Abstract
This second joint document, written by experts from the Brazilian Association of Allergy and Immunology (ASBAI) and Brazilian Society of Anesthesiology (SBA) concerned with perioperative anaphylaxis, aims to review the pathophysiological reaction mechanisms, triggering agents (in adults and children), and the approach for diagnosis during and after an episode of anaphylaxis. As anaphylaxis assessment is extensive, the identification of medications, antiseptics and other substances used at each setting, the comprehensive data documentation, and the use of standardized nomenclature are key points for obtaining more consistent epidemiological information on perioperative anaphylaxis.
Keywords: Allergy and immunology, Hypersensitivity, Anaphylaxis, Perioperative period, Anesthesia, Skin tests
Resumo
Este segundo documento, escrito por especialistas da Associação Brasileira de Alergia e Imunologia (ASBAI) e da Sociedade Brasileira de Anestesiologia (SBA) interessados no tema anafilaxia perioperatória, tem por objetivo revisar os mecanismos fisiopatológicos, agentes desencadeantes (em adultos e crianças), assim como a abordagem diagnóstica durante e após o episódio. Por se tratar de uma avaliação abrangente, a identificação das medicações, antissépticos e outras substâncias usadas em cada região, registros detalhados e nomenclatura padronizada são pontos fundamentais para a obtenção de dados epidemiológicos mais fidedignos sobre a anafilaxia perioperatória.
Palavras-chave: Alergia e imunologia, Hipersensibilidade, Anafilaxia, Período perioperatório, Anestesia, Testes cutâneos
Introduction
Perioperative anaphylaxis is more frequent in adults and is associated with age, presence of cardiovascular diseases, and history of previous reactions to medications. Signs and symptoms often start before or during anesthetic induction.1 The major agents triggering perioperative anaphylaxis are: Neuromuscular Blockers (NMBs), antibiotics, latex, opiates, analgesics, non-steroidal anti-inflammatory drugs, chlorhexidine, contrasts, dyes, ethylene oxide.1, 2 The precipitating agents observed at different locations vary, and there has been an increase in the number of reactions due to antibiotics and a decrease in latex-related reactions.2
Mechanisms of anaphylaxis
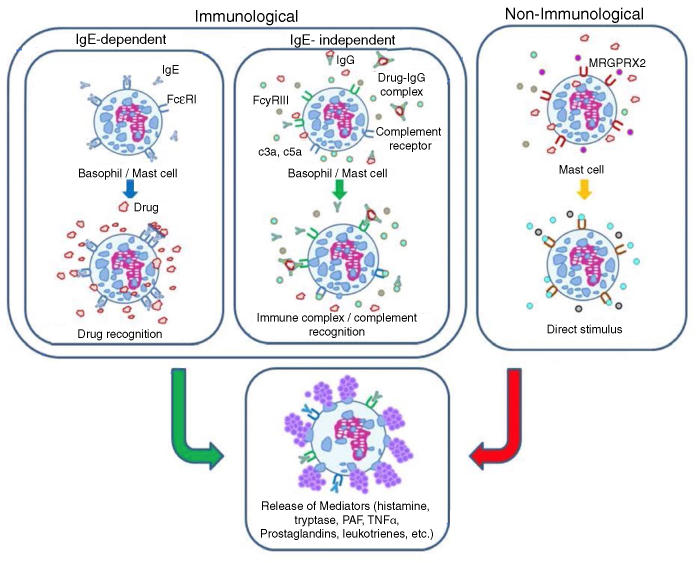
The mechanisms involved in drug-related anaphylaxis can be classified as immunological and non-immunological. Immunological mechanisms include the IgE-dependent and IgE-independent pathways, while non-immunological ones are mediated by direct activation of mast cells (Fig. 1).3, 4 Regardless of the underlying mechanism, the signs and symptoms of allergy are similar, and caused by degranulation of mast cells and basophils and result in release of mediators, such as histamine, tryptase, Platelet Activating Factor (PAF), cysteinyl leukotrienes and others. The mast cell is the main cell in IgE-mediated anaphylaxis. Recent investigations suggest the participation of macrophages and neutrophils in IgE-independent immune anaphylaxis. Basophils are implicated in IgE-dependent and IgE-independent reactions.1
Figure 1.
Mechanisms involved in anaphylaxis.
The chief signs and symptoms of anaphylaxis are mediated by histamine. They include flushing, itching, hives/angioedema, rhinorrhea, sneezing, stridor, cough, wheezing, dyspnea, hypoxia, nausea, vomiting, abdominal pain, diarrhea, tachycardia, hypotension, increased vascular permeability and syncope. Tryptase activates several pathways, including the complement cascade, coagulation and the kinin-kallikrein system, and promotes arterial hypotension and angioedema. Cysteinyl leukotrienes and PAF enhance the increase in vascular permeability and the development of hypotension.4, 5
It is important to underscore that the same etiological agent can trigger anaphylactic reactions by more than one mechanism. For example, radiological contrast media can trigger anaphylaxis by IgE-dependent and IgE-independent immune mechanisms, and by direct activation of mast cells.3, 4
Immune anaphylaxis
IgE-dependent reactions
The most frequent anaphylactic reaction mechanism is the IgE-dependent mechanism, or the classic pathway. It involves the process of sensitization to the allergen, activation of Th2 lymphocytes, and production of specific IgE against the allergen. IgE binds to the high-affinity receptor – FcεRI – present in mast cells and basophils and, at a subsequent contact with the allergen, the cross-linking of two or more of these receptors with the allergen (or carrier-bound hapten) triggers a complex intracellular signaling cascade. The cascade promotes the degranulation and release of preformed mediators, such as histamine and tryptase. Subsequently, there is the release of newly synthetized mediators from the metabolism of arachidonic acid from phospholipids present in the cell membrane (cysteinyl leukotrienes and prostaglandins) and the activation of other inflammatory cells that amplify and potentiate the allergic reaction.3, 4, 5 Penicillin and neuromuscular blockers are considered the main triggers of IgE-mediated anaphylactic reactions to drugs.4
IgE-independent reactions
IgE-independent mechanisms can be mediated by IgG class antibodies or by the complement system. Studies in murine models have shown anaphylactic reactions mediated by the interaction of drugs with specific IgG bonded to the FcεRIII receptor in basophils, macrophages and neutrophils, triggering PAF release.6 Although this model has not yet been proven in humans, studies with infliximab and adalimimab showed the occurrence of anaphylactic reactions without specific IgE and with the detection of high levels of specific IgG against the culprit agent.7, 8
Complement system activation can be induced by IgG-allergen immune complexes and by drugs solubilized in therapeutic liposomes, as well as in lipid excipients. Such mechanism promotes the release of C3a, C5a and C5-b-9, triggering the activation of mast cells, basophils and other cells, leading to degranulation and release of mediators. Among the most common triggers of IgE-independent anaphylactic reactions are radiological contrast media, dextran and heparin contaminated with super-sulfated chondroitin sulfate.3, 4
Non-immunological anaphylaxis
This form of anaphylaxis does not require immune system activation. It is associated with direct stimulation of mast cells, promoting degranulation and release of mediators. Several drugs are related to this mechanism, including radiological contrasts, opioids, NMB, dextran, and vancomycin. Recently, there have been reports that direct degranulation of mast cells associated with opioids, NMBs and quinolones can be mediated by the MAS-Related G Protein-Coupled Receptor-X2 (MRGPRX2).9 The interaction of these drugs with the MRGPRX2 receptor can induce the release of histamine, β-hexosamidase, TNF-α, prostaglandin, and other mediators triggering anaphylaxis.4, 10
Risk factors
Potentiating cofactors and factors may explain how an allergen can be tolerated on some occasions in the same patient, and on others, trigger, from mild reactions to severe anaphylaxis. In the presence of cofactors, the reactions may become more severe and/or occur with smaller amounts of the triggering agent. Cofactors are present in 30% of anaphylaxis cases according to the literature.1, 2, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22
Three categories of risk factors have been suggested: 1) Potentiating factors, which impact the immune mechanism, such as physical exercise, acute infections, drugs (non-steroidal anti-inflammatory drugs, proton pump inhibitors), alcohol, and menstrual period; 2) Comorbidities, which are associated with more severe reactions and increased mortality, such as asthma, mastocytosis, and cardiovascular disease; 3) Cofactors that have no impact on immunological mechanisms, such as beta-blockers, Angiotensin-Converting Enzyme (ACE) inhibitors, and emotional stress.10, 23 However, classifying risk factors is not always easy, as the knowledge on the mechanisms involved is not necessarily clear for all the factors identified.
Epidemiological studies indicate higher frequency of anaphylactic reactions in women. However, the difference only occurs during the female reproductive period, hinting the relationship with sex hormones. In addition, recurrent episodes of anaphylaxis during the menstrual period are described, suggesting estrogens or progesterone as potentiating factors.10, 23 Female susceptibility observed in clinical studies was also demonstrated in a murine model.24
Beta-blockers and ACE inhibitors have been reported in several studies as cofactors in anaphylaxis. The anaphylaxis odds ratio for beta-blockers and ACE inhibitors was estimated at 6.8 and 13, respectively. However, other studies have concluded that the risk of developing anaphylaxis with beta-blockers and ACE inhibitors is only significant when there is simultaneous use of both drugs. Epidemiological studies are required to determine the actual impact of these drugs as risk factors for anaphylaxis.10, 23
Statins can be deemed a risk factor for anaphylaxis since low plasma LDL levels can increase the risk of severe or fatal anaphylaxis. This class of drugs increases the plasma concentration of PAF by reducing the activity of the PAF Acetylhydrolase Enzyme (PAF-HA), which degrades PAF.10, 25
Non-hormonal anti-inflammatory drugs are a well-established factor for anaphylactic reactions, especially in food anaphylaxis. However, the mechanisms involved are not fully understood.10, 23
A recent study on fatal anaphylaxis underscored an increasing incidence of drug-related anaphylaxis, while the food and venoms anaphylaxis rate remained stable. Cardiovascular comorbidities and age over 50 years were identified as risk factors for fatal anaphylaxis, and in these cases, the most involved drugs were beta-lactam antibiotics, NMBs, and contrast media.26
Causal agents
The differences in nomenclature, methods used and regional availability, and preference for certain drugs make the study of the incidence of Perioperative Hypersensitivity Reactions (PHR) a strenuous task.
The wide geographic variability reflects numerous issues, such as anesthesia practice, preparation for diagnosing hypersensitivity reactions, presence of research centers, in addition to gene-environment interaction.27
The several agents involved in perioperative hypersensitivity reactions will be discussed below, without obeying an order of incidence, given we do not have this kind of studies in Brazil yet.
Neuromuscular blockers and sugammadex
Neuromuscular Blockers (NMBs) are the most common cause of perioperative hypersensitivity reactions in France, Norway, and Belgium, and rank second in the UK, but are less common in the US, Sweden, and Denmark.28 In Brazil, although without further diagnostic confirmation by skin tests or in vitro, in response to a questionnaire prepared by allergists on perioperative anaphylaxis, neuromuscular blockers were pointed out by 37.6% as the culprits of reactions observed by anesthesiologists, ranking first among the listed agents.29, 30
Reactions to NMBs can occur by mechanisms mediated by IgE or not. Studies focusing on the structure-activity relationship have established that the IgE recognition site for the NMB comprises groups of tertiary and quaternary ammonium, and adjacent molecules.30 The substituted groups of tertiary and quaternary ammonium also present in cosmetics, disinfectants, and foods and could explain the sensitization presented by patients who have never been previously exposed to NMBs.31 The hypothesis of folcodine – an antitussive that contains allergenic epitopes that would cross-react with BNMs32 – could explain the reaction to the first exposure to NMBs.33
A prospective, case-control study, initiated in France in 2015 and expected to last 4 years (ALlergie aux curares et exposition à la PHOlcodine – ALPHO), may elucidate many issues.27 NMBs can also activate mast cells, regardless of the presence of IgE, by activating the MRGPRX234 membrane receptor. The binding of the MRGPRX2 receptor by several NMBs could be an alternative explanation for the cross sensitivity among the different NMBs.9
From what was described above, both due to the structure-activity relationship, as well as the MRGPRX2 receptor discovery, the need for systematic investigation of cross-reactivity among the available NMBs is justified, aiming to provide a safe alternative for patients who experienced PHR and will have to face anesthetic procedures in the future.35
It is necessary to underscore that when a trigger agent (NMB) is clearly identified and other NMBs result in negative skin tests, they are considered to be normally safe options, but it is impossible to entirely rule out a new reaction.36
Negative immediate hypersensitivity skin tests to NMB, followed by exposure without a hypersensitivity reaction, should be recorded on the medical chart.37
Sugammadex, a modified cyclodextrin that selectively binds to steroidal NMBs, has been suggested as a possible treatment for anaphylaxis to rocuronium. Experimental studies have demonstrated, through the expression of CD63, a marker of basophil activation, that activation could not be blocked by sugammadex after being initiated.38 Another study, using a skin model, also concluded that modification of the clinical response was unlikely if the allergic reaction had already been established.39 The retrospective analysis of 13 cases of anaphylaxis (by rocuronium and antibiotics) has not shown any change in the clinical course by sugammadex itself.40 In addition to these studies, cases of hypersensitivity to sugammadex have been described.41 For the moment, the recommendation is that sugammadex has no role in the treatment of anaphylaxis.42
Latex
The decrease in reactions to natural rubber latex from Hevea brasiliensis has been observed as a result of several measures, such as identification of risk groups and use of preventive measures, correct labeling of medical devices regarding presence of latex,43 and use of powder free gloves.44
In special groups of children undergoing multiple surgeries (with diagnoses of spina bifida, myelomeningocele, urological conditions), latex ranked first as a cause of perioperative anaphylaxis.45 For this group of high-risk patients, primary prevention is proposed, so early sensitization to latex should be avoided. Recommendations are to use latex-free medical materials/devices from birth, perform surgery in a safe environment regarding presence of latex,46, 47 and schedule surgery as the first case of the day.48, 49
Despite the relevance of the subject there is no quest for primary prevention measures, that is changes in operating room routine by the Brazilian Society of Pediatrics (SBP, www.sbp.com.br) and its Neonatology Department, by the Brazilian Allergy and Immunology Association (ASBAI, www.asbai.org.br), and by the Brazilian Society of Anesthesiology (SBA, www.sbahq.org). In the Brazilian literature, the only recommendation on primary prophylaxis by exclusion of latex from birth, was found in Soares.50
In Brazil, the National Health Surveillance Agency (ANVISA) issued the Collegiate Board Resolution, RDC 37/2015, regarding information on presence of latex in medical supplies.51
Reactions to latex tend to occur later in surgery, after significant contact with mucous membranes.43, 49 Latex sensitization evaluation by the allergist preoperatively is indicated for patients presenting an uninvestigated suspected history of hypersensitivity reaction in previous anesthesia and for:52 patients with manifestations of hypersensitivity to latex, regardless of circumstances; children submitted to several surgeries, especially those with spina bifida, myelomenigocele, as they present a high rate of latex allergy; patients with a history of clinical manifestations after eating avocado, banana, kiwi, and others, due to the high incidence of cross reactions with latex.52
Opioids
Natural (morphine and codeine) and semi-synthetic (folcodine, hydromorphone and diamorphine) opioids are potent direct histamine releasing medications. They mainly promote cutaneous manifestations that include hives, itching and flushing.53 High doses of morphine used during cardiac surgery do not cause bronchospasm or angioedema.54
Reactions related to direct histamine release are much more prevalent than reactions mediated by IgE, with the former probably resulting more from the occupation of MRGPRX29 receptors of mast cells than from binding to the μ receptor.55
Semi-synthetic phenylpiperidine class opioids (alfentanil, fentanyl, remifentanil) are not histamine releasing medications, and there seems to be no cross sensitivity between them and the derivatives of morphine, diphenyl-heptanes and phenanthrenics.56 Confirmed diagnosis of opioid reaction is difficult due to unreliability of skin tests, the potential for histamine releasing by some of these medications,57 and the unavailability of specific validated and reliable IgE assays.58 Still, reactions to these agents are exceptionally rare, considering their extensive use in anesthesia.27
Hypnotics
Reactions to hypnotics were frequently associated to thiopental (currently in disuse) and to propofol, when it was formulated with the solubilizer Cremophor EL.59, 60
Propofol has two isopropyl groups that act as antigenic determinants, in addition to presenting, in its current formulation, a lipid solution with soy oil and egg lecithin.60 Lecithin is derived from egg yolk and is highly purified. Patients allergic to egg tend to show sensitization and reaction to the egg white protein.54 The soy oil used for the propofol solution is refined and its allergenic proteins are removed at the end of the process.60
The safety alert pediatric reference always cited is a case report of a child with anaphylaxis to egg and who presented a generalized urticaria reaction after the use of propofol, with a positive immediate hypersensitivity skin test.61 A retrospective observational study of children with eosinophilic esophagitis, a distinct non-IgE mediated phenotype, found no difference in the rate of propofol-related complications.62
The American Academy of Allergy, Asthma and Immunology recently decided that patients allergic to egg can safely receive anesthesia without any precautions, and the guideline was reaffirmed in a recent review.47 Etomidate, ketamine, and benzodiazepines are rarely involved; among the latter, midazolam is the most frequent agent.11
Local anesthetics
Local anesthetics are widely used and rarely promote anaphylactic reactions regardless of the mechanism.63, 64, 65 Despite the rarity, local anesthetic reaction can occur even when the substance is not suspected.66
Several manifestations considered as local anesthetic hypersensitivity may have other etiologies, such as vasovagal reaction, accidental intravascular overdose, symptoms related to use of vasopressors or reaction to the concurrent exposure to another agent (latex, antibiotics, non-steroid anti-inflammatory drugs, chlorhexidine, additives, and preservatives).67
According to Kvisselgaard et al.,65 extra knowledge on how to recognize anaphylaxis is required for health professionals. The authors draw attention to the use of adrenaline in cases of vasovagal reaction. These episodes are often labeled as anaphylaxis, without being the case.65
Evaluation of suspected hypersensitivity reactions to local anesthetics is performed with immediate-type reaction skin tests (prick and intradermal tests), followed by subcutaneous challenge tests.65, 66, 67, 68 Investigating the suspected agent is important to establish a definitive diagnosis69 in order to offer at least one alternative37, 67 or options for future anesthetic procedures.67
Skin and intradermal tests should be performed with preparations without vasoconstrictors due to the high likelihood of a false-negative result.37 Cross-reactivity is more common among esters (not available in Brazil) than among amino-amides or amine-esters.68, 69
Local anesthetic allergy can be caused by methylparaben, paraben, or metabisulfite used as preservatives. Although there are preservative-free local anesthetics, they may not be easy to obtain.69
Obstetric patients represent a particular situation for testing a history of hypersensitivity to local anesthetics. In this circumstance, the test should be performed on the day of delivery, at the maternity center. The allergist performs the skin tests and, if they are negative, neuraxial anesthesia is performed with local anesthetic. In case of any reaction, the obstetric team will be ready to perform the necessary procedures.68
Ester-type local anesthetics (chloroprocaine, procaine, tetracaine) are considered more antigenic than the amide-type (lidocaine, bupivacaine, ropivacaine, levobupivacaine). Para-aminobenzoic acid, resulting from the metabolism of esters, is thought to be responsible for the greater antigenic properties of esters.20
Antiseptics, disinfectants and sterilizing agents
Chlorhexidine
Chlorhexidine is an antiseptic widely used perioperatively acting as a component of skin preparation solutions, local anesthetics gels, lubricants, dressing compresses, ophthalmic solutions, or chlorhexidine-impregnated central venous catheters. In the extra-hospital environment chlorhexidine is present in mouthwashes, dentifrices, make-up removers, dressings and household antiseptic solutions.43 The patient may have used them previously and shown no reaction to these products. In the perioperative period, reactions may occur abruptly, during central venous catheter placement70 or within 20–40 minutes after initiating the procedure.71 Absorption through the mucosal surface or incised skin increases exposure. Skin tests are shown to be predictive of allergic sensitivity and are correlated with the levels of specific IgE.72
Chlorhexidine may be hidden in several perioperative products and should be part of the anaphylaxis investigation, including reactions in which there is no description of the agents used.37, 71
Povidone
Povidone is also present in soaps and ophthalmic antiseptic solutions. Reactions attributed to povidone are rare, but some have been published describing skin tests associated with the measurement of the tryptase level.73 It is important to emphasize that the main antigen identified is povidone, and not iodine,74 and there is no cross reaction with iodinated contrast agents.49 Skin tests are recommended for diagnosis and dilutions are standardized.28, 37
Ethylene oxide
It is a gas used to sterilize a wide range of medical supplies. It has rarely caused perioperative anaphylaxis.75 Reaction has been restricted mainly to high-risk groups, such as patients undergoing dialysis, with myelomeningocele and ventriculoperitoneal shunts.76, 77, 78 It is described as an agent that is practically impossible to eliminate completely,28 thus measures that minimize exposure are recommended.79
The diagnosis is made only with specific IgE,77 and pretreatment with omalizumab has been reported to be effective.80, 81
Glutaraldehyde and orthophthaldehyde
Glutaraldehyde and Orthophthaldehyde (OPA) are disinfectants used for reprocessing dental, medical, and hospital supplies that are sensitive to heat. Glutaraldehyde has been attributed to late hypersensitivity reactions (contact dermatitis). Immediate-type hypersensitivity reactions during cystoscopy and laryngoscopy with orthophthaldehyde have been reported.82, 83, 84
The OPA manufacturer, after receiving numerous complaints of reactions, contraindicated its use in cancer patients undergoing recurrent cystoscopies.85
Colloids
Colloids, or plasma volume expanders, are usually administered to patients with hypotension, so to correctly diagnose the hypersensitivity reaction is challenging. Signs may appear within 20–30 minutes after the infusion has started.86
Gelatins are responsible for most reactions, followed by dextrans.49 Reactions to dextran solutions are IgG-mediated due to the activation of complement.87 Due to the high risk of reactions to hydroxyethyl starch, it has been withdrawn from some countries and has not been included in more recently published studies,27 although it is still marketed in Brazil.
While gelatins can induce reactions by unspecific histamine release, IgE-dependent reactions have been reported. In addition, gelatins can trigger reactions in children as “hidden” agents in hemostatic products that are derivatives of bovine or porcine gelatin.88 In addition to allergy to gelatin derived from bone protein present in plasma expanders or in hemostatic topical agents,89, 90 there is allergy to alpha-gal (alpha-gal syndrome). It is described as an IgE-mediated allergy to red meat. More specifically, the allergy involves an oligosaccharide present in many foods from animal origin (mammals), such as meat and jellies.91, 92 In summary, patients with alpha-gal syndrome should not receive gelatin-based colloids, unless they have negative skin tests.47
Blood and blood products
Hypersensitivity reactions can occur to a heterogeneous group of blood components, with a wide range of risk levels. The major challenge in diagnosing perioperative reactions to blood and blood components is the absence of confirming skin tests.27
Urticarial reactions occur in 0.5% of all transfusions of fresh frozen plasma. As in all blood components there is always a small amount of plasma, reactions can also occur with packed red blood cells and platelets.41
To better understand reactions, they can be divided into those related to the recipient or related to the donor. The best example of recipient-related reactions is the patient (recipient) with IgA deficiency, whose anti-IgA antibodies react to the donor antigen. The assessment of IgA levels of recipient patients should be part of the investigation of hypersensitivity reactions in transfused patients.93
When blood and blood components have been administered prior to the perioperative hypersensitivity reaction, the blood bank must be notified. The possibility of obtaining positive tests for blood and/or blood components is restricted; thus, the patient should be referred to an allergy specialist assessment. The presence of negative allergic tests leads to an exclusion diagnosis of reaction to blood or its products.76
In addition to ABO incompatibility and hypovolemia, other causes, such as bacterial contamination and accumulation of bradykinin of the transfused blood, may explain hemodynamic instability during transfusion.94
Regarding tests, they should also include the search for hidden allergens, that is, substances administered with the blood, such as methylene blue or hemostatic agents.78
Involving an allergy specialist in the assessment of the case should be pursued for proper investigation.95
Radiological contrasts
The different iodinated contrast media have a common structure: an aromatic benzene ring with attached iodine atoms and whose allergenic sequence has not yet been identified, although iodine is not involved.74, 96
Iodine is a bioelement essential to life and does not constitute an “antigenic sequence”. It does not correspond to any documented clinical entity, therefore, the expression “allergic to iodine” should be abandoned.96, 97
Concerning fish, the identified allergenic sequence is parvalbumin, a muscle tissue protein, as well as the glycolysis enzymes enolases and aldolases.98 Tropomyosin is considered the largest pan-allergen from crustaceans, mollusks and arthropods (for example, mites).99, 100
These considerations are necessary to eliminate the misconception of cross-reaction between iodinated contrasts and fish/seafood.47, 74, 97, 101,102 Similar reasoning applies to povidone-iodine, where the allergenic determinant is povidone, and there is no cross-reactivity with seafood.47, 97
Iodinated contrast media can trigger both IgE dependent reactions103 and delayed hypersensitivity reactions.104 Cross-reactivity appears to be low, despite the similar molecular structure. Skin tests are performed after a hypersensitivity reaction to confirm the suspected agent and to offer a safe alternative. Alternative contrasts that result in negative skin tests have been safely used in patients with previous reactions to iodinated contrasts.105
Treatment prior to exposure remains a controversial issue. Although it is recommended in protocols in some countries, such as the US, in Europe it is not recommended. It is reserved for patients who have suffered previous severe reactions, not mediated by IgE, mastocytosis.106 Others restrict the recommendation to patients with mastocytosis and chronic urticaria, who require contrast to which skin tests have been negative.103 In summary, premedication does not prevent IgE-mediated reactions, and its effectiveness in preventing moderate to severe reactions, immediate or late, has not been proven. The essential is that all those who administer contrasts know how to properly recognize and treat anaphylaxis.105, 106, 107
Paramagnetic contrasts
Contrast media used in magnetic resonance are called paramagnetic contrasts. Quite often they are large, complex or chelating molecules containing gadolinium. The symptoms described for immediate reactions are remarkably like those described for iodinated contrast reactions. To date, reports of late reactions are not known, although they cannot be ruled out.108
The pathophysiological mechanisms have not been well established. In some cases, the involvement of specific IgE is suggested, based on positive skin tests performed on patients with anaphylaxis to these contrast media.109, 110
Dyes
Dyes have often become described as etiologic agents of hypersensitivity reactions. In a recent study they rank as the fourth identified cause in the UK, only behind antibiotics, NMBs and chlorhexidine.64
There are three commonly used blue dyes: patent blue V and isosulphan blue that are structurally related, and methylene blue not structurally related to the previous two. In breast cancer surgery and melanomas, they are used for searching and mapping lymphatic drainage and identifying the sentinel nodule.27
Anaphylaxis to dyes occurs later in relation to perioperative intravenous injection of the antigen, due to the slow absorption by subcutaneous and lymphatic tissues.111 It is necessary to highlight the prolonged interference with pulse oximetry readings, causing a misleading drop in oximetry values,27 which can be measured by arterial blood gas analysis.112, 113
Hypersensitivity reactions to fluorescein, a dye used for retina angiography and other procedures, have been reported.114, 115
Other agents
Aprotinin
Aprotinin, a bovine derivative, is an inhibitor of serum proteases and has anti-fibrinolytic activity. It is used intravenously or as a component of topical hemostatic agents, to reduce bleeding mainly in orthopedic or cardiac surgery.116 There is a higher prevalence of aprotinin reactions in patients with previous exposure, explaining why intervals shorter than 6 months between aprotinin exposures are considered a relative contraindication.117
In Brazil, the registration of intravenous aprotinin expired in 2016 and had not been renewed until the publication of this article. However, aprotinin is a constituent of products marketed in Brazil, such as topical hemostatic agents and biological glues.
Protamine
Protamine sulfate is a highly alkaline polypeptide used to reverse the anticoagulant effects of heparin. It also forms a complex with insulin (NPH or Neutral Protamine Hagedorn) delaying absorption and prolonging the pharmacological effect of insulin. There have been reports of hypersensitivity reactions mediated by IgG, IgE, complement activation and non-specific release of histamine.118
Although protamine is produced by recombinant technology, initially it was isolated from fish sperm, and protamine was supposed to promote cross-reaction in patients allergic to fish. No evidence was found to justify this hypothesis.119
Previous exposure to NPH insulin was also deemed a risk factor for using protamine. Although the rate of reactions is higher in diabetic patients using NPH insulin, evidence for IgE-mediated allergy is extremely low.120
Three cases of allergy to protamine have recently been reported.121 However, two of these patients receiving NPH insulin were re-exposed to protamine with no events, showing that other non-IgE mechanisms may be involved.122
Thus, there is no evidence to date to avoid the use of protamine in patients with allergy to fish and in those using NPH insulin.47
Tranexamic acid
It is an antifibrinolytic agent recommended in specific situations,123 although rare hypersensitivity reactions are reported both in children and adolescents,124 and in adults. Skin and provocation tests have been suggested.125
Hyaluronidase
It is a bovine or ovine enzyme that degrades hyaluronic acid and can be used as an adjunct to local anesthetics facilitating tissue penetration. Immediate reactions to hyaluronidase have been described.126 Even late reactions can have disastrous consequences due to edema and compression of important anatomical structures as, for example, in ophthalmic surgery.127, 128 The differential diagnosis with early orbital cellulitis must be made in cases presenting edema and hyperemia, due to the risk of compartmental compression and consequent visual loss associated with delayed diagnosis.129
A previous history of allergy to wasp and bee stings (for possible cross-reactivity with the hyaluronidase of the insect venom) and prior use of hyaluronic acid fillers for cosmetic purposes should be investigated before deciding to use hyaluronidase to perform regional eye blocks.130
Hidden antigens
Some agents are not among the drugs, substances and devices described on the anesthesia record or surgery report. Thus, they go unnoticed and are omitted in post-crisis investigation. An example, Polyethylene Glycols (PEGs). PEGs and their derivatives are nonionic polymers of ethylene oxide, commercially available in a wide range of molecular weights. PEGs are extensively used in regular pharmaceutical products, in medications for cancer, gout and immunotherapies, in cosmetics and food products.131 The lack of nomenclature standardization and the low rate of hypersensitivity warnings for adjuvants and excipients can render them even more concealed, making investigation challenging.132, 133
Some examples are listed below, and some alerts are offered in the reference article:78 ethylene oxide (sterilizing gas), related to reactions in ventriculoperitoneal shunt surgeries; antibiotics in bone cement and eye drops; mannitol as an adjunct to some intravenous medications; methylcellulose in artificial tears; polyethylene glycol gel, local anesthetics spray, bone cements.78
Cooperation among allergists, anesthesiologists and all members of the surgical team is essential to research other substances that are not always evident upon an initial investigation.
Non-steroidal anti-inflammatory drugs
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are also widely used in surgical procedures (pre, intra and postoperative). Estimates from Europe, for example, reveal that almost all anesthesiologists (99.1%) use this class of medication at some point during perioperative management.134
In most cases of hypersensitivity to NSAIDs, including anaphylaxis, there is no involvement of an immunological mechanism. In these situations, NSAIDs' own mechanism of action on the Cyclooxygenase (COX) enzyme isoforms COX1, COX2 and COX3 deviates the arachidonic acid metabolism toward the lipoxygenase pathway and causes larger production of leukotriene and lipoxins. Leukotrienes are potent vasodilators; increase vascular permeability, inducing edema of nasal and bronchial mucosae, increase glandular mucous secretion, and act on bronchial smooth muscle resulting in bronchoconstriction. In addition, there is also a reduction in the synthesis of Prostaglandin E2 (PGE2). PGE2 has vasodilation and bronchodilation properties and stabilizes mast cells.135, 136 Reactions involving this mechanism are considered non-selective, since they are not specific to a certain molecular structure, but are rather related to the potency with which NSAID inhibits COX-1. In this way, individuals can react to several NSAIDs (with distinct structures) and the more potent the drug in inhibiting COX-1, greater the risk and severity of the reaction.
Conditions presenting an immediate immunological involvement, such as anaphylaxis, start with type I hypersensitivity mechanism and synthesis of specific IgE against a particular NSAID or against NSAIDs with a similar chemical structure. These reactions are considered selective since the patient tolerates anti-inflammatory drugs with a different chemical structure. About 24% of hypersensitivity reactions to NSAIDs are selective.137 Derivatives of pyrazolone (dipyrone, metamizole), diclofenac, and propionic acid derivatives (ibuprofen, ketoprofen, naproxen) are the agents most frequently implicated in these reactions138 according to patient history, in vivo confirmation after positive Immediate Hypersensitivity Skin Tests (IHST), and in vitro confirmation using basophil activation test.
Although NSAIDs are the main cause of drug-related anaphylaxis in Brazil, in adults and children139, 140 they are considered a rare cause of perioperative anaphylaxis.27
In Spain, a five-year study showed the presence of dipyrone-specific IgE (positive IHST) in two cases, both occurring during anesthesia recovery.141 In Germany, investigation with skin tests in 53 patients showed five IgE-mediated reactions to metamizole and two to phenylbutazone.142 One case of ibuprofen anaphylaxis was revealed from data of the 6th National Audit Project (NAP6), carried out in 356 hospitals in England, Wales, and Northern Ireland.2, 143
In France, only three cases of perioperative hypersensitivity to immunologically mediated NSAIDs were identified in a nationwide study over an 8-year period.22
Despite the low frequency of perioperative reactions to NSAIDs, in cases of previous non-investigated perioperative reaction, the use of paracetamol and selective COX-2 inhibitors has been suggested, as they are recognized as having a low association with either immunological or non-immunological mechanism hypersensitivity reactions to NSAIDs.52
In pediatrics, a review of original articles meeting the criteria of dipyrone use as analgesics in children up to 17 years of age, identified two, out of four studies, whose main objective was to determine side effects or serious adverse reactions related to dipyrone.144 Besides itching, edema, rash, and vomiting, no serious adverse events were reported. The study concluded that the odds for serious adverse reactions related to dipyrone (hemodynamic, anaphylactic, or respiratory reactions) is less than 0.3%.145, 146
These are the relevant summarized recommendations from articles and guidelines from expert opinions:147 a) Increased prevalence of hypersensitivity reactions to NSAIDs (in children) than previously reported; b) The natural history of these reactions in children is still unknown, requiring regular reassessments; c) Provocation test is the gold standard, and d) COX2 inhibitors, although not yet approved, have proved to be useful alternatives.147
Antibiotics
Antibiotics are used throughout the perioperative period, either for prophylaxis or treatment of infections. It is estimated that circa 15% of all antibiotics used in hospitals are prescribed for surgical antimicrobial prophylaxis.148, 149
They are well-known and a common cause of perioperative anaphylaxis, and the reactions to this class of medication have increased markedly in many countries in recent decades. It is true that the antimicrobial agents involved in perioperative anaphylaxis vary depending on local use.150 However, the evidence indicates clear predominance of β-lactam involvement, particularly penicillin and cephalosporin.141 In these situations, there is a participation of the mechanism of hypersensitivity type I described by Gell and Coombs with synthesis of specific IgE and sensitization to the β-lactam ring or to the β-lactam side chains. It is worth pointing out that sensitization to the ring establishes the risk of reaction to any antibiotic in the group, for all β-lactams share the ring structure. Sensitization to side chains, on the other hand, makes it possible for reactions by cross-reactivity between β-lactams depicting analogous side chains (e.g. amoxicillin and cefadroxil).151 Thus, it is known, for example, that cefazolin, a cephalosporin commonly used as surgical prophylaxis, has side chains distinct from all other β-lactams, so that patients with reactions to cefazolin tolerate other drugs in the group.152
Evidence also indicates that there may be differences in the pattern of sensitization depending on the population group analyzed. It is known, for example, that most patients with β-lactam allergy in the US and Europe are sensitized, respectively, to the β-lactam ring and the side chains.141, 153, 154, 155, 156
The spurious labeling of allergy to β-lactams, notably to penicillin, is an issue worthwhile discussing.
Penicillin allergy is reported in approximately 10% of the population and in 20% of inpatients. However, this label could be removed in at least 90% of them, by detailed history or by evaluation by an allergist.157
In children, allergy to antibiotics is reported in 5% to 10% of cases, but it seems that roughly 90% of these have been incorrectly labelled.158
The implications of an incorrect diagnosis are the use of alternative antibiotics, causing high rates of surgical site infection, bacterial resistance, hospital infection, prolonged hospital stay, and increased costs.157, 159, 160, 161 There is great need for studies aiming to remove inadequate labeling, minimizing its consequences.157, 162, 163
There are also reports of perioperative reactions to vancomycin and quinolones. However, confirmation of their role as the etiological agent using skin tests for specific IgE investigation is severely impaired, since such drugs cause direct mast cell granulation and can release histamine, regardless of specific IgE production.164
How to investigate?
At the operating room
After the clinical diagnosis is established and therapeutic measures are taken, without waiting for the results of laboratory tests, which will be useful to confirm the diagnosis of immediate hypersensitivity.
The most recent recommendations suggest obtaining the first sample within 1 hour after the reaction starts, followed by collection of a second sample within 2–4 hours after the first.42 If it is not possible to obtain both samples, withdrawing a single sample within 1–4 hours is adequate.28, 52, 64 The baseline sample for comparison should be collected 24 hours after the reaction or, later, when performing the skin tests.28, 52, 64
The recently validated algorithm from an international consensus is also the most effective tool for analyzing the results. Based on the algorithm, tryptase levels after reaction must be higher than [(1.2 × basal tryptase) + 2] µg.L-1, to differentiate, in the perioperative scenario, between anaphylactic and non-anaphylactic event.165, 166
Post-mortem samples can be used due to the high stability of tryptase.167 It is noteworthy that now, tryptase level is the only test to be collected during a crisis, for afterward quantification. Histamine has a short-lived half-life, declining rapidly to normal values, and is not part of routine tests. Its use is restricted to a few specialized centers.52
At the allergist office
The perioperative reaction investigation goal is to identify the culprit agent and to provide alternatives for a safe future anesthesia, even when no culprit is identified. This investigation requires a systematic approach and, ideally, should be a team effort, combining the expertise of allergists/immunologists together with anesthesiologists with experience in anesthesia allergy investigation. The anesthesiologist understands the perioperative scenario and the several differential diagnoses, can better analyze the anesthesia record, and can help identify possible culprits, even those not documented in the anesthesia record. On the other hand, the allergist/immunologist is aware of the available tests and their limitations.28 Ideally, all patients who present immediate reactions should be referred to an allergy outpatient clinic for investigation, both patients that presented only skin reactions (Grade I), and those who had the most severe manifestations (Grade II to IV). 28, 42, 168
In Brazil, it is uncommon for patients to seek an allergist spontaneously, without having been referred, which can lead to management challenges and greater delay in obtaining the diagnosis.
For the allergist, a detailed anamnesis of the event is a crucial tool. All contacts up to 2 hours before the onset of reaction28 can be relevant and complete documentation, with chronological order of events, is essential. Documentation must include the anesthetic record, all medical records (preoperative, operating room and post-anesthetic recovery), notes by the anesthesiologist, details of any surgical or other perioperative exposures (disinfectants, local anesthetic spray/gels, dyes, cements), and details of all procedures (venous and urinary catheters, stents). Planning the investigation based only on information from a referral letter is not recommended.28
Latex and antiseptics (mainly chlorhexidine) are on the list of products to be investigated, but these are often not recorded in medical records. Other substances less commonly associated with reactions are local anesthetics and ethylene oxide, which should also be listed for investigation. It should be noted that regarding ethylene oxide, the currently available diagnosis is a specific IgE.77
Thus, all agents present in the pre-, intra and immediate postoperative period should be investigated.27, 28, 49
Conversely, there is no indication for testing agents whose pharmacological groups have not been used (e.g., etomidate should not be tested in patients sedated with propofol and/or midazolam).28
Another important measure is to check the subsequent exposure to agents used perioperatively. There is no need to test agents used at the time of the index reaction, and which in a subsequent exposure did not cause a new reaction. However, it is worth noting that agents that were maintained or reused shortly after recovery from reaction should still be considered for investigation, as the patient may be in a refractory period or under the effect of medications that mask a new reaction, such as antihistamines or corticosteroids.28
In cases of patients for whom access to their medical record/anesthetic record is impossible, usually due to very remote previous reactions (over 10 years), the investigation of latex, chlorhexidine, ethylene oxide, propofol, fentanyl, remifentanil, and a NMB is recommended to warrant safe use in future anesthesia.28, 52 It is also suggested to investigate cefazolin, midazolam and include succinylcholine as an option for NMB. Cefazolin is the most commonly used antibiotic in the perioperative period and, although it does not seem to cross-react with other cephalosporins and beta-lactams, it is not yet appropriate to declare it safe for use without complete negative investigation (skin tests followed by provocation).169 Midazolam, although described as a very rare cause of perioperative reactions,27, 28, 52 was a frequent cause of positive skin tests in a Brazilian population study sample,11 which may suggest a different sensitization profile for this population.
Once the agents to be investigated in the first consultation with the allergist are defined, skin tests must be scheduled in a hospital environment, and initial laboratory tests can help to elucidate the agent and even avoid unnecessary in vivo tests. A still controversial issue is the ideal time and time limit for investigation. Although in vitro and skin test positivity has a tendency to decrease over time, there is no maximum time limit for the request, since a positive result must be valued.27, 28, 37, 49,168 Conversely, the “ideal time” is unknown. Waiting a minimum of 4 to 6 weeks after the event is suggested but avoiding more than 4 months apart. Alternatively, UK guidelines suggest the possibility of investigation right after the event, although the possibility of some false-negative result in this period cannot be ruled out.37, 52, 168, 170,171
For skin tests, antihistamines should ideally be suspended at least 5 days before the procedure. High doses of systemic corticosteroids and drugs with antihistamine action (antidepressants and antipsychotics) can influence results,37 but should only be suspended if prescribing physicians consider it safe. To this point, as it may increase patient cardiovascular risk, there is no evidence of benefit in suspending angiotensin-converting enzyme inhibitors, or beta-blockers. Therefore, they should not be interrupted as a routine.28
Thus, when scheduling skin tests, the physician must already adjust oral medications according to the day of the procedure and request in vitro tests so that they can be checked before scheduled in vivo tests.
In vitro tests
In vitro tests mainly comprise serum tryptase, specific IgE (sIgE) and Basophil Activation Tests (BAT). In vitro tests can add accuracy combined with safety to the investigation of hypersensitivity reactions. However, they are not performed in isolation and are part of a diagnostic algorithm, being evaluated according to other additional tests. Although there are several in vitro tests, largely they are only available in research centers, and not in the clinical routine.172
a) Serum tryptase: Serum tryptase is the best biomarker for mast cell degranulation, both by IgE and non-IgE mediated mechanisms. Because it is only available in a few centers, the immediate result of the sample taken in the acute phase of a perioperative reaction is rarely obtained. Ideally, collecting two samples is recommended. The first is collected at the acute phase and the second sample, for obtaining the baseline value, should be withdrawn 24 hours after the reaction.28 The recently published British national study NAP6 showed that collecting a sample immediately after stabilization of the patient increases the accuracy for detecting significant increase.2 Tryptase level above 2 mcg.L-1 + 1.2 × basal tryptase value (in mcg.L-1) is considered a significant increase.165, 166
Even when the acute phase sample is not obtained, the isolated basal tryptase measurement is useful as screening for cases of conditions presenting mast cell activation, such as mastocytosis.28
b) Specific serum IgE (sIgE): When combined with other tests, sIgE measurement is a crucial complementary test to check an immediate hypersensitivity reaction. The positive specific IgE result to a particular agent confirms sensitization to that agent, but not necessarily “allergy” to it.
The availability of the test is restricted to a limited number of agents and its predictive value is not absolute. For many agents, trials have not yet been properly validated because of inadequate number of patients with accurate phenotyping and exposed or provoked control individuals.173
The available sIgE assays are mainly for NMBs, beta-lactam antibiotics, latex, chlorhexidine, and ethylene oxide, but not all are marketed in Brazil.
The sIgE measurement is important in cases requiring early reintervention (surgeries below 4 weeks of the suspected crisis), when sIgE positivity will alert for the culprit agent. If not, the test must be repeated after 1–2 months.174
c) Basophil Activation Test (BAT): BAT is a flow cytometry assay that evaluates activation and degranulation markers present on the basophil membrane surface.174 It has been compared to in vivo tests, showing high diagnostic accuracy for cases of perioperative hypersensitivity reactions.175
BAT can potentially be performed for any agent, comprising NMBs, antibiotics, latex, opioids, and opiates.174
For validating BAT use to diagnose suspected perioperative hypersensitivity reactions large studies are still required, with consistent controls and unified diagnosis criteria for the US, Europe and other regions of the world.172 In Brazil, like in many countries, BAT is still unavailable for clinical routine.
In vivo tests
In vivo drug tests include skin and provocation tests. Skin tests confirm the IgE-mediated mechanism and provocation tests are considered the gold standard in the diagnosis of hypersensitivity reactions to drugs.176, 177 However, in the context of perioperative reactions, the indications and contraindications of the tests are particular and should be thoroughly considered.
a) Skin tests: The skin tests performed in the investigation of immediate perioperative reactions are the Skin Prick Test (SPT) and the Intradermal Test (IDT). Both tests provide immediate reading (15–20 min) to confirm the IgE-mediated mechanism. It is recommended that the tests be performed by a qualified allergist.35, 52, 176
Despite efforts to standardize the concentrations used for skin testing, the non-irritating concentrations of some drugs, such as opioids and NMBs, for example, are still a matter of debate. So far, the dilutions suggested by the European Academy are used, which are summarized in Table 1.177
Table 1.
Suggested non-irritating concentrations for skin tests with drugs to investigate perioperative reactions.28, 179
| Drug | Skin prick test | Intradermal test |
|---|---|---|
| Cephalosporins | 20 mg.mL-1 | 20 mg.mL-1 (Cefepime 2 mg.mL-1) |
| Thiopental | 25 mg.mL-1 | 2.5 mg.mL-1 |
| Propofol | 10 mg.mL-1 | 1 mg.mL-1 |
| Ketamine | 10 mg.mL-1 | 1 mg.mL-1 |
| Etomidate | 2 mg.mL-1 | 0.2 mg.mL-1 |
| Midazolam | 5 mg.mL-1 | 0.05 mg.mL-1 |
| Fentanyl | 0.05 mg.mL-1 | 0.005 mg.mL-1 |
| Alfentanil | 0.5 mg.mL-1 | 0.05 mg.mL-1 |
| Sufentanil | 0.005 mg.mL-1 | 0.0005 mg.mL-1 |
| Remifentanil | 0.05 mg.mL-1 | 0.005 mg.mL-1 |
| Morphine | 1 mg.mL-1 | 0.01 mg.mL-1 |
| Atracurium | 1 mg.mL-1 | 0.01 mg.mL-1 |
| Cisatracurium | 2 mg.mL-1 | 0.02 mg.mL-1 |
| Mivacurium | 0.2 mg.mL-1 | 0.002 mg.mL-1 |
| Rocurorium | 10 mg.mL-1 | 0.05 mg.mL-1 |
| Vecuronium | 4 mg.mL-1 | 0.4 mg.mL-1 |
| Pancuronium | 2 mg.mL-1 | 0.2 mg.mL-1 |
| Suxamethonium | 10 mg.mL-1 | 0.1 mg.mL-1 |
| Pyrazolones | 0.1 to 2 mg.mL-1 | 0.1 to 2 mg.mL-1 |
| Others NSAIDs | 0.1 mg.mL-1 | 0.1 mg.mL-1 |
| Local anesthetics | Pure | 1/10 |
| Patent blue | 25 mg.mL-1 | 0.25 mg.mL-1 |
| Methylene blue | 10 mg.mL-1 | 0.01 mg.mL-1 |
| Chlorhexidine | 5 mg.mL-1 | 0.002 mg.mL-1 (sterile, colorless, alcohol-free solution) |
| Povidone | 100 mg.mL-1 | Not to be performed |
SPT should always be performed first, usually on the volar surface of the forearm, with negative (saline or diluent) and positive (histamine) controls. The results are read after 15 to 20 minutes, and a wheal ≥ 3 mm in relation to the negative control is considered positive. When SPT is negative or inconclusive, the IDT is performed, preferably also on the forearm.
The technique and interpretation of immediate reading IDT has also been subject of debate in recent years. The 2011 international guidelines suggested the administration of a volume between 0.02 and 0.05 mL, and the initial papule should be marked with a fine point pen. A positive IDT was considered when the final size of the papule was at least twofold the size of the initial papule.52 According to the most recent guidelines, a test in which a thin papule is 3 mm larger than the initial papule should be considered positive, and there is no need to double the diameter.28, 49 However, the skin injection should have volumes of only 0.02 to 0.03 mL, to initially produce a small 3 to 5-mm diameter papule. Inconclusive test results can be repeated on the contralateral limb.28, 176
Albeit extremely low, it is worth noting that there is a risk of systemic reaction, even anaphylactic, associated with the IDT test. Thus, besides the fact that only parenteral presentation drugs can be used, it is recommended to perform the tests only in a hospital environment, under the supervision of an allergist with expertise in the procedure and in the management of serious reactions.176
Concerning which agent to be elected for skin tests, besides those exposed at the time of the reaction, in cases presenting positive skin test for one NMB, it is recommended to include all NMB available for investigation of cross-reactivity.28, 37, 49 Similar recommendation applies to a positive investigation for cefazolin. It is suggested to investigate other beta-lactams due to the theoretical possibility for cross-reactivity (penicillin or ampicillin and another intravenous cephalosporin).49 As for latex, a very important sensitizing agent in Brazil, skin testing should be restricted to SPT without performing IDT, but when available, it should be performed with commercial extract and in the prick-to-prick method with a latex glove. If SPT is negative, in a controlled environment we can perform the use test, which although an immediate reading contact test, works as a provocation for latex.178
b) Drug Provocation Tests (DPT): DPT is the gold standard in the investigation of immediate hypersensitivity reactions to drugs.177, 180, 181 However, in cases of perioperative anaphylactic reactions, the use of DPT is restricted by the major effects of drugs used perioperatively, such as, respiratory depression, muscle paralysis and anesthesia.28 In principle, general indications and contraindications for DPT in patients with drug hypersensitivity can be respected, so when skin tests are inconclusive or negative, DPT can be performed to exclude drug sensitization, or to test a safe alternative.177, 180, 181 In addition, for drugs, such as NSAIDs and opioids, whose majority of reactions are usually not IgE-mediated, DPT may be the only reliable test.28
Anaphylaxis is a contraindication related to the performance of DPT, and the test is more often used to find a safe therapeutic alternative than for diagnostic confirmation.182 This is due to the higher possibility that DPT could induce new anaphylaxis. Thus, DPT for intraoperative anaphylaxis investigation is a high-risk procedure, and therefore, it should only be performed in prepared facilities and by allergists with experience in the procedure. In addition, full-dose DPT cannot be performed due to the potent pharmacological actions of several perioperative drugs, notably BNMs and hypnotics.51, 183 In Europe, DPTs with BNMs, potent opioids and sedatives have been performed in prepared environments, such as operating room, post-anesthesia care unit and intensive care unit in the presence of the anesthetist.49, 184, 185
In Brazil, so far, there are no reports of DPT with such drugs, since the tests are not carried out for safety reasons and because most centers do not have such well-controlled environments. Therefore, so far, the recommendation is that DPT for perioperative reactions should be restricted to latex (use test), local anesthetics, antibiotics, NSAIDs and others (antiemetics, proton pump inhibitors, morphine and weak opioids).
In relation to beta-lactams, it is necessary to underscore the issue of cefazolin, the drug most used in Brazil for antibiotic prophylaxis of surgical site infection in clean surgeries. In addition, recent data suggest that cefazolin has little or no cross-reactivity with other antibiotics of the same class.169 Thus, in cases of intraoperative anaphylaxis, in which skin tests were inconclusive for the culprit agent, the investigation with provocation using beta-lactams is required. However, the choice between DPT using either cefazolin itself or another cephalosporin should be made after risk-benefit ratio considerations. DPT with the suspected drug (cefazolin), if negative, allows for the future safe use of all antibiotics of the class. If the DPT is positive, an additional challenge with a distinct cephalosporin should be performed on another occasion, in order to avoid the unnecessary exclusion of the whole class. Conversely, if the team chooses not to provoke with cefazolin, but directly jump to an alternative antibiotic, the negative DPT will indicate tolerance to other cephalosporins, but in the final report, cefazolin should continue being described as suspected or inconclusive investigation, and it cannot be considered safe for future use. On the other hand, if the investigation with skin tests of all agents was conclusive (e.g., positive for rocuronium, but already with safe NMB options) and the IDT with 20 mg.mL-1 cefazolin was negative, the provocation with cefazolin itself is probably safer and more assertive (rocuronium was the probable agent).
Preoperative assessment – indications
There is no scientific basis for submitting the general population to diagnostic tests for drugs and substances used in anesthesia (screening tests).28, 37, 186 Such tests, with the aim of preventing hypersensitivity reactions, constitute a measure to unlikely reduce the incidence of these episodes.168, 187
For the occurrence of anaphylaxis, in addition to the triggering substances, other elements also contribute (such as amplifying cofactors),188 and not all these factors may be present in previous tests, which could explain the discrepancies. In addition, there is not enough knowledge yet on the positive and negative predictive strength of the tests in the general population.52
However, there are scenarios in which an assessment may be necessary and is indicated:53 a) Patients who have experienced hypersensitivity reactions in a previous surgery; b) Patients who have suffered a reaction to medications that can be used in surgery; c) Patients with a history of latex allergy; d) Patients with a history of reaction to food that is latex cross-reactive, such as banana, avocado, kiwi, manioc; e) Pediatric patients undergoing several surgeries, especially those with spina bifida or myelomeningocele, due to the high incidence of latex allergy.
In the last three items (c, d, e), the investigation is conducted only for latex.48
The correct investigation when indicated can increase safety in subsequent surgeries. In a US study, from 2003 to 2012, 73 patients who suffered an anaphylactic reaction were referred for allergy evaluation. An IgE-mediated mechanism was confirmed in 13 patients and 43 of the 73 patients had to undergo further surgery. In 45% of these, the procedure occurred without incidents, following the guidance based on the evaluation. Two patients who experienced recurrence of reaction presented mast cell disease.189
In another retrospective British study190 (70 patients evaluated between 2002–2015), 67 of them subsequently underwent anesthesia without complications. Of the three that presented new episodes, in two the cause was the lack of data related to the substances used. These were not anesthetics, but disinfectant and colloid solution, and the third patient was diagnosed with systemic mastocytosis, later confirmed by biopsy.191
The relevance of an adequate assessment of patients with pre-existing conditions, such as mastocytosis, angioedema by bradykinin, among others, should be emphasized.43
Special situations
Bradykinin angioedema
In addition to histamine-mediated angioedema, often triggered by antibiotics, NMBs, opioids, latex and radiographic contrast media, there are bradykinin related reactions. Although reactions by bradykinin are rarer, they also manifest themselves by angioedema, usually restricted, particularly to the extremities, face and airways. The reaction can progress to respiratory failure, like anaphylaxis, but does not respond to adrenaline.192, 193
Bradykinergic angioedema can occur due to deficiency or functional alteration of the C1 esterase inhibitor, Hereditary (HAE) or Acquired (AAE), and due to angiotensin Converting Enzyme Inhibitors (ACEI) and angiotensin receptor blocker medications. Angioedema can compromise the airways during the perioperative period, affecting up to two thirds of patients with HAE and AAE, and can lead to death in 15% to 33% of cases.192, 193
Prophylaxis, in this case considered short-term, is always indicated in surgical or anesthetic procedures, particularly those involving the cervicofacial region, such as tonsillectomy, tooth extraction, facial surgery, or that require tracheal intubation. In addition, procedures such as endoscopy and bronchoscopy must be performed in the operating room and require short-term prophylaxis.194
To date, there are no controlled studies evaluating the efficacy of the different drugs used in short-term prophylaxis in AEH and AAE. Therefore, current recommendations are based on expert opinion and small, uncontrolled trials.195
The most indicated agents for short-term prophylaxis are the intravenous C1 esterase inhibitor concentrates that should be used 1 to 6 hours before the procedure. Currently, in Brazil, the only C1 esterase inhibitor licensed by ANVISA is Berinert®, at the dose of 20 U. kg-1.196 In addition, it may be required to repeat the dose in the case of complex and long surgery and when there is extensive blood loss. When C1 esterase inhibitor is not available, administration of fresh frozen plasma should also be considered, at a dose of 10 mL.kg-1 (2–4 units for an adult), 1 to 6 hours before procedure.197 However, in some cases, worsening of angioedema may supervene, given plasma provides complement substrate.
Additionally, an alternative is the use of danazol, at a dose of 10 mg.kg-1.day-1 (maximum 600 mg.day-1, divided into 3 × 200 mg doses) during 5–7 days preoperatively. The drug should be maintained for another 3–5 days after the procedure, and can be used along with the C1 esterase inhibitor.198 Antifibrinolytics showed less documented efficacy in relation to the three previous agents, thus tranexamic acid should only be used if the previous medications are not available. Tranexamic acid should be used at a dose of 25 mg.kg-1.day-1 (maximum 3–6 g.day-1), divided into 2 to 3 times a day, 5 days before and maintained for 2–5 days after procedure.196 Short-term prophylaxis can be omitted for cases in which risk is considered minimal and when there is access to drugs indicated in the crisis, such as icatibant (bradykinin receptor inhibitor) or C1 esterase inhibitor.
In the case of acquired angioedema types I and II, prevention and treatment are based on management of hereditary angioedema patients.199 However, patients with acquired angioedema may not respond to attenuated androgens and benefit from antifibrinolytic agents. Likewise, in the acute crisis of acquired angioedema, there seems to be greater resistance to treatment with C1 esterase inhibitor and better response to the bradykinin receptor inhibitor.200
Patients must remain under close observation for 36 hours and medication be readily available if a crisis develops.201
Mastocytosis
Mastocytosis is a clonal disease characterized by the proliferation and accumulation of mast cells in several tissues, most commonly in the skin and bone marrow.202 The excess number of accumulated mast cells with greater capacity of degranulation can increase the rate and severity of immediate hypersensitivity reactions, and 22% to 49% of adults with mastocytosis can present anaphylaxis.203 The literature on anesthesia in patients with mastocytosis is very limited, and no studies have described the incidence of reactions during general anesthesia in these patients.204 Cases of fatal anaphylaxis, particularly after hymenopteran bites, however, may occasionally occur after intake of drugs such as NSAIDs, opioids and others drugs used in the perioperative period.205
However, presently there is no evidence of higher prevalence of drug mediated IgE or non-IgE reactions in patients with mastocytosis than in the general population.204, 206
In the case of anesthesia, several other factors that are components of the general care of the patient, such as proper positioning on the operating table (avoiding undue pressure zones), room temperature (danger of hypothermia) and anxiety control, should be valued in addition to the choice of drugs, a component that seems to be overestimated in some situations.43, 206
Nonetheless, the recommendation of caution stands for NMBs from the benzylisoquinoline group, due to their ability to release histamine.43 For the same reason, it is also suggested to use fentanyl and analogs instead of morphine.207
Regarding pre-treatment, although there is no evaluation for this measure,208 many centers recommend the use of antihistamines and systemic corticosteroids before procedures and/or surgeries,204, 206 since there are no recommendations otherwise.43 Therefore, on the day of surgery, pre-treatment with intramuscular antihistamine 1 hour before surgery, prednisone 50 mg 13 h, 7 h and 1 hour before surgery is suggested, in addition to benzodiazepines to reduce anxiety and psychological symptoms.207
Medications for mast cell stabilization should also be maintained until surgery.43, 206 In case of suspected hypersensitivity perioperative reaction, the modified Ring and Messner scale for characterizing the condition is used, and the treatment is carried out according to patient clinical manifestations and existing protocols.206 ENDA/EAACI204 has stated that there is no evidence of a higher risk of anaphylaxis to beta lactam antibiotics in patients with mastocytosis. In addition, it also suggests that patients who tolerate NSAIDs do not need to discontinue their use, as there are no studies showing increased risk of anaphylaxis to NSAIDs in these patients.204 Parturients and children have been present in series that report uneventful procedures in patients with mastocytosis.209, 210
IgA deficiency
Patients with IgA deficiency are at risk of anaphylactic reactions after being transfused with blood, plasma or receiving IV immunoglobulin, since they can produce anti-IgA antibodies, and therefore develop a reaction when they receive products containing IgA. Recently, a study evaluating 229 severe allergic or anaphylactic reactions reported by the US and Canada hemovigilance, between 2003 and 2012, showed that only 3 (1.3%) of the patients with IgA deficiency had anti-IgA. Whereas anaphylactic reactions associated with anti-IgA antibodies are rare, there is a possibility that these reactions may develop after blood produc transfusions, therefore, clinicians should try to identify the presence of these antibodies prior to the administration of blood products.211
Final considerations
Of the NAP6 recommendations,64 it is important to highlight one that requires every anesthesia department to sign up one anesthetist as responsible for perioperative anaphylaxis, and that the professional should be offered hours and conditions to carry out that role.
An appropriate communication channel between this professional and the allergy immunology service is recommended, with the exchange of e-mails and contact phone.
Attempts should be made to establish local, regional, national and international networks of centers for the investigation of perioperative anaphylaxis, targeting to increase the capacity to conduct large studies, share experiences and offer better quality care to these complex patients.28
Finally, it is desirable to have, in each regional of the Brazilian Society of Anesthesiology and the Brazilian Association of Allergy and Immunology, a specialist responsible for perioperative anaphylaxis matters.
The combination of anesthesia effects, surgical procedure and differential diagnoses makes it challenging to assess perioperative events. The multidisciplinary approach is critical to proceed with the diagnostic investigation and determination of the etiological agent of the reaction, provide patient orientation to avoid the inappropriate exclusion of useful agents, and avert exposure to non-identified harmful agents.
The joint effort will render future procedures safer for patients.
Glossary
AAE, Acquired Angioedema; HAE, Hereditary Angioedema; NSAI, Non-steroid Anti-inflammatory; BAT, Basophil Activation Test; NMB, Neuromuscular Blocker; ACE, Angiotensin Converting Enzyme; IDT, Intradermal Test; ACEI, Angiotensin Converting Enzyme Inhibitor; NPH, Neutral Protamine Hagedorn; PAF; Platelet Activating Factor; PEG, Polyethylene Glycol; PHR, Perioperative Hypersensitivity Reaction; sIgE, specific serum IgE; SPT, Skin Prick Test; DRT, Drug Provocation Test.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
This document was published in Arquivos de Asma, Alergia e Imunologia DOI: 10.5935/2526-5393.20200003 with the consent of authors and editors.
References
- 1.Moneret-Vautrin DA, Mertes PM. Anaphylaxis to general anesthetics. Chem Immunol Allergy. 2010;95:180–189. doi: 10.1159/000315951. [DOI] [PubMed] [Google Scholar]
- 2.Harper N.J.N., Cook T.M., Garcez T., et al. Anaesthesia, surgery, and life-threatening allergic reactions: epidemiology and clinical features of perioperative anaphylaxis in the 6th National Audit Project (NAP6) Br J Anaesth. 2018;121:159–171. doi: 10.1016/j.bja.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010;125:S161–81. doi: 10.1016/j.jaci.2009.12.981. [DOI] [PubMed] [Google Scholar]
- 4.Montañez M.I., Mayorga C., Bogas G., et al. Epidemiology, mechanisms, and diagnosis of Drug-induced anaphylaxis. Front Immunol. 2017;8:614. doi: 10.3389/fimmu.2017.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reber L.L., Hernandez J.D., Galli S.J. The pathophysiology of anaphylaxis. J Allergy Clin Immunol. 2017;140:335–348. doi: 10.1016/j.jaci.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finkelman F.D., Rothenberg M.E., Brandt E.B., Morris S.C., Strait R.T. Molecular mechanisms of anaphylaxis: Lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:409–457. doi: 10.1016/j.jaci.2004.12.1125. [DOI] [PubMed] [Google Scholar]
- 7.Steenholdt C, Svenson M, Bendtzen K, Thomsen O.Ø, Brynskov J, Ainsworth Ma. Acute and delayed hypersensitivity reactions to infliximab and adalimumab in a patient with Crohn’s disease. J Crohns Colitis. 2012;6:108–111. doi: 10.1016/j.crohns.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Cheifetz A., Smedley M., Martin S., et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315–1324. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 9.Navines-Ferrer A., Serrano-Candelas E., Lafuente A., Munoz-Cano R., Martin M., Gastaminza G. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci Report. 2018;8:11628. doi: 10.1038/s41598-018-29965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munõz-Cano R., Pascal M., Araujo G., et al. Mechanisms, cofactors, and augmenting factors involved in anaphylaxis. Front Immunol. 2017;8:1193. doi: 10.3389/fimmu.2017.01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvão V.R., Giavina-Bianchi P., Castells M. Perioperative anaphylaxis. Curr Allergy Asthma Rep. 2014;14:452. doi: 10.1007/s11882-014-0452-6. [DOI] [PubMed] [Google Scholar]
- 12.Rocha JF. Cómo hacer frente a una reacción alérgica en el perioperatorio: del rash a la anafilaxia. Rev Med Aeronaut. 2013;36:S288–90. [Google Scholar]
- 13.Soetens FM. Anaphylaxis during anaesthesia: diagnosis and treatment. Acta Anaesthesiol Belg. 2004;55:229–237. [PubMed] [Google Scholar]
- 14.Kannan JA, Bernstein JA. Perioperative anaphylaxis: diagnosis, evaluation, and management. Immunol Allergy Clin North Am. 2015;35:321–334. doi: 10.1016/j.iac.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Dewachter P., Mouton-Faivre C., Hepner D.L. Perioperative anaphylaxis: what should be known? Curr Allergy Asthma Rep. 2015;15:21. doi: 10.1007/s11882-015-0522-4. [DOI] [PubMed] [Google Scholar]
- 16.McAleer P.T., McNicol L., Rose M.A. Perioperative anaphylaxis: progress, prevention and pholcodine policy. Anaesth Intensive Care. 2017;45:147–150. doi: 10.1177/0310057X1704500203. [DOI] [PubMed] [Google Scholar]
- 17.Worm M. Epidemiology of anaphylaxis. Chem Immunol Allergy. 2010;95:12–21. doi: 10.1159/000315935. [DOI] [PubMed] [Google Scholar]
- 18.Freundlich R.E., Duggal N.M., Housey M., Tremper T.T., Engoren M.C., Kheterpal S. Intraoperative medications associated with hemodynamically significant anaphylaxis. J Clin Anesth. 2016;35:415–423. doi: 10.1016/j.jclinane.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Escolano F., Valero A., Huguet J., et al. Prospective epidemiologic study of perioperative anaphylactoid reactions occurring in Catalonia (1996-7) Rev Esp Anestesiol Reanim. 2002;49:286–293. [PubMed] [Google Scholar]
- 20.Mertes P.M., Lambert M., Guéant-Rodriguez R.M., et al. Perioperative anaphylaxis. Immunol Allergy Clin N Am. 2009;29:429–451. doi: 10.1016/j.iac.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Freeman S.G., Love N.J., Misbah S.A., Pollard R.C. Impact of national guidelines on reporting anaphylaxis during anaesthesia – an outcome audit. Acta Anaesthesiol Scand. 2013;57:1287–1292. doi: 10.1111/aas.12173. [DOI] [PubMed] [Google Scholar]
- 22.Mertes P.M., Alla F., Tréchot P., Auroy Y., Jougla E., Groupe d’Etudes des Réactions Anaphylactoïdes Peranesthésiques Anaphylaxis during anesthesia in France: An 8-year national survey. J Allergy Clin Immunol. 2011;128:366–373. doi: 10.1016/j.jaci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Niggemann B., Beyer K. Factors augmenting allergic reactions. Allergy. 2014;69:1582–1587. doi: 10.1111/all.12532. [DOI] [PubMed] [Google Scholar]
- 24.Hox V., Desai A., Bandara G., Gilfillan A.M., Metcalfe D.D., Olivera A. Estrogen increases the severity of anaphylaxis in female mice through enhanced endothelial nitric oxide synthase expression and nitric oxide production. J Allergy Clin Immunol. 2015;135:729–736. doi: 10.1016/j.jaci.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vadas P., Gold M., Perelman B., et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 26.Turner P., Jerschow E., Umasunthar T., Lin R., Campbell D.E., Boy R.J. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017;5:1169–1178. doi: 10.1016/j.jaip.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mertes P.M., Ebo D.G., Garcez T., et al. Comparative epidemiology of suspected perioperative hypersensitivity reactions. Br J Anaesth. 2019;123:e16–e28. doi: 10.1016/j.bja.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Garvey L.H., Ebo D.G., Mertes P.M., et al. An EAACI position paper on the investigation of perioperative immediate hypersensitivity reactions. Allergy. 2019;74:1872–1884. doi: 10.1111/all.13820. [DOI] [PubMed] [Google Scholar]
- 29.Garro L.S., Aun M.V., Soares I.S., et al. Specific questionnaire detects a high incidence of intra-operative hypersensitivity reactions. Clinics. 2018;113:1202–1212. doi: 10.6061/clinics/2018/e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldo BA, Fisher MM. Substituted ammonium ions as allergenic determinants in drug allergy. Nature. 1983;306:262–264. doi: 10.1038/306262a0. [DOI] [PubMed] [Google Scholar]
- 31.Baldo BA, Fisher MM. On the origin and specificity of antibodies to neuromuscular blocking (muscle relaxant) drugs: an immunochemical perspective. Clin Exp Allergy. 2009;39:325–344. doi: 10.1111/j.1365-2222.2008.03171.x. [DOI] [PubMed] [Google Scholar]
- 32.Florvaag E, Johansson SGO. The pholcodine case. Cough medicines, IgE-sensitization and anaphylaxis: a devious connection. World Allergy Organ J. 2012;5:73–78. doi: 10.1097/WOX.0b013e318261eccc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antunes J., Kochuyt A.M., Ceuppens JL. Perioperative allergic reactions: experience in a Flemish referral centre. Allergol Immunopathol (Madr). 2014;42:348–354. doi: 10.1016/j.aller.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Spoerl D., NIgolian H., Czarnetzki C., Harr T. Reclassifying anaphylaxis to neuromuscular blocking agents based on the presumed patho-mechanism: IgE-mediated, pharmacological adverse reaction or “innate hypersensitivity”? Int J Mol Sci. 2017;18:1223. doi: 10.3390/ijms18061223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiriac Am, Tacquard C, Fadhel Bn, et al. Safety of subsequent general anesthesia in patient allergic to neuromuscular blocking agents: value of allergy skin testing. Br J Anaesth. 2018;120:1437–1440. doi: 10.1016/j.bja.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Fraser BA, Smart JA. Anaphylaxis to cisatracurium following negative skin testing. Anaesth Intensive Care. 2005;33:816–819. doi: 10.1177/0310057X0503300620. [DOI] [PubMed] [Google Scholar]
- 37.Scolaro R.J., Crilly H.M., Maycock E.J., et al. The Australian and New Zealand anaesthetic allergy group perioperative anaphylaxis investigation guidelines. Anaesth Intensive Care. 2017;45:543–555. doi: 10.1177/0310057X1704500504. [DOI] [PubMed] [Google Scholar]
- 38.Leysen J., Bridts C.H., De Clerk L.S., Ebo D.G. Rocuronium – induced anaphylaxis is probably not mitigated by sugammadex: evidence from an in vitro experiment. Anaesthesia. 2011;66:526–527. doi: 10.1111/j.1365-2044.2011.06729.x. [DOI] [PubMed] [Google Scholar]
- 39.Clarke R.C., Sadleir P.H., Platt P.R. The role of sugammadex in the development and modification of an allergic response to rocuronium: evidence from a cutaneous model. Anaesthesia. 2012;67:266–273. doi: 10.1111/j.1365-2044.2011.06995.x. [DOI] [PubMed] [Google Scholar]
- 40.Platt P.R., Clarke R.C., Jonson G.H., Sadleir P.H. Efficacy of sugammadex in rocuronium-induced or antibiotic-induced anaphylaxis. A case-control study. Anaesthesia. 2015;70:1264–1267. doi: 10.1111/anae.13178. [DOI] [PubMed] [Google Scholar]
- 41.Tsur A., Kalansky A. Hypersensitivity associated with sugammadex administration: a systematic review. Anaesthesia. 2014;69:1251–1257. doi: 10.1111/anae.12736. [DOI] [PubMed] [Google Scholar]
- 42.Garvey L.H., Dewachter P., Hepner D.L., et al. Management of suspected immediate perioperative allergic reactions: an international overview and consensus recommendations. Br J Anaesth. 2019;123:e50–e64. doi: 10.1016/j.bja.2019.04.044. [DOI] [PubMed] [Google Scholar]
- 43.Volcheck GW, Hepner DL. Identification and management of perioperative anaphylaxis. J Allergy Clin Immunol Pract. 2019 doi: 10.1016/j.jaip.2019.05.033. S2213-2198(19)30490-30498. [DOI] [PubMed] [Google Scholar]
- 44.Blaabjerg M.S., Andersen K.E., Bindeslev-Jensen C., Mortz C.G. Decrease in the rate of sensitization and clinical allergy to natural rubber latex. Contact Dematitis. 2015;73:21–28. doi: 10.1111/cod.12386. [DOI] [PubMed] [Google Scholar]
- 45.Cremer R., Lorbacher M., Hering F., Engelskirchen R. Natural rubber latex sensitization and allergy in patients with spina bífida, urogenital disorders and oesophageal atresia compared with normal paediatric population. Eur J Pediatr Surg. 2007;17:194–198. doi: 10.1055/s-2007-965144. [DOI] [PubMed] [Google Scholar]
- 46.Michavila Gomez A.V., Belver Gonzalez M.T., Alvarez N.C., et al. Perioperative anaphylactic reactions: Review and procedure protocol in paediatrics. Allergol Immunopathol (Madr). 2015;43:203–214. doi: 10.1016/j.aller.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Dewachter P., Kopac P., Laguna J.J., et al. Anaesthetic management of patients with pre-existing conditions: a narrative review. Br J Anaesth. 2019;123:e65–81. doi: 10.1016/j.bja.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 48.Société Française d’Anesthesie et de Réanimation Société Française d”Allergologie. Reducing the risk of anaphylaxis during anaesthesia. Short text. Ann Fr Anesth Reanim. 2011;30:212–222. doi: 10.1016/j.annfar.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Laguna J.J., Archilla J., Doña I., et al. Practical guidelines for perioperative hypersensitivity reactions. J Investig Allergol Immunol. 2018;28:216–232. doi: 10.18176/jiaci.0236. [DOI] [PubMed] [Google Scholar]
- 50.Soares ISC. Faculdade de Medicina; São Paulo: 2016. Sensibilização ao látex em pacientes com mielomeningocele na Urologia do HC-FMUSP: prevalência e fatores associados [tese] [DOI] [Google Scholar]
- 51.Silva Jr JB. Resolução RDC nº 37, de 26 de agosto de 2015. Ministério da Saúde/Agência Nacional de Vigilância Sanitária/Diretoria Colegiada. Dispõe sobre a padronização de frases de declaração de conteúdo de látex de borracha natural em rótulos de dispositivos médicos. Diário Oficial da União. Edição 164, Seção 1, Página 46.
- 52.Mertes P.M., Malinovsky J.M., Jouffroy L, et al. Reducing the risk of anaphylaxis during anesthesia: 2011 update guidelines for clinical practice. J Investig Allergol Clin Immunol. 2011;21:442–453. [PubMed] [Google Scholar]
- 53.Baldo BA, Pham NH. Histamine-releasing and allergenic properties of opioid analgesic drugs: resolving the two. Anaesth Intensive Care. 2012;40:216–235. doi: 10.1177/0310057X1204000204. [DOI] [PubMed] [Google Scholar]
- 54.Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381–1395. doi: 10.1213/01.ANE.0000082993.84883.7D. [DOI] [PubMed] [Google Scholar]
- 55.Blunk J.A., Schmelz M., Zeck S., Skov P., Likar R., Koppert W. Opioid–induced mast cell activation and vascular response: an in vivo microdialysis study in human skin. Ansth Analg. 2004;98:364–370. doi: 10.1213/01.ANE.0000097168.32472.0D. [DOI] [PubMed] [Google Scholar]
- 56.Ebo D.G., Fischer M.M., Hagendorens M.M., Bridts C.H., Stevens W.J. Anaphylaxis during anaesthesia: diagnostic approach. Allergy. 2007;62:471–487. doi: 10.1111/j.1398-9995.2007.01347.x. [DOI] [PubMed] [Google Scholar]
- 57.Nasser SM, Ewan PW. Opiate-sensitivity: clinical characteristics and the role of skin prick testing. Clin Exp Allergy. 2001;31:1014–1020. doi: 10.1046/j.1365-2222.2001.01090.x. [DOI] [PubMed] [Google Scholar]
- 58.Van Gasse A.L., Hagendorens M.M., Sabato V., Bridts C.H., De Clerck L.S., Ebo D.G. IgE to poppy seed and morphine are not useful tools to diagnose opiate allergy. J Allergy Clin Immunol. 2015;3:396–399. doi: 10.1016/j.jaip.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Baker M.T.N., Aguib M. Propofol: the challenges of formulation. Anesthesiology. 2005;103:860–876. doi: 10.1097/00000542-200510000-00026. [DOI] [PubMed] [Google Scholar]
- 60.Asseroj LL, Mosbech H, Kroigaard M, Garvey LH. No evidence for contraindications to the use of propofol in adults allergic to egg, soy and peanuts. Br J Anaesth. 2016;116:77–82. doi: 10.1093/bja/aev360. [DOI] [PubMed] [Google Scholar]
- 61.Murphy A., Campbell D.E., Baines D., Mehr S. Allergic reactions to propofol in egg-allergic children. Anesth Analg. 2011;113:140–144. doi: 10.1213/ANE.0b013e31821b450f. [DOI] [PubMed] [Google Scholar]
- 62.Mehta P., Sundaram S.S., Furuta G.T., Pan Z., Atkins D., Markowitz S. Propofol use in pediatric patients with food allergy and eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2017;64:546–549. doi: 10.1097/MPG.0000000000001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tacquard C., Collange O., Gomis P., et al. Anaesthetic hypersensitivity reactions in France between 2011 and 2012: the 10th GERAP epidemiology survey. Acta Anaesthesiol Scand. 2017;61:290–299. doi: 10.1111/aas.12855. [DOI] [PubMed] [Google Scholar]
- 64.Harper N.J.N., Cook T.M., Garcez T., et al. Anaesthesia, surgery, and life-threatening allergic reactions: management and outcomes in the 6th National Audit Project (NAP6) Br J Anaesth. 2018;121:172–188. doi: 10.1016/j.bja.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 65.Kvisselgaard A.D., Mosbech H.F., Fransson S., Garvey L.H. Risk of Immediate-type to local anesthetic is overestimated-results from 5 years of provocation testing in a Danish allergy clinic. J Allergy Clin Immunol Pract. 2018;6:1217–1223. doi: 10.1016/j.jaip.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Kvisselgaard A.D., Melchiors B.B., Kroigaard M., Garvey LH. lidocaine as a rare and hidden allergen in the perioperative setting: a case report. Anesth Analg Pract. 2019;12:430–432. doi: 10.1213/XAA.0000000000000955. [DOI] [PubMed] [Google Scholar]
- 67.Bhole M.V., Manson A.L., Seneviratne S.L., Misbah S.A. IgE-mediate allergy to local anaesthetic: separating fact from perception: a UK perspective. Br J Anaesth. 2012;108:903–911. doi: 10.1093/bja/aes162. [DOI] [PubMed] [Google Scholar]
- 68.Malinovsky J.M., Chiriac A.M., Tacquard C., Mertes P.M., Demoly P. Allergy to local anesthetics: Reality or mith? Presse Med. 2016;45:753–757. doi: 10.1016/j.lpm.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 69.Volcheck GW, Mertes PM. Local and general anesthetics immediate hypersensitivity reactions. Immunol Allergy Clin N Am. 2014;34:525–546. doi: 10.1016/j.iac.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Rose M.A., Garcez T., Savic S., Garvey L.H. Chlorhexidine allergy in the perioperative setting: a narrative review. Br J Anaesth. 2019;123:e95–e103. doi: 10.1016/j.bja.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 71.Garvey L.H., Roed-Petersen J., Husum B. Anaphylactic reactions in anaesthetized patients - four cases of chlorhexidine allergy. Acta Anaesthesiol Scand. 2001;45:1290–1294. doi: 10.1034/j.1399-6576.2001.451020.x. [DOI] [PubMed] [Google Scholar]
- 72.Opstrup M.S., Malling H.J., Kroigaard M., et al. Standardized testing with chlorhexidine in perioperative allergy - a large single - centre evaluation. Allergy. 2014;69:1390–1396. doi: 10.1111/all.12466. [DOI] [PubMed] [Google Scholar]
- 73.Palobart C., Cros J., Orsel I., Nathan N. Choc anaphylactique à la povidone iodée. Ann Fr Anesth Reanim. 2009;28:168–170. doi: 10.1016/j.annfar.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 74.Dewachter P., Mouton-Faivre C. Allergie aux medicaments et aliments iodés: la sequence allergénique n’est pas l’iode. Presse Med. 2015;44:1136–1145. doi: 10.1016/j.lpm.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 75.Mertes P.M., Demoly P., Malinovsky J.M. Hypersensitivity reactions in the anesthesia setting/allergic reactions to anesthetics. Curr Opin Allergy Clin Immunol. 2012;12:361–368. doi: 10.1097/ACI.0b013e328355b82f. [DOI] [PubMed] [Google Scholar]
- 76.Garvey LH. Practical aspects of perioperative anaphylaxis. Trends Anaesth Crit Care. 2013;3:320–326. [Google Scholar]
- 77.Opstrup M.S., Mosbech H., Garvey L.H. Allergic sensitization to ethyelene oxide in patients with suspected allergic reactions during surgery and anesthesia. J Investig Allergol Clin Immunol. 2010;20:69–70. [PubMed] [Google Scholar]
- 78.Garvey LH. Old, new and hidden causes of perioperative hypersensitivity. Curr Pharm Des. 2016;22:6814–6824. doi: 10.2174/1381612822666161004125143. [DOI] [PubMed] [Google Scholar]
- 79.Bache S., Petersen J.T., Garvey L.H. Anaphylaxis to ethylene oxide - a rare and overlooked phenomenon? Acta Anaesthesiol Scand. 2011;55:1279–1282. doi: 10.1111/j.1399-6576.2011.02504.x. [DOI] [PubMed] [Google Scholar]
- 80.Listyo A, Hofmaeier Ks, Bandschapp O, Erb T, Hasler CC, Bircher AJ. Severe anaphylactic shock due to ethylene oxide in a patient with myelomeningocele: successful exposure prevention and pretreatment with omalizumab. Anesth Analg Case Rep. 2014;2:3–6. doi: 10.1097/ACC.0b013e3182a08ff1. [DOI] [PubMed] [Google Scholar]
- 81.Hamad A., Iweala O.I., Henderson C., et al. Recurrent anaphylaxis during cardiac catheterization. J Allergy Clin Immunol Pract. 2018;6:2148–2150. doi: 10.1016/j.jaip.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sokol WN. Nine episodes of anaphylaxis following cystoscopy caused by Cidex OPA (Ortho phthaladehyde) high level desinfectatnt in 4 patients after ciystoscopy. J Allergy Clin Immunol. 2004;114:392–397. doi: 10.1016/j.jaci.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 83.Atiych K., Chitkara A., Achlatis S., Branski R.C., Amin M.R. Allergic reaction to orthophtalaldehyde following flexible laryngoscope. Laryngoscope. 2015;125:2349–2352. doi: 10.1002/lary.25421. [DOI] [PubMed] [Google Scholar]
- 84.Larrauri B.J., Torre M.G., Malbran E., Juri M.C., Fernández-Romero D.S., Málbran A. Anafilaxias y reaccciones alérgicas durante cirurgías y procedimientos médicos. Medicina (Buenos Aires). 2017;77:382–387. [PubMed] [Google Scholar]
- 85.Cooper D.E., White A.A., Werkema A.N., Auge B.K. Anaphylaxis following cystoscopy with equipment sterilized with Cidex OPA (orthophthalaldehyde): a review of two cases. J Endourol. 2008;22:2181–2184. doi: 10.1089/end.2007.0358. [DOI] [PubMed] [Google Scholar]
- 86.Laxenaire M.C., Mertes P.M., Goupe d’Etudes des Réactions Anaphylacoïdes Peranesthésiques Anaphylaxis during anaesthesia. Results of two-year survey in France. Br J Anaesth. 2001;87:549–558. doi: 10.1093/bja/87.4.549. [DOI] [PubMed] [Google Scholar]
- 87.Laxenaire M.C., Charpentier C., Feldman L. Anaphylactoid reactions to clloid plasma substitutes: incidence, risk factors, mechanisms. A French multicenter prospective study. Ann Fr Anesth Reanim. 1994;13:301–310. doi: 10.1016/s0750-7658(94)80038-3. [DOI] [PubMed] [Google Scholar]
- 88.Agarwal N.S., Spalding C., Nassef M. Life threating intraoperative anaphylaxis to gelatin in Floseal during pediatric spinal surgery. J Allergy Clin Immunol Pract. 2015;3:110–111. doi: 10.1016/j.jaip.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 89.Shah G., Scadding G., Nguyen-Lu N., et al. Perio-perative cardiac arrest with ST elevation secondary to gelofusin anaphylaxis-Kounis syndrome in the anaesthetic room. Int J Cardiol. 2013;164:e22–6. doi: 10.1016/j.ijcard.2012.09.166. [DOI] [PubMed] [Google Scholar]
- 90.Spencer H.T., Hsu J.T., McDonald D.R., Karlin L.L. Intraoperative anaphylaxis to gelatina in topical hemostatic agentes during anterior spinal fusion: a case report. Spine J. 2012;12:e1–6. doi: 10.1016/j.spinee.2012.08.425. [DOI] [PubMed] [Google Scholar]
- 91.Uyttebroeck A, Sabato V, Bridts CH, De Clerck LS, Ebo DG. Anaphylaxis to sccinylated gelatin in a patient with a meat allergy: galactose-alfa (1,3)-galactose (alfa-gal) as antigenic determinant. J Clin Anesth. 2014;26:574–576. doi: 10.1016/j.jclinane.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 92.Wilson JM, Platts-Mills TAE. Meat Allergy and allergens. Mol Immunol. 2018;100:107–112. doi: 10.1016/j.molimm.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tacquard C., Boudjedir K., Carlier M., Muller J.Y., Gornis P., Mertes P.M. Hypersensitivity transfusion reactions due to IgA deficiency are rare according to French hemovigilance date. J Allergy Clin Immunol. 2017;140:884–885. doi: 10.1016/j.jaci.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 94.Squire JE. Risks of transfusion. South Med J. 2011;104:762–769. doi: 10.1097/SMJ.0b013e31823213b6. [DOI] [PubMed] [Google Scholar]
- 95.Tinegate H., Birchall J., Gray A., Haggas R., Massey E., Norfolk D., et al. Guideline on the investigation and management of acute transfusion reaction. Prepared by the BCSH Blood Transfusion Task Force. Br J Haematol. 2012;159(2):143–153. doi: 10.1111/bjh.12017. [DOI] [PubMed] [Google Scholar]
- 96.Dewachter P., Mouton-Faivre C., Laroche D., Clément O. Allergie immediate aux produits de contraste iodés et prevention des reactions. Rev Med Interne. 2009;30:872–881. doi: 10.1016/j.revmed.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 97.Dewachter P, Mouton-Faivre C, Castells M, Hepner DL. Anesthesia in the patient with multiple drug allergies: are all allergies the same? Curr Opin Anesthesiol. 2011;24:320–325. doi: 10.1097/ACO.0b013e3283466c13. [DOI] [PubMed] [Google Scholar]
- 98.Kuhen A., Hilger C., Lehners-Weber C., et al. Identification of enolases and aldolases as important fish allergens in cod, salmon and tuna: component resolved diagnosis using paravalbumin and the new allergens. Clin Exp Allergy. 2013;43:811–822. doi: 10.1111/cea.12117. [DOI] [PubMed] [Google Scholar]
- 99.Lopata A.L., O’Hehir R.E., Leherer S.B. Shellfish allergy. Clin Exp Allergy. 2010;40:850–858. doi: 10.1111/j.1365-2222.2010.03513.x. [DOI] [PubMed] [Google Scholar]
- 100.Ruethers T., Taki A.C., Johnston E.B., Nugraha R., Le TTK Kalic T, et al. Seafood allergy: a comprehensive review of fish and sellfish allergy. Mol Immunol. 2018;100:28–57. doi: 10.1016/j.molimm.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 101.Huang SW. Seafood and Iodine: An Analysis of a Medical Myth. Allergy Asthma Proc. 2005;26:468–469. [PubMed] [Google Scholar]
- 102.Beaty A.D., Lieberman P.L., Slavin R.G. Seafood allergy and radiocontrast media: are physicians propagating a myth? J Med. 2008;121 doi: 10.1016/j.amjmed.2007.08.025. 158.e1-4. [DOI] [PubMed] [Google Scholar]
- 103.Schrijvers R., Breynaert C., Ahmedali Y., Bourrain J.L., Demoly P., Chiriac A.M. Skin testing for suspected iodinated contrast media hypersensitivity. J Allergy Immunol Pract. 2018;6:1246–1254. doi: 10.1016/j.jaip.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 104.Torres M.J., Gomez F., Doña I., et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity to iodinated contrast media. Allergy. 2012;67:929–935. doi: 10.1111/j.1398-9995.2012.02840.x. [DOI] [PubMed] [Google Scholar]
- 105.Dewachter P., Laroche D., Mouton-Faivre C., et al. Immediate reactions following iodinated contrast media injection: a study of 38 cases. Eur J Radiol. 2011;77:495–501. doi: 10.1016/j.ejrad.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 106.Sánchez-Borges M., Aberer W., Brockow K., et al. Controversies in drug allergy: radiographic contrast media. J Allergy Clin Pract. 2019;7:60–64. doi: 10.1016/j.jaip.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 107.Bartlett M.J., Bynevelt M. Acute contrast reaction management by radiologists: a local audit. Australas Radiol. 2003;47:363–367. doi: 10.1046/j.1440-1673.2003.01203.x. [DOI] [PubMed] [Google Scholar]
- 108.Rosado Ingelmo A., Doña Diaz I., Cabãnas Moreno R., et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. 2016;26:144–155. doi: 10.18176/jiaci.0058. [DOI] [PubMed] [Google Scholar]
- 109.Li A., Wong C.S., Wong M.K., Lee C.M., Au Yeung M.C. Acute adverse reactions to magnetic contrast media-gadolinium chelates. Br J Radiol. 2006;79 doi: 10.1259/bjr/88469693. 386-71. [DOI] [PubMed] [Google Scholar]
- 110.Schiavino D., Murzilli F., Del Ninno M., et al. Demonstration of an IgE-mediated immunological pathogenesis of a severe adverse reaction to gadopentato dimeglumine. J Investig Allergol Clin Immunol. 2003;13:140–142. [PubMed] [Google Scholar]
- 111.Mertes P.M., Malinovsky J.M., Mouton-Faivre, et al. Anaphylaxis to dyes during the perioperative period: reports of 14 clinical cases. J Allergy Clin Immunol. 2008;122:348–352. doi: 10.1016/j.jaci.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 112.Ishiyama T., Kotoda M., Asano N., Ikimoto K., Mitisui K., Sato H. Blue dye on peripheral and cerebral oxyhemoglobin saturations. Anaesthesia. 2015;70:429–433. doi: 10.1111/anae.12932. [DOI] [PubMed] [Google Scholar]
- 113.Batti MACSB, Mendes A., Pinheiro J.T., Frode T.S. Reação alérgica ao azul patente. Rev Bras Anest. 2004;54:180. [Google Scholar]
- 114.Xu K., Tzankowa V., Li C., Sharma S. Intravenous fluorescein angiograhy- associated adverse reactions. Can J Ophtalmol. 2016;51:321–325. doi: 10.1016/j.jcjo.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 115.Lee T., Sanderson D., Doyle P., Buchsbaum G. Anaphylactic Shock After Intravenous Fluorescein Administration for Intraoperative Cystoscopy. Obstet Gynecol. 2018;131:727–729. doi: 10.1097/AOG.0000000000002519. [DOI] [PubMed] [Google Scholar]
- 116.Levy JH, Adkinson NF. Anaphylaxis during cardiac surgery: implications for clinicians. Anesth Analg. 2008;106:392–403. doi: 10.1213/ane.0b013e3181602e0d. [DOI] [PubMed] [Google Scholar]
- 117.Dietrich W., Ebell A., Busley R., Boulesteix AL. Aprotinin and anaphylaxis: analysis of 12.403 exposures to aprotinin in cardiac surgery. Ann Thorac Surg. 2007;84:1144–1150. doi: 10.1016/j.athoracsur.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 118.Nybo M, Madsen JS. Serious anaphylactic reactions due to protamine sulfate: a systematic literature review. Basic Clin Pharmacol Toxicol. 2008;103:192–196. doi: 10.1111/j.1742-7843.2008.00274.x. [DOI] [PubMed] [Google Scholar]
- 119.Greenberger P.A., Patterson R., Tobin M.C., Liotta J.L., Roberts M. Lack of cross-reactivity between IgE to salmon and protamine sulfate. Am J Med Sci. 1989;298:104–108. doi: 10.1097/00000441-198908000-00006. [DOI] [PubMed] [Google Scholar]
- 120.Levy J.H., Scwieger I.M., Zaidan J.R., Faraj B.A., Weintraub W.S. Evaluation of patients at risk for protamine reactions. J Thorac Cardiovasc Surg. 1989;98:200–204. [PubMed] [Google Scholar]
- 121.Valchanov K., Falter F., George S., et al. Three cases of anaphylaxis to protamine: management of anticoagulation reversal. J Cardiothorac Vasc Anesth. 2019;33:482–486. doi: 10.1053/j.jvca.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 122.Levy JH, Bartz RR. Protamine, is something fishy abaout it? The spectre of anaphylaxis continus. J Cardiothorac Vasc Anesth. 2019;33:487–488. doi: 10.1053/j.jvca.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Marinho S., Kemp H., Cook T.M., et al. Cross-sectional study of perioperative drug and allergen exposure in UK practice in 2016: the 6th National Audit Project (NAP6) Allergen Survey. Br J Anaesth. 2018;121:146–158. doi: 10.1016/j.bja.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 124.Chiem J., Ivanova I., Parker A., Krengel W, 3rd, Jimenez N. Anaphylactic reaction to tranexamic acid in an adolescent undergoing posterior spinal fusion. Paediatr Anaesth. 2017;27:774–775. doi: 10.1111/pan.13141. [DOI] [PubMed] [Google Scholar]
- 125.Li P.H., Trigg C., Rutkowski R., Rutkowski K. Anaphylaxis to tranexamic acid - a rare reaction to a common drug. J Allergy Clin Immunol Pract. 2017;5:839–841. doi: 10.1016/j.jaip.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 126.Lee H.K., Choi E.J., Lee P.B., Nahm F.S. Anaphylactic shock caused by the epidurally-administered hyaluronidase. Korean J Pain. 2011;24:221–225. doi: 10.3344/kjp.2011.24.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Raichura N.D., Alam M.S., Jaichandran V.V., Mistry S., Mukherjee B. Hyaluronidade allergy mimicking orbital cellulitis. Orbit. 2018;37:149–153. doi: 10.1080/01676830.2017.1383465. [DOI] [PubMed] [Google Scholar]
- 128.Alcubierre R., Sanchez-Dalmau B.F., Mousavi K. Compressive optic neuropathy secondary toa n allergic reaction to hyaluronidase. Arch Soc Esp Oftalmol. 2019 doi: 10.1016/j.oftal.2019.03.017. doi 10.16/j.oftal,2019.03.017. [DOI] [PubMed] [Google Scholar]
- 129.Halliday L., Sia P.I., Durkin S., Selva D. Atypical case of hyaluronidase allergy with orbital compartment syndrome and visual loss. Clin Exp Ophtalmology. 2018;46:563–564. doi: 10.1111/ceo.13128. [DOI] [PubMed] [Google Scholar]
- 130.Tan P., Malhotra R. Hyaluronic acid fillers and hyaluronidase use in regional eye blocks. Anaesthesia. 2016;71:988–989. doi: 10.1111/anae.13597. [DOI] [PubMed] [Google Scholar]
- 131.Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46:907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 132.Cerdá V.J., Pacheco R.R., Witek J.D. de la Calle FMM, Fernández MLS. Immediate hypersensitivity to polyethylene glycols in unrelated products: when standardization in the nomenclature of the components of drugs, cosmetics and food become necessary. Allergy Asthma Clin Immunol. 2019;15:9. doi: 10.1186/s13223-019-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Farber MK, Angelo TE, Castells M, Tsen LC. Anesthetic management of patient with an allergy to propylene glycol and parabens. Anesth Analg. 2010;110:839–842. doi: 10.1213/ANE.0b013e3181cde5a5. [DOI] [PubMed] [Google Scholar]
- 134.Witschi L., Reist L., Stammschulte T., Erlenwein J., Becke K., Stamer U. Perioperative Anwendung von Metamizol und anderen Nichtopioidanalgetika bei KindernPerioperative use of metamizole and other nonopioid analgesics in children. Anaesthesist. 2019;68:152–160. doi: 10.1007/s00101-018-0532-4. [DOI] [PubMed] [Google Scholar]
- 135.Varalda DB, Motta AA. Adverse reactions to nonsteroidal anti-inflammatory. Rev Bras Alerg Imunopatol. 2009;32:27–34. [Google Scholar]
- 136.Torres M.J., Barrionuevo E., Kowalski M., Blanca M. Hypersensitivity reactions to nonsteroidal anti-inflammatory drugs. Immunol Allergy Clin North Am. 2014;34:507–524. doi: 10.1016/j.iac.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 137.Doña I., Blanca-López N., Cornejo-García J.A., et al. Characteristics of subjects experiencing hypersensitivity to non-steroidal anti-inflammatory drugs: Patterns of response. Clin Exp Allergy. 2011;41:86–95. doi: 10.1111/j.1365-2222.2010.03651.x. [DOI] [PubMed] [Google Scholar]
- 138.Wöhrl S. NSAID hypersensitivity – recommendations for diagnostic work up and patient management. Allergo J Int. 2018;27:114–121. doi: 10.1007/s40629-018-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aun M.V., Blanca M., Garro L.S., et al. Nonsteroidal anti-inflammatory drugs are major causes of drug-induced anaphylaxis. J Allergy Clin Immunol Pract. 2014;2:414–420. doi: 10.1016/j.jaip.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 140.Ensina L.F., Bastos P.G.A., de Lacerda A.E., de Araujo C.A., Camelo-Nunes I., Solé D. Comments on Balp et al. Pediatr Allergy Immunol. 2018;29:669–670. doi: 10.1111/pai.12950. [DOI] [PubMed] [Google Scholar]
- 141.Lobera T.I., Audicana M.T., Pozo M.D., et al. Study of hypersensitivity reactions and anaphylaxis during anesthesia in Spain. J Investig Allergol Clin Immunol. 2008;18:350–356. [PubMed] [Google Scholar]
- 142.Trautmann A., Seidl C., Stoevesandt J., Seitz C.S. General anaesthesia-induced anaphylaxis: Impact of allergy testing on subsequent anaesthesia. Clin Exp Allergy. 2016;46:125–132. doi: 10.1111/cea.12632. [DOI] [PubMed] [Google Scholar]
- 143.Kemp H.I., Cook T.M., Thomas M., Harper N.J.N. UK anaesthetists’ perspectives and experiences of severe perioperative anaphylaxis: NAP6 baseline survey. Br J Anaesth. 2017;119:132–139. doi: 10.1093/bja/aex124. [DOI] [PubMed] [Google Scholar]
- 144.Mertes PM, Laxenaire MC. Anaphylactic and anaphylactoid reactions occurring during anaesthesia in France seventh epidemiologic survey. Ann Fr Anesth Reanim. 2004;23:1133–1143. doi: 10.1016/j.annfar.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 145.Fieler M., Eich C., Becke K., et al. Metamizole for postoperative pain therapy in 1177 children: a prospective, multicentre, observational, post authorisation safety study. Eur J Anaesthesiol. 2015;32:839–843. doi: 10.1097/EJA.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 146.De Leeuw T.G., Dirckx M., Gonzalez C., et al. The use of dipyrone (metamizol) as an analgesic in children: What is the evidence? A review. Paediatr Anaesth. 2017;27:1193–1201. doi: 10.1111/pan.13257. [DOI] [PubMed] [Google Scholar]
- 147.Kidon M., Blanca-Lopez N., Gomes E., et al. EAACI/ENDA position paper: diagnosis and management of hypersensitivity reactions to non-steroidal anti-inflammatory drugs (NSAIDs) in children and adolescents. Pediatr Allergy Immunol. 2018;29:469–480. doi: 10.1111/pai.12915. [DOI] [PubMed] [Google Scholar]
- 148.Ansari F., Erntell M., Goossens H., Davey P. The European Surveillance of Antimicrobial Consumption (ESAC) Point‐Prevalence Survey of Antibacterial Use in 20 European Hospitals in 2006. Clin Infect Dis. 2009;49:1496–1504. doi: 10.1086/644617. [DOI] [PubMed] [Google Scholar]
- 149.Robert J., Péan Y., Varon E., et al. Point prevalence survey of antibiotic use in French hospitals in 2009. J Antimicrob Chemother. 2012;67:1020–1026. doi: 10.1093/jac/dkr571. [DOI] [PubMed] [Google Scholar]
- 150.Garvey LH, Hunter JM. Changing culprits in perioperative anaphylaxis. Br J Anaesth. 2018;121:114–117. doi: 10.1016/j.bja.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 151.Harper N., Cook T. Anaesthesia, surgery and life-threatening allergic reactions: from the Report and findings of the 6th National Audit Project; Royal College of Anaesthetists; 2018. pp. 183–191. [DOI] [PubMed] [Google Scholar]
- 152.Trubiano J.A., Stone C.A., Grayson M.L., et al. The 3 Cs of antibiotic allergy–classification, cross-reactivity, and collaboration. J Allergy Clin Immunol Pract. 2017;5:1532–1542. doi: 10.1016/j.jaip.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Romano A., Warrington R. Antibiotic allergy. Immunol Allergy Clin North Am. 2014;34:489–506. doi: 10.1016/j.iac.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 154.Gonzalez-Estrada A., Pien L.C., Zell K., Wang X.F., Lang D.M. Antibiotics are an important identifiable cause of perioperative anaphylaxis in the United States. J Allergy Clin Immunol Pract. 2015;3 doi: 10.1016/j.jaip.2014.11.005. 101-105.e1. [DOI] [PubMed] [Google Scholar]
- 155.Kuhlen J.L., Camargo C.A., Balekian D.S., et al. Antibiotics are the most commonly identified cause of perioperative hypersensitivity reactions. J Allergy Clin Immunol Pract. 2016;4:697–704. doi: 10.1016/j.jaip.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Savic L.C., Garcez T., Hopkins P.M., Harper N.J.N., Savic S. Teicoplanin allergy - An emerging problem in the anaesthetic allergy clinic. Br J Anaesth. 2015;115:595–600. doi: 10.1093/bja/aev307. [DOI] [PubMed] [Google Scholar]
- 157.Penicillin Allergy in Antibiotic Resistance Workgroup Penicillin allergy testing should be performed routinely in patients with self-reported penicillin allergy. J Allergy Clin Immunol Pract. 2017;5:333–334. doi: 10.1016/j.jaip.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 158.Lucas M., Arnold A., Sommerfield A., et al. Antibiotic allergy labels in children are associated with adverse clinical outcomes. J Allergy Clin Immunol Pract. 2019;7:975–982. doi: 10.1016/j.jaip.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 159.Macy E., Contreras R. Healthcare utilization and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790–796. doi: 10.1016/j.jaci.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 160.Vorobeichik L., Weber E.A., Tarshis J. Misconceptions surroundingpenicillin allergy: implications for Anesthesiologists. Anest Analg. 2018;127:642–649. doi: 10.1213/ANE.0000000000003419. [DOI] [PubMed] [Google Scholar]
- 161.Har D., Solensky R. Penicillin and beta-lactam hypersensitivity. Immunol Allergy Clin North Am. 2017;37:643–666. doi: 10.1016/j.iac.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 162.Savic LC, Khan DA, Kopac P, et al. Management of a surgical patient with a label of penicillin allergy: narrative review and consensus recommendations. Br J Anaesth. 2019;123:e82–e94. doi: 10.1016/j.bja.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 163.Moussa Y., Shuster J., Matte G., Sullivan A., Goldstein R.H., Cunningham D., et al. De-labeling of β-lactam allergy reduces intraoperative time and optimizes choice in antibiotic prophylaxis. Surgery. 2018 doi: 10.1016/j.surg.2018.03.004. pii: S0039-6060(18)30127-2. [DOI] [PubMed] [Google Scholar]
- 164.Nel L., Eren E. Peri-operative anaphylaxis. Br J Pharmacol. 2011;71:647–658. doi: 10.1111/j.1365-2125.2011.03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vitte J., Amadei L., Gouitaa M., et al. Paired acute-baseline serum tryptase levels in perioperative anaphylaxis: an observational study. Allergy. 2019;74:1157–1165. doi: 10.1111/all.13752. [DOI] [PubMed] [Google Scholar]
- 166.Baretto R.L., Beck S, Heslegrave J., et al. Validation of international consensus equation for acute serum total tryptase in mast cell activation: a perioperative perspective. Allergy. 2017;72:20314. doi: 10.1111/all.13226. [DOI] [PubMed] [Google Scholar]
- 167.Laroche D., Gomis P., Gallimidi E., Malinowsky J.M., Mertes P.M. Diagnostic value of histamine and tryptase concentrations in severe anaphylaxis with shock or cardiac arrest during anesthesia. Anesthesiology. 2014;121:272–279. doi: 10.1097/ALN.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 168.Ewan P.W., Dugué P., Mirakian R., Dixon T.A., Harper J.N., Nasser S.M. BSACI guidelines for the investigation of suspected anaphylaxis during general anesthesia. Clin Exp Allergy. 2010;40:15–31. doi: 10.1111/j.1365-2222.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 169.Uyttebroek A.P., Decuyper I.I., Bridts C.H., et al. Cefazolin hypersensitivity: toward optimized diagnosis. J Allergy Clin Immunol Pract. 2016;4:1232–1236. doi: 10.1016/j.jaip.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 170.Opstrup M.S., Poulsen L.K., Malling H.J., Jensen B.M., Garvey L.H. Dynamics of specific IgE in chlorhexidine allergic patients with and without accidental re‐exposure. Clin Exp Allergy. 2016;46:1090–1098. doi: 10.1111/cea.12743. [DOI] [PubMed] [Google Scholar]
- 171.Soetens F., Rose M., Fisher M. Timing of skin testing after a suspected anaphylactic reaction during anaesthesia. Acta Anaesthesiol Scand. 2012;56:1042–1046. doi: 10.1111/j.1399-6576.2011.02643.x. [DOI] [PubMed] [Google Scholar]
- 172.Mayorga C., Ebo D.G., Lang D.M., et al. Controversies in drug allergy: In vitro testing. J Allergy Clin Immunol. 2019;143:56–65. doi: 10.1016/j.jaci.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 173.Decuyper I.I., Mangodt E.A., Van Gasse A.L., et al. In vitro diagnosis of immediate drug hypersensitivity anno 2017: potentials and limitations. Drugs R D. 2017;17:265–278. doi: 10.1007/s40268-017-0176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Takazawa T., Sabato V., Ebo D.G. In vitro diagnostic tests for perioperative hypersensitivity, a narrative review: potential, limitations, and perspectives. Br J Anaesth. 2019;123:e117–e125. doi: 10.1016/j.bja.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 175.Ebo D.G., Faber M., Elst J., et al. In vitro diagnosis of immediate drug hypersensitivity during anesthesia: a review of the literature. J Allergy Clin Immunol Pract. 2018;6:1176–1184. doi: 10.1016/j.jaip.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 176.Aun M.V., Malaman M.F., Felix M.M.R., et al. In vivo tests in hypersensitivity drug reactions - Part I: skin tests. Arq Asma Alerg Imunol. 2018;2:390–398. [Google Scholar]
- 177.Aun M.V., Malaman M.F., Felix M.M.R., et al. In vivo tests in hypersensitivity drug reactions - Part II: provocation tests. Arq Asma Alerg Imunol. 2019;3:7–12. [Google Scholar]
- 178.Sá AB, Garro LS, Fernandes FR, et al. Recomendações para o diagnóstico de alergia ao látex. Rev Bras Alerg Imunopatol. 2012;35:183–189. [Google Scholar]
- 179.Brockow K., Garvey L.H., Aberer W., et al. Skin test concentrations for systemically administered drugs – an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68:702–712. doi: 10.1111/all.12142. [DOI] [PubMed] [Google Scholar]
- 180.Aberer W., Bircher A., Romano A., et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58:854–863. doi: 10.1034/j.1398-9995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 181.Kroigaard M., Garvey L.H., Menné T., Husum B. Allergic reactions in anaesthesia: are suspected causes confirmed on subsequent testing? Br J Anaesth. 2005;95 doi: 10.1093/bja/aei198. 486-71. [DOI] [PubMed] [Google Scholar]
- 182.Aun M.V., Kalil J., Giavina-Bianchi P. Drug-induced anaphylaxis. Immunol Allergy Clin N Am. 2017;37:629–641. doi: 10.1016/j.iac.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 183.Garvey L.H., Ebo D.G., Krøigaard M., et al. The use of drug provocation testing in the investigation of suspected immediate perioperative allergic reactions: current status. Br J Anaesth. 2019;123:e126–e134. doi: 10.1016/j.bja.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 184.Melchiors B.L.B., Kroigaard M., Garvey L.H. Intravenous provocation with Neuromuscular Blocking Agents in the investigation of perioperative anaphylaxis – preliminary findings from the Danish Anaesthesia Allergy Centre (DAAC) Eur J Anaesthesiol. 2018;35:318. [Google Scholar]
- 185.van Cuilenborg V.R., Hermanides J., Bos E.M.E., Hollmann M.W., Kooij F.O., Terreehorst I. Awake intravenous provocation with small doses of neuromuscular blocking agent in patients with suspected allergy: experiences from the Dutch Perioperative Allergy Centre. Br J Anaesth. 2019;123:e153–e155. doi: 10.1016/j.bja.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 186.Laxenaire MC. Management of the allergic patient. Ann Fr Anesth Reanim. 2002;21:93–96. [PubMed] [Google Scholar]
- 187.Fischer MM, Doig GS. Prevention of anaphylactic reactions to anesthetic drugs. Drug Saf. 2004;27:393–410. doi: 10.2165/00002018-200427060-00004. [DOI] [PubMed] [Google Scholar]
- 188.Simmons F.E.R., Ebisawa M., Sanchez-Borges M., et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Org J. 2015;8:32. doi: 10.1186/s40413-015-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guyer A.C., Saff R.R., Conroy M., et al. Comprehensive allergy evaluation is useful in the subsequent care of patients with drug hypersensitivity reactions during anesthesia. J Allergy Clin Immunol Pract. 2015;3:94–100. doi: 10.1016/j.jaip.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 190.Miller J., Clough S.B., Pollard R.C., Misbah S.A. Outcome of repeat anaesthesia after investigation for perioperative anaphylaxis. Br J Anaesth. 2018;120:1195–1201. doi: 10.1016/j.bja.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 191.Miller J, Misbah SA. Mast Cells, Mastocytosis, and Related Disorders. N Engl J Med. 2015;373:1884. doi: 10.1056/NEJMc1510021. [DOI] [PubMed] [Google Scholar]
- 192.Szema A., Paz G., Merriam L., Stellaccio F., Jen J. Modern preoperative and intraoperative management of hereditary angioedema. Allergy Asthma Proc. 2009;30:338–342. doi: 10.2500/aap.2009.30.3225. [DOI] [PubMed] [Google Scholar]
- 193.Levy J., Freiberger D., Roback J. Hereditary angioedema: current and emerging treatment options. Anesth Analg. 2010;110:1271–1280. doi: 10.1213/ANE.0b013e3181d7ac98. [DOI] [PubMed] [Google Scholar]
- 194.Aygoren-Pursun E., Martinez-Saguer I., Kreuz W., Klingebiel T., Schwabe D. Risk of angioedema following invasive or surgical procedures in HAE type I and II - the natural history. Allergy. 2013;68:1034–1039. doi: 10.1111/all.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cicardi M., Bork K., Caballero T., et al. Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2012;67:147–157. doi: 10.1111/j.1398-9995.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 196.Farkas H., Zotter Z., Csuka D., et al. Short-term prophylaxis in hereditary angioedema due to deficiency of the C1-inhibitor - a long-term survey. Allergy. 2012;67:1586–1593. doi: 10.1111/all.12032. [DOI] [PubMed] [Google Scholar]
- 197.Galan H.L., Reedy M.B., Starr J., Knight A.B. Fresh frozen plasma prophylaxis for hereditary angioedema during pregnancy. A case report. J Reprod Med. 1996;41:541–544. [PubMed] [Google Scholar]
- 198.Birjmohun R.S., Kees Hovingh G., Stroes E.S.G., et al. Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: two clinical trials in healthy volunteers and patients with hereditary angioedema. CLITHE. 2008;30:2314–2323. doi: 10.1016/j.clinthera.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 199.Bouillet L., Ponard D., Drouet C., et al. Acquired angioneurotic edema. Clinical and biological characteristics in 9 patients. Presse Med. 2000;29:640–644. [PubMed] [Google Scholar]
- 200.Cicardi M., Zingale L.C., Pappalardo E., Folcioni A., Agostoni A. Autoantobodies and lymphoproliferative diseases in acquired C1-inhibitor deficiencies. Medicine (Baltimore). 2003;82:274–281. doi: 10.1097/01.md.0000085055.63483.09. [DOI] [PubMed] [Google Scholar]
- 201.Giavina-Bianchi P., Arruda L.K., Aun M.V., et al. Diretrizes brasileiras para o diagnóstico e tratamento do angioedema hereditário – 2017. Arq Asma Alerg Imunol. 2017;1:23–48. [Google Scholar]
- 202.Valent P., Horny H.P., Escribano L., et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 203.Brockow K., Jofer C., Behrendt H., Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–232. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 204.Bonadonna P., Pagani M., Aberer W., et al. Drug hypersensitivity in clonal mast cell disorders: ENDA/EAACI position paper. Allergy. 2015;70:755–763. doi: 10.1111/all.12617. [DOI] [PubMed] [Google Scholar]
- 205.Ahmad N., Evans P., Lloyd-Thomas AR. Anesthesia in children with mastocytosis – a case based review. Pediatr Anesth. 2009;19:97–107. doi: 10.1111/j.1460-9592.2008.02904.x. [DOI] [PubMed] [Google Scholar]
- 206.Dewachter P., Castells M.C., Hepner D.L., Mouton-Faivre C. Perioperative management of patients with mastocytosis. Anesthesiology. 2014;120:753–759. doi: 10.1097/ALN.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 207.Escribano L, de Gonzalez Olano D, de La Hoz Caballer B, Esteban Lopez I, Sanchez Fernandez I. In: Tratado de Alergologıa. 1 ed. Pelaez Hernandez A, Davila-Gonzalez IJ, editors. Ergon; Madrid: 2007. Mastocitosis: guıas para su diagnostico y tratamiento; pp. 1241–1262. [Google Scholar]
- 208.Hermans M.A.W., Arends N.J.T., Gerth van Wijk R, et al. Management around invasive procedures in mastocytosis: An update. Ann Allergy Asthma Immunol. 2017;119:304–309. doi: 10.1016/j.anai.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 209.Ciach K., Niedoszytko M., Abacjew-Chmylko A., et al. Pregnancy and delivery in patients with mastocytosis treated at the polish center of the European Competence Network on Mastocytosis (ECNM) PLoS One. 2016;11:e0146924. doi: 10.1371/journal.pone.0146924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Carter M.C., Uzzaman A., Scott L.M., Metcalfe D.D., Quezado Z. Pediatric mastocytosis: routine anesthetic management for a complex disease. Anesth Analg. 2008;107:422–427. doi: 10.1213/ane.0b013e31817e6d7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sandler S.G., Eder A.F., Goldman M., Winters J.L. The entity of immunoglobulin A-related anaphylactic transfusion reactions is not evidence based. Transfusion. 2015;55:199–204. doi: 10.1111/trf.12796. [DOI] [PubMed] [Google Scholar]