Abstract
Background and objectives
Abdominal Hysterectomy (AH) is associated with significant inflammatory response and can result in moderate to severe postoperative pain. This study aimed to evaluate the efficacy of magnesium infusion in reducing postoperative pain and analgesic consumption after AH under spinal anesthesia with Intrathecal Morphine (ITM).
Method
Eighty-six patients were included in this clinical, controlled, randomized, double-blind study. Patients received in Group Mg, MgSO4 50 mg kg−1 for 15 minutes followed by 15 mg kg−1 h−1 until the end of the surgery; and in Group C, (control) the same volume of isotonic saline. Both groups received 100 μg of ITM. All patients received dipyrone + ketoprofen intraoperatively and postoperatively, and dexamethasone intraoperatively only. We evaluated the intensity of pain, tramadol consumption, and adverse events 24 hours postoperatively.
Results
Serum magnesium concentrations were higher in Group Mg at the end, and one hour after the operation (p = 0.000). Postoperative pain scores were reduced in Group Mg at 6 hours at rest and on movement (p < 0.05). Tramadol consumption did not show a statistically significant difference between Group Mg and Group C (15.5 ± 36.6 mg and 29.2 ± 67.8 mg respectively, p = 0.53). Hemodynamic variables, the incidence of pruritus, nausea, and vomiting were similar in the two groups.
Conclusion
Infusion of MgSO4 during AH undergoing spinal anesthesia with ITM reduced at 6 hours at rest and on movement. More studies should be performed to evaluate the potential antinociceptive effect of MgSO4 in scenarios where a multimodal analgesia approach was employed.
Keywords: Hysterectomy, Magnesium sulfate, Tramadol, Postoperative pain, Spinal anesthesia, Morphine
Introduction
Hysterectomy is the most common major gynecologic operation and is usually performed through laparotomy.1 Abdominal Hysterectomy (AH) is associated with an intense inflammatory response that can result in moderate to severe postoperative pain, sometimes difficult to control.2, 3 Adequate analgesia that allows early mobilization with fewer complications is essential for improved postoperative recovery.4, 5 Perioperative regional anesthesia is often used in fast-track programs. Spinal anesthesia with intrathecally applied opioids may further optimize postoperative pain management after AH.6, 7 The most commonly used doses of morphine range from 100 to 200 μg, with higher doses increasing the adverse effects, especially nausea, vomiting, and postoperative itching.7, 8 To minimize these adverse effects and optimize analgesia, it has been attempted to associate adjuvants.9, 10 Intravenous (IV) magnesium is used as an adjuvant due to its analgesic properties. Although the exact mechanism is not fully understood, the analgesic properties of magnesium are based on acting as a non-competitive antagonist of N-Methyl-D-Aspartate (NMDA) receptors in central nervous system and regulating the calcium influx into the cell. These properties avoid the central sensitization mechanisms due to the stimulation of peripheral nociceptive nerves.11 To our knowledge, no previous studies have addressed the use intravenous (IV) of magnesium in patients undergoing hysterectomy under spinal anesthesia with Intrathecal Morphine (ITM), which may be a more efficacious alternative for the postoperative analgesia. Therefore, we conducted this study to evaluate the efficacy of the magnesium to the analgesia after abdominal hysterectomy under spinal anesthesia with ITM.
Methods
The Research Ethics Committee of the institution approved this study. This trial was registered in the Brazilian Clinical Trials database (RBR-7B5X5K). We obtained written, informed consent from all patients. Between October 2016 and December 2018, patients aged between 18 and 65 years, ASA (American Society of Anesthesiologists) physical status I to III and scheduled to undergo abdominal hysterectomy were eligible to participate in this prospective, randomized, controlled, double-blind study. Exclusion criteria included Body Mass Index (BMI) ≥ 40 kg m−2, previous abdominal surgery (except cesarean delivery), oncological surgery, severe cardiovascular, renal, and hepatic dysfunction, neuromuscular diseases, using calcium channel blockers, and inappropriate for spinal anesthesia.
Randomization lists were computer-generated. During preparation for surgery, a nurse, educated on how to prepare the drugs, opened the sealed black envelopes containing the groups, and prepared the infusions. In this manner, patients were randomized to receive one of two regimens: in the Group Mg, IV Magnesium Sulfate (MgSO4) 50 mg kg−1 (regarding the ideal body weight) in 100 mL of isotonic saline over 15 minutes immediately before spinal anesthesia, and then 15 mg kg.−1 h−1 until the end of the operation (MgSO4 at a concentration of 100 mg mL−1); in the Group C (control), the same volume of isotonic saline over the same period. For example, a patient weighing 80 kg received 40 mL of the solution in 15 minutes, followed by 12 mL h−1 of the same solution until the end of the operation, either in the intervention group or in the control group. Patients, anesthesiologists, and surgeons were unaware of the randomization process or the types of infusion used. An observer who was blinded to both groups recorded the study data.
Anesthesia and operative techniques
The patients had nothing by mouth overnight. In the operation room, routine monitorization including electrocardiography, pulse oximetry, and noninvasive blood pressure were implemented. Then, an 18G IV cannula was inserted. Oxygen 3–4 L min−1 was administered via a nasal catheter. Then, each patient received boluses of 0.03 mg kg−1 of midazolam and 0.25 μg kg−1 of fentanyl for sedation, aiming to a Ramsay Sedation Scale score of 3.12 Before anesthesia onset, all patients received 500 mL of Ringer's lactate solution and 10 mL kg−1 h−1 of the same solution until the end of the operation. An anesthesiologist performed spinal anesthesia through L3–4 or L4–5 interspace in lateral decubitus position and using 25G or 27G Quincke needles. After confirming the cerebrospinal fluid flow, 3.5 mL of 0.5% hyperbaric bupivacaine +100 μg of morphine was administered over 30 seconds. Immediately after the blockade, patients were placed in horizontal dorsal decubitus. The loss of pinprick sensation evaluated the level of sensory block. The start of the operation was authorized as soon as the T6 dermatome was reached. In operation, the Pfannenstiel incision was used. Hypotension was defined as a decrease in systolic arterial blood pressure by 20% from baseline values, and it was treated with ephedrine 5 mg IV. Bradycardia was defined as heart rate decreases to less than 50 bpm, and it was treated with atropine 0.5 mg IV. All the patients received cefazolin 2 g, dexamethasone 10 mg, ketoprofen 100 mg, and dipyrone 2 g IV at the beginning of the surgery and ondansetron 4 mg IV at the end of surgery. Ketoprofen 100 mg and dipyrone 2 g IV were maintained every 6 and 8 hours after surgery, respectively. Pain scores were evaluated using a 0–10 cm visual analog scale (VAS, from 0, no pain, to 10, worst pain imaginable). In the Post-Anesthesia Care Unit (PACU) and after discharge to the ward (discharge readiness from the PACU was assessed by using the modified Aldrete's score13 every 15 minutes until patients met discharge criteria (score = 10), tramadol 100 mg IV was administered when VAS scores were > 3, or the patient requested analgesic. Nausea and Vomiting (NVPO) were treated with 4 mg ondansetron intravenously.
Outcome variables
The primary outcomes of this study were the pain intensity assessed at rest and on movement (from lying to sitting on the bed) at 6 and 24 hours after the operation, using the VAS score. Secondary endpoints included the tramadol consumption and occurrence of hypotension, bradycardia, NVPO, and pruritus 24 hours postoperatively. Demographic variables, ASA physical status, previous cesarean delivery, comorbidities, and duration of surgery were recorded for each patient. Hemodynamic variables: Mean Arterial Blood Pressures (MAP) and Heart Rates (HR) were recorded at time 0 (before the study drug infusion), 15, 30, 60, 90, and 120 minutes during the surgery, and one hour after admission in the PACU. The serum level of magnesium was assessed immediately before, in the end, and one hour after the operation.
Statistical analysis
The sample size calculation was based on the mean ± Standard Deviation (SD) 4.2 cm (1.9) pain scores on movement from a pilot study involving hysterectomies under spinal anesthesia. We considered a reduction in the mean intensity pain score of 1.26 cm as clinically significant. A sample size, taking into consideration an α error of 5% and a β error of 20%, was calculated as at least 36 patients per group. Continuous data with normal distribution were analyzed by the Student's t test for independent samples. Continuous data without normal distribution or discrete data were analyzed using the Mann-Whitney test. For categorical data, the Chi-Square test or Fisher's exact test was used. Generalized estimating equations model was used for comparative analysis of the evolution of the variables MAP and HR over time between groups. A significant difference was set at p-value < 0.05. For the statistical analysis, SPSS version 21.0 was used.
Results
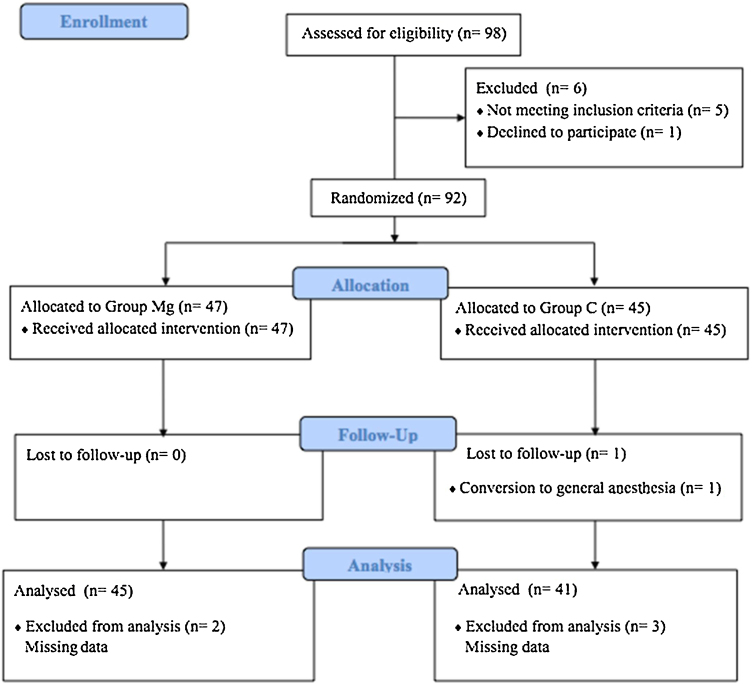
Ninety-eight patients were recruited for the study. Ninety-two patients were randomized to the study. One patient was excluded due to conversion to general anesthesia. Five patients were excluded due to missing data. The data of 86 patients were analyzed. The flow diagram of recruitment, allocation, follow-up, patient analysis, and causes of exclusion is shown in Figure 1.
Figure 1.
The flow chart of the study.
There were no significant differences between groups regarding patient demographics, ASA physical status, comorbidities, the occurrence of previous cesarean delivery, and duration of surgery (Table 1).
Table 1.
Baseline characteristics of the patients in the groups.
| Group Mg | Group C | p-value | |
|---|---|---|---|
| (n = 45) | (n = 41) | ||
| Age (years)a | 44.5 ± 6.7 | 45.9 ± 7.7 | 0.39 |
| Weight (Kg)a | 70.0 ± 12.8 | 73.0 ± 13.8 | 0.29 |
| Height (cm)a | 161.3 ± 5.6 | 162.0 ± 6.7 | 0.59 |
| ASA (I/II/III)b | 20/24/1 | 18/22/1 | 0.99 |
| Previous CD (Yes/No)b | 22/23 | 27/14 | 0.16 |
| Duration of surgeryb | 107.4 ± 28.8 | 103.9 ± 28.5 | 0.57 |
| Comorbitiesb | |||
| SAH (n, %) | 13 (28.8) | 12 (29.2) | 0.92 |
| DM (n, %) | 3 (6.6) | 2 (4.8) | |
| Other (n, %) | 6 (13.3) | 4 (9.6) |
Values are mean ± standard deviation, n or n (%). ASA, American Society of Anesthesiologists physical Status; CD, Cesarean Delivery; SAH, Systemic Arterial Hypertension; DM, Diabetes Mellitus.
Student's t test.
Chi-square test.
Postoperative serum magnesium concentrations in Group Mg were significantly higher than those in Group C immediately after surgery, and at one hour after surgery (p = 0.000) (Table 2).
Table 2.
Serum magnesium concentration (mEq.L−1) in the study groups.
| Group Mg | Group C | p-value | |
|---|---|---|---|
| (n = 45) | (n = 41) | ||
| Preoperative | 2.0 (0.9–3.0) | 1.8 (0.8–2.7) | 0.63 |
| Immediately after operation | 3.1 (1.5–4.8) | 1.9 (0.9–2.8) | 0.000 |
| One hour after operation | 3.2 (1.7–4.2) | 1.9 (0.9–2.8) | 0.000 |
Values are mean (minimum – maximum). Student's t test.
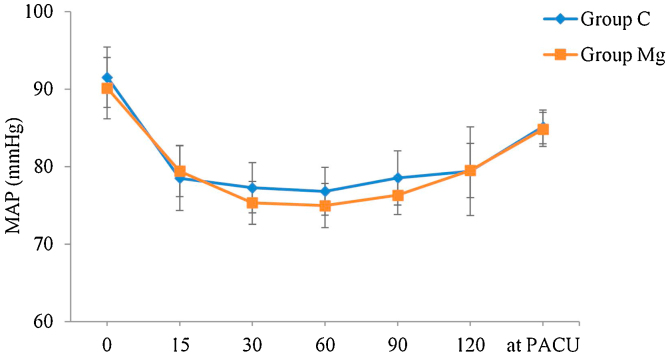
MAP was similar in the two groups (p = 0.59). There was no interaction between group and time (p = 0.73), but there was a decrease in MAP over time (baseline > 15, 30, 60, 90 minutes, and at PACU) with progressive recovery at the end of the observed period (at PACU > 30, 60, and 120 minutes), p = 0.000 (Figure 2).
Figure 2.
Mean arterial pressure (mean and 95% confidence interval) according to group and time. Horizontal axis: 0 (baseline), 15, 30, 60, 90, 120 minutes, and at Postanesthesia Care Unit (PACU). Variable group (p = 0.59); variable time (p = 0.000); variable time versus group (p = 0.73). Generalized estimating equations model.
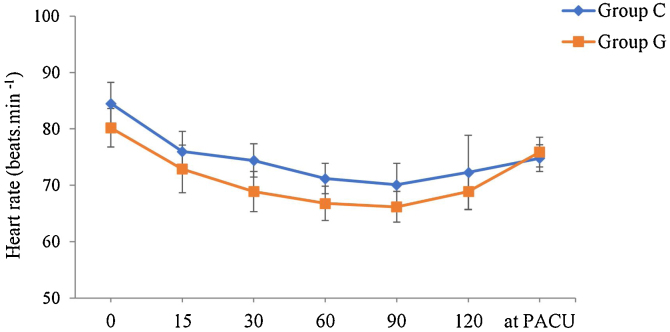
Mean HR was similar in the two groups (p = 0.054). There was also no interaction between group and time (p = 0.12), but there was a decrease in HR over time (baseline > 15, 30, 60, 90 minutes, and at PACU) with progressive recovery at the end of the observed period (at PACU > 30, 60, and 90 minutes), p = 0.000 (Fig. 3).
Figure 3.
Heart rate (mean and 95% Confidence Interval) according to group and time. Horizontal axis: 0 (baseline), 15, 30, 60, 90, 120 minutes, and at Postanesthesia Care Unit (PACU). Variable group (p = 0.054); variable time (p = 0.000); variable time versus group (p = 0.12). Generalized estimating equations model.
The incidence of hypotension was similar between the two groups: Group Mg, 31.1%, and Group C, 17.1%, (p = 0.14). The incidence of bradycardia was similar between groups: Group Mg, 11.1%, and Group C, 0% (p = 0.05).
Postoperative VAS scores were lower in Group Mg at rest and on movement than in Group C at 6 hours (p = 0.02 and p = 0.03, respectively). However, VAS scores were similar at 24 hours at rest (p = 0.12) and on movement (p = 0.29). There was no significant difference between Group Mg and Group C concerning the tramadol consumption (15.5 ± 36.6 mg and 29.2 ± 67.8 mg respectively, p = 0.53) (Table 3).
Table 3.
Average VAS pain scores and tramadol consumption 24 h after surgery.
| Group Mg | Group C | p-value | |
|---|---|---|---|
| (n = 45) | (n = 41) | ||
| VAS at rest 6h after surgery | 2 (0–3.6) | 3 (2–5) | 0.02 |
| VAS on movement 6h after surgery | 4 (2–5) | 5 (5–7) | 0.03 |
| VAS at rest 24h after surgery | 1.2 (0–3) | 2 (0–4.5) | 0.12 |
| VAS on movement 24h after surgery | 4 (2–6) | 4.8 (3–6) | 0.29 |
| Tramadol consumption (mg) | 15.5 ± 36.6 | 29.2 ± 67.8 | 0.53 |
Data are expressed mean and standard deviation and median (percentile 25–75).
VAS, Visual Analogic Scale. Mann-Whitney test.
The incidence of NVPO was similar between the two groups: Group Mg, 8.9%, and Group C, 17.1%, (p = 0.33). The incidence of pruritus (moderate and severe) was similar between the two groups: Group Mg, 6.7%, and Group C, 0% (p = 0.24).
Discussion
This study showed that MgSO4 infusion during abdominal hysterectomy under spinal anesthesia with 100 μg ITM reduced postoperative pain scores at 6 hours but did not reduce at 24 hours. We also observed that MgSO4 had no impact on tramadol consumption, and we did not find any notable adverse events.
Regional anesthesia can be preferred to general anesthesia during the perioperative period.6, 8, 14 In our service, abdominal hysterectomy is performed under spinal anesthesia with 17.5 mg hyperbaric bupivacaine and 100 μg of ITM, unless contraindicated. Although this scheme is adequate, some patients report pain of moderate to severe intensity. Pain after abdominal hysterectomy can be multifactorial: from a surgical incision, visceral pain, and particularly dynamic pain, such as during coughing and mobilization. Accordingly, several strategies for pain management have been introduced during the intraoperative and postoperative period. The administration of adjuvants to optimize postoperative analgesia and reduce painful stimuli to prevent central sensitization and amplification of postoperative pain has been used. Intravenous magnesium has been used in obstetrics for a long time to safely treat pregnant women with eclampsia and preeclampsia. It has recently been used as an adjuvant due to its analgesic properties. Although the exact mechanism is not fully understood, the analgesic properties of magnesium are based on acting as a non-competitive antagonist of NMDA receptors in the central nervous system and in the peripheral tissues.15 It also acts by regulating the influx of calcium into the cell.11 Moreover, it was suggested that NMDA receptor antagonists might enhance the analgesic effect of opioids by delaying or reducing the development of acute tolerance.11, 16
The normal range of magnesium in plasma is 1.5–2.5 mEq.L−1. Hypermagnesemia (serum concentrations more than 4 mEq.L−1) rarely occurs in clinical medicine unless the renal function of the patient is compromised. Further increasing magnesium serum concentrations may result in hypotension, loss of deep tendon reflex, and dizziness. Respiratory arrest and cardiac arrest occur at blood concentrations greater than 12 mEq.L−1.11 In this study, with MgSO4 in the loading dose (50 mg kg−1 in for minutes) and maintenance (15 mg kg−1.h−1), six patients with the serum concentration exceeded 4 mEq.L−1 (4.03–4.89), and none of them presented any complications.
In this study, the MAP and HR were uniforms over time between the two groups, without significant hemodynamic changes. These results are similar to studies of Hwang et al.17 and Agrawal et al.18 According to these authors, the pre-hydration with 500 mL of Ringer's lactate and the slow infusion of the medication could explain this hemodynamic stability. In contrast, Ryu et al.19 and Seyhan et al.20 reported lower MAP and HR values in patients who received MgSO4. Five cases of bradycardia (with immediate recovery after administration of atropine) were recorded in Group Mg and no case in Group C (p = 0.05). However, bradycardia was never observed in concomitance to hypotension. According to Albrecht et al.,21 the incidence of bradycardia was higher in the magnesium group (RR = 1.76; 95% CI 1.01–3.07; p = 0.04), but without an increased incidence of hypotension (RR = 1.49; 95% CI 0.88–2.52; p = 0.14), and they reported that although bradycardia was more common after magnesium administration, there were no reports of persistent hemodynamic instability or bradycardia that did not respond to first-line pharmacologic therapy.
A systematic review carried out in 2013 by Albrecht et al.,21 that included two studies in which patients received spinal anesthesia and 23 in which they received general anesthesia, concluded that perioperative IV magnesium reduces opioid consumption and, to a lesser extent, pain scores in the first 24 postoperative hours. Recently, several studies have reported the benefits of IV magnesium infusion in the postoperative pain scores and opioid consumption of patients who undergone surgery under general anesthesia.22, 23, 24, 25, 26, 27 However, the effects of magnesium on pain scores and the consumption of opioids in patients undergoing spinal anesthesia is still debated. In some studies, the patients received only bupivacaine,28, 29, 30 and in other studies, the patients received only bupivacaine or bupivacaine + 10–20 μg fentanyl18, 31 and showed a consistent decrease in opioid consumption, but with a decrease in pain scores up to 4,28, 29 10,30 and 48 hours31 postoperatively. In our study, there was a decrease in pain scores only in the first 6 hours postoperatively, which may reflect a more limited effect of magnesium sulfate infusion in our patients. It is noteworthy that in the study conducted by Agrawal et al.,18 in patients undergoing lower limb orthopedic surgeries, the consumption of tramadol in the group that received magnesium compared to the group that received saline was (190 ± 30 mg vs. 265 ± 48 mg; p = 0.000). This result differs significantly from the result of our study (15.5 ± 36.6 mg vs. 29.2 ± 67.8 mg versus, p = 0.53). We can suppose that the inflammatory response in these orthopedic surgeries is very different from hysterectomies.
To the best of our knowledge, we found only one prospective study in which patients received magnesium infusion that underwent spinal anesthesia with hyperbaric bupivacaine plus ITM, but none of them did hysterectomies. In a randomized, double-blind study, Frassanito et al.32 evaluated 40 patients undergoing total knee arthroplasty under spinal anesthesia with hyperbaric bupivacaine mixed with 100 μg ITM – 20 received magnesium infusion and 20 received saline. Ketorolac and paracetamol were routinely prescribed and received analgesic rescue with received patient-controlled analgesia with morphine in the postoperative period. These authors found neither decreased pain scores nor a decrease in morphine consumption and concluded that a multimodal analgesic approach could partially hide the effect of MgSO4. We conjecture that, in our study, multimodal analgesic approach to pain, which included the administration of dexamethasone 10 mg in a single dose, in addition to the administration of ketoprofen 100 mg and dipyrone 2 g at regular intervals within 24 hours of the operation may have mitigated the effects of infusion of MgSO4.
Our study showed an incidence of PONV of approximately 9% in the Group Mg and 17% in the Group C (p = 0.33). Interestingly, the systematic review carried out in 2018 by Peng et al.,33 suggested that the administration of IV magnesium in patients undergoing orthopedic surgeries could decrease nausea (Relative Risk – RR = 0.32, Confidence Interval – 95% CI 0.12–0.82) and vomiting (RR = 0.38, 95% CI 0.15–0.92).
The present study has some limitations. First, it was decided not to exclude women with previous cesarean delivery, but this decision may hinder the external validation of our results. Second, it was not evaluated the effect of magnesium on inflammatory activity. Third, it was not investigated the potential action of magnesium ion on prolonging the duration of sensory block of spinal anesthesia with bupivacaine. Finally, it was analyzed the data at a single medical center; therefore, the generalizability of this study may be limited.
Conclusion
In conclusion, we found that intraoperative administration of MgSO4 (50 mg kg−1 boluses followed by 15 mg kg−1 h−1 continuous infusions) during AH under spinal anesthesia with 100 μg ITM reduced postoperative pain scores at 6 hours (at rest and on movement) but did not reduce at 24 hours. We also found that magnesium sulfate had no impact on tramadol consumption, with any notable adverse events. More studies should be performed to evaluate the potential antinociceptive effect of MgSO4, in scenarios where a multimodal analgesia approach was employed.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Aarts J.W., Nieboer T.E., Johnson N., et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015;2015:CD003677. doi: 10.1002/14651858.CD003677.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandsborg B. Pain following hysterectomy: epidemiological and clinical aspects. Dan Med J. 2012;59:B4374. [PubMed] [Google Scholar]
- 3.Kim T.K., Yoon J.R. Comparison of the neuroendocrine and inflammatory responses after laparoscopic and abdominal hysterectomy. Korean J Anesthesiol. 2010;59:265–269. doi: 10.4097/kjae.2010.59.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beverly A., Kaye A.D., Ljungqvist O., Urman R.D. Essential Elements of Multimodal Analgesia in Enhanced Recovery After Surgery (ERAS) Guidelines. Anesthesiol Clin. 2017;35:e115–e143. doi: 10.1016/j.anclin.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Azari L., Santoso J.T., Osborne S.E. Optimal pain management in total abdominal hysterectomy. Obstet Gynecol Surv. 2013;68:215–227. doi: 10.1097/OGX.0b013e31827f5119. [DOI] [PubMed] [Google Scholar]
- 6.Wodlin N.B., Nilsson L., Arestedt K.al. Mode of anesthesia and postoperative symptoms following abdominal hysterectomy in a fast-track setting. Acta Obstet Gynecol Scand. 2011;90:369–379. doi: 10.1111/j.1600-0412.2010.01059.x. [DOI] [PubMed] [Google Scholar]
- 7.Hein A., Rösblad P., Gillis-Haegerstrand, et al. Low dose intrathecal morphine effects on post-hysterectomy pain: a randomized placebo-controlled study. Anaesthesiol Scand. 2012;56:102–109. doi: 10.1111/j.1399-6576.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 8.Borendal Wodlin N., Nilsson L., Carlsson P., Kjølhede P. Cost-effectiveness of general anesthesia vs spinal anesthesia in fast-track abdominal benign hysterectomy. Am J Obstet Gynecol. 2011;205 doi: 10.1016/j.ajog.2011.05.043. 326.e1326.e3267. [DOI] [PubMed] [Google Scholar]
- 9.Baldini G., Carli F. Anesthetic and adjunctive drugs for fast-track surgery. Curr Drug Targets. 2009;10:667–686. doi: 10.2174/138945009788982504. [DOI] [PubMed] [Google Scholar]
- 10.Brown E.N., Pavone K.J., Naranjo M. Multimodal General Anesthesia: Theory and Practice. Anesth Analg. 2018;127:12461258. doi: 10.1213/ANE.0000000000003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sang-Hwan Do. Magnesium: a versatile drug for anesthesiologists Korean J Anesthesiol. 2013;65:4–8. doi: 10.4097/kjae.2013.65.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsay M.A., Savege T.M., Simpson B.R., Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldrete J.A. The Post-Anesthetic Recovery Score Revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 14.Catro-Alves L.J., De Azevedo V.L., De Freitas Braga T.F., Goncalves A.C., De Oliveira G.S., Jr. The effect of neuraxial versus general anesthesia techniques on postoperative quality of recovery and analgesia after abdominal hysterectomy: a prospective, randomized, controlled trial. Anesth Analg. 2011;113:1480–1486. doi: 10.1213/ANE.0b013e3182334d8b. [DOI] [PubMed] [Google Scholar]
- 15.Khorasanizadeh S., Panahi M., Mohseni G., et al. Comparison of analgesia in subcutaneous infiltration of ropivacaine and magnesium sulfate for postoperative pain control of cholecystectomy. Novel Biomed. 2020;8:13–19. [Google Scholar]
- 16.Bujalska-Zadrożny M., Tatarkiewicz J., Kulik K., Filip M., Naruszewicz M. Magnesium enhances opioid-induced analgesia – What we have learnt in the past decades? Eur J Pharm Sci. 2017;99:113–127. doi: 10.1016/j.ejps.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Hwang J.Y., Na H.S., Jeon Y.T., et al. Infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. Br J Anaesth. 2009;103:861–866. doi: 10.1093/bja/aep334. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal A., Agrawal S., payal Y.S. Effect of continuous magnesium sulfate infusion on spinal block characteristics: A prospective study. Saudi J Anaesth. 2014;8:78–82. doi: 10.4103/1658-354X.125945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu J.H., Kang M.H., Park K.S., Do S.H. Effects of magnesium sulphate on intraoperative anesthetic requirements and postoperative analgesia in gynecology patients receiving total intravenous anesthesia. Br J Anaesth. 2008;100:397–403. doi: 10.1093/bja/aem407. [DOI] [PubMed] [Google Scholar]
- 20.Seyhan T.O., Tugrul M., Sungur M.O., et al. Effects of three different dose regimens of magnesium on propofol requirements, haemodynamic variables and postoperative pain relief in gynaecological surgery. Br J Anaesth. 2006;96:247–252. doi: 10.1093/bja/aei291. [DOI] [PubMed] [Google Scholar]
- 21.Albrecht E., Kirkham K.R., Liu S.S., Brull R. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: a meta-analysis. Anaesthesia. 2013;68:79–90. doi: 10.1111/j.1365-2044.2012.07335.x. [DOI] [PubMed] [Google Scholar]
- 22.Taheri A., Haryalchi K., Mansour Ghanaie M., Habibi Arejan N. Effect of low-dose (single-dose) magnesium sulfate on postoperative analgesia in hysterectomy patients receiving balanced general anesthesia. Anesthesiol Res Pract. 2015;2015:306145. doi: 10.1155/2015/306145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asadollah S., Vahdat M., Yazdkhasti P., et al. Magnesium sulphate on postoperative analgesia requirements. 2015 J Turk Soc Obstet Gynecol. 2015;1:34–37. doi: 10.4274/tjod.02439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haryalchi K., Abedinzade M., Khanaki K., et al. Whether preventive low dose magnesium sulphate infusion has an influence on postoperative pain perception and the level of serum beta-endorphin throughout the total abdominal hysterectomy. Rev Esp Anestesiol Reanim. 2017;64:384–390. doi: 10.1016/j.redar.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Ghaffaripour S., Mahmoudi H., Eghbal H., et al. The effect of intravenous magnesium sulfate on post-operative analgesia during laminectomy. Cureus. 2016;8:e626. doi: 10.7759/cureus.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghezel-Ahmadi V., Ghezel-Ahmadi D., Schirren J., et al. Perioperative systemic magnesium sulphate to minimize acute and chronic post-thoracotomy pain: a prospective observational study. J Thorac Dis. 2019;11:418–426. doi: 10.21037/jtd.2019.01.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C., Tao R. The impact of magnesium sulfate on pain control after 28. laparoscopic cholecystectomy: a meta-analysis of randomized controlled studies. Surg Laparosc Endosc Percutan Tech. 2018;28:349–353. doi: 10.1097/SLE.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 28.Kahraman F., Eroglu A. The effect of intravenous magnesium sulfate infusion on sensory spinal block and postoperative pain score in abdominal hysterectomy. BioMed Research International. 2014;2014:236024. doi: 10.1155/2014/236024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah P.N., Dhengle Y. Magnesium sulfate for postoperative analgesia after surgery under spinal anesthesia. Acta Anaesthesiol Taiwan. 2016;54:62–64. doi: 10.1016/j.aat.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhong H.Y., Zhang W.P. Effect of intravenous magnesium sulfate on bupivacaine spinal anesthesia in preeclamptic patients. Biomed Pharmacother. 2018;108:1289–1293. doi: 10.1016/j.biopha.2018.09.157. [DOI] [PubMed] [Google Scholar]
- 31.Shin H.J., Kim E.Y., Na H.S., Kim T.K., Kim M.H., Do S.H. Magnesium sulphate attenuates acute postoperative pain and increased pain intensity after surgical injury in staged bilateral total knee arthroplasty: a randomized, double-blinded, placebo-controlled trial. Br J Anaesth. 2016;117:497–503. doi: 10.1093/bja/aew227. [DOI] [PubMed] [Google Scholar]
- 32.Frassanito L., Messina A., Vergari A., et al. Intravenous infusion of magnesium sulfate and postoperative analgesia in total knee arthroplasty. Minerva Anestesiol. 2015;81:1184–1191. [PubMed] [Google Scholar]
- 33.Peng Y.N., Sung F.C., Huang M.L., et al. The use of intravenous magnesium sulfate on postoperative analgesia in orthopedic surgery A systematic review of randomized controlled trial. Medicine (Baltimore). 2018;97:e13583. doi: 10.1097/MD.0000000000013583. [DOI] [PMC free article] [PubMed] [Google Scholar]