Abstract
Dermatological reactions have been reported following Shingrix vaccine administration in previous published cases, but dermatomal rash after Shingrix vaccination has not been reported in the United States. This case describes a 73-year-old immunocompetent woman with a dermatomal rash after Shingrix recombinant vaccine administration. This case highlights the rare possibility of an acute reaction after Shingrix vaccine administration, which should be recognized. Nonetheless, the vaccine has been shown to be very effective at preventing varicella zoster virus reactivation and postherpetic neuralgia, so the benefit of receiving the vaccination significantly outweighs the risk.
Keywords: Dermatomal rash, herpes zoster, Shingrix, vaccine
Herpes zoster (Shingles) is a dermatomal rash caused by reactivation of the varicella zoster virus (VZV). VZV usually occurs in childhood, presenting as chickenpox, and remains dormant in the dorsal root ganglia.1 It is often reactivated later in life from stress, aging, or immunodeficiency. Zoster presents as a maculopapular rash that can evolve into vesicular lesions with unilateral dermatomal distribution. It can be further complicated by postherpetic neuralgia or herpes zoster encephalitis.2 The Advisory Committee on Immunization Practices currently recommends adjuvanted recombinant Shingrix vaccines in two doses for all adults over 50 to reduce the incidence of zoster and/or postherpetic neuralgia.3 While Zostavax was a live vaccine with limited efficacy in the older age group, Shingrix is an adjuvanted recombinant zoster vaccine that is widely sought due to its strong efficacy with a sustained antibody response.3
CASE PRESENTATION
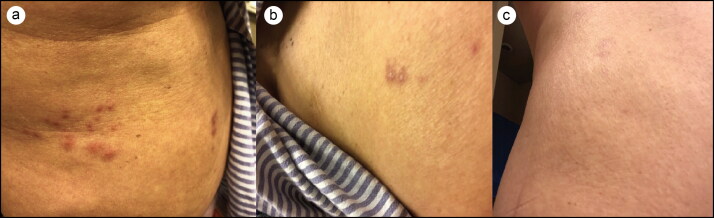
A 73-year-old woman with known hypertension, hypothyroidism, and stage IIA infiltrating ductal breast cancer status post bilateral mastectomy on letrozole for 3 years reported mild, itchy rash 3 days after receiving the first dose of the Shingrix vaccine. It started with vesicular lesions on the right side of her abdominal wall, which over the next few days progressed to her back in the L3–L4 dermatome (Figure 1a, 1b). She described the rash as tingly, itchy, and tender with no discharge from vesicular lesions. The day after rash onset, she flew to the Philippines and returned 7 days later. She received the flu vaccine at the same time as Shingrix. She had chickenpox as a child and had received the Zostavax vaccine about 10 years earlier with no complications. Upon returning from the Philippines, she contacted the internal medicine clinic. Since the vesicular lesions had resolved, no samples could be sent for Tzank smear. Her rash completely resolved over the next few days with no sequela of pain (Figure 1c).
Figure 1.
(a, b) Dermatomal herpes zoster rash after Shingrix administration. (c) Resolution of the rash.
DISCUSSION
Shingrix is an adjuvanted subunit vaccine made up of a single recombinant VZV antigen, glycoprotein E, and the AS01B adjuvant system.4 Glycoprotein E elicits anti-VZV immunity while the adjuvant system stimulates VZV-specific antibody and a CD4 T-cell response.5 A review of multiple trials suggested strong immune response and significant efficacy with minimal serious adverse events to prevent herpes zoster in immunocompetent adults.6 Similarly, in immunocompromised patients, Shingrix vaccination resulted in improved antibody response with significant disease reduction, further establishing its efficacy and safety.7,8
There are no published data in the United States on Shingrix-associated VZV rash, unlike the Zostavax live vaccine, which has had serious side effects in the immunocompromised.9 The components of this recombinant vaccine are not known to cause a reaction, and the rationale for dermatomal rash after vaccination in an immunocompromised individual is not known.
In our case, the patient was administered seasonal influenza vaccine on the same day she received the Shingrix vaccine. The data suggest that Shingrix can be safely administered with the influenza vaccine without affecting the immune response of either vaccine.10 The most common side effects reported with Shingrix are pain, redness, and swelling at the injection site.11 The most common systemic effects in the trials were myalgia (44.7%), fever (20.5%), headache (37.7%), shivering (26.8%), fatigue (44.5%), and gastrointestinal symptoms (17.3%).3 However, there have been a few case reports mentioning a dermatological reaction to Shingrix. Thompson et al described a bullous rash 2 days after receiving the second dose of Shingrix vaccine.12 Another report mentioned a 74-year-old woman with known ulcerative colitis presenting with a blistering autoimmune skin reaction following Shingrix administration.13 A review was available about a case report in German suggesting occurrence of dermatomal rash after Shingrix.14 Although dermatomal reactions after Shingrix are rare, we need to recognize them.
Shingrix vaccine causing herpes zoster was not observed in study trials. It is possible that the rash resulted from reduced efficacy of previous Zostavax administration (10 years earlier). Further studies are needed with laboratory data including antibody levels and Tzank smear to identify the pathophysiology. This case report should not deter physicians from administering Shingrix, given its strong efficacy in preventing morbidity from zoster rash and postherpetic neuralgia. In a 2021 update, the Advisory Committee on Immunization Practices recommended two doses of Shingrix for adults 19 or older who are or will be immunodeficient or immunosuppressed due to disease or therapy.15
References
- 1.Longo DL, Fauci AS, Kasper D, Hauser S, Jameson J, Loscalzo J.. Harrison's Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012. [Google Scholar]
- 2.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–108. doi: 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lecrenier N, Beukelaers P, Colindres R, et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Rev Vacc. 2018;17(7):619–634. doi: 10.1080/14760584.2018.1495565. [DOI] [PubMed] [Google Scholar]
- 5.Heineman TC, Cunningham A, Levin M.. Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine. Curr Opin Immunol. 2019;59:42–48. doi: 10.1016/j.coi.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 6.James SF, Chahine EB, Sucher AJ, Hanna C.. Shingrix: the new adjuvanted recombinant herpes zoster vaccine. Ann Pharmacother. 2018;52(7):673–680. doi: 10.1177/1060028018758431. [DOI] [PubMed] [Google Scholar]
- 7.Bastidas A, Serna J, Idrissi ME, et al; ZOE-HSCT Study Group Collaborators. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123–133. doi: 10.1001/jama.2019.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagnew AF, Ilhan O, Lee W, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988–1000. doi: 10.1016/S1473-3099(19)30163-X. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz-Brizuela E, Leal-Vega F, Cuellar-Rodriguez J, Bobadilla-Del-Valle M, Ponce-de-Leon A.. Vaccine-derived varicella zoster infection in a kidney transplant recipient after zoster vaccine live administration. Vaccine. 2019;37(27):3576–3579. doi: 10.1016/j.vaccine.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz TF, Aggarwal N, Moeckesch B, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit vaccine coadministered with seasonal influenza vaccine in adults aged 50 years or older. J Infect Dis. 2017;216(11):1352–1361. doi: 10.1093/infdis/jix481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 12.Thompson H, Nichols L, Gonzalez Santiago T.. Bullous fixed drug eruption following administration of the recombinant adjuvant Shingrix vaccine. BMJ Case Rep. 2021;14(8):e241293. doi: 10.1136/bcr-2020-241293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell H, Kamal N, Wong U.. Blistering autoimmune skin reaction following SHINGRIX vaccination in an ulcerative colitis patient: case report and literature review. Vaccine. 2020;38(47):7455–7457. doi: 10.1016/j.vaccine.2020.09.073. [DOI] [PubMed] [Google Scholar]
- 14.Kohn D, Wetzig T.. Zostererkrankung nach Shingrix-Impfung [Zoster disease after Shingrix vaccination]. Hautarzt. 2021;72(8):729–732. doi: 10.1007/s00105-020-04738-5. [DOI] [PubMed] [Google Scholar]
- 15.Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–84. doi: 10.15585/mmwr.mm7103a2. [DOI] [PMC free article] [PubMed] [Google Scholar]