Abstract
Objective
Baclofen and γ-Hydroxybutyrate (GHB) exert GABAB receptor agonism and have therapeutic utility but possess different pharmacological activities. We examined whether separate groups of mice could be trained to discriminate either baclofen or GHB, and the contribution of GABAB receptors to discriminative stimulus effects.
Methods
Male C57BL/6J mice were trained to discriminate either baclofen (3.2 mg/kg, intraperitoneal), or GHB (178 mg/kg, intraperitoneal) from saline under a fixed-ratio 10 schedule. The GABAB antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP 35348) was used to pharmacologically assess GABAB receptor involvement. The selectivity of the resulting discriminations was assessed with the opioid agonist morphine and the benzodiazepine midazolam.
Results
In baclofen-trained mice, both baclofen and GHB were readily discriminated. Baclofen produced a maximum of 86% baclofen-appropriate responding. CGP 35348 (320 mg/kg, i.p.) produced a 4.7-fold rightward shift in the dose-effect function. GHB produced a maximum of 85.8% baclofen-appropriate responding. In GHB-trained mice, both GHB and baclofen were readily discriminated. In GHB-trained mice, GHB produced a maximum of 85.3% drug-appropriate responding; CGP 35348 (320 mg/kg, i.p.) produced a 1.8-fold rightward shift in the GHB discrimination dose-effect function. Baclofen produced up to 70.0% GHB-appropriate responding. CGP 35348 (320 mg/kg, i.p.) significantly antagonized baclofen discrimination and baclofen produced up to 37% GHB-appropriate responding up to doses that disrupted operant responding. Morphine did not produce substitution for either baclofen or GHB. Midazolam produced partial substitution for both.
Conclusion
GHB and baclofen discrimination assays in mice provide a useful approach for examining different receptor types mediating the effects of these two drugs.
Keywords: Drug discrimination, GABAB receptor, GHB, mice, baclofen
Introduction
Agonists of the γ-aminobutyric acid (GABA)B receptor may have multiple therapeutic applications. Baclofen, a GABAB receptor agonist, is prescribed for muscle spasticity and pain associated with multiple sclerosis (Sadiq & Poopatana, 2007), and GABAB receptor agonists may also produce analgesia in other diseases where pain is often a symptom (Hwang & Yaksh, 1997; Salte et al., 2016; Zemoura et al., 2016; Deseure & Hans 2017). Numerous studies suggest baclofen and other GABAB agonists possess relatively low abuse potential and may be useful as therapeutics to treat drug abuse. Specifically, baclofen decreased the reinforcing effects of opioids in rat self-administration procedures (Xi & Stein,1999; Ramshini et al., 2013), and abolished morphine preference in the place conditioning assay in mice (Meng et al., 2014). These effects are attributed to the actions of GABAB receptor stimulation to inhibit dopamine release (Fadda et al., 2003; Fu et al., 2012; Klitenick et al., 1992; Xi & Stein, 1999). Baclofen has also been found to reduce behavioral signs of morphine withdrawal in mice (Pedron et al., 2016) and rats (Topkara et al., 2017) as well as morphine-induced nausea and vomiting in ferrets (Suzuki et al., 2005). Additional studies have shown that baclofen can decrease the rewarding effects of other commonly abused drugs such as nicotine, cocaine, and methamphetamine (Ranaldi & Poeggel 2002; Fadda et al., 2003). However, clinically, baclofen is often associated with drowsiness, dizziness, nausea, vomiting, and in some cases, seizures (Chou et al., 2004).
γ-Hydroxybutyric acid (GHB) was first designed synthetically in 1964 as a GABA analog (Laborit, 1964). It was later found to be both a precursor and a metabolite of γ-aminobutyric acid (GABA), and expressed exogenously within the central nervous system by hippocampal midbrain, diencephalon, and cerebellum neurons (Roth, 1970; Maitre, 1997). Clinically, GHB is sold under the name Xyrem, prescribed as a narcolepsy therapeutic (Boscolo-Berto et al., 2012) as well as Alcover, prescribed as an alcohol addiction and dependence therapeutic (Addolorato et al., 2009). Unlike baclofen, GHB has been characterized to possess drug dependence liability (Carter et al., 2009; Gonzalez & Nutt, 2005). The exact pharmacological mechanisms that underlie the in vivo effects of GHB are not fully established though are linked to actions at GABAB receptors. GHB shares several effects with baclofen including cataleptic, ataxic, hypolocomotor, and discriminative stimulus effects; these and other effects (i.e., decreases in operant responding) of GHB are attenuated by GABAB receptor antagonists (Carter et al., 2005; Goodwin et al., 2005; Kaupmann et al., 2003; Koek et al., 2004; 2006; 2007). However, in these studies, the potency of a GABAB receptor antagonist to attenuate the effects of GHB is less than the potency of the same antagonist to attenuate the effects of baclofen, suggesting other receptors are involved. Previous studies in mice demonstrate that GHB can be reliably discriminated (Cook et al., 2006). Thus, although pharmacologically distinct, baclofen and GHB share some pharmacological characteristics across multiple species.
The present study sought to expand upon previous studies in other animal species by characterizing the cross-substitution of GHB and baclofen with one another using drug discrimination assays in mice. We also examined the extent to which the GABAB selective antagonist CGP 35348 attenuates the discriminative stimulus effects of GHB and baclofen.
Methods
All data in the present study were collected in the Department of Pharmacology, The University of Texas Health Science Center, San Antonio, TX.
Subjects.
Sixteen male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were received at eight weeks of age. Mice were individually housed under a 14/10-h light/dark cycle (i.e., lights on at 5 am and off at 7 pm) and maintained in home cages at 85% of free-feeding body weight according to the growth curve provided by the commercial breeder. Mice were fed 2.5 g of food (Dustless Precision Pellets 500 mg, Rodent Grain-Based Diet, Bio-Serv, Frenchtown, NJ) immediately after experimental sessions. Water was available ad libitum in each home cage. The experiments, which were conducted seven days per week, were approved by The University of Texas Health Science Center at San Antonio’s Institutional Animal Care and Use Committee. The National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 2011) was followed.
Drugs.
The compounds used in the present study were as follows: (±)-baclofen (Sigma-Aldrich, St. Louis, MO), CGP35348 [synthesized by J. Again at the University of Texas Health Science Center (San Antonio, TX), in the manner of Froestl and purity checked as described (Froestl et al., 1995)], GHB (National Institute on Drug Abuse), midazolam hydrochloride (Bedford Laboratories; Bedford, OH), and (−)-Morphine sulfate pentahydrate (National Institute on Drug Abuse). All compounds were dissolved in sterile water or saline. All compounds were administered intraperitoneally (i.p.) in a volume of saline equivalent to 10 mL/kg, 20 min prior to testing (i.e., being placed in the operant chamber), except CGP35348, which had a 5 min pretreatment time, and midazolam and morphine, which was administered immediately before each subject was placed in the operant conditioning chamber. Doses were selected based on previous studies (Koek et al., 2013).
Apparatus.
Operant conditioning chambers (ENV-307A-CT, Med Associates Inc., St. Albans, VT) were stored in cubicles that attenuated external noise; each cubicle was equipped with an exhaust fan. External noise was masked further with a white-noise generator. Each chamber contained a stand-alone light on one wall and a 2.2 cm-diameter hole recessed on the same wall for presentation of liquid food. On the opposite wall, three identical nose-poking holes were horizontally arranged and spaced 5.5 cm apart. A stimulus light-emitting diode (LED) was installed in each nose-poking hole. The center of each hole was 1.6 cm above from the floor. When one or both the left and right holes were illuminated, disruption of a photobeam in one of the holes resulted in access to 0.01 cc of 50% v/v unsweetened condensed milk/water through the dispenser hole on the opposite wall. The operant conditioning chambers were connected through an interface (MED-SYST-8, Med Associates Inc.) to a PC. Med-PC software (Med Associates Inc.) was used to control experimental events and provide a record of responses.
Discrimination Training.
The 16 mice used in these studies were divided into two separate groups, one trained to discriminate baclofen the other trained to discriminate GHB. After seven days of habituating the mice to the housing room, experimental sessions were conducted approximately at the same time during the light period each day. Initially, mice were placed into the operant conditioning chamber for 60 min with both left and right holes illuminated. A photobeam disruption in either hole resulted in 10-s access to the condensed milk through presentation of a dipper. For 10 s, the milk dipper was presented to the mouse, the light on the wall was illuminated, and the lights inside the nose-poking holes were extinguished. Any disruption of a photobeam during milk dipper presentation was not associated with a programmed consequence. After 100 reinforcers per session were earned for four consecutive sessions, session duration was shortened to 25 min and the response requirement was systematically increased to a fixed ratio 10-response (FR10) schedule of reinforcement. Experimental sessions were then divided into a 10-min timeout; during the timeout, any disruption of a photobeam had no programmed consequence. Following the timeout, milk was available under the FR10 schedule for a total of 15 min.
Drug discrimination training was initiated by administering 3.2 mg/kg baclofen, 178 mg/kg GHB, or saline 20 min prior to the timeout during training sessions. During the 10-min timeout, all stimulus and house lights in the operant conditioning chamber were off. Following the 10-min timeout, illumination of the lights in the left and right nose-poke holes provided a stimulus indicating that milk was available for delivery pending completion of the FR10. During each training session, ten responses in the correct hole (left following baclofen or GHB for half of the mice; right following baclofen or GHB for the other half) resulted in presentation of milk. During saline-training sessions, ten responses in the opposite hole were required to obtain milk delivery. Correct holes were permanently assigned. The sequence of training included two consecutive days of baclofen or GHB training followed by two consecutive days of saline training. To pass a training session, two criteria had to be satisfied: 1) a minimum of 80% of the total responses during the 15-min response period needed to be correct; and 2) any incorrect responses made prior to delivery of the first reinforcer needed to be less than ten (i.e., the first reinforcer had to be obtained with fewer than a total of 20 responses in both holes). Responses in the incorrect hole had no scheduled consequences, i.e., did not reset the ratio requirement. Testing commenced once the criteria were satisfied in five consecutive or six out of seven training sessions. After the first test, inter-test training alternated between one baclofen- or GHB- and one saline-training session; however, the first training condition (i.e., baclofen/GHB or saline) following each test session varied non-systematically. If a mouse failed to meet the criteria, a training condition was repeated. However, the next training session was switched to the alternative training condition to avoid more than two consecutive days of a particular training condition. Subsequent test sessions were conducted after the mouse passed at least three consecutive training sessions.
Discrimination Testing.
Test sessions were the same as training sessions, except that ten responses in either hole resulted in milk presentation. Dose-effect tests were conducted with baclofen or GHB, followed by GHB (for baclofen-trained mice) or baclofen (for GHB-trained mice). Within each drug test series, the doses of each were studied in ascending order. The control compounds midazolam and morphine were studied next. Drugs were administered from doses that produced less than 20% drug-appropriate responding up to doses that produced greater than or equal to 80% drug-appropriate responding, antagonized drug-appropriate responding produced by a test drug, decreased response rates to less than 20% of the saline control rates achieved during saline training or were deemed potentially toxic or up to the maximum solubility. Dose-effect functions for all substituted compounds were conducted prior to testing with CGP 35348. Dose-effect functions for each training drug were determined twice: once before and again after completion of all tests with other drugs.
Data analyses.
All data are expressed as the mean ± standard error of the mean (S.E.M.). The discrimination data were expressed as the percentage of drug-appropriate responses out of the total number of responses per subject. The response rate data were expressed as a percentage of control for each animal, defined as the mean response rate from the five saline training sessions immediately preceding the test. Some animals lost their respective discriminative stimulus for the training drug dose during the experimental testing and were removed from the study. In some conditions, this led to an uneven experimental group number reported. Statistical analyses were conducted using SigmaPlot version 14.0 (Systat Software Inc., San Jose, CA). Dose-effect data were fitted with straight lines using linear regression. The linear portion of a dose-effect function, as determined per individual mouse, included only one dose that produced a less than 20% effect, and one dose greater than 50% effect. If these criteria were not met, (i.e., midazolam in GHB-discriminating mice) then the linear portion of a dose-effect function was unable to be determined. The slopes of different functions were determined by grouping individual data in a single analysis and were compared with one way (repeated-measures) analysis of variance (ANOVA), with post-hoc Bonferroni tests. When the mean of drug-appropriate responding was greater than 50%, the ED50 values and corresponding 95% confidence limits were calculated according to Tallarida (2000).
Results
The discriminative stimulus effects of baclofen: substitution and antagonism.
Saline (i.p.) produced 4.7% (S.E.M: 1.7%) drug-appropriate responding in mice trained with 3.2 mg/kg baclofen. The dose-effect functions determined for baclofen, determined once at the beginning and again at the end of the study, were not significantly different from each other (p values > 0.05); these were averaged for further analyses (Figure 1A, symbols above Saline). In baclofen-trained mice, baclofen produced a maximum of 86% baclofen-appropriate responding at the training dose of 3.2 mg/kg; a dose of 5.6 mg/kg baclofen decreased response rate to 15% of control (Figure 1A and 1B, respectively, filled circles). The ED50 values (95% confidence limits) of baclofen to produce discriminative stimulus and rate-decreasing effects were 1.87 (1.36 ― 2.42) and 3.61 (2.51 ― 5.08) mg/kg (i.p.), respectively. Both doses of CGP 35348 studied (100, 320 mg/kg, i.p.) produced dose-dependent rightward shifts up to 4.7-fold in the baclofen discriminative stimulus dose-effect function (Figure 1A, open triangles). The ED50 values (95% confidence limits) of baclofen to produce discriminative stimulus effects in the presence of 100 and 320 mg/kg CGP 35348 were 6.68 (4.93 ― 17.6) and 8.87 (2.76 ― 14.3) mg/kg (i.p.), respectively. Likewise, both doses of CGP 35348 also produced dose-dependent rightward shifts for the effects of baclofen to decrease operant response rates (Figure 1B, open triangles). The ED50 values (95% confidence limits) of baclofen to decrease operant response rates in the presence of 100 and 320 mg/kg CGP 35348 were 6.23 (2.29 ― 30.9) and 15.0 (10.7 ― 63.1) mg/kg (i.p.), respectively.
Figure 1. Discriminative stimulus (top row) and rate-decreasing (bottom row) effects in mice trained to discriminate 3.2 mg/kg baclofen from saline.
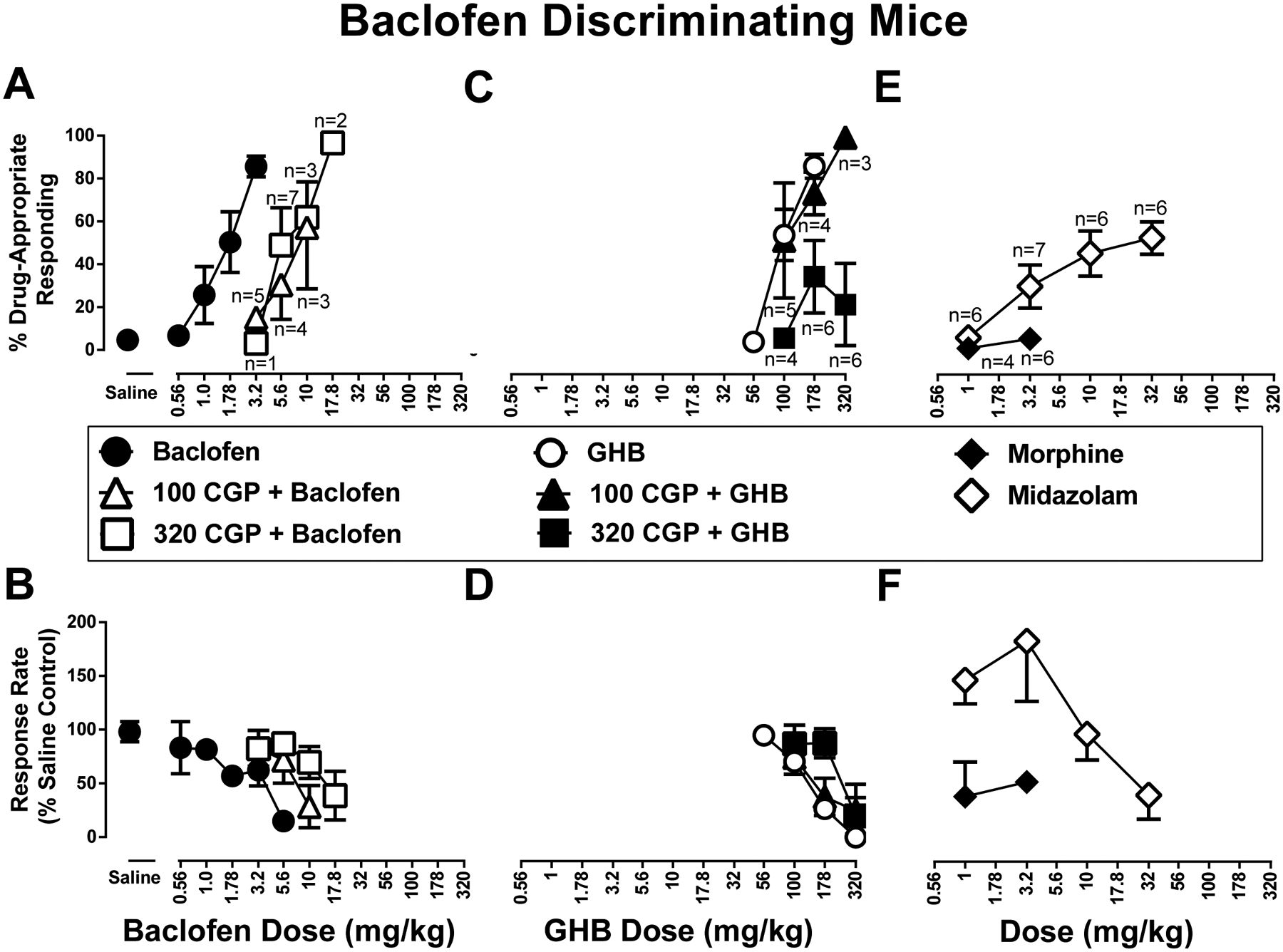
A.) CGP 35348 (100 mg/kg, open upward facing triangles, 320 mg/kg open squares) antagonizes baclofen discriminative stimulus (closed circles) and B.) rate-decreasing effects. C.) At a lower dose CGP 35348 (100 mg/kg, closed upward facing triangles) does not antagonize GHB (open circles) discriminative stimulus and D.) rate-decreasing effects; at a higher dose (320 mg/kg closed squares) it does. E.) Discriminative stimulus and F.) rate-decreasing effects of morphine (closed diamonds) and midazolam (open diamonds). Top panels show drug-appropriate responding on the ordinates and drug dose in mg/kg (log scale) on the abscissae. Bottom panels show response rate normalized to saline control on the ordinates as function of drug dose in mg/kg (log scale) on the abscissae. All data points represent the mean and SEM of 8 independent determinations, except as listed in the top panels. These reduced animal numbers are also reflected in the respective lower panel data.
In baclofen-trained mice, GHB produced a maximum of 86% baclofen-appropriate responding at 178 mg/kg; the dose of 320 mg/kg GHB abolished responding (Figure 1C,D, respectively, open circles). The ED50 values (95% confidence limits) of GHB to produce baclofen-like discriminative stimulus and rate-decreasing effects were 112 (95.8 ― 134) and 149 (122 ― 183) mg/kg (i.p.), respectively. The dose of 100 mg/kg CGP 35348 did not significantly antagonize the either the discriminative stimulus or rate decreasing effects of GHB (Figure 1C, closed triangles). The ED50 values (95% confidence limits) of GHB to produce baclofen-like discriminative stimulus and rate-decreasing effects in the presence of 100 mg/kg CGP 35348 were 159 (2.99 ― 234) and 161 (49.1 ― 248) mg/kg (i.p.), respectively. However, 320 mg/kg CGP 35348 produced antagonism of both the GHB discrimination dose-effect function as well as GHB rate-decreasing effects (Figure 1C and 1D, respectively, filled downward-facing triangles). The ED50 value (95% confidence limits) of GHB to produce rate-decreasing effects in the presence of 320 mg/kg CGP 35348 was 189 (77.1 ― 276) mg/kg (i.p.).
Morphine produced a maximum of 5.4% baclofen-appropriate responding at 3.2 mg/kg; the dose of 3.2 mg/kg morphine decreased response rate to 51% (S.E.M: 7.9%) of control (Figure 1E and 1F, filled squares). The benzodiazepine midazolam produced a maximum of 52% baclofen-appropriate responding at 32 mg/kg; a dose of 100 mg/kg midazolam abolished responding (Figure 1E and 1F, open squares). The ED50 values (95% confidence limits) of midazolam to produce discriminative stimulus and rate-decreasing effects were 21.5 (12.3 ― 202) and 48.8 (27.5 ― 171) mg/kg, respectively.
The discriminative stimulus effects of GHB: substitution and antagonism.
Saline (i.p.) produced 4.3% (SEM 0.9%) drug-appropriate responding in mice trained with 178 mg/kg GHB. The dose-effect functions determined for GHB, once at the beginning and again at the end of the study, were not significantly different from each other (p value > 0.05); these were averaged for further analyses (Figure 2A and 2B, symbols above Saline). In GHB-trained mice, GHB produced a maximum of 85% GHB-appropriate responding at the training dose of 178 mg/kg GHB; a dose of 320 mg/kg GHB abolished responding (Figure 2A and 2B, closed circles). The ED50 values (95% confidence limits) of GHB to produce discriminative stimulus and rate-decreasing effects were 142 (135 ― 150) and 190 (130 ― 250) mg/kg, respectively. In mice trained with GHB, 100 mg/kg CGP 35348 did not significantly modify either the GHB discriminative stimulus dose-effect function or rate decreasing effects of GHB (Figure 2A, and 2B, closed upward facing triangles). The ED50 values (95% confidence limits) of GHB to produce GHB-like discriminative stimulus and rate-decreasing effects in the presence of 100 mg/kg CGP 35348 were 161 (122 ― 213) and 208 (174 ― 248) mg/kg (i.p.), respectively. However, 320 mg/kg CGP 35348 produced a 1.8-fold and 1.5-fold rightward shift in the GHB discriminative stimulus dose-effect function and rate decreasing effects of GHB, respectively (Figure 2A, and 2B, closed downward facing triangles). The ED50 values (95% confidence limits) of GHB to produce GHB-like discriminative stimulus and rate-decreasing effects in the presence of 320 mg/kg CGP 35348 were 260 (210 ― 391) and 282 (222 ― 450) mg/kg (i.p.), respectively.
Figure 2. Discriminative stimulus (top row) and rate-decreasing (bottom row) effects in mice trained to discriminate 178 mg/kg GHB from saline.
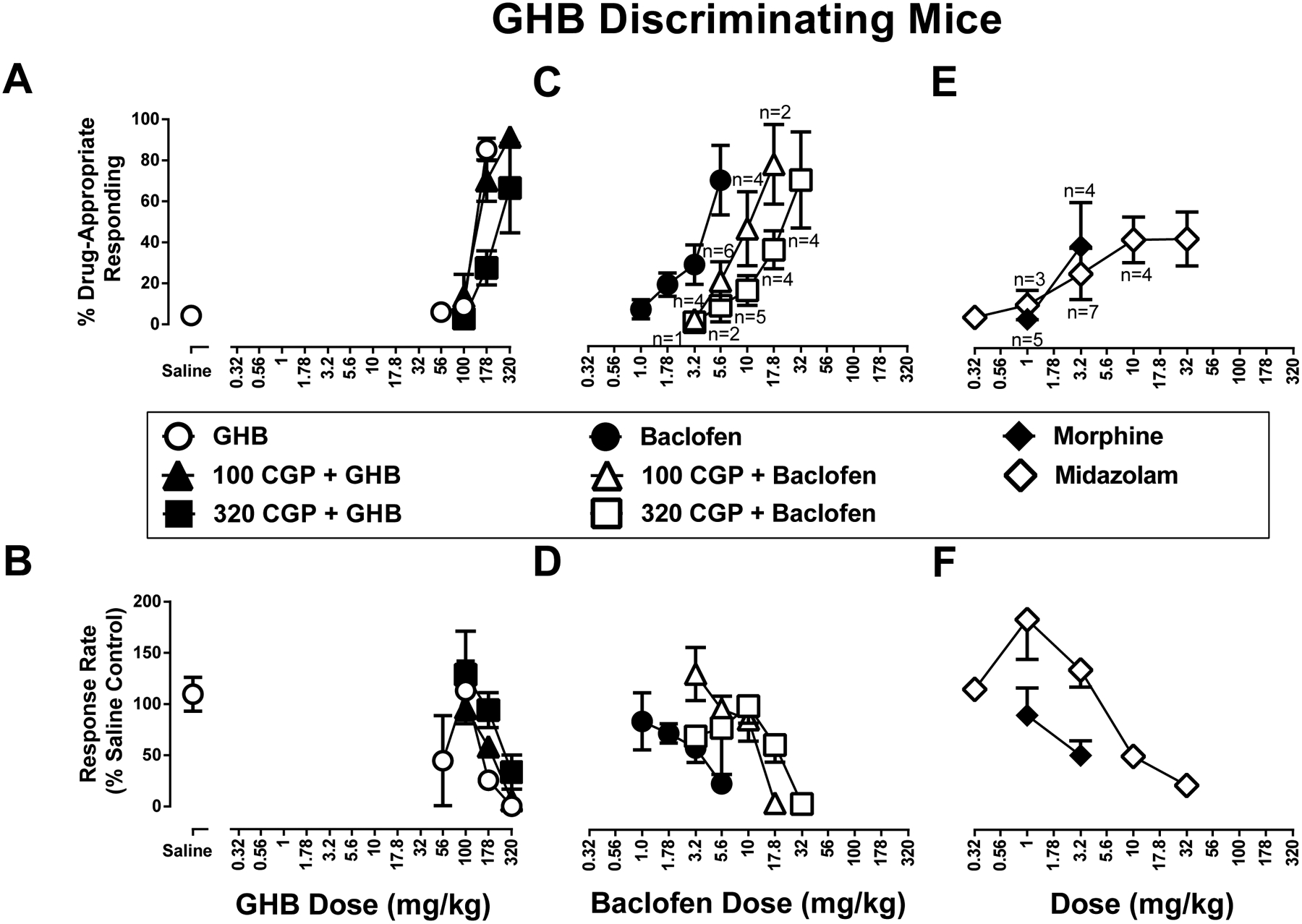
A.) CGP 35348 (100 mg/kg, closed upward facing triangles, 320 mg/kg closed squares) does not significantly antagonize either GHB discriminative stimulus (open circles) or B.) rate-decreasing effects. C.) CGP 35348 (100 mg/kg, open upward facing triangles, 320 mg/kg open squares) antagonizes baclofen discriminative stimulus (closed circles) and D.) rate-decreasing effects. E.) Discriminative stimulus and F.) rate-decreasing effects of morphine (closed diamonds) and midazolam (open diamonds) in mice trained to discriminate 178 mg/kg GHB from saline. Top panels show drug-appropriate responding on the ordinates and drug dose in mg/kg (log scale) on the abscissae. Bottom panels show response rate normalized to saline control on the ordinates as function of drug dose in mg/kg (log scale) on the abscissae. All data points represent the mean and SEM of 8 independent determinations, except as listed in the top panels. These reduced animal numbers are also reflected in the respective lower panel data.
In GHB-trained mice, baclofen produced a maximum of 70% GHB-appropriate responding at 5.6 mg/kg; a dose of 10 mg/kg baclofen abolished responding (Figure 2C and 2D, closed circles). The ED50 values (95% confidence limits) of baclofen to produce GHB-like discriminative stimulus and rate-decreasing effects were 4.45 (3.57 ― 6.78) and 3.61 (2.41 ― 83.6) mg/kg, respectively. In mice trained with GHB, CGP 35348 produced dose-dependent rightward shifts in the baclofen discriminative stimulus dose-effect function (Figure 2C, open triangles). When combined with CGP 35348 doses of 100 and 320 mg/kg, maximum GHB-appropriate responding was 46.3% and 36.9%, respectively. Likewise, CGP 35348 (100, 320 mg/kg, i.p.) produced dose-dependent rightward shifts in the baclofen response rate dose-effect function (Figure 2D, open triangles). The ED50 values (95% confidence limits) of baclofen rate-decreasing effects in the presence of 100 or 320 mg/kg CGP 35348 were 12.8 (9.89 ― 20.8) and 20.0 (13.8 ― 33.4) mg/kg (i.p.), respectively.
Morphine produced a maximum of 28% GHB-appropriate responding; the dose of 3.2 mg/kg morphine decreased response rate to 50% of control (S.E.M: 14.2%) (Figure 2E and 2F, closed squares). Midazolam produced a maximum of 42% GHB-appropriate responding at 32 mg/kg (Figure 2E and 2F, open squares). The ED50 value (95% confidence limits) of midazolam to decease response rate was 40.7 (23.8 ― 69.8) mg/kg.
Discussion
Separate groups of mice can be trained to discriminate either 178 mg/kg i.p. GHB or 3.2 mg/kg i.p. baclofen. The observed partial cross-substitution, i.e., GHB partially substituted for the baclofen discriminative stimulus, and baclofen partially substituted for the GHB discriminative stimulus, suggests that these drugs produce discriminative stimulus effects through overlapping pharmacological mechanisms. Antagonism of both drugs by the GABAB receptor antagonist CGP 35348 demonstrates the involvement of GABAB receptors in mediating the discriminative stimulus effects of both drugs. These results are consistent with and extend the results of previous drug discrimination studies in other species (Koek et al., 2004; 2006). These results also extend the noted differences in the potency of GABAB receptor antagonists in the behavioral effects of baclofen and GHB in a number of different assays including the discriminative stimulus properties of these two drugs (Koek et al., 2007; Koek & France, 2008).
These results add to a growing literature showing that GHB possesses a complex pharmacology. GHB exerts GABAB receptor agonist activity, albeit and apparently as part of a complex pharmacology that includes mechanisms in addition to GABAB receptors. The additional pharmacological activity of GHB has been suggested to include actions at GABAA receptors as well as a putative GHB receptor (Bay et al., 2014; van Nieuwenhuijzen & McGregor, 2009; Connelly et al., 2013; Molnár et al., 2009). Previous studies in mice demonstrate that GHB shares many similar properties to alcohol, but the two drugs do not fully substitute for one another (Cook et al., 2006). Although outside the scope of this short report, additional mouse drug discrimination studies investigating the relative contribution of GABAA receptors and the putative GHB receptor would provide additional insights into GHB-related pharmacology.
In the design of future baclofen and GHB drug discrimination assays, the dose of the training drug should be considered. Here, we based our training doses of baclofen and GHB off of previous reports in pigeons (Koek et al., 2013). One aspect of drug discrimination is the dose of the drug selected for training as a discriminative stimulus is known to impact the pharmacological selectivity of the resulting discrimination. For example, the discrimination of a relatively small training dose can lack pharmacological selectivity because the magnitude of the difference between the presence of a “drug effect” versus its absence is relatively small and difficult to detect. Lack of pharmacological selectivity is evidenced by substitution of test drugs with mechanisms of action distinct from the training drug. This may be one explanation for the similar but not overlapping baclofen and GHB discriminative effects in these current studies. In contrast, sufficiently large training doses can result in discriminations that are relatively selective for test drugs that share a mechanism of action with the training drug (Smith and Stolerman, 2009; Cunningham and McMahon, 2013). Thus, it will be important to look at higher GHB and baclofen training doses in future studies. In these studies, morphine produced some discriminative stimulus effects similar to GHB but not baclofen. One possible explanation for this observation may be that the mice were perhaps partially discriminating a sedative-like effect. Without further information, only a limited interpretation of these data is possible. Thus, additional substitution studies with different drug classes or other drugs within the same drug class, as well as additional antagonism studies, are needed to evaluate the complex pharmacology of GHB and baclofen.
The current in vivo literature establishing the GABAB receptor pharmacology of GHB has relied primarily on receptor antagonists and agonists. These current results showing that mice can reliably discriminate either baclofen or GHB underscore the potential utility of integrating drug discrimination assays, pharmacological tools, and genetic knockout or transgenic strategies currently most readily available in mice to interrogate the complex receptor pharmacology of GHB.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Colin Cunningham, Mr. David Schulze, and Mr. Armia Zaki for technical assistance.
Funding:
This work was supported by the National Institute on Drug Abuse DA25267 and DA48353 (LRM)
Non-standard abbreviations:
- CGP 35348
γ-aminobutyric acid (GABA), (3-Aminopropyl)(diethoxymethyl)phosphinic acid
- GHB
gamma hydroxybutyrate
- FR
fixed ratio
Footnotes
Conflicts of Interests or Disclaimers: None
References
- Addolorato G, Leggio L, Ferrulli A, Caputo F, and Gasbarrini A (2009) The therapeutic potential of gamma-hydroxybutyric acid for alcohol dependence: balancing the risks and benefits. A focus on clinical data. Expert Opin Investig Drugs 18:675–686. [DOI] [PubMed] [Google Scholar]
- Bay T, Eghorn LF, Klein AB, and Wellendorph P (2014) GHB receptor targets in the CNS: focus on high-affinity binding sites. Biochem Pharmacol 87:220–228. [DOI] [PubMed] [Google Scholar]
- Boscolo-Berto R, Viel G, Montagnese S, Raduazzo DI, Ferrara SD, and Dauvilliers Y (2012) Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev 16:431–443. [DOI] [PubMed] [Google Scholar]
- Carter LP, Pardi D, Gorsline J, and Griffiths R (2009) Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse. Drug Alcohol Depend 104:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Wu H, Chen W, Matthews MM, Mehta AK, Hernandez RJ, Thomson JA, Ticku MK, Coop A, Koek W, et al. (2005) Novel gamma-hydroxybutyric acid (GHB) analogs share some, but not all, of the behavioral effects of GHB and GABAB receptor agonists. J Pharmacol Exp Ther 313:1314–1323. [DOI] [PubMed] [Google Scholar]
- Chou R, Peterson K, and Helfand M. (2004) Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage 28:140–75. [DOI] [PubMed] [Google Scholar]
- Connelly WM, Errington AC, and Crunelli V (2013) γ-Hydroxybutyric acid (GHB) is not an agonist of extrasynaptic GABAA receptors. PLoS One 8:e79062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CD, Biddlestone L, Coop A, and Beardsley PM (2006). Effects of combining ethanol (EtOH) with gamma-hydroxybutyrate (GHB) on the discriminative stimulus, locomotor, and motor-impairing functions of GHB in mice. Psychopharmacology, 185:112–122. [DOI] [PubMed] [Google Scholar]
- Cunningham CS, McMahon LR. (2013) Multiple nicotine training doses in mice as a basis for differentiating the effects of smoking cessation aids. Psychopharmacology (Berl) 228:321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deseure K and Hans GH (2017) Differential drug effects on spontaneous and evoked pain behavior in a model of trigeminal neuropathic pain. J Pain Res 10:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Fresu A, Collu M and Fratta W (2003) Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse 50:1–6. [DOI] [PubMed] [Google Scholar]
- Froestl W, Mickel SJ, Von Sprecher G, Strub D, Baumann PA, Brugger F, Gentsch C, Jaekel J, Olpe HR, Rihs G, et al. (1995) Phosphinic acid analogues of GABA: 2. Selective, orally active GABAB antagonists. J Med Chem 38:3313–3331. [DOI] [PubMed] [Google Scholar]
- Fu Z, Yang H, Xiao Y, Zhao G and Huang H (2012) The gamma-aminobutyric acid type B (GABAB) receptor agonist baclofen inhibits morphine sensitization by decreasing the dopamine level in rat nucleus accumbens. Behav Brain Funct 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A and Nutt DJ (2005) Gamma hydroxy butyrate abuse and dependency. J Psychopharmacol 19:195–204. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Froestl W, and Weerts EM (2005) Involvement of gamma-hydroxybutyrate (GHB) and GABA-B receptors in the acute behavioral effects of GHB in baboons. Psychopharmacology (Berl) 180:342–351. [DOI] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals 8th ed. Institute for Laboratory Animal Research, Division of Earth and Life Sciences, National Research Council, (2011) Washington, DC. [Google Scholar]
- Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, Schmutz M, Froestl W, van der Putten H, Mosbacher J, et al. (2003) Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci 18:2722–2730. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, DeWitte P and Kalivas PW (1992) Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci 12:2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Chen W, Mercer SL, Coop A, and France CP (2006) Discriminative stimulus effects of γ-hydroxybutyrate: role of training dose. J Pharmacol Exp Ther 317:409–417. [DOI] [PubMed] [Google Scholar]
- Koek W, Cheng K and Rice KC (2013) Discriminative stimulus effects of the GABAB receptor-positive modulator rac-BHFF: comparison with GABAB receptor agonists and drugs of abuse. J Pharmacol Exp Ther 344:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Flores LR, Carter LP, Lamb RJ, Chen W, Wu H, Coop A, and France CP (2004) Discriminative stimulus effects of γ-hydroxybutyrate in pigeons: role of diazepam-sensitive and -insensitive GABAA and GABAB receptors. J Pharmacol Exp Ther 308:904–911. [DOI] [PubMed] [Google Scholar]
- Koek W and France CP (2008) Cataleptic effects of gamma-hydroxybutyrate (GHB) and baclofen in mice: Mediation by GABA(B) receptors, but differential enhancement by N-methyl-d-aspartate (NMDA) receptor antagonists. Psychopharmacology (Berl) 199:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Mercer SL, and Coop A (2007) Cataleptic effects of gamma-hydroxybutyrate (GHB), its precursor gamma-butyrolactone (GBL), and GABAB receptor agonists in mice: differential antagonism by the GABAB receptor antagonist CGP35348. Psychopharmacology (Berl) 192:407–414. [DOI] [PubMed] [Google Scholar]
- Laborit H (1964) Sodium 4-hydroxybutyrate. Int J Neuropharmacol 3:433–451 [DOI] [PubMed] [Google Scholar]
- Maitre M (1997) The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol 51:337–361. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Masciadri R, Norcross RD, Knoflach F, Kratzeisen C, Zenner MT, Kolb Y, Marcuz A, Huwyler J, Nakagawa T, Porter RH, Thomas AW, Wettstein JG, Sleight AJ, Spooren W and Prinssen EP (2008) Characterization of (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one as a positive allosteric modulator of GABAB receptors. Br J Pharmacol 154:797–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S, Quan W, Qi X, Su Z and Yang S (2014) Effect of baclofen on morphine-induced conditioned place preference, extinction, and stress-induced reinstatement in chronically stressed mice. Psychopharmacology (Berl) 231:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár T, Antal K, Nyitrai G, and Emri Z (2009) Gamma-hydroxybutyrate (GHB) induces GABAB receptor independent intracellular Ca2+ transients in astrocytes, but has no effect on GHB or GABAB receptors of medium spiny neurons in the nucleus accumbens. Neuroscience 162:268–281. [DOI] [PubMed] [Google Scholar]
- Pedron VT, Varani AP and Balerio GN (2016) Baclofen prevents the elevated plus maze behavior and BDNF expression during naloxone precipitated morphine withdrawal in male and female mice. Synapse 70:187–197. [DOI] [PubMed] [Google Scholar]
- Ramshini E, Alaei H, Reisi P, Alaei S and Shahidani S (2013) The Role of GABAB Receptors in Morphine Self-Administration. Int J Prev Med 4:158–164. [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R and Poeggel K (2002) Baclofen decreases methamphetamine self-administration in rats. Neuroreport 13:1107–1110. [DOI] [PubMed] [Google Scholar]
- Roth RH (1970) Formation and regional distribution of gamma-hydroxybutyric acid in mammalian brain. Biochem Pharmacol 19:3013–9. [DOI] [PubMed] [Google Scholar]
- Sadiq SA and Poopatana CA (2007) Intrathecal baclofen and morphine in multiple sclerosis patients with severe pain and spasticity. J Neurol 254:1464–1465. [DOI] [PubMed] [Google Scholar]
- Salte K, Lea G, Franek M and Vaculin S (2016) Baclofen reversed thermal place preference in rats with chronic constriction injury. Physiol Res 65:349–355. [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. (2009) Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol 295–333. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nurrochmad A, Ozaki M, Khotib J, Nakamura A, Imai S, Shibasaki M, Yajima Y and Narita M (2005) Effect of a selective GABA(B) receptor agonist baclofen on the mu-opioid receptor agonist-induced antinociceptive, emetic and rewarding effects. Neuropharmacology 49:1121–1131. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ (2000) Dose-response analysis, in Drug Synergism and Dose-Effect Data Analysis, pp 44–50, Chapman & Hall/CRC, Boca Raton, FL. [Google Scholar]
- Topkara B, Yananli HR, Sakalli E and Demirkapu MJ (2017) Effects of Injection of Gamma-Aminobutyric Acid Agonists into the Nucleus Accumbens on Naloxone-Induced Morphine Withdrawal. Pharmacology 100:131–138. [DOI] [PubMed] [Google Scholar]
- van Nieuwenhuijzen PS, and McGregor IS (2009) Sedative and hypothermic effects of gamma-hydroxybutyrate (GHB) in rats alone and in combination with other drugs: assessment using biotelemetry. Drug Alcohol Depend 103:137–147. [DOI] [PubMed] [Google Scholar]
- Xi ZX and Stein EA (1999) Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J Pharmacol Exp Ther 290:1369–1374. [PubMed] [Google Scholar]
- Zemoura K, Ralvenius WT, Malherbe P and Benke D (2016) The positive allosteric GABAB receptor modulator rac-BHFF enhances baclofen-mediated analgesia in neuropathic mice. Neuropharmacology 108:172–178. [DOI] [PubMed] [Google Scholar]


