (orf2::Tn10d-cat) phage lysate. Overnight cultures of reference strain MAK757, transformed with different plasmids or lysogenized with different phages, were diluted 1:100 in LB containing 10 mM CaCl2 and the corresponding antibiotics. Then 150 μl of cells (OD600 of 0.3) was incubated with 150 μl of phage lysate (107 to 108 PFU/ml) for 30 min at 37°C. Cells were diluted and subsequently plated onto LB agar plates containing the antibiotic corresponding to the plasmid used and ampicillin (50 μg/ml) or chloramphenicol (1.5 μg/ml) to select for lysogens formed by phage K139.A (ampicillin resistant [Apr]) or K139.cm2 (Cmr). Viable cells were determined by plating suitable dilutions of the same samples under nonselective conditions. For the Glo overexpression experiments, MAK757 cells transformed with pTrcglokan and pTrckan were grown to an OD600 of 0.3 and induced with 1 mM IPTG for 2 h. Cells were then diluted and used in phage infection assays as described above.
Nucleotide sequence accession number.
The sequence presented in Fig. 1 has been assigned GenBank accession no. AF125163.
RESULTS
DNA sequence analysis of the major control region of K139.
A 5,031-bp segment of DNA sequence containing the glo gene and flanking regions was determined by sequencing both strands of the isolated K139 phage genome. The region from bp 1 to 690 was found to be identical with the 3′ end of a more than 5-kbp DNA sequence submission (AF008938) of V. cholerae phage V86 by Das et al. (13a). In Fig. 1, the DNA sequence is shown together with the six predicted open reading frames (ORFs). The region from bp 851 to 314 encodes an ORF with partial similarity (27% identity in 169 amino acids [aa]) to the CII protein of E. coli phage P186 (26). Another ORF (bp 1174 to 964) shows similarity to the Cox proteins of phages HP1 (17) (28% identity in 69 aa) and S2 (33% identity in 60 aa) of Haemophilus influenzae (53a). Finally, four ORFs are encoded in the opposite direction. The first ORF (bp 1319 to 1967) shows 26% identity in 112 amino acids to CI of phage P186 (26) and was named cI. Downstream of cI was found an ORF, named orf2 (bp 1993 to 2896), encoding a deduced product with no detectable similarity to known proteins. Base pairs 2898 to 3308 encode the previously identified gene glo, and downstream of glo the complete phage integrase gene int was identified (bp 3311 to 4348). The deduced amino acid sequence of the Int protein shows consistent similarity to the superfamily of phage integrases (7), and 36% identity to the integrases of phages P186 and P2 (26, 67).
The putative intergenic control region, containing two promoters, is located between cox and cI. Nonradioactive primer extension analysis (3) was performed on the PL promoter (transcriptional direction of cI, orf2, glo, and int), which resulted in the identification of the mRNA start site (data not shown) at position bp 1222 (Fig. 1). A possible promoter element for RNA polymerase recognition (−10 and −35) is shown in Fig. 1 (bp 1184 to 1189 and 1208 to 1213). Features of the putative promoter PR (cox direction) and ribosomal binding sites (52) are indicated in Fig. 1 (bp 1302 to 1307 and 1325 to 1330) and are based on similarity analysis.
Enrichment of Glo and detection of its cellular localization.
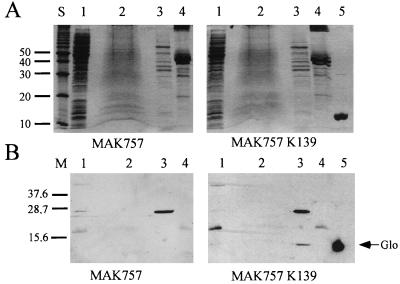
In an earlier report, we showed that a Glo-Bla fusion hybrid protein was exported outside the cytoplasmic membrane (47). As bacteriophages often encode exported proteins, such as extracellular enzymes (e.g., cholera toxin [63]), outer membrane proteins (e.g., Bor of phage λ [8]), lipoproteins (e.g., Llp of phage T5 [14]), or periplasmic proteins (e.g., Sim of phage P1 [35]), we were interested in the cellular localization of Glo. Overexpression of glo in V. cholerae MAK757, utilizing plasmid pTrcglo, resulted in the production of Glo observed in periplasmic extracts (data not shown). The Glo protein purified from E. coli (Fig. 3A, lane 5) was used to generate Glo-specific rabbit antiserum as described in Materials and Methods.
FIG. 3.
Cellular localization of Glo. V. cholerae MAK757 and MAK757 K139 were cell fractionated. (A) SDS-polyacrylamide gels (15%) stained with Coomassie brilliant blue. (B) Corresponding Western blots after reaction with a Glo-specific polyclonal rabbit antiserum. Lanes 1 to 4, cytoplasmic, inner membrane, periplasmic, and outer membrane extracts, respectively; lane 5, purified Glo protein; lane S, 10-kDa molecular mass ladder (Gibco-Life Technologies); lane M, Kaleidoscope polypeptide standard (Bio-Rad). Sizes are indicated in kilodaltons.
Using the Glo-specific antiserum, it was possible to detect the Glo protein in K139 lysogenic wt V. cholerae cells. Cell fractionation of V. cholerae strains MAK757 K139 and MAK757 into membrane and soluble fractions was performed. Subsequent Western blot analysis using a preadsorbed Glo-specific antiserum revealed that the Glo protein was located in the periplasm (Fig. 3B, lane 3, MAK757 K139), with no Glo detectable in the supernatant (data not shown). The same result was obtained with the natural K139 lysogenic strain MO10 in cell fractionation experiments (data not shown).
To identify any posttranscriptional modifications, the purified protein was subjected to automated protein sequencing and ion spray mass spectrometry. The N′-terminal sequence of the mature protein was found to be STD(X)EKA. Therefore, a signal sequence is encoded by aa 1 to 17, with a cleavage site of the precursor protein between aa A17 and S18 (Fig. 1). The theoretical molecular mass of the mature Glo protein is 13,650 Da, calculated with the Genetics Computer Group Wisconsin Package. This value is in agreement with the experimentally determined mass of 13,648 Da, and therefore mature Glo seems to be unmodified.
Phenotypic characterization of phage-encoded genes involved in immunity and exclusion.
To characterize the function of the phage-encoded genes cI, orf2, and glo, spontaneous phage release was determined and superinfection experiments (survival and a lysogeny plating and plaque assay) were established. To use Cmr-selectable challenge phages, Tn10d-cat insertions were generated in the K139 phage genome (see Materials and Methods). The Tn10d-cat transposon is believed not to contain any promoters in the flanking IS10 elements of 29 bp (47). However, this element encodes the cat gene associated promoter along with the cat gene without transcriptional termination elements (30). Two Tn10d-cat insertions were found to be located within and transcribed divergently from orf2 (Fig. 2). One isolate, phage K139.cm9 (orf2::Tn10d-cat, inserted at bp 2081 [Fig. 1]), showed increased lytic activity (clear, larger plaques) and drastically decreased ability to form lysogenic colonies. We interpret this phenotype as produced by the promoter activity of the cat promoter, which may interfere with cI transcription, resulting in cI antisense RNA production, or alternatively caused by an artificial activation of PR. However, additional mutations may also contribute to this phenotype. We have chosen this phage as a lytic challenger phage to investigate the protection effect of Glo and Orf2, since there was no evidence that phage K139.cm9 acted differently than the wt phage in the initial infection process (adsorption and infection of DNA). Another isolate, phage K139.cm2 (orf2::Tn10d-cat, inserted at bp 2863 [Fig. 1]), and the previously isolated phage K139.A (glo::Tn10d-bla) (47) showed moderate lytic activity, corresponding with the wt phage activity (data not shown). Both antibiotic resistance-converting phages were used to determine lysogenic colonies.
Strong evidence for a phage-specific function of the periplasmic protein Glo was found and suggests its participation in phage exclusion. A first indication of a Glo-mediated protective effect was obtained in survival assays using the lytic phage K139.cm9. While reference strain MAK757 transformed with the control plasmid was killed by K139.cm9 (not observed with wt phage), Glo-expressing cells were viable (Table 2). To further address Glo’s function in prevention of the phage infection process, we investigated the formation of lysogens and plaques. Expressing Glo from plasmid pTrcglokan caused 16-fold (uninduced) and 166-fold (IPTG-induced) reductions of lysogen formation (Table 3) compared with MAK757 transformed with control plasmid pTrckan. A similar reduction (sevenfold, uninduced [Table 3]) of plaque formation was observed with the lytic phage K139.cm9. These plaques exhibited changed morphology (turbid and smaller). It was subsequently shown that wt phage K139 in such experiments did not allow the monitoring of plaques in Glo-expressing cells.
TABLE 2.
Viabilitya of Glo-expressing cells after infection with wt phage K139 or K139.cm9
| Strain | Infection | No. of survivors/ml |
|---|---|---|
| MAK757 | None | 3.0 × 107 |
| K139 | 5.0 × 107 | |
| MAK757/pTrckan | None | 1.5 × 107 |
| K139.cm9 | 3.0 × 103 | |
| MAK757/pTrcglokan | None | 1.0 × 107 |
| K139.cm9 | 1.7 × 107 |
Log-phase cells (107/ml) were infected with K139 or K139.cm9 (multiplicity of infection of about 10) and incubated for 1 h at 37°C. Dilutions were plated on LB plates or LB plates containing 50 μg of kanamycin per ml, respectively.
TABLE 3.
Protection effect versus superinfecting phage in the presence of cI, orf2, and glo and determination of lysogeny and plaque formationa
| Strain, plasmid and/or phage | Relevant genotype | Plaque formation with K139.cm9 (%) | Lysogeny formation (%) with:
|
|
|---|---|---|---|---|
| K139.A | K139.cm2 | |||
| MAK757, pACYC184 | 100 | 100 | ||
| MAK757, pJBcII-orf2 | cI+ | 35.5 ± 14.1 | 1.12 ± 0.42 | |
| MAK757, pJBcII-glo | cI+ orf2+ | 0 | 0.208 ± 0.038 | |
| MAK757, pJBcII-int | cI+ orf2+ glo+ | 0 | 0.010 ± 0.006 | |
| MAK757, pTrckan | 100 | 100 | ||
| MAK757, pTrcglokan | glo+ | 14.2 ± 4.7 | 6.00 ± 1.57 | |
| MAK757, pTrcglokanb | glo+ | NDc | 0.62 ± 0.03 | |
| MAK757 | 100 | 100 | ||
| MAK757, K139 | 0 | 1.44 ± 0.19 | ||
| MAK757, K139.A | glo | 4.85 ± 1.48 | 2.81 ± 0.69d | |
| MAK757, K139.A, pTrcglokan | glo+ | 0 | 0.12 ± 0.03d | |
The lysis and lysogeny assays were repeated at least three times. To enable comparison of ratios, plaque number (PFU per milliliter) and formation of lysogens, using strain MAK757 or control plasmid-transformed derivatives (reference strains), have been set to 100%. The plaque number was about 107 to 108 PFU/ml for the reference strains. Lysogeny formation was determined by the ratio between all viable cells and lysogenic cells (Apr or Cmr) and was found to be between 1/12 and 1/200 (lysogen/viable cells) for the reference strains.
Cells were grown under glo-inducing conditions (1 mM IPTG).
ND, not determined.
Cells represent Apr Cmr double lysogens.
More evidence of Glo’s function was obtained in additional experiments. Reduction of lysogeny was observed when glo was encoded together with cI and orf2 (pJBcII-int [Table 3]). Furthermore, a glo mutation in strain MAK757 K139 glo::Tn10d-bla showed an increased level of being lysogenized by challenge phage K139.cm2 (Table 3) compared with K139 wt lysogenic cells. These glo lysogenic cells also allowed challenge phage K139.cm9 to produce plaques, which was not observed with lysogenic strain MAK757 K139 as the recipient strain. Both glo mutant phenotypes, (i) the increased level of plaque formation and (ii) the increased amount of lysogeny formation of the superinfecting challenge phages, could be complemented by a plasmid expressing Glo (Table 3). To exclude that Glo is involved in lysis control or phage maturation, the spontaneous release of phage K139 from cells expressing a plasmid encoding glo was determined. In these experiments, similar lysis rates were observed in cells expressing Glo from pTrcglokan (under noninduced and induced conditions) and cells harboring control plasmids (data not shown).
In contrast, spontaneous K139 phage release in CI-expressing cells (pJBcII-orf2) was found to be about 23-fold lower than in control plasmid-harboring cells (data not shown). Furthermore, no plaques were detected in recipient cells encoding cI from plasmid pJBcII-orf2 (Fig. 2) and challenged with wt phage K139 (data not shown). Both observations are in agreement with the function of the transcriptional regulator protein CI repressor of phage P186 (15), to which K139 CI shows considerable similarity. However, in lysogeny plating assays using phage K139.A as a challenge phage, the formation of Apr lysogenic colonies was reduced about 100-fold in the presence of CI (Table 3). This effect might be the result of a feedback inhibition on the expression of the int gene, due to higher cI copy numbers (pJBcII-orf2). By challenging strain MAK757 pJBcII-orf2 with the highly lytic phage K139.cm9, plaque formation was no longer prevented, but plaques appeared smaller. This finding indicates that the lytic development of this superinfecting phage could not be effectively repressed by the CI repressor, as explained above.
The Orf2 phenotype was also assumed to be involved in preventing superinfection. It was shown that if cI and orf2 were present on plasmids (pJBcII-glo [Fig. 2]), no plaques were observed upon challenge with the wt phage or with phage K139.cm9 (Table 3). Also, the formation of lysogeny was reduced in cells expressing orf2 (pJBcII-glo) compared with MAK757 pJBcII-orf2 (Table 3). Analysis of the spontaneous release of phage K139 in cI and orf2-expressing cells (pJBcII-glo) showed the same effect as seen with cI-expressing cells (pJBcII-orf2) (data not shown), indicating that orf2 does not interfere with phage induction or maturation processes.
Site-specific integration of phage K139 and characterization of the att site.
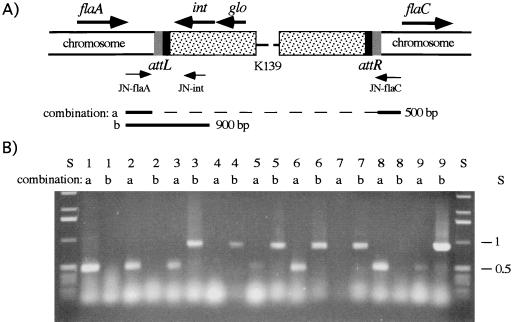
The first indication about the location of the attP site came from previous DNA hybridization experiments (47). In these analyses, the attP site was predicted to be located downstream of the int gene. DNA comparison of this region (bp 4348 to 5031) with the GenBank library resulted in the identification of a 24-bp sequence which is also found in a region located between the flagellin genes flaA and flaC of V. cholerae (29). To determine whether this region might be the attB site, the synthetic primers JN-flaA, JN-flaC and JN-int (Fig. 4A) were designed to amplify specific fragments consisting of the host attB or phage host attL site. Investigation of different strains revealed the presence of two types of PCR fragments indicating the absence or presence of integrated phage K139 between flaA and flaC. A 0.9-kb chromosomal phage junction PCR fragment was synthesized with JN-int and JN-flaA primers, indicating the integration of phage K139. A 0.5-kb chromosomal DNA PCR fragment was produced by JN-flaA and JN-flaC primers (Fig. 4A) if no phage was integrated. The 0.9-kb PCR fragments from two lysogenic strains were sequenced with primer seq 14 (Fig. 1). As a result, the predicted formation of the sequence structure int-attL-flaA was obtained. Subsequently, a typical dyad symmetry-structured att site (13) was identified as GAAANNG6CNNTTTTC, also seen in Fig. 1 (bp 4557 to 4579).
FIG. 4.
Identification of the phage and bacterial attachment site. (A) Chromosomal organization of integrated phage K139 between flaA and flaC and locations of the primers. Also indicated are the orientations of phage-harbored int and glo genes, attL and attR, and the PCR products expected from the primer combinations a (JN-flaA/JN-flaC) and b (JN-flaA/JN-int). (B) A 0.7% agarose gel shows the PCR products generated with primer combinations a and b. Lane S, 1-kb ladder DNA standard (Gibco Life Technologies); lane 1, classical O1 V. cholerae strain O395; lanes 2 to 7, O1 El Tor V. cholerae strains MAK757, MAK757 K139, M799, M804, M807, and M817, respectively; lanes 8 and 9, O139 V. cholerae strains AI1838 and MO10, respectively. PCR was performed for 30 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min.
In addition, several pathogenic O1 V. cholerae strains (M799, M804, M807, and M817) isolated from different time points and regions of cholera epidemics (27) were investigated for K139 lysogeny (Table 4). It was shown that these strains, including O139 (MO10) (64) and lysogenized MAK757 K139, were phage productive, immune to K139 infection, and positive for glo in PCR (data not shown) and Western blot (data not shown) analyses. Furthermore, the phage in these strains was found to be integrated at the attB site within the flagellin gene cluster (Fig. 4B). However, probably due to spontaneous phage curing, both PCR fragments (0.5 and 0.9 kbp) can be observed for several strains (Fig. 4B). O1 strain MAK757 and O139 strain AI1838 (64), previously described as not associated with K139 (47), were used as controls. In addition, classical O1 strain O395 (37) was found to be phage resistant, and glo+, but no phage production was observed. These results and results obtained earlier (21, 47) indicate that classical strain O395 harbors a defective kappa prophage variant which was not found to be integrated between the flagellin genes (Fig. 4B).
TABLE 4.
Association and characterization of K139 lysogeny among pathogenic V. cholerae strains
| Strain | Description; isolation | K139 plating | Phage release | glo (PCR) | Glo (Western) | Integration |
|---|---|---|---|---|---|---|
| O395 | O1 classical; India, 1964 | − | − | + | + | Not in flagellin genes |
| MAK757 | O1 El Tor; Celebes, 1937 | + | − | − | − | |
| MAK757 K139 | MAK757 K139 lysogen | − | + | + | + | Flagellin genes |
| M799 | O1 El Tor; Hong Kong, 1989 | − | + | + | + | Flagellin genes |
| M804 | O1 El Tor; India, 1962 | − | + | + | NDa | Flagellin genes |
| M807 | O1 El Tor; Vietnam, 1966 | − | + | + | ND | Flagellin genes |
| M817 | O1 El Tor; Chad, 1974 | − | + | + | ND | Flagellin genes |
| AI1838 | O139; Bangladesh, 1993 | − | − | − | − | |
| MO10 | O139; India, 1992 | − | + | + | + | Flagellin genes |
ND, not determined.
DISCUSSION
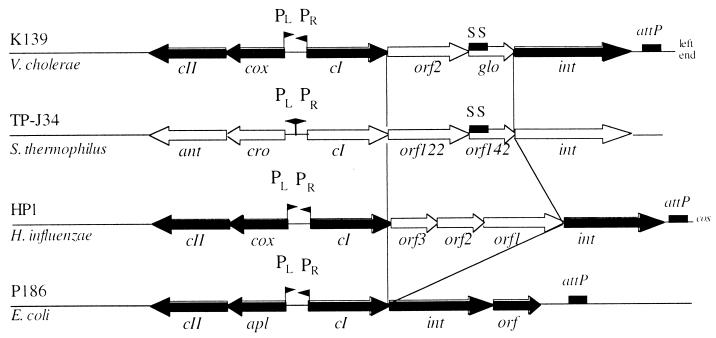
In this report, we present data addressing the characterization of genes which are encoded in the major control region of V. cholerae phage K139. This region was also characterized by sequencing, and it was found that the deduced ORFs and gene order showed significant similarities to the phage P2 family (10). In Fig. 5, this region is aligned to the lysogeny-lysis switch (major control) region of temperate phages P186 (26), HP1 (17), and TP-J34 (43). Similar to P186 and HP1, K139 contains divergently encoded lytic and lysogenic operons that appear to be transcribed from oppositely located promoters (PL and PR). In the transcriptional direction of the putative lytic promoter (PR), homologs of genes cox/apl and cII are encoded. Both Cox (HP1) and Apl (P186) have dual roles in prophage induction, acting directly in prophage excision and in repression of CI transcription from PL during prophage induction (18, 19, 46). The cII gene product of P186 is required for the establishment of lysogeny (42). K139 Cox and CII show some amino acid similarity to H. influenzae phage HP1 (Cox) and E. coli phage P186 (CII) and may therefore have analogous functions, which remains to be established experimentally. In addition, both putative proteins CII and Cox contain a weak helix-turn-helix motif in the N-terminal portion with SD scores (scores expressed in standard deviation units relative to the appropriate mean) of 3.15 and 2.48, respectively (16) (Fig. 1). This indicates that these proteins, like their homologs in phages P186 and HP1, may be DNA-binding proteins (14a).
FIG. 5.
Comparison of the major control regions of different phages. Sequence similarity between phages K139 of V. cholerae, HP1 of H. influenzae, and P186 of E. coli are indicated for the ORFs CII, Cox, CI, and Int as black arrows; light arrows show the corresponding genes for TP-J34 and HP1 without detectable sequence similarity. SS (black boxes), signal sequence which can also be found in Orf142 of phage TP-J34, encoding a putative lipoprotein (43); attP, phage attachment site; PL and PR, locations of promoter structures.
In the lysogenic state, transcription of the genes cI, orf2, glo, and int is probably initiated by the mapped promoter PL. The proximal ORF of the PL transcript, CI, is representing the putative CI repressor, since it shares similarity with P186 CI (26). Given the demonstrated phenotype of preventing superinfection and hindering spontaneous phage release, it seems reasonable to speculate that cI encodes the repressor responsible for controlling lytic development. However, the proposed function remains to be tested experimentally. The promoter-distal ORF in this region is the int gene, which shows consistent homology to phage integrases (7).
Two remaining genes (orf2 and glo) were found between cI and int. Orf2 showed no significant similarity to other proteins, whereas Glo harbors some features also found in eukaryotic G proteins, as reported earlier (47). However, in phages HP1 and TP-J34 (Fig. 5), additional ORFs of unknown function can be found between cI and int. Because of the position of orf2 and glo within two conserved functional phage gene products (cI and int), we investigated the involvement of these proteins in preventing superinfection. The results indicate that Orf2 helps to protect a lysogenic cell against superinfecting K139 phages. However, evidence that Glo is involved in hindering phage infection processes was also presented.
Certain phage-encoded resistance mechanisms are known to abort infection at an early step. Proteins which alter the cell surface in lysogenic strains and prevent adsorption to the phage receptor, a process known as lysogenic conversion, have been identified (54, 62). Other phage proteins are known to act in superinfection exclusion, by inhibiting the DNA transfer from the adsorbed phage particle into the cytoplasm. Examples of gene products which are involved in superinfection exclusion are represented by SieA (54, 55) and Sim (28) of temperate phages P22 and P1 and by Imm and Sp of the lytic phage T4 (34). Our investigations suggest that the Glo protein of phage K139 may exclude superinfecting phages either by superinfection exclusion or lysogenic conversion. Several facts account for that assumption. First, data from a combination of protein and cell fractionation analyses indicated that Glo is an exported protein, localized as a soluble protein in the periplasm. Therefore, we speculate that the exclusion function of Glo may be based on protein-protein interaction with yet unidentified targets or may be membrane associated. Second, plaque and lysogeny formation after superinfection with challenge phages was significantly reduced in the presence of Glo expressed from a plasmid. Consequently, a missing glo function in a lysogenic cell allowed increased plaque and lysogeny formation after superinfection with the challenge phage. This effect could be complemented in trans by a plasmid-encoded glo gene. Third, in killing experiments using K139.cm9, it was shown that Glo-expressing cells were significantly protected. Fourth, in contrast to cI-harboring plasmids, the glo-harboring plasmid did not interfere with phage induction or lysis control, as demonstrated by the quantification of spontaneously released phage particles. In future analyses, we will investigate the molecular basis of the predicted exclusion function of the Glo protein. A comparison of the gene region between cI and int with the corresponding regions of other temperate phages (Fig. 5) suggests that the other unassigned ORFs on such phages may also be associated with functions related to immunity and exclusion.
Previous analysis suggested a participation of Glo in O1 V. cholerae virulence in the infant mice assay (47). Our results presented here shed new light on Glo’s function, suggesting that Glo encodes a soluble periplasmic protein involved in phage exclusion. One explanation for the observed attenuated virulence phenotype of glo lysogenic V. cholerae cells may be that an elevated superinfection susceptibility led to a decreased viable V. cholerae cell number. However, we cannot exclude the possibility that Glo is released from the periplasm, for example, during phage-mediated cell lysis, allowing a direct interaction with host tissues. Recently, phage lysis was proposed as a plausible releasing mechanism for Shiga toxin Stx-I or Stx-II in an H-19B lysogenic E. coli strain (41). Future studies will investigate whether the glo-associated virulence effect is caused indirectly by a phage exclusion function or by direct interaction of the Glo protein with the host systems. Other phage-specific proteins which seem to be involved in both phage viability and virulence are known. For example, Ace of phage CTXØ is a minor coat protein (63) as well as an enterotoxin (60). Another example is represented by a Salmonella phage P22-encoded enzyme, which is responsible for alteration of the lipopolysaccharide structure, preventing phage adsorption and hence altering the antigenic properties (54, 66).
A 24-bp attP core site was found to be located in the distal part of the int gene. The corresponding attB site in the V. cholerae chromosome was found to be located in a noncoding region between the flagellin genes flaA and flaC. Recently, Klose and Mekalanos (29) presented sequence data identifying a transposon located downstream of the flaC region in O1 classical V. cholerae strains. Motility contributes to the virulence of V. cholerae (20); thus, one can speculate that the fla genes have been acquired by horizontal gene transfer, e.g., by phage transduction or conjugative transposons. Investigating several K139 lysogenic El Tor isolates, we always found K139 integrated into the flaAC region but not in an O1 classical isolate.
Besides the specific characterizations of the components of the major control region, some interesting questions regarding the relationship between pathogenic V. cholerae strains and the kappa phage family remain to be answered. Does phage K139 somehow contribute to the fitness and pathogenicity of O1 V. cholerae cells? Also, might K139 lysogeny support V. cholerae in its other, less well investigated ecological niches, e.g., the seawater environment or within shellfish?
ACKNOWLEDGMENTS
We thank I. B. Dodd, K. E. Klose, and J. Morschhäuser for many helpful comments, critical reading, and suggestions. We also thank S. Kahn for expert help in protein purification and A. Friedlein for mass spectroscopy analysis. For clinical V. cholerae strains used in this study, we thank J. J. Mekalanos and D. K. Karaolis.
This work was funded by BMBF grant 01KI8906.
REFERENCES
- 1.Ackermann H W, Kasatiya S S, Kawata T, Koga T, Lee J V, Mbiguino A, Newman F S, Vieu J F, Zachary A. Classification of Vibrio bacteriophages. Intervirology. 1984;22:61–71. doi: 10.1159/000149535. [DOI] [PubMed] [Google Scholar]
- 2.Albert J B, Siddinque A K, Islam M S, Faruquwe A S G, Anzarzzaman M, Faruque S M, Sack R. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet. 1993;341:704. doi: 10.1016/0140-6736(93)90481-u. [DOI] [PubMed] [Google Scholar]
- 3.Altermann E, Klein J R, Henrich B. Synthesis and automated detection of fluorescently labeled primer extension products. BioTechniques. 1998;26:96–98. doi: 10.2144/99261st03. [DOI] [PubMed] [Google Scholar]
- 4.Altschul S F, Madden T L, Schaffer A A, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amman E, Ochs B, Abel K. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 6.Aono R, Tsukagoshi N, Yamamoto N. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvements of organic solvent tolerance of Escherichia coli. J Bacteriol. 1998;180:938–944. doi: 10.1128/jb.180.4.938-944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argos P, Landy A, Abremski K, Egan J B, Haggard-Ljungquist E, Hoess R H, Kahn M L, Kalionis B, Narayana S V, Pierson III L S, Sternberg N, Leong J M. The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986;5:433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barondess J, Beckwith J. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature. 1990;346:871–874. doi: 10.1038/346871a0. [DOI] [PubMed] [Google Scholar]
- 9.Basu S, Mukerjee S. Bacteriophage typing of Vibrio El-Tor. Experientia. 1968;24:299–300. doi: 10.1007/BF02152832. [DOI] [PubMed] [Google Scholar]
- 10.Bertani L E, Six E W. The P2-like phages and their parasite, P4. In: Calendar R, editor. The bacteriophages. 2nd ed. New York, N.Y: Plenum Press; 1988. pp. 73–143. [Google Scholar]
- 11.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Bradley D E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967;31:230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell A M. Chromosomal insertion sites for phages and plasmids. J Bacteriol. 1992;174:7495–7499. doi: 10.1128/jb.174.23.7495-7499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Das, M., and A. K. Chopra, J. W. Peterson. 1997. Direct submission to GenBank.
- 14.Decker K, Kaul V, Meesmann A, Heller K J. Lytic conversion of Escherichia coli by bacteriophage T5: blocking of the FhuA receptor protein by a lipoprotein expressed early during infection. Mol Microbiol. 1994;12:321–332. doi: 10.1111/j.1365-2958.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 14a.Dodd, I. B. Personal communication.
- 15.Dodd I B, Egan J B. DNA binding by the coliphage 186 repressor protein CI. J Biol Chem. 1996;271:11532–11540. doi: 10.1074/jbc.271.19.11532. [DOI] [PubMed] [Google Scholar]
- 16.Dodd I B, Egan J B. Improved detection of helix-turn-helix DNA binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito D, Fitzmaurice W P, Benjamin R C, Goodman S D, Waldman A S, Scocca J J. The complete nucleotide sequence of bacteriophage HP1 DNA. Nucleic Acids Res. 1996;24:2360–2368. doi: 10.1093/nar/24.12.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esposito D, Scocca J J. Identification of an HP1 phage protein required for site-specific excision. Mol Microbiol. 1994;13:685–695. doi: 10.1111/j.1365-2958.1994.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 19.Esposito D, Wilson J C E, Scocca J J. Reciprocal regulation of the early promoter region of bacteriophage HP1 by the Cox and CI proteins. Virology. 1997;234:267–276. doi: 10.1006/viro.1997.8646. [DOI] [PubMed] [Google Scholar]
- 20.Gardel C L, Mekalanos J J. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect Immun. 1996;64:2246–2255. doi: 10.1128/iai.64.6.2246-2255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerdes J C, Romig W R. Complete and defective bacteriophages of classical Vibrio cholerae: relationship to the kappa-type bacteriophage. J Virol. 1975;15:1231–1238. doi: 10.1128/jvi.15.5.1231-1238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinrich J, Velleman M, Schuster H. The tripartite immunity system of phages P1 and P7. FEMS Microbiol Rev. 1995;17:121–126. doi: 10.1111/j.1574-6976.1995.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 23.Higa N, Honma Y, Albert J M, Iwanaga M. Characterization of Vibrio cholerae O139 synonym Bengal isolated from patients with cholera-like disease in Bangladesh. Microbiol Immunol. 1993;37:971–974. doi: 10.1111/j.1348-0421.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 24.Hovde C J, Calderwood S B, Mekalanos J J, Collier R J. Evidence that glutamic acid 167 is an active site residue of shiga-like toxin I. Proc Natl Acad Sci USA. 1988;85:2568–2572. doi: 10.1073/pnas.85.8.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson A D, Poteete A R, Lauer G, Sauer R T, Ackers G K, Ptashne M. Lambda repressor and cro components of an efficient molecular switch. Nature. 1981;294:217–223. doi: 10.1038/294217a0. [DOI] [PubMed] [Google Scholar]
- 26.Kalionis B, Dodd I B, Egan J B. Control of gene expression in the P2-related template coliphages. III. DNA sequence of the major control region of phage 186. J Mol Biol. 1986;191:199–209. doi: 10.1016/0022-2836(86)90257-3. [DOI] [PubMed] [Google Scholar]
- 27.Karaolis D K, Lan R, Reeves P R. Molecular evolution of the seventh-pandemic clone of Vibrio cholerae and its relationship to other pandemic and epidemic V. cholerae isolates. J Bacteriol. 1994;176:6199–6206. doi: 10.1128/jb.176.20.6199-6206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kliem M, Dreiseikelmann B. The superimmunity gene sim of bacteriophage P1 causes superinfection exclusion. Virology. 1989;171:350–355. doi: 10.1016/0042-6822(89)90602-8. [DOI] [PubMed] [Google Scholar]
- 29.Klose K E, Mekalanos J J. Differential regulation of multiple flagellins in Vibrio cholerae. J Bacteriol. 1998;180:303–316. doi: 10.1128/jb.180.2.303-316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraiß A, Schloer S, Reidl J. In vivo transposon mutagenesis in Haemophilus influenzae. Appl Environ Microbiol. 1998;64:4697–4702. doi: 10.1128/aem.64.12.4697-4702.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Lamont I, Richardson H, Carter D R, Egan J B. Genes for the establishment and maintenance of lysogeny by the temperate coliphage 186. J Bacteriol. 1993;175:5286–5288. doi: 10.1128/jb.175.16.5286-5288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J V, Furniss A L. The phage typing of Vibrio cholerae serovar O1. In: Holme T, Holmgren J, Merson M H, Molby R, editors. Acute enteric infections in children: new prospects for treatment and prevention. Amsterdam, The Netherlands: Elsevier/North Holland Biomedical Press; 1981. pp. 119–122. [Google Scholar]
- 34.Lu M J, Henning U. Superinfection exclusion by T-even-type coliphages. Trends Microbiol. 1994;2:137–139. doi: 10.1016/0966-842x(94)90601-7. [DOI] [PubMed] [Google Scholar]
- 35.Maillou J, Dreiseikelmann B. The sim gene of Escherichia coli phage P1: nucleotide sequence and purification of the processed protein. Virology. 1990;175:500–507. doi: 10.1016/0042-6822(90)90434-s. [DOI] [PubMed] [Google Scholar]
- 36.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 37.Mekalanos J J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 38.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 39.Mulligan M E, Hawley D K, Entriken R, McClure W R. E. coli promoter sequence predict in vitro RNA-polymerase selectivity. Nucleic Acids Res. 1984;12:789–797. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullis K B, Faloona F. Specific synthesis of DNA in vitro via a polymerase chain reaction. Methods Enzymol. 1987;155:335–340. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 41.Neely M N, Friedman D I. Functional and genetic analysis of regulatory regions of coliphage H-19B: location of shiga-like toxin and lysis genes suggest a role for phage function in toxin release. Mol Microbiol. 1998;28:1255–1267. doi: 10.1046/j.1365-2958.1998.00890.x. [DOI] [PubMed] [Google Scholar]
- 42.Neufing P J, Shearwin K E, Camerotto J, Egan J B. The CII protein of bacteriophage 186 establishes lysogeny by activating a promoter upstream of the lysogenic promoter. Mol Microbiol. 1996;21:751–761. doi: 10.1046/j.1365-2958.1996.351394.x. [DOI] [PubMed] [Google Scholar]
- 43.Neve H, Zenz K I, Desiere F, Koch A, Heller K J, Brüssow H. Comparison of the lysogeny modules from the temperate Streptococcus thermophilus bacteriophages TP-J34 and Sfi21: implications for the modular theory of phage evolution. Virology. 1998;241:61–72. doi: 10.1006/viro.1997.8960. [DOI] [PubMed] [Google Scholar]
- 44.Nicolle P, Gallut J, Schraen M F. Diversite des etats lysogenes parmi les vibrions choleriques El Tor de provenances varies. Bull Soc Pathol Exot. 1971;604:603–612. [PubMed] [Google Scholar]
- 45.Ramamurthy T, Grag S, Sharma R. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993;341:703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- 46.Reed M R, Shearwin K E, Pell L M, Egan J B. The dual role of Apl in prophage induction of coliphage 186. Mol Microbiol. 1997;23:669–681. doi: 10.1046/j.1365-2958.1997.2521620.x. [DOI] [PubMed] [Google Scholar]
- 47.Reidl J, Mekalanos J J. Characterization of Vibrio cholerae bacteriophage K139 and use of a novel mini transposon to identify a phage-encoded virulence factor. Mol Microbiol. 1995;18:685–701. doi: 10.1111/j.1365-2958.1995.mmi_18040685.x. [DOI] [PubMed] [Google Scholar]
- 48.Rose R E. The nucleotide sequence of pACYC177. Nucleic Acids Res. 1988;16:356. doi: 10.1093/nar/16.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:356. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimada T, Nair G B, Deb B C, Albert M J, Sack R B, Takeda Y. Outbreak of Vibrio cholerae non-O1 in India and Bangladesh. Lancet. 1993;341:1347. doi: 10.1016/0140-6736(93)90855-b. [DOI] [PubMed] [Google Scholar]
- 52.Shine J, Dalgarno L. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA. 1974;71:1341–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 53a.Skowronek, K. 1996. Direct submission to GenBank.
- 54.Susskind M M, Botstein D. Molecular genetics of bacteriophage P22. Microbiol Rev. 1978;42:385–413. doi: 10.1128/mr.42.2.385-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Susskind M M, Wright A, Botstein D. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. II. Genetic evidence for two exclusion systems. Virology. 1971;45:638–662. doi: 10.1016/0042-6822(71)90178-4. [DOI] [PubMed] [Google Scholar]
- 56.Synder L. Phage-exclusion enzymes: a bonanza of biochemical and cell biology reagents? Mol Microbiol. 1995;15:415–420. doi: 10.1111/j.1365-2958.1995.tb02255.x. [DOI] [PubMed] [Google Scholar]
- 57.Takeya K, Shimodori S. “Prophage typing” of El-Tor vibrios. J Bacteriol. 1963;85:957–958. doi: 10.1128/jb.85.4.957-958.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeya K, Zinnaka Y, Shimodori S, Nakayama Y, Amako K, Tida K. Lysogeny in El-Tor Vibrios. In: Bushnell O S, Brookhyser C S, editors. Proceedings of the Cholera Research Symposium, Honolulu. U.S. Washington, D.C: Department of Health, Education, and Welfare; 1965. pp. 24–29. [Google Scholar]
- 59.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trucksis M, Galen J E, Michalski J, Fasano A, Kaper J B. Accessory cholera enterotoxin (Ace), the third toxin of Vibrio cholerae virulence cassette. Proc Natl Acad Sci USA. 1993;90:5267–5271. doi: 10.1073/pnas.90.11.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Gabain A, Belasco J G, Schottel J L, Chang A C Y, Cohen S N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci USA. 1983;80:653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vostrov A A, Vostrukhina O A, Svarchevsky A N, Rybchin V N. Proteins responsible for lysogenic conversion caused by coliphages N15 and φ80 are highly homologous. J Bacteriol. 1996;178:1484–1486. doi: 10.1128/jb.178.5.1484-1486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waldor K W, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 64.Waldor M K, Mekalanos J J. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect Immun. 1994;62:72–78. doi: 10.1128/iai.62.1.72-78.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson N. A new revision of the sequence of plasmid pBR322. Gene. 1988;70:399–403. doi: 10.1016/0378-1119(88)90212-0. [DOI] [PubMed] [Google Scholar]
- 66.Wright A. Mechanism of conversion of the Salmonella O antigen by bacteriophage ɛ34. J Bacteriol. 1971;105:927–936. doi: 10.1128/jb.105.3.927-936.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu A, Bertani L E, Haggard-Ljungquist E. Control of prophage integration and excision in bacteriophage P2: nucleotide sequence of the int gene and att sites. Gene. 1989;80:1–12. doi: 10.1016/0378-1119(89)90244-8. [DOI] [PubMed] [Google Scholar]