Abstract
Background and objectives
Limited data are present on safety and efficiency of epinephrine for the prophylaxis and treatment of spinal-hypotension. This study was conducted to compare the effect of epinephrine with norepinephrine and phenylephrine on the treatment of spinal-hypotension and ephedrine requirement during cesarean delivery.
Methods
One hundred and sixty parturients with uncomplicated pregnancies undergoing elective cesarean delivery under spinal anesthesia were recruited. They were allocated randomly to receive norepinephrine 5 μg.mL−1 (n = 40), epinephrine 5 μg.mL−1 (n = 40), phenylephrine 100 μg.mL−1 (n = 40) or 0.9% saline infusions (n = 40) immediately after induction of spinal anesthesia. Whenever systolic blood pressure drops to less than 80% of baseline, 5 mg of intravenous ephedrine was administered as rescue vasopressor. The incidence of hypotension, total number of hypotension episodes, the number of patients requiring ephedrine, the mean amount of ephedrine consumption and side effects were recorded.
Results
There was no statistically significant difference in incidence of maternal hypotension between groups. The number of patients requiring ephedrine was significantly greater in group saline than in group phenylephrine (p < 0.001). However, it was similar between phenylephrine, norepinephrine, and epinephrine groups. The mean ephedrine consumption was significantly higher in group saline than in norepinephrine, epinephrine, phenylephrine groups (p = 0.001).
Conclusion
There is no statistically significant difference in incidence of hypotension and ephedrine consumption during spinal anesthesia for cesarean delivery with the use of epinephrine when compared to norepinephrine or phenylephrine. Epinephrine can be considered an alternative agent for management of spinal hypotension.
Keywords: Spinal anesthesia, Hypotension, Epinephrine, Norepinephrine, Phenylephrine
Resumo
Justificativa e objetivos
Existem dados limitados sobre segurança e eficiência da epinefrina na profilaxia e tratamento da hipotensão arterial associada a raquianestesia. O presente estudo foi realizado para comparar o efeito da epinefrina com norepinefrina e fenilefrina no tratamento da hipotensão após raquianestesia e necessidade de efedrina durante o parto cesáreo.
Método
Foram recrutadas 160 parturientes com gestações não complicadas, submetidas a cesariana eletiva sob raquianestesia. Elas foram alocadas aleatoriamente para receber norepinefrina 5 μg.mL−1 (n = 40), epinefrina 5 μg.mL−1 (n = 40), fenilefrina 100 μg.mL−1 (n = 40) ou infusão de solução fisiológica NaCl a 0,9% (n = 40) imediatamente após a indução da raquianestesia. Sempre que houvesse redução da pressão arterial sistólica para valor inferior a 80% da linha de base, 5 mg de efedrina intravenosa eram administrados como vasopressor de resgate. A incidência de hipotensão, o número total de episódios de hipotensão, o número de pacientes que necessitaram de efedrina, o consumo médio de efedrina e os efeitos colaterais foram registrados.
Resultados
Não houve diferença estatisticamente significante na incidência de hipotensão materna entre os grupos. O número de pacientes que necessitaram de efedrina foi significantemente maior no grupo solução fisiológica do que no grupo fenilefrina (p < 0,001). No entanto, foi semelhante entre os grupos fenilefrina, norepinefrina e epinefrina. O consumo médio de efedrina foi significantemente maior no grupo solução fisiológica do que nos grupos norepinefrina, epinefrina e fenilefrina (p = 0,001).
Conclusão
Não houve diferença estatisticamente significante na incidência de hipotensão e consumo de efedrina durante raquianestesia para parto cesáreo com uso de epinefrina quando comparada a norepinefrina ou fenilefrina. A epinefrina pode ser considerada como agente alternativo para o tratamento da hipotensão após raquianestesia.
Palavras-chave: Raquianestesia, Hipotensão, Epinefrina, Norepinefrina, Fenilefrina
Introduction
Several studies have demonstrated that α-agonist vasopressors are still the most reliable drugs for the management of spinal hypotension during cesarean delivery.1, 2, 3 Currently, phenylephrine is the vasopressor of choice for the prevention and treatment of spinal-induced hypotension, thanks to its pure α-agonistic effect.4 However, the drug can lead to bradycardia and reduced Cardiac Output (CO), even at clinically effective doses.2, 3
Norepinephrine maintains blood pressure as effectively as phenylephrine, and preserves Heart Rate (HR) and CO within normal ranges, thanks to additional β-effects.5 However, norepinephrine has been reported to exert minimal effect on CO and HR, as it increases afterload from α-1 stimulation, resulting in reflex bradycardia.6 Therefore, the drug has been advocated for use in the management of spinal-induced hypotension, during cesarean delivery. Equally, in North America, norepinephrine, ephedrine and epinephrine have also been recommended as first-choice vasopressors for the treatment of spinal hypotension during cesarean delivery.7 Additionally, in an international consensus guideline statement, epinephrine was recommended for circulatory collapse only.4
In fact, in the obstetric setting, epinephrine is a less familiar drug to anesthesia providers for preventing and treating spinal hypotension during cesarean delivery. In the literature, there is only one study comparing the effect of epinephrine with phenylephrine in the treatment of hypotension after hyperbaric tetracaine spinal anesthesia.8
Although some studies have compared the effect of phenylephrine and norepinephrine for the treatment of spinal hypotension,5, 9 there is limited data regarding the effect of epinephrine for the treatment of spinal hypotension, and comparisons with other vasopressors. However, debates and trials determining the best vasopressors for preventing and treating spinal hypotension during cesarean delivery are still ongoing.
Our hypothesis was that epinephrine would be more effective than norepinephrine and phenylephrine in the management of spinal hypotension during cesarean delivery. Thus, the primary outcome of this study was the incidence of intraoperative maternal hypotension, and the secondary outcomes were total ephedrine consumption and maternal and neonatal outcomes.
Material and methods
This study was approved by the University's Institutional Review Board (IRB decision number; 64/6). Written informed consent was obtained from all participating subjects. The trial was registered prior to patient enrollment at clinicaltrials.gov (NCT03163914) and conducted between July 2017 and March 2018.
Study format
This prospective, double-blinded, randomized, controlled, phase IV study was conducted to compare the effect of epinephrine with norepinephrine and phenylephrine, on the treatment of spinal hypotension, and ephedrine requirements during cesarean delivery. After obtaining written informed patient consent, 160 patients with American Society of Anesthesiologists (ASA) physical status I–II were recruited. Patients aged between 18–42 years were selected for elective cesarean section under spinal anesthesia. Exclusion criteria included the presence of respiratory, hepatic, renal, and cardiovascular disease, patients with a Body Mass Index (BMI) > 45 kg.m−2, and any contraindication to regional anesthesia. A 6–8 hours fasting period was enforced and premedication was not administered before surgery. An intravenous (iv) route was established with an 18G iv catheter into an upper limb vein.
Monitoring and measurements
All patients were monitored using Noninvasive Arterial Blood Pressure (NIBP), Electrocardiogram (ECG), and Peripheral Oxygen Saturation (SpO2). Upon arrival in the operating room, patients were placed in a supine position with left lateral tilt. HR and Systolic Blood Pressures (SBP) were measured three times, and the mean HR and SBP were accepted as baseline values. SBP, Diastolic Blood Pressure (DBP), Mean Blood Pressure (MBP), and HR were measured preoperatively, and at 1-minute intervals for 15 minutes, and then at 2.5 minutes intervals thereafter until the end of surgery.
Spinal anesthesia
Spinal anesthesia was performed on all patients with a combination of hyperbaric bupivacaine (10 mg) and fentanyl (20 μg). Drugs were applied to the patients in a sitting position with a 25 G pencil-point needle, through the L3–L4 or L4–L5 intervertebral space. Immediately after spinal anesthesia injection, the patient was placed in a 15°–20° left lateral supine position, and the study drugs were initiated. Oxygen was not administered unless the peripheral oxygen saturation level fell below 94%. The maximal sensory block height was assessed by an investigator once every 3 minutes, until the same dermatome level was confirmed on three consecutive evaluations. This dermatomal level was accepted as the maximal sensory block height. Surgery commenced when the block height was higher than T5. The investigator was unaware of study agent/group allocation.
Randomization
Patients were randomly assigned to one of four study agents/groups, using computer-generated schedule permuted blocks with size of eight. Four injectors were prepared by an investigator blinded to the study groups, and these were tagged only with a coded label to preserve the double-blinded nature of the study. Drugs were diluted in 5% dextrose solution, in 50 mL injectors that were labelled “study drug” and contained a 30 mL volume. The injectors were identical and consisted of 150 μg norepinephrine (5 μg.mL−1), 150 μg epinephrine (5 μg.mL−1), 3 mg phenylephrine (100 μg.mL−1), and the same volume of saline solution (30 mL).
Study design
According to the study protocol and randomization procedures, solutions of 5 μg.mL−1 norepinephrine (group norepinephrine), 5 μg.mL−1 epinephrine (group epinephrine), 100 μg.mL−1 phenylephrine (group phenylephrine) and 0.9% saline (group saline) were infused immediately after spinal anesthesia induction. All study drugs were infused at a 30 mL.h−1 fixed rate and continued at this rate until the end of surgery. Rapid iv co-hydration was administered using Ringer’s Lactate (RL) solution, with the clamp fully open to a maximum of 2L, after which infusion was reduced to a slow maintenance rate.
An SBP < 80% of baseline (prenatal) SBP was regarded as hypotension. In the event SBP dropped to < 80% of baseline, 5 mg iv ephedrine was administered as a rescue vasopressor, regardless of the group. Incidences for hypotension, the total number of hypotension episodes during surgery (the magnitude of hypotension), the number of patients requiring ephedrine, the mean amount of ephedrine and atropine consumption, and adverse effects such as bradycardia and hypertension were recorded during the study. Bradycardia was defined as a HR < 60 beats/min and was corrected with a 0.5 mg iv bolus dose of atropine sulfate. Hypertension, regarded as SBP > 120% of baseline, was treated by discontinuation of vasopressor infusion. The study drug infusion was recommenced when the SBP decreased below the maximal level of the target range (< 80% of baseline SBP).
Data collection
Two investigators participated as the anesthesia provider and data collector. The first investigator, who was in charge of the case, applied the spinal block, administered study agents and fluids, supervised patients, and recorded the maximal sensory block height. The other investigator, who was blinded to the study drugs, recorded hemodynamic parameters, ephedrine consumption, and adverse effects.
Demographic data (age, height, and weight), parturient characteristics (parity, BMI, and indication for cesarean), and surgery duration were collected. The time from intrathecal injection of local anesthetic + fentanyl, to skin incision, and time from skin incision to fetal delivery were calculated and recorded. Newborns were assessed by a pediatrician after delivery, and Apgar scores were recorded at the first and the fifth minutes. Fetal blood samples were taken from the umbilical cord vein, to measure umbilical blood gases. All blood gas samples were measured immediately using an ABL800 FlexQ device (XLab solution, Denmark).
Postoperative recovery
Patients were transported to the Post Anesthesia Care Unit (PACU) for follow-up after surgery. In the PACU, hemodynamic variables, regression of motor and sensory blockage, and adverse effects were assessed. Discharge criteria were stable vital signs, completely resolved motor blockade, and adequate analgesia (verbal pain scores < 4), absence of nausea, vomiting and pruritus.
Power analysis
To determine the power of the study, we referred to the trial of Tsen et al., as it was conducted in a similar clinical setting.10 Using data from that trial, the primary endpoint of this study was the incidence of intraoperative maternal hypotension. We determined that 38 patients from each group would be needed to determine a 35% difference in the incidence of hypotension between the saline group (the incidence of hypotension: 70%)10 and vasopressor groups (the incidence of hypotension: 35% expected in each group) (β = 0.1, α = 0.05, 0.35:0.35:0.35:0.70). However, to compensate for losses and improve statistical power, 40 patients were recruited to each group.
Statistical analysis
Categorical variables are presented as number and percentages, whereas continuous variables are expressed as the mean with standard deviation. The Chi-Square test was chosen to compare categorical variables between groups. The normality of distribution for continuous variables was confirmed using the Shapiro-Wilk test. For group comparisons, one-way analysis of variance (ANOVA) or the Kruskal-Wallis test was chosen, depending on whether statistical hypotheses were fulfilled. For normally distributed data, regarding homogeneity of variances, Tukey or Games & Howell tests were used for multiple group comparisons. For non-normal distributed data, the Bonferroni corrected Mann-Whitney U test was used for multiple group comparisons. A p < 0.05 value was considered statistically significant. Statistical analyses were performed using the IBM SPSS Statistics Version 20.0 statistical software package (IBM Corp., Armonk, NY, USA, released 2011).
Results
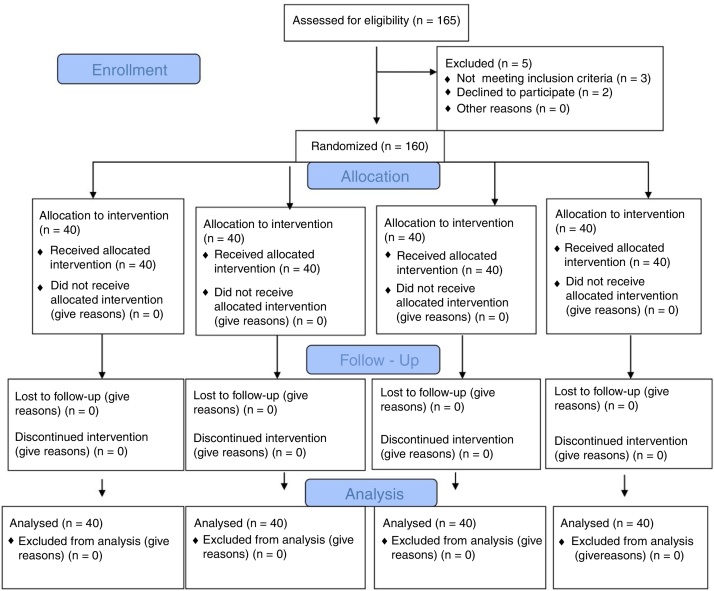
One hundred sixty patients were enrolled, and all study protocol completed (Fig. 1). Demographic data (age, weight, and height), parturient characteristics (BMI, parity, and indication of cesarean) and duration of surgery were presented in Table 1. The maximum sensory block level, time between intrathecal injection to skin incision, and time between skin incision to fetal delivery were similar (Table 2).
Figure 1.
Flow diagram.
Table 1.
Demographics, parturient characteristics, duration of surgery data in groups.
| Group NE (n = 40) | Group E (n = 40) | Group PE (n = 40) | Group S (n = 40) | |
|---|---|---|---|---|
| Age (years), mean ± SD | 30.8 ± 5.3 | 30 ± 4.5 | 31.5 ± 5.9 | 31.9 ± 6 |
| Weight (kg), mean ± SD | 79.8 ± 9.9 | 83.4 ± 10.9 | 82.5 ± 9.6 | 82.4 ± 12.3 |
| Height (cm), mean ± SD | 1.62 ± 0.04 | 1.64 ± 0.04 | 1.63 ± 0.03 | 1.63 ± 0.04 |
| BMI (kg.cm−2), mean ± SD | 29.9 ± 2.6 | 30.8 ± 3.1 | 30.8 ± 2.5 | 30.5 ± 3.1 |
| Parity median (IQR) | 2 (1) | 2.5 (1.75) | 2.5 (1) | 3 (1) |
| Duration of surgery (min) (mean ± SD) | 31.4 ± 5.5 | 33.2 ± 5.5 | 29.75 ± 4.9 | 30.8 ± 6.7 |
| Indication of cesarean | ||||
| Cesarean history | 34 (85%) | 35 (87%) | 35 (87%) | 36 (90%) |
| AFD | 4 (10%) | 3 (7.5%) | 4 (10%) | 3 (7.5%) |
| Others | 2 (5%) | 2 (5%) | 1 (2.5%) | 1 (2.5) |
Data were presented as Mean ± SD or Median; IQR, Interquartile Range; BMI, Body Mass Index; AFD, Acute Fetal Distress; NE, Norepinephrine; E, Epinephrine; PE, Phenylephrine; S, Saline.
Table 2.
Apgar scores and umbilical cord gas data in groups.
| Group NE (n = 40) | Group E (n = 40) | Group PE (n = 40) | Group S (n = 40) | p | |
|---|---|---|---|---|---|
| Apgar Scores Median (IQR) | |||||
| 1 min | 8 (1) | 9 (1)a | 8 (1) | 8 (2) | 0.001 |
| 5 min | 9 (1) | 10 (1)a | 9.5 (1) | 9 (1) | 0.016 |
| Umbilical venous blood gas median (IQR) | |||||
| pH | 7.34 (0.06)a | 7.32 (0.05)a | 7.31 (0.03)a | 7.3 (0.06) | 0.001 |
| PCO2 (mmHg) | 39.8 (3.48) | 37.8 (5.88) | 39.8 (3.5) | 39.1 (5.1) | 0.151 |
| pO2 (mmHg) | 24.2 (7.48) | 28.6 (7.45) | 28.4 (5.3) | 27.1 (7.6) | 0.273 |
| Base excess (mmoL.L-1) | −1.25 (3.75)a | −3.35 (4.48)a | −4.3 (1.28)a | −4.7 (2) | 0.001 |
Data were presented as Median (IQR, Interquartile range).
ap < 0.05 compared with group S.
pO2, Partial Oxygen Pressure; pCO2, Partial Carbon Dioxide pressure; NE, Norepinephrine; E: Epinephrine; PE, Phenylephrine; S, Saline.
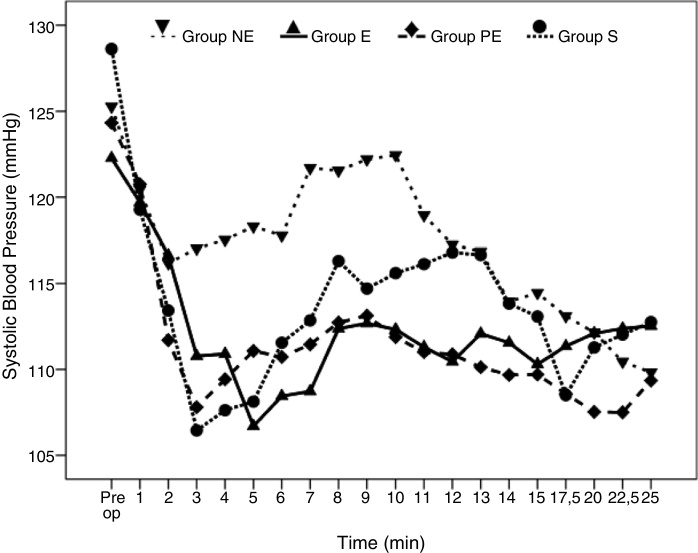
Perioperative hemodynamic SBP variables are shown (Fig. 2). Transient decreases and increases in blood pressure values were observed throughout the study. These variables (SBP, DBP, and MBP) were similar among groups, suggesting SBP were maintained by study drugs and/or ephedrine to baseline levels.
Figure 2.
Systolic Blood Pressure variables in the groups. Figure 2 shows changes in systolic arterial pressure in the groups at the first 25 min. after the induction of spinal anesthesia. (NE, Norepinephrine; E, Epinephrine; PE, Phenylephrine; S, Saline).
Hypotension incidences are shown (Table 3). There were no statistically significant differences in the incidence of intraoperative maternal hypotension among the groups (p = 0.625). Twenty-eight (70%) patients in group norepinephrine, 29 (72.5%) patients in group epinephrine, 27 (67.5%) patients in group phenylephrine versus 32 (80%) patients in group saline experienced one or more hypotension event. Only 17 (42.5%) patients in the norepinephrine group, 18 (45%) patients in the epinephrine group, 9 (22.5%) patients in the phenylephrine group, and 26 (65%) patients in the saline group required rescue ephedrine for spinal-hypotension correction. There were significant differences between the saline and the phenylephrine groups in terms of the number of patients requiring ephedrine (p = 0.001). However, there was no significant difference between phenylephrine, norepinephrine, and epinephrine groups.
Table 3.
Comparison of maternal outcomes and side effects.
| Group NE (n = 40) | Group E (n = 40) | Group PE (n = 40) | Group S (n = 40) | p | |
|---|---|---|---|---|---|
| Maximal sensory block height n (%) | 0.805 | ||||
| T3–4 | 23 (57%) | 19 (47%) | 19 (47%) | 21 (53%) | |
| T4–5 | 17 (43%) | 21 (53%) | 21 (53%) | 19 (47%) | |
| Intrathecal injection to skin incision (min) | 7 ± 0.6 | 6.9 ± 0.5 | 6.9 ± 0.5 | 6.8 ± 0.3 | 0.466 |
| Skin incision to fetal delivery (min) | 4.5 ± 0.4 | 4.7 ± 0.5 | 4.6 ± 0.4 | 4.6 ± 0.4 | 0.228 |
| Incidence of hypotension n (%) | 28 (70%) | 29 (72.5%) | 27 (67.5%) | 32 (80%) | 0.625 |
| The mean number of hypotension episodesa | 3.53 ± 4.4 | 3.2 ± 4.2 | 2.88 ± 3.6 | 4.8 ± 5.2 | 0.228 |
| The number of patients requiring ephedrine n (%) | 17 (42.5%) | 18 (45%) | 9 (22.5%)b | 26 (65%) | 0.001 |
| Mean ephedrine consumption (mg) | 3.7 ± 5.1b | 3.5 ± 4.4b | 2.1 ± 4.7b | 9.1 ± 8 | 0.001 |
| Total atropine dose (μg) | 62.5 ± 167 | 37.5 ± 133 | 75 ± 180 | 62.5 ± 167 | 0.770 |
| Side effects | |||||
| Bradycardia | 5 (12.5%) | 3 (7.5%) | 6 (15%) | 5 (12.5%) | 0.752 |
| Nausea | 7 (17.5%) | 5 (12.5%) | 5 (12.5%) | 8 (20%) | 0.734 |
| Vomiting | 3 (8%) | 2 (12.5%) | 3 (7.5%) | 6 (15%) | 0.452 |
Data are presented as n (%) or mean ± SD.
aThe magnitude of hypotension.
bp < 0.05 compared with group S, Mann Whitney-U.
NE, Norepinephrine; E, Epinephrine; PE, Phenylephrine; S, Saline; min, minutes; mg, milligrams; μg, micrograms.
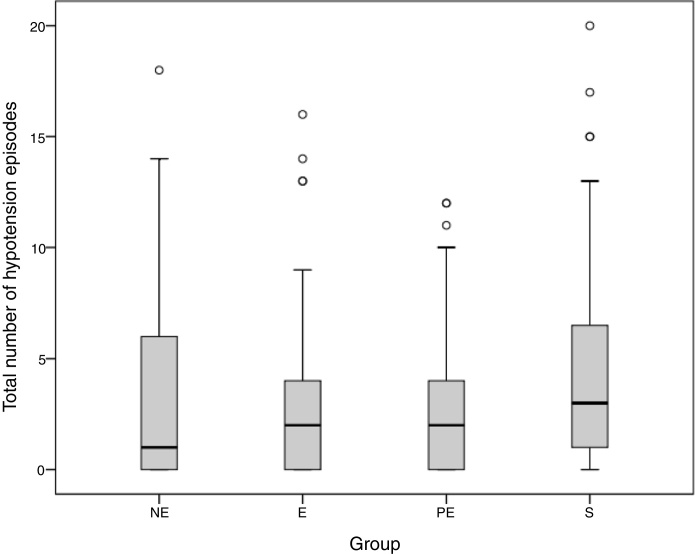
The mean number of hypotension episodes during anesthesia is shown (Table 3). There were no statistically significant differences in the number of hypotension episodes between groups (Fig. 3). Mean ephedrine consumption was significantly higher in the saline group, when compared to the norepinephrine, epinephrine, and phenylephrine groups (p = 0.001) (Table 3). Although the phenylephrine group consumed the least ephedrine when compared with the other groups, there were no significant differences between phenylephrine, norepinephrine, and epinephrine groups.
Figure 3.
The total number of hypotension episodes. The total number of hypotension episodes shows the magnitude of hypotension in the groups. (NE, Norepinephrine; E, Epinephrine; PE, Phenylephrine; S, Saline).
There were no statistically significant differences for bradycardia incidences and mean atropine consumption between groups (Table 3). The mean norepinephrine, epinephrine, and phenylephrine consumption in groups during anesthesia was 78.6 ± 13.7 μg, 87 ± 14.2 μg and 1487 ± 249 μg, respectively.
Apgar scores and umbilical venous blood gas analyses are shown (Table 2). Side effect incidences (hypertension, bradycardia, nausea, vomiting) between groups were similar, with no significant differences (Table 3).
Discussion
Our results showed that the epinephrine group had a similar incidence of hypotension and ephedrine consumption, when compared with norepinephrine and phenylephrine groups during spinal anesthesia for cesarean section. The saline group consumed significantly more ephedrine when compared with the vasopressor groups, suggesting hypotension was significantly more pronounced in this group.
Although structurally similar, epinephrine and norepinephrine exert different effects in their affinities for adrenergic receptor subtypes. Norepinephrine acts mainly by stimulation of α receptors, but it also has weak β adrenergic effect.5 Epinephrine interacts with the same receptors as norepinephrine, but it has greater affinity to both α and β receptors when compared with norepinephrine.11 Phenylephrine is a pure α-adrenergic receptor agonist, and is as pharmacologically effective as norepinephrine.1 Thus, norepinephrine and epinephrine are powerful vasopressors and may have theoretical advantages of added β-effects when compared with phenylephrine.
To establish placental perfusion and oxygen delivery to the fetus during spinal anesthesia, effective management of hypotension is crucial. Studies have demonstrated that placental perfusion and oxygen delivery to the fetus are primarily related to maternal CO and HR, rather than SBP.5, 12 Preliminary studies comparing norepinephrine with phenylephrine have demonstrated that norepinephrine preserves placental blood flow better than phenylephrine.5, 13 In 2015, Nagn Kee et al. suggested that this effect was mainly attributed to the additional β-effects of norepinephrine.5 However, in their previous study, combinations of ephedrine and phenylephrine were not found to be superior to phenylephrine alone.14 In this study, the lowest number of patients requiring ephedrine and least ephedrine use in group phenylephrine suggested that the additional β-effect of a vasopressor may be indispensable.
Using different relative concentrations or doses, several studies have compared the potency of phenylephrine and norepinephrine in patients undergoing spinal anesthesia.5, 15 Ngan Kee et al., previously described infusions of phenylephrine 100 μg.mL−1 versus norepinephrine at 5 μg.mL−1 administered by computer-controlled infusion, and suggested an approximate potency ratio of 20:1.5 When we began this trial, the updated potency ratio of norepinephrine:phenylephrine was 20:1;5 therefore, used a potency ratio of 20:1. However, the same authors in their more recent study revised the potency ratio as 13.1:1.0.9
A limited study has been published on the efficacy of epinephrine for the management of hypotension during regional anesthesia.8, 16 Brooker et al. compared the effect of epinephrine with phenylephrine in the treatment of hypotension after hyperbaric tetracaine spinal anesthesia.8 In their study, patients were given both bolus and infusion doses of either epinephrine or phenylephrine, in the ratio of 10:1. In our study, we chose a potency ratio of 1:1:20 for epinephrine:norepinephrine:phenylephrine, respectively. Notwithstanding these discrepancies, further dose-finding studies are required to identify exact potency ratios for these drugs.
Prophylactic fixed rate infusions of vasopressors reduce the severity and incidence of maternal hypotension; data from a dose-finding study suggested that 25 and 50 μg.min−1 may be preferable starting dosage ranges for prophylactic phenylephrine infusions, when compared with initial infusion ranges of 5 and 100 μg.min−1.17 Accordingly, in our study, we used 50 μg min−1 (3 mg.hour−1) infusion doses of phenylephrine. Epinephrine and norepinephrine infusion rates were also determined according to the potency ratio of phenylephrine, at 2.5 μg.min−1. Different relative concentrations or doses should be explored in further studies.
We found no significant differences between the four groups for spinal hypotension incidences; however, ephedrine consumption was significantly higher in the saline group than in vasopressor groups. This increased ephedrine consumption was attributed to the deeper magnitude of spinal hypotension in this group. Therefore, this study supports the concept that ephedrine consumption may not always be related to spinal hypotension incidences.
Patients in all groups consumed more atropine than the usual; possibly because we defined bradycardia with a HR lower than 60 beats.min−1 instead of 50 beats.min−1. Additionally, patients in the epinephrine groups required less atropine than the other vasopressor groups, possibly because of this drug strong β1 effects and less reflex bradycardia.
Although the safety of any new proposed vasopressor is always a concern, this study evaluated the safety of epinephrine infusion by way of fetal umbilical blood gases and Apgar scores. Base excess and pH values from umbilical venous blood gas analyses were significantly lower in the saline group than in vasopressor groups. The lower umbilical venous pH and BE in the saline group may be related to decreased utero-placental oxygen delivery, when compared with the vasopressor groups. Low et al. stated that a pH < 7.1 and less than BE-12 mEq.L−1 might be a cause for concern.18 In our study, pH and BE values were significantly higher (pH > 7.1 and > BE 0–12 mEq.L−1) than clinically critical values in all groups.
There were some limitations to our study. First, we found no significant differences between the four groups for spinal hypotension incidences. If we increased patient numbers or vasopressor agent doses, we may have found such significant differences between the saline and vasopressor groups. Secondly, we did not measure CO changes during drug infusions, because we did not have access to a CO instrument. Studies evaluating CO changes associated with phenylephrine have demonstrated that CO changes run parallel with HR changes.1, 19, 20 In our study, HR changes were similar between groups. Finally, we did not assess catecholamine safety metrics. Neonatal safety profiles of maternal catecholamines in pregnancy are potentially concerning, however, catecholamines do not cross the placenta in significant quantities, thanks to placental breakdown of catecholamines.21, 22 Furthermore, human trial data on the fetal effects of catecholamines are reassuring.21
In conclusion, there were no statistically significant differences in the incidences of hypotension and ephedrine consumption during spinal anesthesia for cesarean delivery with epinephrine, when compared to norepinephrine or phenylephrine. Epinephrine may be considered as an alternative agent for the management of spinal hypotension. Further and larger study trials are warranted to demonstrate the safety and efficacy of epinephrine.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Habib A.S. A review of the impact of phenylephrine administration on maternal hemodynamics and maternal and neonatal outcomes in women undergoing cesarean delivery under spinal anesthesia. Anesth Analg. 2012;114:377–390. doi: 10.1213/ANE.0b013e3182373a3e. [DOI] [PubMed] [Google Scholar]
- 2.Ngan Kee W.D., Khaw K.S., Ng F.F. Comparison of phenylephrine infusion regimens for maintaining maternal blood pres-sure during spinal anaesthesia for Caesarean section. Br J Anaesth. 2004;92:469–474. doi: 10.1093/bja/aeh088. [DOI] [PubMed] [Google Scholar]
- 3.Stewart A., Fernando R., McDonald S., et al. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg. 2010;111:1230–1237. doi: 10.1213/ANE.0b013e3181f2eae1. [DOI] [PubMed] [Google Scholar]
- 4.Kinsella S.M., Carvalho B., Dyer R.A., et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73:71–92. doi: 10.1111/anae.14080. [DOI] [PubMed] [Google Scholar]
- 5.Ngan Kee W., Lee S., Ng F., et al. Randomized doubleblinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122:736–745. doi: 10.1097/ALN.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 6.Severs W.B., Summy-Long J.Y. Norepinephrine-induced reflex bradycardia after central administration of angiotensin II. Pharmacology. 1977;15:428–435. doi: 10.1159/000136719. [DOI] [PubMed] [Google Scholar]
- 7.Macarthur A., Riley E.T. Obstetric anesthesia controversies: vasopressor choice for postspinal hypotension during cesarean delivery. Int Anesthesiol Clin. 2007;45:115–132. doi: 10.1097/AIA.0b013e31802b8d53. [DOI] [PubMed] [Google Scholar]
- 8.Brooker R.F., Butterworth J.F., 4th, Kitzman D.W., et al. Treatment of hypotension after hyperbaric tetracaine spinal anesthesia. A randomized, double-blind, cross-over comparison of phenylephrine and epinephrine. Anesthesiology. 1997;86:797–805. doi: 10.1097/00000542-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Ngan Kee W.D. A Random-allocation Graded Dose-Response Study of Norepinephrine and Phenylephrine for Treating Hypotension during Spinal Anesthesia for Cesarean Delivery. Anesthesiology. 2017;127:934–941. doi: 10.1097/ALN.0000000000001880. [DOI] [PubMed] [Google Scholar]
- 10.Tsen L.C., Boosalis P., Segal S., et al. Hemodynamic effects of simultaneous administration of intravenous ephedrine and spinal anesthesia for cesarean delivery. J Clin Anesth. 2000;12:378–382. doi: 10.1016/s0952-8180(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 11.Zaritsky A.L. In: The pharmacologic approach to the critically ill patient. Chernow B., editor. Williams and Wilkins; Baltimore: 1994. Catecholamines, inotropic medications, and vasopressor agents; pp. 387–404. [Google Scholar]
- 12.Veeser M., Hofmann T., Roth R., et al. Vasopressors for the management of hypotension after spinal anesthesia for elective caesarean section. Systematic review and cumulative meta-analysis. Acta Anaesthesiol Scand. 2012;56:810–816. doi: 10.1111/j.1399-6576.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson-Myrthil N. Vasopressor use in adult patients. Cardiol Rev. 2012;20:153–158. doi: 10.1097/CRD.0b013e31824e2294. [DOI] [PubMed] [Google Scholar]
- 14.Ngan Kee W.D., Lee A., Khaw K.S., et al. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: the effects on fetal acid-base status and hemodynamic control. Anesth Analg. 2008;107:1295–1302. doi: 10.1213/ane.0b013e31818065bc. [DOI] [PubMed] [Google Scholar]
- 15.Onwochei D.N., Ngan Kee W.D., Fung L., et al. Norepinephrine intermittent intravenous boluses to prevent hypotension during spinal anesthesia for cesarean delivery: a sequential allocation dose-finding study. Anesth Analg. 2017;125:212–218. doi: 10.1213/ANE.0000000000001846. [DOI] [PubMed] [Google Scholar]
- 16.Ottesen S., Renck H., Jynge P. Cardiovascular effects of epidural analgesia. II. Haemodynamic alterations secondary to lumbar epidural analgesia and their modification by plasma expansion and adrenaline administration. Acta Anaesthesiol Scand. 1978;69:17–31. doi: 10.1111/j.1399-6576.1978.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharrock N.E., Urquhart B. Hemodynamic response to low-dose epinephrine infusion during hypotension epidural anesthesia for total hip replacement. Reg Anesth. 1990;15:295–299. [PubMed] [Google Scholar]
- 18.Low J.A., Lindsay B.G., Derrick E.J. Threshold of metabolic acidosis associated with newborn complications. Am J Obstet Gynecol. 1997;177:1391–1394. doi: 10.1016/s0002-9378(97)70080-2. [DOI] [PubMed] [Google Scholar]
- 19.Dyer R.A., Reed A.R., Van Dyk D., et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology. 2009;111:753–765. doi: 10.1097/ALN.0b013e3181b437e0. [DOI] [PubMed] [Google Scholar]
- 20.Thomas D.G., Robson S.C., Redfern N., et al. Randomized trial of bolus phenylephrine or ephedrine for maintenance of arterial pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 1996;76:61–65. doi: 10.1093/bja/76.1.61. [DOI] [PubMed] [Google Scholar]
- 21.Vallejo M.J., Zakowski M.I. Old ways do not open new doors: norepinephrine for first-line treatment of spinal hypotension. Anesth Analg. 2018;126:1809–1811. doi: 10.1213/ANE.0000000000002491. [DOI] [PubMed] [Google Scholar]
- 22.Puolakka J., Kauppila A., Tuimala R., et al. The effect of parturition on umbilical blood plasma levels of norepinephrine. Obstet Gyneco. 1983;61:19–21. [PubMed] [Google Scholar]