Abstract
Acquired hemophilia A is a rare bleeding diathesis most typically seen in systemic rheumatic disease, solid and hematologic malignancies, and pregnancy. We present a case of this condition that occurred immediately after vaccination against SARS-CoV-2 with the mRNA-1273 (Moderna) vaccine.
Keywords: Acquired hemophilia A, COVID-19, mRNA vaccination, SARS-CoV-2, vaccine reactions
Widely available mRNA vaccines have shown impressive efficacy against SARS-CoV-2. Significant adverse reactions have been rare. We report one such potential reaction, a case of acquired hemophilia A (AHA) that occurred 2 weeks after administration of a second Moderna mRNA-1273 inoculation.
CASE DESCRIPTION
A 61-year-old woman presented to our institution after developing diffuse ecchymosis, most predominantly in her lower extremities, along with severe anemia. She reported a history of rheumatoid arthritis for which she was previously given methotrexate, but denied any current or recent symmetric synovitis and had not received treatment for it in over 10 years. Her rheumatoid factor was normal at 11 IU/mL, with a negative antinuclear antibody screen, but her cyclic citrullinated peptide antibody was mildly elevated at 32, with an elevated quantitative C-reactive protein of 1.7 mg/dL. Her past medical history was otherwise negative. She was (and remains) a very active fitness instructor with no prior bruising or bleeding issues, including during previous bilateral knee replacement surgeries.
She had her first mRNA-1273 vaccination against SARS-CoV-2 on April 15, 2021, and her second 6 weeks later. Two weeks after her second shot, she developed bruising on her inner thigh (Figure 1), as well as rapidly worsening exertional dyspnea. After seeing her primary care physician, she was sent to her local emergency department, where she was found to have a hemoglobin of 6.6 g/dL with a mean corpuscular volume of 69 fL. Platelets were elevated at 894 K/μL. She had no prior history of gastrointestinal bleeding, and her hemoglobin and platelets had been within normal limits on routine laboratory tests 9 months earlier. She had never previously undergone any type of endoscopy. Subsequent studies confirmed iron deficiency. Over the next 2 months, she was admitted multiple times with recurrent bruising and bleeding, with hemoglobin as low as 5.4 g/dL, and received a total of 11 packed red blood cell units. Workup revealed an elevated activated partial thromboplastin time (aPTT) of 42 seconds with a significantly diminished Factor VIII activity level of 12%. Initially, a Factor VIII inhibitor was not detected, and mixing of her serum with normal plasma showed correction of aPTT, which would typically be associated with deficiency rather than any inhibition. One subsequent study did identify an inhibitor, but no titer was given. Von Willebrand labs were unremarkable. She was subsequently started on human plasma-derived von Willebrand Factor (Humate P) with rapid improvement in her hematomas and stabilization of her hemoglobin. Subsequent evaluation including computed tomography of the chest, abdomen, and pelvis was negative for malignancy.
Figure 1.
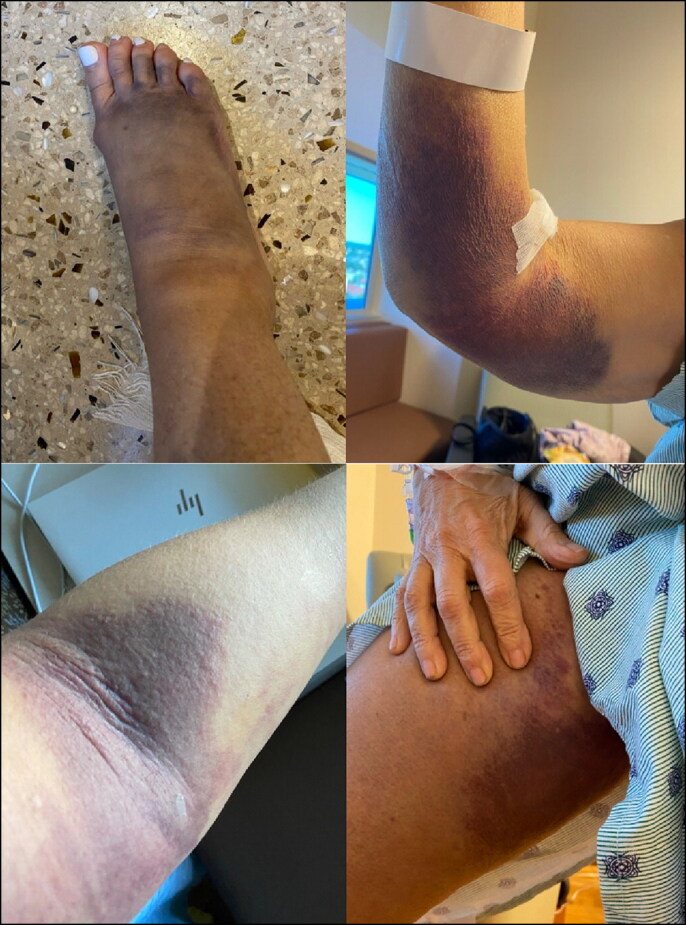
Our patient’s arms and legs after presentation.
She was again discharged but presented 2 weeks later with upper gastrointestinal bleeding. Laboratory tests showed that her aPTT had normalized at 26.5 seconds, but her Factor VIII activity level remained diminished at 16% (Table 1). She was given recombinant Factor VIIa and underwent endoscopic cauterization of a Dieulafoy lesion. Due to the concern for a possible inhibitor, she then received four weekly rituximab infusions in combination with a slow prednisone taper. Her Factor VIII activity level subsequently normalized and a Factor VIII inhibitor has not been detected again. She has had no further bleeding or bruising episodes. We have advised that she receive no further SARS CoV-2 mRNA vaccinations.
Table 1.
Relevant laboratory data from our patient
| Date | Factor VIII activity (%) [50–200]* | Factor VIII inhibitor panel | aPTT (sec) [22–32] | Hemoglobin (g/dL) [12–16] | Platelets (K/μL) [140–440] |
|---|---|---|---|---|---|
| 11/16/20 | 13.9 | 389 | |||
| 8/6/21 | 39.6 | 6.6 | 894 | ||
| 9/14/21 | 12 | Not detected | 41.7 | 5.1 | 658 |
| 9/29/21 | 8 | Detected | 32.2 | 9.6 | 642 |
| 10/22/21 | 16 | 26.5 | 11.0 | 388 | |
| 11/5/21 | 53 | Not detected | 24.3 | 11.9 | 503 |
| 12/22/21 | 184 | 13.9 | 401 | ||
| 4/8/22 | 12.9 | 329 |
Values in brackets represent reference range. aPTT indicates activated partial thromboplastin time.
DISCUSSION
AHA is an unusual and potentially lethal bleeding diathesis that results from the development of autoantibodies against endogenous Factor VIII, sometimes producing a clinical scenario similar to that of congenital hemophilia A. While it is most typically seen in patients with systemic rheumatic disease, solid and hematologic malignancies, and pregnancy, approximately half of all AHA cases remain idiopathic.1 This condition has rarely been associated with infection with SARS-CoV-2,2 and we have found only a few reports of it being attributed to vaccination against this novel coronavirus.3–6 We believe we have identified an additional case. Our patient was administered the mRNA-1273 version developed by Moderna. The foregoing reports have involved both this vaccine and the one developed by Pfizer-BioNTech.
To date, over 470 million doses of mRNA vaccines have been administered. Common side effects have included fevers, chills, headaches, myalgias, and injection site discomfort. Anaphylaxis can also rarely occur.7 Adenoviral vector-based SARS-CoV-2 vaccines have rarely been associated with a different bleeding diathesis, thrombotic thrombocytopenia.8 We are not aware of any reported cases of this reaction attributed to mRNA vaccines. Other vaccinations, including ones for influenza, have been associated with autoimmune reactions, including AHA.9 Given the rarity of these events and the large number of vaccination episodes, for most vaccines, any true association with autoimmunity remains speculative, as does any mechanism. In these highly unusual events, it may be that vaccines induce immune system cross-reactivity due to similarity between vaccine and host antigens, a process sometimes called molecular mimicry. While not definitive, one recent study found minimal overlap between the amino acid sequences of Factor VIII and the SARS-CoV-2 spike protein, suggesting that this may not be the pathogenic mechanism in these cases.10 Another proposed mechanism is vaccine-induced bystander activation of self-antigen–expressing antigen-presenting cells.11
We recognize that it would be difficult to establish conclusively that vaccination of our patient with mRNA-1273 caused her AHA. She did report a history of rheumatoid arthritis, which has been associated with this condition. Moreover, the majority of our studies did not detect a Factor VIII inhibitor, something we would have expected regardless of what caused the decrease in her Factor VIII activity. However, she did not meet American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for rheumatoid arthritis, and she reported no prior bleeding issues despite multiple surgical challenges and a very active lifestyle. Further, one of our studies did detect an inhibitor. Given the timing of this finding so soon after her second inoculation, we believe the most plausible explanation for her dramatic clinical and laboratory findings was a vaccine-induced inhibitor that was present at a level sufficient to precipitate her AHA but low enough to evade the majority but not all of our testing.
References
- 1.Franchini M, Lippi G.. Acquired factor VIII inhibitors. Blood. 2008;112(2):250–255. doi: 10.1182/blood-2008-03-143586. [DOI] [PubMed] [Google Scholar]
- 2.Franchini M, Glingani C, De Donno G, et al. The first case of acquired hemophilia A associated with SARS-CoV-2 infection. Am J Hematol. 2020;95(8):E197–E198. doi: 10.1002/ajh.25865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radwi M, Farsi S.. A case report of acquired hemophilia following COVID-19 vaccine. J Thromb Haemost. 2021;19(6):1515–1518. doi: 10.1111/jth.15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez R, Gutierrez-Nunez J, Fonseca Ferrer V, Torres G, Alvarez Cardin N.. Dark skin-acquired hemophilia A after Pfizer-Biontech Covid-19 vaccine. Chest. 2021;160(4):A1384. doi: 10.1016/j.chest.2021.07.1265. [DOI] [Google Scholar]
- 5.Cittone MG, Battegay R, Condoluci A, et al. The statistical risk of diagnosing coincidental acquired hemophilia A following anti-SARS-CoV-2 vaccination. J Thromb Haemost. 2021;19(9):2360–2362. doi: 10.1111/jth.15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leone MC, Canovi S, Pilia A, et al. Four cases of acquired hemophilia A following immunization with mRNA BNT162b2 SARS-CoV-2 vaccine. Thromb Res. 2022;211:60–62. doi: 10.1016/j.thromres.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erdeljic Turk V. Anaphylaxis associated with the mRNA COVID-19 vaccines: approach to allergy investigation. Clin Immunol. 2021;227:108748. doi: 10.1016/j.clim.2021.108748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S.. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulis G, Pugnet G, Bagheri H, et al. Acquired factor VIII haemophilia following influenza vaccination. Eur J Clin Pharmacol. 2010;66(10):1069–1070. doi: 10.1007/s00228-010-0852-z. [DOI] [PubMed] [Google Scholar]
- 10.Hirsiger JR, Martinez M, Tsakiris DA, et al. Investigating potential mechanisms underlying FVIII inhibition in acquired hemophilia A associated with mRNA COVID-19 vaccines. J Thromb Haemost. 2022;20(4):1015–1018. doi: 10.1111/jth.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wraith DC, Goldman M, Lambert PH.. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362(9396):1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]


