Abstract
Objective
To observe the effects of preoperative right stellate ganglion block on perioperative atrial fibrillation in patients undergoing lung lobectomy.
Methods
Two hundred patients who underwent a scheduled lobectomy were randomly divided into the S and C groups. The S group was injected with 4 mL of 0.2% ropivacaine under ultrasound guidance, and the C group did not receive stellate ganglion block. The patients underwent continuous ECG monitoring, and the incidences of atrial fibrillation and other types of arrhythmias were recorded from the start of surgery to 24 hours after surgery.
Results
The respective incidences of atrial fibrillation in the S group and the C group were 3% and 10% (p = 0.045); other atrial arrhythmias were 20% and 38% (p = 0.005); and ventricular arrhythmia were 28% and 39% (p = 0.09).
Conclusions
The results of the study indicated that preoperative right stellate ganglion block can effectively reduce the incidence of intraoperative and postoperative atrial fibrillation.
Keywords: Atrial fibrillation, Lobectomy, Autonomic function, Stellate ganglion block
Resumo
Objetivo
Observar os efeitos do bloqueio do gânglio estrelado na fibrilação atrial no período perioperatório em pacientes submetidos a lobectomia pulmonar.
Método
Duzentos pacientes programados para lobectomia foram divididos aleatoriamente nos grupos S e C. O grupo S recebeu infusão de 4 mL de ropivacaína a 0,2% orientada por ultrasom e o grupo C não foi submetido a bloqueio do gânglio estrelado. Os pacientes foram submetidos à monitoração contínua de ECG, e as incidências de fibrilação atrial e outros tipos de arritmias foram registradas do início da cirurgia até 24 horas depois da cirurgia.
Resultados
As incidências de fibrilação atrial no grupo S e no grupo C foram 3% e 10%, respectivamente (p = 0,045); as de outras arritmias atriais foram 20% e 38% (p = 0,005); e de arritmias ventriculares 28% e 39% (p = 0,09).
Conclusões
Os resultados do estudo indicaram que o bloqueio do gânglio estrelado no pré-operatório pode ser efetivo na redução da incidência de fibrilação atrial nos períodos intra- e pós-operatório.
PALAVRAS-CHAVE: Fibrilação atrial, Lobectomia, Função autonômica, Bloqueio do gânglio estrelado
Introduction
Surveys have shown that the incidence and mortality of lung cancer are at the forefront of various types of cancer, and surgical treatment is currently the preferred method to improve the cure rate and reduce mortality.1 Postoperative Atrial Fibrillation (POAF) is a frequent postoperative complication after noncardiac thoracic surgery, in which the incidence of atrial fibrillation is 4% to 37%.2, 3 POAF is not only associated with poorer perioperative outcomes and hospital mortality but also linked to reduced long-term survival after surgery. Thus, prevention of POAF is one of the most important objectives of postoperative patient care.
Age, sex, hypertension, diabetes and chronic obstructive pulmonary disease are closely related to POAF following thoracic surgery.4, 5 Meanwhile, a number of efforts have been made at identifying mechanisms of POAF; other than direct injury to the cardiac conduction system in surgical patients, these mechanisms are as follows: cardiac stimulation from exogenous catecholamines, endogenous sympathetic activation that occurs following volume loss or pain, and both local and systemic inflammation.6, 7, 8, 9 More recently, some preventive medications, such as amiodarone and metoprolol, have been introduced to diminish POAF.10 Correspondingly, it has also been helpful to reduce POAF by adjusting anesthetic drugs and methods and improving postoperative analgesia patterns and other related aspects.11
Stellate Ganglion Block (SGB), a cervical sympathetic blockade, has been widely used to treat some refractory arrhythmia storms.12, 13 Studies have shown that blocking or resecting the stellate ganglion can inhibit the occurrence and maintenance of Atrial Fibrillation (AF), possibly by regulating the autonomic and immune systems.7, 14 However, the reported changes in cardiac function with LSGB in the literature have been contradictory. Costa et al.15 showed that LSGB prolonged ventricular isovolume relaxation time and reduced ventricular contraction rate, resulting in a decrease in cardiac ejection fraction. Compared with traditional blind puncture, the ultrasound-guided technique has made the block more precise and effective, with a small volume of injection and improved tolerability, by direct visualization and reduction in the risk of esophageal, vascular, or neuronal injury.16, 17
Despite the abovementioned benefits, the contribution of RSGB in decreasing POAF has never been evaluated. Thus, a randomized controlled trial was conducted to evaluate whether RSGB is beneficial for reducing the incidence of intraoperative and postoperative atrial fibrillation in patients undergoing lung lobectomy.
Methods
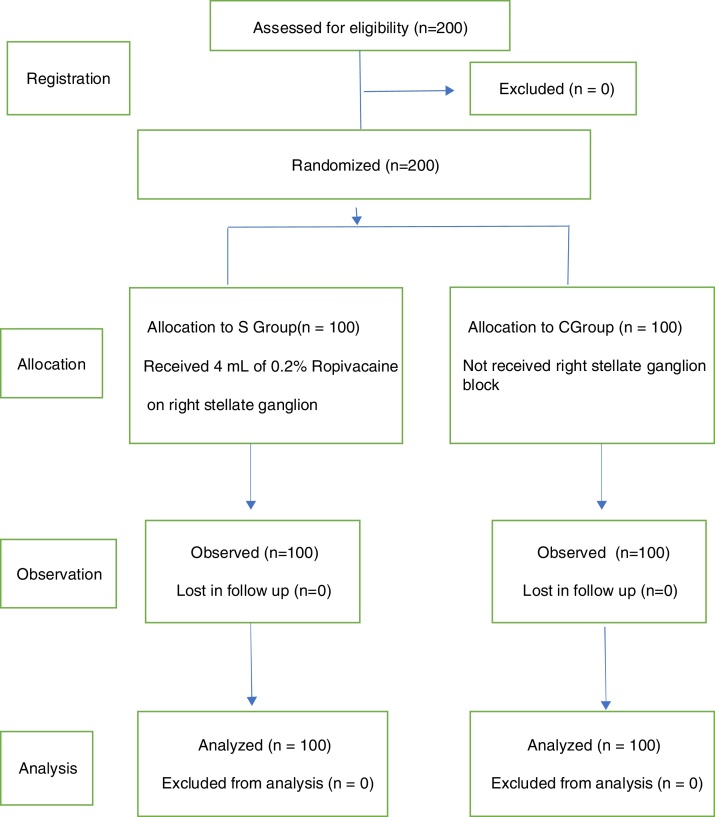
This randomized controlled trial was approved by the Medical Ethics Committee, and it was conducted in the anesthesia and thoracic surgery intensive care unit departments of the First Affiliated Hospital of Nanchang University. Free and informed consent forms were signed by the patients' legal guardians prior to randomization. Two hundred patients aged 55 to 80 years, with ASA physical status II-III and scheduled surgical treatment for lung cancer, were recruited in two groups (Fig. 1). The exclusion criteria included severe organ dysfunction, anesthetic drug allergy, and other types of arrhythmias before surgery. All patients underwent routine preoperative examinations, and were instructed to quit smoking and engaged in a bedside respiratory function exercise.
Figure 1.
Study Flowchart.
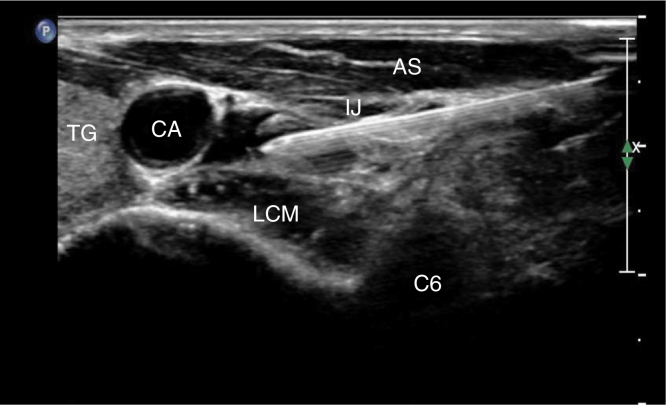
The 200 patients were randomly divided into two groups using computer-generated random numbers. Group S received RSGB under ultrasound guidance before general anesthesia, and the Group C did not receive this treatment. After establishing a standard monitoring procedure and care for general anesthesia, the radial artery and right internal jugular vein catheter were placed under local anesthesia. At the same time, 5 mL of venous blood was taken and sent to the laboratory for routine blood tests. Invasive Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP), Heart Rate (HR), Oxygen Saturation (SPO2), Electrocardiogram (ECG) and Bispectral Index (Bis) were monitored with a multifunction monitor and a small portable dynamic heart electrical monitor (PC-80A), which performed continuous ECG recording. Dexmedetomidine was intravenously infused into every patient over 10 min. After the above procedure, a trained anesthesiologist conducted an RSGB under ultrasound guidance with a 5 to 10 MHz probe (Sonosite M-Turbo) using the in-the-plane approach in Group S. With a size 7 puncture needle, 4 mL of 0.2% ropivacaine was injected at the C6 level below the fascia of the longus colli muscle (Fig. 2). Horner's syndrome on the right side of the patients indicated that the block was successful; Group C did not undergo this process.
Figure 2.
Note: TG, Thyroid Gland; CA, Carotid Artery; IJ, Internal Jugular vein; AS, Anterior Scalene; LCM, Longus Collis Muscle.
The two groups of patients were treated with propofol 1.5‒2.0 mg kg-1, rocuronium 0.6 mg kg-1, and sufentanil 0.4‒0.6 μg kg-1 for intravenous induction; they underwent visual laryngoscope and fiber bronchoscopy-assisted double-lumen bronchial intubation; and had mechanical ventilation performed with maintenance of the Pressure of End-Tidal Carbon dioxide (PETCO2) at 35‒45 mmHg. The Bis value was maintained at 45‒55 with propofol 5 mg kg-1 h-1, remifentanil 8 to 10 μg kg-1 h-1, cis-atracurium 0.04 to 0.05 mg kg-1 h-1. During the surgery, single lung ventilation, sucking and other related measures were needed. After the surgery ended, patients were sent to the ICU to continue dynamic ECG monitoring. The tube was withdrawn by the ICU doctor after the indication for extubation was met.
Observation indexes
We recorded preoperative and operative clinical factors, including sex, age, pack-years of smoking, preoperative respiratory function, history of ischemic heart disease, tumor location, operation time, amount of bleeding and replenishing, adverse events and the quantity of vasoactive drugs. The incidences of AF and other types of arrhythmias were recorded from the start of the surgery to 24 hours after surgery. At the same time, the changes in White Blood Cell (WBC) counts were evaluated to compare the inflammatory response between the two groups after the lobectomy.
Sample size and statistical analysis
Using the incidence (11.8%) from the study by Ivanovic et al.2 for Group C and assuming the expected incidence was 7% after making a stellate ganglion block, with a Type I error of 0.05 and a power of 0.80, the sample size was calculated to be at least 180 individuals for the trial. Taking into consideration the possible elimination of some patients due to changes in their condition, 200 patients were initially recruited for this study.
Categorical data were expressed as numbers or percentages, and quantitative data that followed the normal distribution were expressed as mean and standard deviation. In this context, parametric tests were applied to the data with normal distributions, while data without normal distributions were analyzed with nonparametric tests. Chi-Square tests and Fisher probabilities were used to analyze categorical data. A p-value < 0.05 was our cutoff point for statistical significance. All data were analyzed using the Statistical Package for Social Sciences version 20.0 (SPSS).
Results
Comparison of preoperative and operative clinical factors between the two groups
There were no significant differences in sex, age, pack-years of smoking, preoperative respiratory function, history of ischemic heart disease, or tumor location between the two groups (p > 0.05). There were no significant differences in operation time, amount of bleeding and replenishing, adverse events and quantity of vasoactive drugs between the two groups (p > 0.05) (Table 1).
Table 1.
Comparison of preoperative and operative clinical factors between the two groups.
| Characteristic | S Group (n = 100) | C Group (n = 100) |
|---|---|---|
| Sex | ||
| Male | 61 | 58 |
| Female | 39 | 42 |
| Age (Y) | 64.78 ± 5.744 | 66.04 ± 6.334 |
| Tumor location | ||
| Right | 59 | 61 |
| Upper | 28 | 30 |
| Middle | 10 | 8 |
| Lower | 21 | 23 |
| Left | 41 | 39 |
| Upper | 30 | 31 |
| Lower | 11 | 8 |
| FEV1 (%) | 76.57 ± 3.245 | 76.62 ± 3.362 |
| Hypertension | ||
| Yes | 48 | 51 |
| No | 52 | 49 |
| Diabetes | ||
| Yes | 32 | 29 |
| No | 68 | 71 |
| Urinary volume (mL) | 326.90 ± 89.924 | 331.55 ± 97.513 |
| Blood loss (mL) | 129.40 ± 65.440 | 137.64 ± 56.293 |
| Infusion volume (mL) | ||
| Liquid | 1231.98 ± 147.619 | 1257.67 ± 195.688 |
| Colloid | 525.41 ± 80.767 | 515.59 ± 75.978 |
Comparison of POAF and other types of arrhythmias between the two groups
The respective incidences of AF in Group S and Group C were 3% and 10% (p = 0.045); the incidences of other atrial arrhythmias were 20% and 38% (p = 0.05); and the incidences of ventricular arrhythmia were 28% and 39% (p = 0.09) (Table 2).
Table 2.
Comparison of POAF and other types of arrhythmias between the two groups.
| Type | S Group n = 100 | C Group n = 100 | χ2 | p |
|---|---|---|---|---|
| Atrial fibrillation | 3/100 (3%) | 10/100 (10%) | 4.031 | 0.045 |
| Other atrial arrhythmia | 20/100 (20%) | 38/100 (38%) | 7.868 | 0.005 |
| Ventricular arrhythmia | 28/100 (28%) | 39/100 (39%) | 1.792 | 0.09 |
Note: Qualitative data were expressed as numbers or percentages, and the comparison between the two groups used Chi-Square tests and Fisher probabilities.
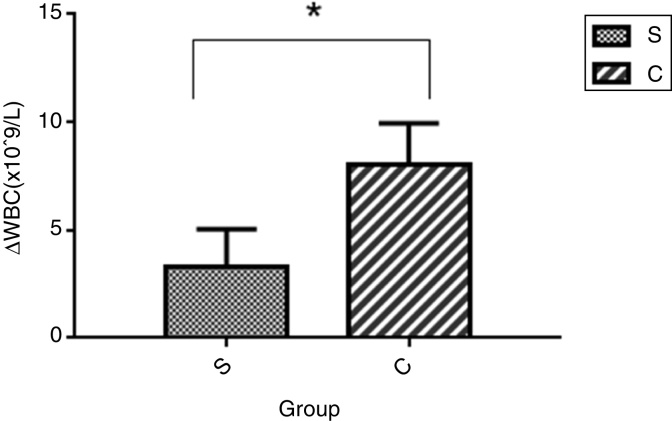
Comparison of differences in White Blood Cell (WBC) counts between the two groups (24 hours after surgery and before surgery)
The difference in WBC counts in Group S was lower than that in Group C, and the difference was statistically significant (p < 0.05) (Fig. 3).
Figure 3.
Note: The data’s followed a normal distribution, and the comparison between the two groups used the parametric test.
Discussion
Stellate Ganglion Blocks (SGB) are widely used in clinics due to the well-developed procedure and ability to control its delivery. In addition, SGB is used for the treatment of a variety of cardiac arrhythmias, possibly by regulating the autonomic and immune systems.7 However, there are differences in the stellate ganglion block on the left and right sides. Studies18 have shown that the left stellate ganglion block inhibits the ventricular ejection fraction and increases myocardial oxygen consumption, but there are no meaningful results relevant to cardiac electrophysiology. The safety and efficacy of ultrasound-guided SGB is widely accepted by clinicians, and there has been extensive research on the dose-effect relationship of ropivacaine. The ED95 of 0.2% ropivacaine is 3.2 mL for ultrasound-guided SGB.19 Therefore, the selected concentration of local anesthetic drugs was based on the literature and preliminary experiments.
POAF is one of the complications of thoracic surgery, and it is possibly attributable to the imbalance between the sympathetic and parasympathetic systems after surgical manipulations. Moreover, changes or imbalances in cardiac sympathetic activation were the mechanism of atrial fibrillation. Vretzakis et al.20 pointed out that high sympathetic tone can induce activation of the vagus nerve, and the synergistic effect of the two triggers the occurrence of POAF in patients undergoing lobectomy. The sympathetic nervous system, which derives from the nucleus coeruleus in the brainstem, plays an important role in the interaction of the neural and immune systems. The postganglionic sympathetic nerves (stellate ganglion), which travel through the paravertebral ganglia and prevertebral ganglia, release noradrenaline. Swissa et al.21 proposed that the enhancement of sympathetic nerves was accompanied by the release of norepinephrine, that induces adenylate cyclase to increase cAMP levels, which is an important mechanism for the occurrence of atrial fibrillation.
This study considered whether RSGB can reduce the incidence of atrial fibrillation in patients undergoing lobectomy. To our knowledge, this was the first study to evaluate the benefits of RSGB in terms of postoperative atrial fibrillation. Our study found that the incidence of AF was reduced in Group S compared with Group C, confirming that general anesthesia combined with RSGB can reduce the occurrence of AF by regulating the balance of cardiac autonomic nerves. We believe that RSGB can inhibit the release of norepinephrine and reduce catecholamine content, which prolongs the Atrial Effective Refractory Period (AEPR) and increases the electrophysiological stability of the heart. Furthermore, RSGB can inhibit the stress response and reduce the production of related inflammatory factors, which inhibit the electrical remodeling and structural remodeling of cardiomyocytes, thereby reducing the occurrence of AF.7, 9, 22, 23 At the same time, we found that the WBC counts in Group S group were significantly lower than those in Group C at the 24th hour after surgery. This result confirmed the previously mentioned mechanism that RSGB can reduce the perioperative inflammatory response. This study found that the occurrence of other types of atrial arrhythmias was more often in the process of lymph node dissection, especially when the surrounding tissues were heavier, but the incidence of atrial arrhythmia in Group S was significantly lower than that in Group C. The reason may be that RSGB dilated the coronary arteries, improving the protection of cardiomyocyte oxygen supply.24
Blocking was successful in our patients because we performed RSGB with ultrasound guidance by the precise visualization of needle-tip placement and solution expansion. In our study, there were no significant hemodynamic changes or other adverse effects, such as nerve injury or esophageal puncture, after RSGB.
This study has some limitations. First, we did not measure the autonomic nerve changes by electrophysiological techniques. In fact, Heart Rate Variability (HRV) is the marker of neural autonomic control computed from HRV time series, and we did not detect electrophysiological parameters in our patients. Second, the study pointed out that the inflammatory response was a major mechanism of POAF, but assessing the white blood cell counts alone is not a specific measure. Finally, considering that AF may occur at any time after surgery, the endpoint of this study should be extended.
Conclusions
Preoperative Right Stellate Ganglion Block (RSGB) can effectively reduce the incidence of intraoperative and postoperative atrial fibrillation.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 66:7-30. [DOI] [PubMed]
- 2.Ivanovic J., Maziak D.E., Ramzan S., et al. Incidence, severity and perioperative risk factors for atrial fibrillation following pulmonary resection. Interact Cardiovasc Thorac Surg. 2014;18:340–346. doi: 10.1093/icvts/ivt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu L., Scherlag B.J., Sha Y., et al. Interactions between atrial electrical remodeling and autonomic remodeling: how to break the vicious cycle. Heart Rhythm. 2012;9:804–809. doi: 10.1016/j.hrthm.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Amar D., Zhang H., Leung D.H., et al. Older age is the strongest predictor of postoperative atrial fibrillation. Anesthesiology. 2002;96:352–356. doi: 10.1097/00000542-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Philip I., Berroeta C., Leblanc I. Perioperative challenges of atrial fibrillation. Curr Opin Anaesthesiol. 2014;27:344–352. doi: 10.1097/ACO.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 6.Kailasam R., Palin C.A., Hogue C.W., et al. Atrial fibrillation after cardiac surgery: an evidence-based approach to prevention. Semin Cardiothorac Vasc Anesth. 2005;9:77–85. doi: 10.1177/108925320500900108. [DOI] [PubMed] [Google Scholar]
- 7.Leftheriotis D., Flevari P., Kossyvakis C., et al. Acute effects of unilateral temporary stellate ganglion block on human atrial electrophysiological properties and atrial fibrillation inducibility. Heart Rhythm. 2016;13:2111–2117. doi: 10.1016/j.hrthm.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Katritsis D.G. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:886. doi: 10.1093/eurheartj/ehi780. [DOI] [PubMed] [Google Scholar]
- 9.Kao Y.H., Chen Y.C., Cheng C.C., et al. Tumor necrosis factor-alpha decreases sarcoplasmic reticulum Ca2+-ATPase expressions via the promoter methylation in cardiomyocytes. Crit Care Med. 2010;38:217–222. doi: 10.1097/CCM.0b013e3181b4a854. [DOI] [PubMed] [Google Scholar]
- 10.Tisdale J.E., Wroblewski H.A., Kesler K.A. Prophylaxis of atrial fibrillation after noncardiac thoracic surgery. Semin Thorac Cardiovasc Surg. 2010;22:310–320. doi: 10.1053/j.semtcvs.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Simeoforidou M., Vretzakis G., Bareka M., et al. Thoracic epidural analgesia with levobupivacaine for 6 postoperative days attenuates sympathetic activation after thoracic surgery. J Cardiothorac Vasc Anesth. 2011;25:817–823. doi: 10.1053/j.jvca.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Przybylski A., Romanek J., Chlebus M., et al. Percutaneous stellate ganglion block as an adjunctive therapy in the treatment of incessant ventricular tachycardia. Kardiol Pol. 2018;76:1018–1020. doi: 10.5603/KP.2018.0120. [DOI] [PubMed] [Google Scholar]
- 13.Zambito P.E., Talreja A., Gundewar S., et al. Severe left ventricular systolic dysfunction increases atrial fibrillation after ablation of atrial flutter. Pacing Clin Electrophysiol. 2005;28:1055–1059. doi: 10.1111/j.1540-8159.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- 14.Xie X., Visweswaran R., Guzman P.A., et al. The effect of cardiac sympathetic denervation through bilateral stellate ganglionectomy on electrical properties of the heart. Am J Physiol Heart Circ Physiol. 2011;301:H192–199. doi: 10.1152/ajpheart.01149.2010. [DOI] [PubMed] [Google Scholar]
- 15.Puente de la Vega Costa K., Gomez Perez M.A., Roqueta C., et al. Effects on hemodynamic variables and echocardiographic parameters after a stellate ganglion block in 15 healthy volunteers. Auton Neurosci. 2016;197:46–55. doi: 10.1016/j.autneu.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Wei K., Feldmann R.E., Jr, Brascher A.K., et al. Ultrasound-guided stellate ganglion blocks combined with pharmacological and occupational therapy in Complex Regional Pain Syndrome (CRPS): a pilot case series ad interim. Pain Med. 2014;15:2120–2127. doi: 10.1111/pme.12473. [DOI] [PubMed] [Google Scholar]
- 17.Lee M.H., Kim K.Y., Song J.H., et al. Minimal volume of local anesthetic required for an ultrasound-guided SGB. Pain Med. 2012;13:1381–1388. doi: 10.1111/j.1526-4637.2012.01495.x. [DOI] [PubMed] [Google Scholar]
- 18.Fujiki A., Masuda A., Inoue H. Effects of unilateral stellate ganglion block on the spectral characteristics of heart rate variability. Jpn Circ J. 1999;63:854–858. doi: 10.1253/jcj.63.854. [DOI] [PubMed] [Google Scholar]
- 19.Jung G., Kim B.S., Shin K.B., et al. The optimal volume of 0.2% ropivacaine required for an ultrasound-guided stellate ganglion block. Korean J Anesthesiol. 2011;60:179–184. doi: 10.4097/kjae.2011.60.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vretzakis G., Simeoforidou M., Stamoulis K., et al. Supraventricular arrhythmias after thoracotomy: is there a role for autonomic imbalance? Anesthesiol Res Pract. 2013 doi: 10.1155/2013/413985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swissa M., Zhou S., Paz O., et al. Canine model of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia. Am J Physiol Heart Circ Physiol. 2005;289:H1851–1857. doi: 10.1152/ajpheart.00083.2005. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L.P., Zhang L.P., Hu K. The relationship between sympathetic nerve sprouting and ventricular arrhythmia after myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:1045–1047. [PubMed] [Google Scholar]
- 23.Qian Y.S., Zhao Q.Y., Zhang S.J., et al. Effect of alpha7nAChR mediated cholinergic anti-inflammatory pathway on inhibition of atrial fibrillation by low-level vagus nerve stimulation. Zhonghua Yi Xue Za Zhi. 2018;98:855–859. doi: 10.3760/cma.j.issn.0376-2491.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y.Q., Xie Y.Y., Wang B., et al. Effect of stellate ganglion block on hemodynamics and stress responses during CO2-pneumoperitoneum in elderly patients. J Clin Anesth. 2017;37:149–153. doi: 10.1016/j.jclinane.2016.12.003. [DOI] [PubMed] [Google Scholar]