Abstract
The polyhydroxyalkanoic acid (PHA) granule-associated 16-kDa protein (GA16 protein) of Paracoccus denitrificans was identified, and its corresponding gene was cloned and analyzed at the molecular level. The N-terminal amino acid sequence of GA16 protein revealed that its structural gene is located downstream from the PHA synthase gene (phaCPd) cloned recently (S. Ueda, T. Yabutani, A. Maehara, and T. Yamane, J. Bacteriol. 178:774–779, 1996). Gene walking around phaCPd revealed two new open reading frames (ORFs) possibly related to PHA synthesis, one of which was the phaPPd gene, encoding GA16 protein, and the other was the phaRPd gene, encoding a protein that is putatively involved in the regulation of the expression of phaPPd. Overproduction of PhaPPd was observed in Escherichia coli carrying phaPPd, but the overproduction was not observed in the presence of phaRPd. Coexpression of phaPPd and PHA biosynthesis genes in E. coli caused increases in both the number of poly-(3-hydroxybutyric acid) (PHB) granules and PHB content and caused decreases in both the size of the granules and the molecular weight of PHB. GA16 protein was considered a phasin protein. The phaRPd gene had significant similarities to stdC, a possible transcriptional factor of Comamonas testosteroni, as well as to other ORFs of unknown function previously found in other PHA-synthetic bacteria.
Polyhydroxyalkanoic acids (PHA) are synthesized by many bacteria and function as intracellular reserves of carbon and energy (1). The polyesters exist in cells as granules coated with proteins and lipids (7). They have received great attention for the production of biodegradable plastic materials (12). The pathways for the biosynthesis of PHA have been studied in many bacteria, and the genes of biosynthetic enzymes have also been investigated (25). The biosynthetic pathway of poly-(3-hydroxybutyric acid) [P(3HB) or PHB] has been best studied in Ralstonia eutropha (formerly designated Alcaligenes eutrophus [33]) (29). The first step is catalysis by a β-ketothiolase (EC 2.3.1.9), which condenses acetyl coenzyme A (acetyl-CoA) to acetoacetyl-CoA. Acetoacetyl-CoA is then reduced to d-(−)-3-hydroxybutyryl-CoA by an acetoacetyl-CoA reductase (EC 1.1.1.36). The last step is catalysis by a PHA synthase, which polymerizes d-(−)-3-hydroxybutyryl-CoA to P(3HB). The genes responsible for PHA synthesis have been designated phaA, phaB, and phaC for β-ketothiolase, acetoacetyl-CoA reductase, and PHA synthase genes, respectively.
Recently, we have cloned and analyzed these three PHA synthesis genes in a facultative methylotrophic bacterium, Paracoccus denitrificans (31, 32), which was able to synthesize the P(3HB), poly(3-hydroxyvaleric acid), and poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) copolyesters from methanol, n-pentanol, and a mixture of both substrates, respectively (34, 35). phaA and phaB formed an operon, while phaC was located at a remote locus on the chromosomal DNA. Gene dosage effects for PHA synthesis were investigated in recombinants of P. denitrificans whose level of expression of each PHA-synthetic enzyme was increased. Among these recombinants, a self-cloning recombinant of phaC showed the highest PHA content and the highest rate of PHA accumulation (16). In the recombinant-enhancing phaC, another protein of about 16 kDa increased. The production of the protein correlated closely with PHA content in the strain. The amount of this protein was found to be proportional to the content of PHA even in the wild-type strain. We expected that the protein was a kind of granule-associated protein, like the GA24 protein of R. eutropha (36), the GA14 protein of Rhodococcus ruber (17, 18), the GA13 protein of Acinetobacter spp. (21), and the GA14 protein of Chromatium vinosum (15). These proteins are called phasins, a new class of protein that forms a layer at the surface of a PHA granule, and they have been shown to influence the size and number of PHA granules (9, 18, 36). In addition, production of phasins is suggested to depend on the presence of an intact PHA biosynthesis apparatus and to be regulated by unknown mechanisms (36).
In this study, we report the identification of a new granule-associated 16-kDa protein from PHA granules of P. denitrificans and the cloning, sequencing, and characterization of its gene (named phaPPd). In addition, we will discuss a possible function of another open reading frame (ORF) (named phaRPd), located downstream from phaPPd, as its gene regulator.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains used in this study were P. denitrificans (ATCC 17741) and Escherichia coli XL1-Blue (3). The plasmids used in this study are listed in Table 1. Inorganic salt medium containing 1% (vol/vol) methanol or 0.1% (vol/vol) n-pentanol (29, 30) was used for growing P. denitrificans. The strain was grown aerobically at 30°C. E. coli was grown at 37°C in Luria-Bertani (LB) medium (19). When needed, kanamycin (50 μg/ml) and/or ampicillin (100 μg/ml) was added to the medium.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant description | Source or reference |
|---|---|---|
| pBluescript II SK(+) | 3.0 kb; Apr; lacPOZ′; cloning vector | Stratagene |
| pTV119N | 3.2 kb; Apr; lacPOZ′; cloning vector | Takara |
| pPDES3.6 | 6.6 kb; pBluescript II SK(+) carrying 3.6-kb fragment containing phaCPd | 31 |
| pPDH6.5 | 9.5 kb; pBluescript II SK(+) carrying 6.5-kb fragment containing phaABPd | 32 |
| pPDPP7.2 | 10.2 kb; pBluescript II SK(+) carrying 7.2-kb fragment containing pha′CPRPd | This study |
| pPDPP2.6 | 5.6 kb; pBluescript II SK(+) carrying 2.6-kb fragment containing ORF1 | This study |
| pPDPA4.5 | 7.4 kb; pBluescript II SK(+) carrying 4.5-kb fragment containing pha′CPRPd | This study |
| pPDPX2.2 | 5.1 kb; pBluescript II SK(+) carrying 2.1-kb fragment containing pha′CPRPd | This study |
| pPDPK1.7 | 4.6 kb; pBluescript II SK(+) carrying 1.7-kb fragment containing pha′CPR′Pd | This study |
| pTVC | 5.2 kb; pTV119N carrying 2.0-kb fragment containing phaCPd | This study |
| pTVCP | 5.7 kb; pTV119N carrying 2.6-kb fragment containing phaCPPd | This study |
| pTVCP4 | 6.4 kb; pTV119N carrying 3.3-kb fragment containing phaCPRPd | This study |
| pBBRKm | 5.8 kb; Kmr Tra+ Mob+; broad-host-range cloning vector from pBBR1MCS (10); not belonging to InqP, InqQ, or InqW groups | 31 |
| pBBRKmAB | 7.3 kb; pBBRKm derivative containing phaABPd | This study |
Isolation of native PHA granules.
PHA granules of P. denitrificans were obtained from cells which were cultivated in a 5-liter fermentor in inorganic salt medium maintained at 0.02% n-pentanol. After 64 h of cultivation, the cells were harvested by centrifugation (8,000 × g; 15 min; 4°C), washed, resuspended in 3 volumes of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0), and then disrupted by sonication (150 W; 1 h). After centrifugation (10,000 × g; 15 min; 4°C), the supernatant and loosely packed material were removed and the pellet of PHA granules was resuspended in TE buffer. The granules were centrifuged again (10,000 × g; 15 min; 4°C), resuspended in TE buffer, and stored at −20°C.
PHB granules of E. coli were obtained from cells which were cultivated in LB medium containing 2% sodium lactate as an excess carbon source. After 30 h of cultivation, the cells were harvested by centrifugation (8,000 × g; 5 min; 4°C), washed and resuspended in 3 volumes of TE buffer, and disrupted by sonication (120 W; 20 min). The subsequent procedure was the same as described above.
DNA manipulation.
The total genomic DNA of P. denitrificans was isolated according to the procedure of Wilson (38). Plasmid DNA isolation, agarose gel electrophoresis, and transformation of E. coli were carried out as described by Sambrook et al. (19). All DNA-manipulating enzymes were used as recommended by the manufacturers.
Southern or dot blot hybridization analysis.
Hybridization was carried out as described by Southern (23). DNA fragments were transferred from agarose gels or from bacterial colonies to nylon membranes after alkali denaturation. Preparation of a digoxigenin-labeled probe and its visualization on the membrane were carried out with a digoxigenin nucleic acid labeling and detection kit (Boehringer Mannheim Biochemicals, Indianapolis, Ind.).
Cloning of phaPPd and other genes.
The gene phaPPd, encoding GA16 protein, was cloned by genome walking with a 1.2-kb HindIII-HindIII fragment (HH12) of phaCPd as a probe. Hybridization analysis gave only one positive signal for each of the KpnI-, XhoI-, SalI-, HindIII-, EcoRI-, and BamHI-digested genomic DNAs of P. denitrificans and gave two positive signals at sizes of 0.7 and 7.2 kb for the PstI-digested DNA. The PstI-digested fragments, around 7.2 kb in size, were recovered from agarose gels and cloned into pBluescript II SK(+). A colony hybridization against E. coli transformants with the probe revealed the presence of one positive clone, which contained a 7.2-kb fragment of P. denitrificans DNA, referred to as PP72.
In order to clone an upstream region of phaCPd, a 1.5-kb SacI-HindIII fragment (SH15) was used as a genome-walking probe. Hybridization analysis gave only one positive signal for each XhoI-, SalI-, HindIII-, EcoRI-, BamHI-, and PstI-digested genomic DNA. For the PstI-digested DNA, a 2.6-kb fragment was hybridized with the probe and cloned into pBluescript II SK(+). The resulting plasmid, pPDPP2.6, contained a 2.6-kb insert of P. denitrificans DNA, referred to as PP26.
Nucleotide sequence analysis.
The DNA fragments to be sequenced were subcloned into pBluescript II SK(+), which was used for making serial deletions. DNA sequencing was carried out with a model 373A automatic DNA sequencer and a dye terminator cycle-sequencing FS ready-reaction kit (Perkin-Elmer, Applied Biosystems Division, Foster City, Calif.). The nucleotide sequence was analyzed with genetic information-processing software (SDC-GENETYX; Software Development Co., Tokyo, Japan).
Electrophoresis of proteins.
Proteins were separated by sodium dodecyl sulfate (SDS)–12.5% polyacrylamide gel electrophoresis (PAGE) and stained with Coomassie brilliant blue (CBB) R-250 as described by Laemmli (11).
N-terminal amino acid sequence analysis.
PHA granule-associated proteins were separated on an SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. After proteins on the membrane were stained with CBB R-250, those protein bands were cut out and subjected to sequence analysis with an Applied Biosystems 477A automatic amino acid sequencer.
Molecular mass determination of protein.
The molecular mass of GA16 protein was determined by electrospray ionization mass spectrometry (ESI-MS) with a VG Platform II (Fisons Instruments, Altrincham, United Kingdom) with horse heart myoglobin as a calibration standard.
Construction of plasmids.
The plasmids constructed are listed in Table 1, and the structures of plasmids for expression experiments in E. coli are shown (see Fig. 2e). Plasmid pPDPA4.5 was constructed from pBluescript II SK(+) and a 4.5-kb PstI-ApaI fragment of pPDPP7.2. Plasmid pPDPX2.2 was constructed by deleting the 2.3-kb XhoI-XhoI region from pPDPA4.5. Plasmid pPDPK1.7 was then obtained by deleting the 0.5-kb KpnI-ApaI fragment from pPDPX2.2.
FIG. 2.
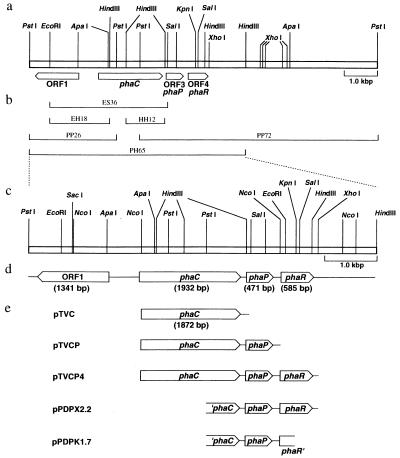
Molecular organization of P. denitrificans PHA biosynthesis genes and restriction endonuclease sites. (a) Restriction map of the chromosomal region surrounding the phaC locus; (b) subfragments already cloned, used as probes, obtained in this study, and registered in DDBJ; (c) restriction map of PH65 region in detail; (d) organization of ORF1, phaCPd, ORF3 (phaPPd), and ORF4 (phaRPd); and (e) structures of plasmids used for expression of phaCPd, phaPPd, and phaRPd in E. coli.
Plasmid pTVC was constructed by the ligation of an NcoI-SalI fragment from plasmid pPDES3.6 (31) into the NcoI and SalI sites of pTV119N. Plasmid pTVCP was constructed as follows. Plasmid pPDPX2.2 was digested with NcoI, filled in with Klenow fragment, and digested with PstI. The 1.4-kb fragment, containing a part of phaC and the full length of phaP, was recovered and ligated into pTVC digested with PstI and SmaI. The resulting plasmid was digested with PstI and ligated to a 0.7-kb fragment obtained from pTVC digested with PstI to make pTVCP. Plasmid pTVCP4 was constructed as follows. Plasmid pPDPX2.2 was digested with XhoI, filled in with Klenow fragment, and digested with PstI. The 2.1-kb fragment, containing a part of phaC and the full length of phaP and of phaR, was recovered and ligated into pTVC digested with PstI and SmaI. The resulting plasmid was digested with PstI and ligated to a 0.7-kb fragment obtained from pTVC digested with PstI to yield plasmid pTVCP4. These plasmids, pTVC, pTVCP, and pTVCP4, contain phaCPd, phaCPPd, and phaCPRPd, respectively, and allow expression of phaCPd under the lac promoter from pTV119N, whereas phaPPd and phaRPd are probably expressed from their own promoters. However, the possibility remains that, in addition to phaCPd, phaPPd and phaRPd are expressed through the lac promoter.
To construct a plasmid for expression of phaABPd, ScaI and HindIII sites were first introduced into the upstream region of phaAPd and the downstream region of phaBPd, respectively, by PCR mutagenesis. The 2,069-bp fragment was amplified with primers USA-ScaI (5′-CGTCAGTACTAAAGCCGTAATCGTTTCCGC-3′) and DSB-HindIII (5′-GGGCAAGCTTCCCAGGACCATGGAATGAGA-3′) (the underlined sequences indicate the restriction sites). The PCR product was purified, digested with ScaI and HindIII, and subcloned into pTV119N at the HindIII site and the blunted NcoI site. The resulting plasmid was digested with PvuII and XbaI, and then the 2.2-kb PvuII-XbaI fragment containing phaABPd with the lac promoter was ligated into pBBRKm digested with EcoRV and XbaI to make pBBRKmAB. pBBRKm is a broad-host-range vector compatible with ColE1 derivatives. Therefore, it was possible that both plasmids, a pTV119N derivative and a pBBRKm derivative, were maintained in the same E. coli cell.
Preparation of antibodies.
The granule-associated proteins were extracted from native PHA granules with SDS-PAGE sample buffer (50 mM Tris-HCl, pH 6.5, 2% SDS, 10% glycerol, 0.002% bromophenol blue). After SDS-PAGE of the extract, a protein band corresponding to the GA16 protein was excised from the gel. The gel piece was crushed, mixed with Freund’s complete adjuvant, and injected into a rabbit. After 2 weeks, the antigen was injected into the same rabbit with Freund’s incomplete adjuvant. After 2 weeks, the immune serum was prepared from the rabbit.
Antibodies raised against PHA synthase, β-ketothiolase, and acetoacetyl-CoA reductase were prepared as described previously (16, 31).
Western blot analysis.
Western blotting was done as described by Burnette (4) with cellulose nitrate membrane. In immunoblot analysis, peroxidase-conjugated anti-rabbit immunoglobulin G (Bio-Rad Laboratories, Richmond, Calif.) was used as the secondary antibody. The blot was developed with 4-chloro-1-naphthol (19).
PHA analysis.
PHA in the cells was analyzed by gas chromatography as previously described (37). The molecular mass of PHA was determined by gel permeation chromatography as previously described (16). Polystyrene standards with a low polydispersity were used to make a calibration curve.
Electron microscopic studies.
Cells were prefixed in the presence of 2% (vol/vol) glutaraldehyde in 50 mM sodium phosphate buffer (pH 7.2) and fixed by 1% osmium tetroxide. After dehydration with a graded ethanol series, the cells were embedded in Spurr’s low-viscosity resin (24). Ultrathin sections were mounted on copper grids treated with neoprene, stained with uranyl acetate and lead citrate, and examined with an electron microscope (model JEM-1210; Jeol Co., Tokyo, Japan).
Nucleotide sequence accession number.
The nucleotide sequence data reported here will appear in the EMBL, GenBank, and DDBJ nucleotide sequence databases with accession no. AB017045.
RESULTS
Identification of granule-associated proteins from PHA granules purified from P. denitrificans.
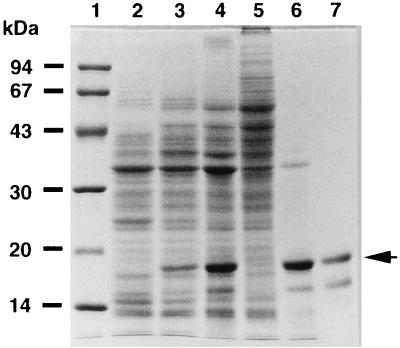
PHA granule-associated proteins in P. denitrificans were analyzed by SDS-PAGE as shown in Fig. 1. The sizes of the proteins extracted from native PHA granules were 17 kDa as a major protein and 15 and 31 kDa as minor proteins on the gel (Fig. 1, lane 6). PHA synthase (70.1 kDa) could not be seen clearly in this SDS-PAGE gel (Fig. 1, lane 6). The N-terminal amino acid sequences of these proteins were determined by automated Edman degradation. The sequence of the 17-kDa protein was AKTPDFTKTMQEVMAKFPVD. Exactly the same sequence was obtained from the 15-kDa protein; thus, the 15-kDa protein was considered to be a proteolytic product possibly digested at its C terminus. The N-terminal sequence of the 31-kDa protein was DVTISGYGRTGVIYYE. This sequence was identified as the N terminus of a porin protein of P. denitrificans (20) by database search. This is possibly due to contamination of the PHA granule fraction by membrane fraction, because most of the membrane was partitioned into a loosely packed fraction by centrifugation of the cell lysate (data not shown).
FIG. 1.
SDS-PAGE analysis of the total cellular and PHA granule-associated proteins from P. denitrificans cells cultivated at 30°C. Lanes: 1, molecular mass standard proteins with the masses indicated on the left (from top to bottom, phosphorylase b, bovine serum albumin, ovalbumin, carbonic anhydrase, soybean trypsin inhibitor, and α-lactalbumin); 2, total cellular proteins after 24 h of cultivation in inorganic salt medium with 1% (vol/vol) methanol; 3, total cellular proteins after 24 h of cultivation in inorganic salt medium with 0.1% n-pentanol; 4, total cellular proteins after 64 h of cultivation in inorganic salt medium maintained at 0.02% n-pentanol; 5, soluble-protein fraction of P. denitrificans after 64 h of cultivation with n-pentanol; 6, granule-associated proteins from P. denitrificans; 7, partially purified GA16 by reversed-phase high-performance liquid chromatography with C18 column. An arrow indicates the intact GA16 protein.
The 17-kDa protein electrophoresed on the gel was partially purified by reversed-phase high-performance liquid chromatography with a C18 column (Fig. 1, lane 7). The molecular mass of the 17-kDa protein excised from the SDS-PAGE gel was then determined by ESI-MS to be 16,377.08 ± 4.85 Da. The 17-kDa protein is referred to as GA16 protein hereafter.
Interestingly, the production of GA16 protein was closely related to the PHA content in P. denitrificans (Fig. 1, lanes 2 to 4). When the cells were cultivated for 24 h in inorganic salt medium with 1% (vol/vol) methanol, the production of GA16 protein was very low and the PHB content of the cells was only 0.5%. However, when the cells were cultivated with 0.1% n-pentanol instead of methanol, the PHA content reached 24% and the amount of GA16 protein increased. The cells cultivated for 64 h in inorganic salt medium maintained at 0.02% n-pentanol accumulated PHA to 55%, and the amount of GA16 protein increased proportionally. Accordingly, PHA granules were prepared from P. denitrificans cultivated for 64 h in inorganic salt medium maintained at 0.02% n-pentanol, and the proteins binding to PHA granules were analyzed by SDS-PAGE (Fig. 1, lane 6).
Gene walking around phaC locus.
When the N terminus of GA16 protein was identified, its corresponding gene was found to be located in the downstream region of phaCPd that had already been cloned (31). Since only a part of the gene had been cloned, upstream and downstream regions of phaCPd were cloned by genome walking as described in Materials and Methods. As a result, a 2.6-kb PstI-PstI fragment (PP26) and a 7.2-kb PstI-PstI fragment (PP72) were obtained as the upstream and downstream regions, respectively, of phaCPd. Fig. 2a and c show the restriction map of the chromosomal region surrounding the phaC locus.
The nucleotide sequence of the 6.5-kb PstI-HindIII region (PH65) shown in Fig. 2 was determined. Four ORFs were identified in the PH65 region by computer analysis, as shown in Fig. 2d. One is phaCPd, a PHA synthase gene previously described (31). We discovered mistakes in the previously published sequence of the phaCPd gene and submitted the correct sequence to the databases. The corrected phaCPd sequence consisted of 1,932 bp, and the molecular mass of the PHA synthase deduced from the DNA sequence was 70.1 kDa.
ORF3 (471 bp), which is located downstream from phaCPd, encodes the GA16 protein, composed of 157 amino acids. Since the N-terminal methionine of the ORF3 product is apparently removed by processing, the molecular mass of this protein (156 amino acids) deduced from the DNA sequence, 16,372.38 Da, agreed well with the molecular mass determined by ESI-MS (16,377.08 ± 4.85 Da). The deduced amino acid sequence of ORF3 was similar to that of an ORF which is located downstream of phaC of Rhodobacter sphaeroides (67.2% identity; 89.8% similarity) (8), although its function is unknown. Because of its granule-associated property and the effect of the gene on PHB biosynthesis in E. coli, as described in the following section, the product of ORF3 was thought to be a phasin of P. denitrificans, and hence, we named its corresponding gene phaPPd. ORF4 (585 bp), which is located downstream of phaPPd, encodes a protein composed of 195 amino acids with a molecular mass of 21.9 kDa. A database search revealed that the ORF4 product is homologous not only with four predicted proteins of unknown function located in the PHA biosynthesis locus of Rhizobium meliloti (33.9% identity; 52.7% similarity) (28), R. eutropha (26.8% identity; 47.0% similarity) (22), C. vinosum (30.1% identity; 49.1% similarity) (13), and Thiocystis violacea (34.8% identity; 54.4% similarity) (14) but also with a protein encoded by stdC, a steroid-inducible gene of Comamonas testosteroni (30.9% identity; 48.6% similarity) (5). Figure 3 shows a multiple alignment of these sequences. ORF4 was named phaRPd, and a detailed explanation is given in the Discussion. In the region upstream of phaCPd, ORF1 (1,341 bp) was oriented in the opposite direction to the other three genes and encoded a protein composed of 447 amino acids with a molecular mass of 49.3 kDa. The deduced amino acid sequence of ORF1 was very similar to that of an ORF which is located upstream of phaC of R. sphaeroides (74.0% identity; 84.3% similarity) (8).
FIG. 3.
Multiple alignment of amino acid sequence of predicted ORF4 (phaR) protein of P. denitrificans with homologs in other bacteria. The asterisks indicate identical residues. The dots indicate similar residues. Shown are products of phaR of P. denitrificans (ORF4Pd) (this study), ORF1 of R. meliloti (ORF1Rm) (28), ORF1 of R. eutropha (ORF1Re) (22), stdC of C. testosteroni (StdCCt) (5), ORF4 of C. vinosum (ORF4Cv) (13), and ORF4 of T. violacea (ORF4Tv) (14). Highly conserved regions (boxes 1 and 2) and a potential helix-turn-helix (HTH) motif structure are boxed.
Effects of phaPPd and phaRPd on PHB biosynthesis in E. coli.
In order to investigate the roles of phaPPd and phaRPd, several plasmids were constructed to express the genes in E. coli, as described in Materials and Methods. Figure 2e shows the structures of these plasmids, pTVC, pTVCP, and pTVCP4, containing phaCPd, phaCPPd, and phaCPRPd, respectively.
The plasmids were retransformed into recombinant E. coli XL1-Blue, which also harbored pBBRKmAB carrying phaABPd. Since these two kinds of plasmids were compatible in E. coli cells, PHA-synthetic genes were maintained in one cell in trans. These recombinant strains were cultivated at 37°C in LB medium containing 2% sodium lactate as an excess carbon source to avoid catabolite repression. After 30 h, the cells were harvested and analyzed. The PHB contents of recombinant E. coli strains containing phaABCPd, phaABCPPd, and phaABCPRPd were 26, 43, and 44%, respectively, as shown in Table 2. The molecular weights of PHB in these strains are also shown in Table 2. The increase in the content of PHB and the decrease in its molecular weight were caused by the presence of the phaPPd or phaPRPd gene.
TABLE 2.
PHB synthesis in recombinant strains of E. coli XL1-Bluea
| Plasmid (relevant markers) | Expression of phaPPd | Size of PHB granule (nm) | PHB content (% of wt) | Mn | Mw | Mw/Mn |
|---|---|---|---|---|---|---|
| pTV119N and pBBRKmAB (phaABPd) | − | 0.28 | NT | NT | NT | |
| pTVC and pBBRKmAB (phaCPd and phaABPd) | − | 70–310 | 26 | 8.22 × 105 | 3.12 × 106 | 3.79 |
| pTVCP and pBBRKmAB (phaCPPd and phaABPd) | +++ | 20–60 | 43 | 5.45 × 105 | 1.37 × 106 | 2.51 |
| pTVCP4 and pBBRKmAB (phaCPRPd and phaABPd) | + | 40–200 | 44 | 3.98 × 105 | 1.19 × 106 | 2.98 |
Cells were cultivated in 300 ml of LB medium containing sodium lactate (2% [vol/vol]) as an excess carbon source for 30 h at 37°C. +, expression level of phaPPd; −, not expressed; NT, not tested; Mn, number-average molecular weight of PHB; Mw, weight-average molecular weight of PHB.
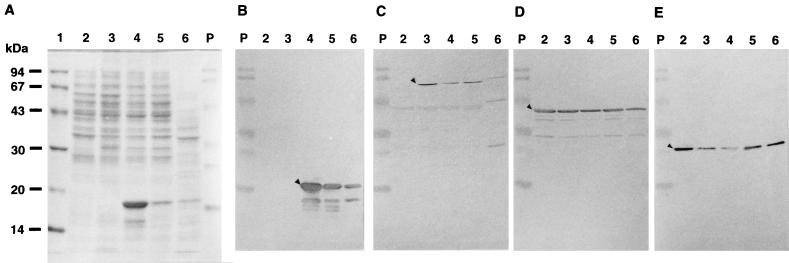
Effects of phaPPd and phaRPd on the amounts of PHA-synthetic enzymes and GA16 protein in E. coli were analyzed by SDS-PAGE and Western blotting with antisera against β-ketothiolase (PhaA), acetoacetyl-CoA reductase (PhaB), PHA synthase (PhaC), and GA16 protein (PhaP), as shown in Fig. 4. Overproduction of PhaP was observed in the strain carrying pTVCP, and the presence of phaRPd in the pTVCP4 strain dramatically decreased the production of PhaP. The data presented in Fig. 4 suggest that phaRPd may also cause a decrease in PhaC accumulation and an increase in PhaB accumulation.
FIG. 4.
Analysis of PHA-synthetic enzymes and granule-associated GA16 protein in recombinants of E. coli and P. denitrificans. E. coli cells were cultivated at 37°C in LB medium with ampicillin and kanamycin. (A) Total cellular proteins (20 μg per lane) were subjected to electrophoresis in an SDS–12.5% polyacrylamide gel and stained with CBB. Western blot analyses with anti-GA16 protein antibody (B), anti-β-galactosidase-PHA synthase fusion protein antibody (C), anti-β-ketothiolase antibody (D), and anti-acetoacetyl-CoA reductase antibody (E) are shown. Total cell proteins used for Western blot analysis with anti-GA16 protein antibody, anti-β-galactosidase-PHA synthase fusion protein antibody, anti-β-ketothiolase antibody, and anti-acetoacetyl-CoA reductase antibody were 4, 20, 10, and 10 μg per lane, respectively. The triangles in panels B, C, D, and E indicate GA16 protein, PHA synthase, β-ketothiolase, and acetoacetyl-CoA reductase, respectively. Lanes: 1, molecular mass standard proteins (same as for Fig. 1); 2, total cellular proteins of E. coli XL1-Blue (pBBRKmAB plus pTV119N); 3, total cellular proteins of E. coli XL1-Blue (pBBRKmAB plus pTVC); 4, total cellular proteins of E. coli XL1-Blue (pBBRKmAB plus pTVCP); 5, total cellular proteins of E. coli XL1-Blue (pBBRKmAB plus pTVCP4); 6, total cellular proteins of P. denitrificans; P, prestained SDS-PAGE standards (from top to bottom, phosphorylase b, bovine serum albumin, ovalbumin, carbonic anhydrase, soybean trypsin inhibitor, and lysozyme; mobilities are not exact).
PHB granules in recombinants of E. coli.
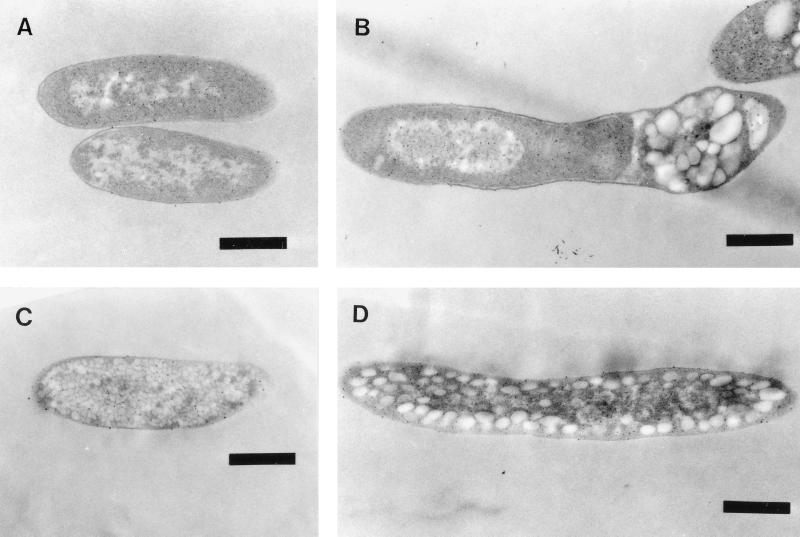
The effects of phaPPd and phaRPd on the size and number of PHB granules synthesized in the recombinants of E. coli XL1-Blue were investigated by using a transmission electron microscope. No PHB granules were found in the cells carrying pBBRKmAB and pTV119N (Fig. 5A) because the strain did not contain phaC. Somewhat large PHB granules (with diameters of approximately 70 to 310 nm, calculated from 25 granules shown in Fig. 5B) were formed in the strain carrying pBBRKmAB and pTVC, preferentially located in groups around the poles of the cells. The PHB granules that accumulated in the strain containing pTVCP, which produced excess GA16 protein, were considerably smaller (approximately 20 to 60 nm in diameter, calculated from more than 400 granules shown in Fig. 5C) and more numerous, and thus spread throughout the cytoplasm. The size (approximately 40 to 200 nm in diameter, calculated from about 80 granules shown in Fig. 5D) and number of PHB granules that accumulated in the strain harboring pTVCP4, which produced a relatively small amount of GA16 protein, were between those of the first two recombinant strains, and they were also distributed throughout the cytoplasm.
FIG. 5.
Electron micrographs demonstrating the sizes and localizations of PHB granules in recombinants of E. coli. The cells were cultivated at 37°C for 30 h in LB medium with sodium lactate, ampicillin, and kanamycin. (A) E. coli XL1-Blue (pBBRKmAB plus pTV119N); (B) E. coli XL1-Blue (pBBRKmAB plus pTVC); (C) E. coli XL1-Blue (pBBRKmAB plus pTVCP); (D) E. coli XL1-Blue (pBBRKmAB plus pTVCP4). Bars, 0.5 μm.
PHB granules that accumulated in the absence of phaPPd were located at the poles of the cells, while PHB granules in the presence of phaPPd were distributed throughout the cytoplasm. GA16 protein bound to the surfaces of PHB granules, and the granules gained an amphipathic layer. Therefore, the dispersion of PHB granules occurred in the strain containing phaPPd.
phaRPd caused a decrease in the accumulation of PhaPPd in E. coli.
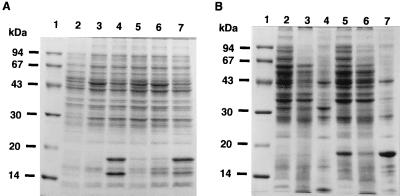
The plasmids pTV119N, pTVC, pTVCP, pTVCP4, pPDPX2.2, and pPDPK1.7 were transformed into E. coli XL1-Blue, and the recombinant strains were cultivated in LB medium. GA16 protein was overproduced in the strains harboring pTVCP or pPDPK1.7 but not in the strain harboring pTVCP4 or pPDPX2.2 carrying phaRPd (Fig. 6A). The plasmid pPDPK1.7 lacks both the 5′-terminal region of phaCPd and the 3′-terminal region of phaRPd but possesses the complete phaPPd, while pPDPX2.2 also lacks the 5′-terminal region of phaCPd but possesses the complete phaPRPd as shown in Fig. 2e.
FIG. 6.
(A) Effect of phaRPd on the expression of phaPPd in E. coli. The cells were cultivated at 37°C in LB medium with ampicillin. Total cellular proteins (20 μg per lane) were subjected to electrophoresis in SDS–12.5% polyacrylamide gels and stained with CBB. Lanes: 1, molecular mass standard proteins (same as for Fig. 1); 2, total cellular proteins of E. coli XL1-Blue (pTV119N); 3, total cellular proteins of E. coli XL1-Blue (pTVC); 4, total cellular proteins of E. coli XL1-Blue (pTVCP); 5, total cellular proteins of E. coli XL1-Blue (pTVCP4); 6, total cellular proteins of E. coli XL1-Blue (pPDPX2.2); 7, total cellular proteins of E. coli XL1-Blue (pPDPK1.7). (B) PHB granule-associated proteins in E. coli. PHB granules were isolated as described in Materials and Methods. Proteins were separated in an SDS–12.5% polyacrylamide gel and stained with CBB. Lanes: 1, molecular mass standard proteins (same as for Fig. 1); 2, total cellular proteins of E. coli XL1-Blue (pBBRKmAB plus pTVC); 3, soluble-protein fractions of E. coli XL1-Blue (pBBRKmAB plus pTVC); 4, granule-associated-protein fractions of E. coli XL1-Blue (pBBRKmAB plus pTVC); 5, total cellular proteins of E. coli XL1-Blue (pBBRKmAB plus pTVCP4); 6, soluble-protein fractions of E. coli XL1-Blue (pBBRKmAB plus pTVCP4); 7, granule-associated-protein fractions of E. coli XL1-Blue (pBBRKmAB plus pTVCP4).
Figure 6A shows that the complete phaRPd gene prevented the cell from overproducing GA16 protein. Exactly the same phenomenon was observed even in the PHA-accumulating E. coli strains carrying pBBRKmAB, as shown in Fig. 4. Therefore, it is likely that ORF4 is a regulator gene of phaPPd, and hence, we named the corresponding gene of ORF4 phaRPd.
Binding of GA16 and other proteins to the surfaces of PHB granules in E. coli.
Proteins associated with PHB granules in E. coli were analyzed by SDS-PAGE. PHB granules that were isolated from the recombinant strains carrying phaABCPd or phaABCPRPd contained a wide variety of proteins (Fig. 6B). The most striking difference in the PHB granule-binding proteins in these strains was the presence of a 32-kDa protein in the first group of strains (Fig. 6B, lane 4) and the presence of GA16 protein in the second group (Fig. 6B, lane 7). PHA synthase (69.1 kDa) could not be seen clearly in this SDS-PAGE gel (Fig. 6B, lanes 4 and 7). The N terminus of the 32-kDa protein was analyzed and found to be SIQHFRVALIPFFAAF?LPV. From comparison with a protein database, this protein was identified as β-lactamase containing the signal sequence, and the molecular mass of 32 kDa calculated by SDS-PAGE was in agreement with the molecular mass of 31,426 Da deduced from the nucleotide sequence of the β-lactamase gene. This indicates that β-lactamase binds to the surfaces of PHB granules before the protein is exported into the periplasm.
DISCUSSION
In this study we identified GA16 as a major protein associated with PHA granules in P. denitrificans. The corresponding gene, designated phaPPd, was also cloned, sequenced, and expressed in E. coli. This GA16 protein is considered a kind of phasin because (i) there is a positive correlation between the accumulation of GA16 protein in P. denitrificans and production of PHA by this strain, (ii) GA16 protein binds PHA granules in P. denitrificans and E. coli, and (iii) the increased number and decreased size of PHA granules correlate with increased levels of GA16 protein in E. coli.
Phasins have been proposed to function as amphiphilic proteins in the interface between the hydrophilic cytoplasm and the hydrophobic core of the PHA inclusion (26). Several phasins of R. eutropha (36), R. ruber (17, 18), Acinetobacter spp. (21), and C. vinosum (15) have been reported, and some candidates for phasins are found in DDBJ, for example, in R. sphaeroides (8) and Aeromonas caviae (6). When homological comparison between GA16 protein and the known phasins was performed, no apparent consensus sequence was found. Since Wieczorek et al. have speculated about the existence of multiple distinct phasins in many bacteria (37), it would not be inconsistent for GA16 protein to be a phasin.
Analysis of the primary sequence of the GA16 protein of P. denitrificans revealed two stretches consisting of hydrophobic or amphiphilic amino acids at the C terminus. Pieper-Fürst et al. and Wieczorek et al. have proposed that two such stretches of GA14 protein from R. ruber and of GA24 protein from R. eutropha are responsible for the binding of phasins to the surfaces of PHA granules (17, 36). Pieper-Fürst et al. have shown that these regions of the GA14 protein are responsible for the binding of phasins to the surfaces of PHA granules (18). Two such stretches were detected between amino acid positions 107 and 118 (VQMDTVDLMLTA) and between positions 144 and 155 (AAAAATSASTVT) of GA16 protein, though the first of these regions did not consist completely of nonhydrophilic amino acids.
An increased number and decreased size of PHB granules correlated with an increased amount of PhaPPd across the series of E. coli strains carrying pTVC, pTVCP4, and pTVCP (Fig. 5A to D). The content of PHA in the strains carrying pTVCP4 increased compared to that in the strains carrying pTVC, but there was no further increase in strains carrying pTVCP (Table 2). Furthermore, the molecular mass of PHB decreased in the strain carrying pTVCP4 compared with that in the strain carrying pTVC, but no further decrease occurred with increased amounts of PhaPPd for the strain carrying pTVCP (Table 2). These results suggest that the threshold for the maximal effect of PhaPPd on PHB levels and molecular mass may be lower than the threshold for the maximal effect of PhaPPd on the number and size of PHB granules. PhaPPd seems to affect the molecular mass of PHB, although it is unclear whether the effect is caused by an interaction between PhaCPd and PhaPPd, by an increase in PHB content, or by something else.
In E. coli XL1-Blue carrying phaABCPd but not phaPPd, β-lactamase containing the signal sequence bound to PHB granules. This protein was probably the same granule-associated 32-kDa protein observed in E. coli XL1-Blue harboring pSK2665 carrying the β-lactamase gene and phaCABRe (26). Since the binding of β-lactamase to PHB granules was not observed in the strains producing GA16 protein, GA16 probably prevented nonspecific binding of other proteins, which existed as minor species in the cell, as well as binding of the precursor of β-lactamase, which existed as a major species.
We identified an ORF, designated phaRPd, as a putative regulator related to PHA synthesis. As shown in Fig. 3, the ORF4 product (PhaRPd) had regions which were highly conserved among four predicted proteins located in the PHA biosynthesis gene loci of C. vinosum (13), R. meliloti (28), T. violacea (14), and R. eutropha (22). This result strongly suggests that PhaRPd contributes to PHA metabolism in P. denitrificans. Interestingly, a steroid-inducible gene (stdC) product in C. testosteroni (5) was found to be similar to PhaRPd. This is considered to be a transcriptional factor involved in steroid degradation. There is a potential helix-turn-helix motif from positions 80 to 99 in the amino acid sequence of PhaRPd of P. denitrificans and its homologs in other bacteria (Fig. 3), if the stereochemical constraints are considered (2), though the sequences of PhaRPd and its homologs do not completely accord with those conditions. Furthermore, phaRPd (ORF4) seemed to regulate the expression of phaPPd in E. coli. Thus, it is possible to form a hypothesis that PhaRPd is a DNA-binding protein possibly related to PHA synthesis. However, the regulatory function of phaRPd was inferred only from E. coli and thus is putative. Further research will be done to elucidate directly in mutant and recombinant P. denitrificans whether phaRPd is involved in the regulation of phaPPd expression, whether phaRPd functions as a global regulator related to PHA synthesis and/or degradation, and whether phaPPd affects the number and size of PHA granules and also the molecular mass of PHA.
Knowledge about the regulation of expression of phasin proteins might be important for establishing the production of PHA not only in bacteria but also in transgenic plants, because phasins affect the size and number of PHA granules and prevent other proteins which are not involved in PHA metabolism from binding nonspecifically to the surfaces of PHA granules. For this reason, further study to make clear the possibility of regulated production of phasins via phaR will be quite useful.
ACKNOWLEDGMENTS
We are grateful to U. Iwasaki, Nagoya University, for his valuable technical advice in determination of amino acid sequences and of the molecular mass of protein, to K. Hirunagi and H. Yamada, Nagoya University, for preparation of electron micrographs, and to Y. Takagi, Nagoya Municipal Industrial Research Institute, for determination of the molecular mass of PHB.
This work was financially supported by the Tokai Foundation for Technology.
REFERENCES
- 1.Anderson A J, Dawes E A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branden C, Tooze J. Introduction to protein structure. New York, N.Y: Garland Publishing Co.; 1991. pp. 87–111. [Google Scholar]
- 3.Bullock W O, Fernandez J M, Stuart J M. XL-1 Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques. 1987;5:376–379. [Google Scholar]
- 4.Burnette W N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 5.Cabrera J E, Panzetta-Dutari G, Pruneda J L, Genti-Raimondi S. A new Comamonas testosteroni steroid-inducible gene: cloning and sequence analysis. J Steroid Biochem Mol Biol. 1997;63:91–98. doi: 10.1016/s0960-0760(97)00078-2. [DOI] [PubMed] [Google Scholar]
- 6.Fukui T, Doi Y. Cloning and analysis of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) biosynthesis genes of Aeromonas caviae. J Bacteriol. 1997;179:4821–4830. doi: 10.1128/jb.179.15.4821-4830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griebel R, Smith Z, Merrick J M. Metabolism of poly-β-hydroxybutyrate. I. Purification, composition, and properties of native poly-β-hydroxybutyrate granules from Bacillus megaterium. Biochemistry. 1968;7:3676–3681. doi: 10.1021/bi00850a047. [DOI] [PubMed] [Google Scholar]
- 8.Hustede E, Steinbüchel A. Characterization of the polyhydroxyalkanoate synthase gene locus of Rhodobacter sphaeroides. Biotechnol Lett. 1993;15:709–714. [Google Scholar]
- 9.Jossek R, Reichelt R, Steinbüchel A. In vitro biosynthesis of poly(3-hydroxybutyric acid) by using purified poly(hydroxyalkanoic acid) synthase of Chromatium vinosum. Appl Microbiol Biotechnol. 1998;49:258–266. doi: 10.1007/s002530051166. [DOI] [PubMed] [Google Scholar]
- 10.Kovach M E, Phillips R W, Elzer P H, Roop II R M, Peterson K M. pBBR1MCS: a broad-host range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Lee S Y. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 1996;14:431–438. [Google Scholar]
- 13.Liebergesell M, Steinbüchel A. Cloning and nucleotide sequences of genes relevant for biosynthesis of poly(3-hydroxybutyric acid) in Chromatium vinosum strain D. Eur J Biochem. 1992;209:135–150. doi: 10.1111/j.1432-1033.1992.tb17270.x. [DOI] [PubMed] [Google Scholar]
- 14.Liebergesell M, Steinbüchel A. Cloning and molecular analysis of the poly(3-hydroxybutyric acid) biosynthesis genes of Thiocystis violacea. Appl Microbiol Biotechnol. 1993;38:493–501. doi: 10.1007/BF00242944. [DOI] [PubMed] [Google Scholar]
- 15.Liebergesell M, Steinbüchel A. New knowledge about the PHA-locus and P(3HB) granule-associated proteins in Chromatium vinosum. Biotechnol Lett. 1996;18:719–724. [Google Scholar]
- 16.Maehara A, Ikai K, Ueda S, Yamane T. Gene dosage effects on polyhydroxyalkanoate synthesis from n-alcohols in Paracoccus denitrificans. Biotechnol Bioeng. 1998;60:61–69. [PubMed] [Google Scholar]
- 17.Pieper-Fürst U, Madkour M H, Mayer F, Steinbüchel A. Purification and characterization of a 14-kilodalton protein that is bound to the surface of polyhydroxyalkanoic acid granules in Rhodococcus ruber. J Bacteriol. 1994;176:4328–4337. doi: 10.1128/jb.176.14.4328-4337.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pieper-Fürst U, Madkour M H, Mayer F, Steinbüchel A. Identification of the region of a 14-kilodalton protein of Rhodococcus ruber that is responsible for the binding of this phasin to polyhydroxyalkanoic acid granules. J Bacteriol. 1995;177:2513–2523. doi: 10.1128/jb.177.9.2513-2523.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Saxena K, Richter O M, Ludwig B, Benz R. Molecular cloning and functional characterization of the Paracoccus denitrificans porin. Eur J Biochem. 1997;245:300–306. doi: 10.1111/j.1432-1033.1997.00300.x. [DOI] [PubMed] [Google Scholar]
- 21.Schembri M A, Woods A A, Bayly R C, Davies J K. Identification of a 13-kDa protein associated with the polyhydroxyalkanoic acid granules from Acinetobacter spp. FEMS Microbiol Lett. 1995;133:277–283. doi: 10.1111/j.1574-6968.1995.tb07897.x. [DOI] [PubMed] [Google Scholar]
- 22.Slater S, Houmiel K L, Tran M, Mitsky T A, Taylor N B, Padgette S R, Gruys K J. Multiple β-ketothiolases mediate poly(β-hydroxyalkanoate) copolymer synthesis in Ralstonia eutropha. J Bacteriol. 1998;180:1979–1987. doi: 10.1128/jb.180.8.1979-1987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 24.Spurr A R. A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 25.Steinbüchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentin H. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev. 1992;103:217–230. doi: 10.1111/j.1574-6968.1992.tb05841.x. [DOI] [PubMed] [Google Scholar]
- 26.Steinbüchel A, Aerts K, Babel W, Föllner C, Liebergesell M, Madkour M H, Mayer F, Pieper-Fürst U, Pries A, Valentin H E, Wieczorek R. Considerations on the structure and biochemistry of bacterial polyhydroxyalkanoic acid inclusions. Can J Microbiol. 1995;41:94–105. doi: 10.1139/m95-175. [DOI] [PubMed] [Google Scholar]
- 27.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tombolini R, Povolo S, Buson A, Squartini A, Nuti M P. Poly-β-hydroxybutyrate (PHB) biosynthetic genes in Rhizobium meliloti 41. Microbiology. 1995;141:2553–2559. doi: 10.1099/13500872-141-10-2553. [DOI] [PubMed] [Google Scholar]
- 29.Ueda S, Matsumoto S, Takagi A, Yamane T. n-Amyl alcohol as a substrate for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by bacteria. FEMS Microbiol Lett. 1992;98:57–60. doi: 10.1128/aem.58.11.3574-3579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda S, Matsumoto S, Takagi A, Yamane T. Synthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from methanol and n-amyl alcohol by the methylotrophic bacteria Paracoccus denitrificans and Methylobacterium extorquens. Appl Environ Microbiol. 1992;58:3574–3579. doi: 10.1128/aem.58.11.3574-3579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda S, Yabutani T, Maehara A, Yamane T. Molecular analysis of the poly(3-hydroxyalkanoate) synthase gene from a methylotrophic bacterium, Paracoccus denitrificans. J Bacteriol. 1996;178:774–779. doi: 10.1128/jb.178.3.774-779.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yabutani T, Maehara A, Ueda S, Yamane T. Analysis of β-ketothiolase and acetoacetyl-CoA reductase genes of a methylotrophic bacterium, Paracoccus denitrificans, and their expression in Escherichia coli. FEMS Microbiol Lett. 1995;133:85–90. doi: 10.1111/j.1574-6968.1995.tb07865.x. [DOI] [PubMed] [Google Scholar]
- 33.Yabuuchi E, Kosano Y, Yano I, Hotta H, Nishiuchi Y. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. nov.: proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. nov., Ralstonia solanacearum (Smith 1896) comb. nov. and Ralstonia eutropha (Davis 1969) comb. nov. Microbiol Immunol. 1995;39:897–904. doi: 10.1111/j.1348-0421.1995.tb03275.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamane T, Chen X F, Ueda S. Polyhydroxyalkanoate synthesis from alcohols during the growth of Paracoccus denitrificans. FEMS Microbiol Lett. 1996;135:207–211. [Google Scholar]
- 35.Yamane T, Chen X F, Ueda S. Growth-associated production of poly(3-hydroxyvalerate) from n-pentanol by a methylotrophic bacterium, Paracoccus denitrificans. Appl Environ Microbiol. 1996;62:380–384. doi: 10.1128/aem.62.2.380-384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wieczorek R, Pries A, Steinbüchel A, Mayer F. Analysis of a 24-kilodalton protein associated with the polyhydroxyalkanoic acid granules in Alcaligenes eutrophus. J Bacteriol. 1995;177:2425–2435. doi: 10.1128/jb.177.9.2425-2435.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wieczorek R, Steinbüchel A, Schmidt B. Occurrence of polyhydroxyalkanoic acid granule-associated proteins related to the Alcaligenes eutrophus H16 GA24 protein in other bacteria. FEMS Microbiol Lett. 1996;135:23–30. doi: 10.1111/j.1574-6968.1996.tb07961.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1987. [DOI] [PubMed] [Google Scholar]