Abstract
Influenza virus infection is a rare cause of neurological complications, with acute necrotizing encephalopathy (ANE) being among the deadliest. Due to the low incidence of ANE, literature about its association with influenza B infection is limited. We present the case of a 29-year-old previously healthy man with an imaging and clinical diagnosis of influenza B virus infection and sudden decline in mental status. Magnetic resonance imaging showed multifocal areas of abnormal T2 FLAIR signal and restricted diffusion without significant enhancement, with negative mircobiological studies of cerebrospinal fluid. The patient died despite multiple treatments including an antiviral, steroids, and intravenous immunoglobulin. Due to ANE’s more common presentation during childhood, this case report represents one of the few available publications in the adult population.
KEYWORDS: acute necrotizing encephalitis, influenza B, neurological complications
Influenza virus infection can cause neurological complications, acute necrotizing encephalopathy (ANE) being one of the deadliest. ANE can be caused by other viral infections such as varicella zoster, human herpesvirus-6, enterovirus, and COVID-19.1 First described by Mizuguchi et al in 1995 in the pediatric population, ANE is characterized by symmetric, multifocal lesions in the brain that mainly affect bilateral thalami, putamen, internal and external capsules, and the cerebellum. Mortality is typically high, approximately 30%, with a complete recovery rate <10%.2 Pathophysiology remains unclear, but it is proposed to involve immune-mediated reactions secondary to viral infection.3
CASE PRESENTATION
A 29-year-old previously healthy man, with no history of toxic habits, an unknown vaccination status, and no family history of neurological diseases, presented with fever, fatigue, headache, and vomiting for 1 week. Two days earlier, he was diagnosed with an influenza B virus infection. He was hospitalized for a new diagnosis of diabetes mellitus associated with ketoacidosis and was fully oriented without neurologic deficit at admission. His mental status suddenly declined, and he had absent brainstem reflexes and was unresponsive to painful stimuli. A noncontrast head computed tomography scan revealed diffuse cerebral edema (Figure 1).
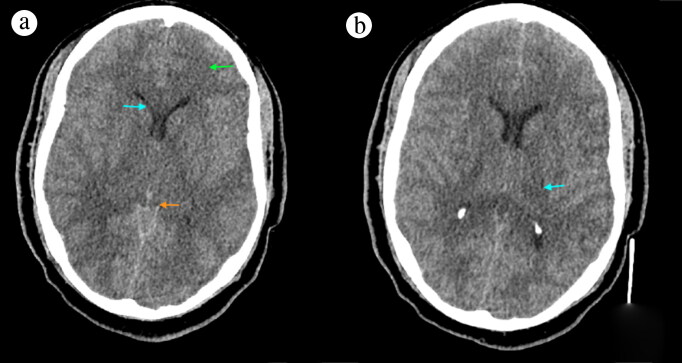
Figure 1.
Noncontrast CT (a) at the level of the basal cistern revealed extensive hypodensity in the white matter, basal ganglia, and thalami consistent with a global insult (green arrow), as well as narrow ventricles (blue arrow) and effacement of the basal cisterns (orange arrow). The (b) thalamus level showed mild hypodensity on the left thalamus.
An external ventricular device was placed to monitor intracranial pressure with an opening pressure of 24 mm Hg. The patient was started on methylprednisolone, hypertonic saline, and oseltamivir. A COVID-19 test was negative, and a liver panel showed abnormal markers. Electroencephalography suggested generalized encephalopathy without epileptiform discharge. Cerebrospinal fluid (CSF) analysis revealed reddish color, red blood cells of 5406/mm3, white blood cells of 8/mm3 (neutrophils 75%, lymphocytes 12%), glucose of 220 mg/dL, and protein of 71 mg/dL. A CSF gram stain, IgM and IgG for West Nile virus, and tests for herpes simplex viruses 1 and 2, measles, and mumps were negative.
Three days after admission, the serum sodium level surged to 170 mEq/L, hypertonic saline was discontinued, and normal saline was initiated along with vasopressin. Four days after admission, magnetic resonance imaging (MRI) showed multifocal areas of T2 FLAIR hyperintensity and abnormal restricted diffusion on bilateral basal ganglia, thalami, splenium of the corpus callosum, hippocampal regions, right temporal lobe, midbrain, and middle cerebral peduncles with multiple microhemorrhages on the bilateral basal ganglia without abnormal enhancement. Magnetic resonance venography of the brain was unremarkable, and imaging findings were compatible with ANE (Figure 2). Intravenous immunoglobulin was started on the fifth day of hospitalization4–6 without any clinical improvement, and the patient expired on the eighth day of admission.
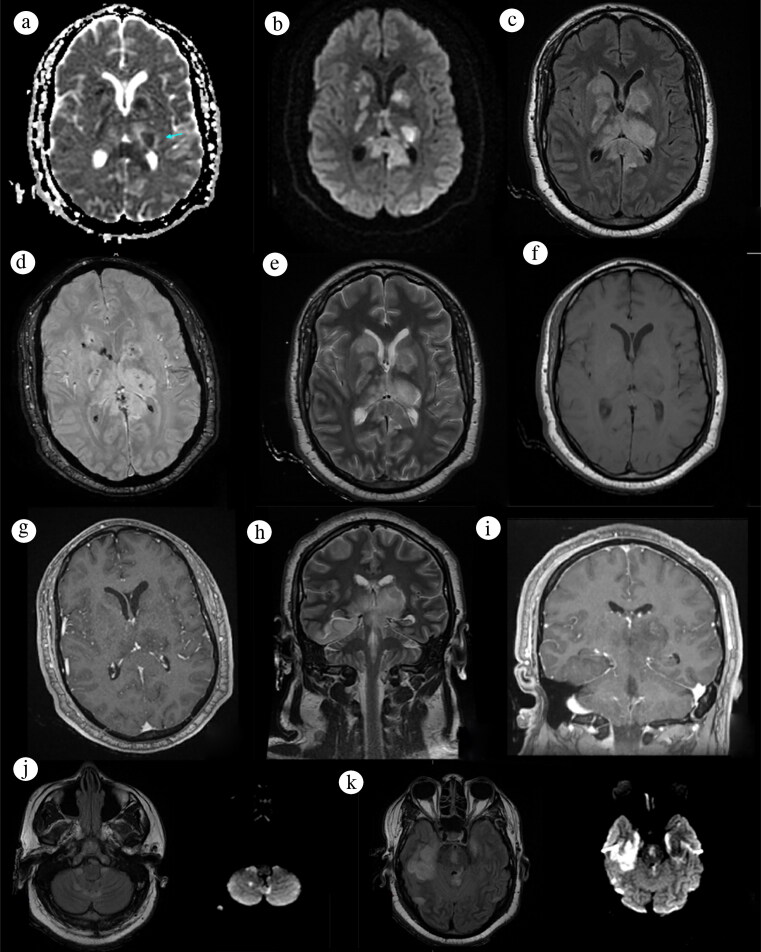
Figure 2.
MRI brain in an axial view at the level of the basal ganglia on (a) ADC, (b) DWI, (c) FLAIR, (d) SWAN, (e) T2, (f) T1, and (g) T1 contrast. A coronal view shows other affected areas on (h) T2 and (i) contrast. A trilaminar pattern (blue arrow) of the thalamus in (a) ADC can be seen with a central high ADC value and a middle low ADC value, suggesting cytotoxic edema with peripheral high ADC suggesting vasogenic edema, with (b) diffusion restriction on these areas. Symmetrical (c) FLAIR hyperintense lesions are also seen on the basal ganglia. Hypointense lesions on (d) SWAN represented hemorrhages. (j, k) FLAIR hyperintense lesions with diffusion restriction on DWI were detected on the cerebellar, brainstem, and temporal region.
DISCUSSION
Neurological complications of viral infections ranging from febrile seizures to ANE are rare, and they are reported more often in children than in adults. Approximately 21% to 45% of patients with neurological complications from influenza experience moderate to severe neurological disability.6 The incidence of influenza-associated encephalitis is about 0.21 per million.7 The differential diagnosis includes Reye syndrome, acute disseminated encephalomyelitis, Wernicke’s encephalopathy, as well as ANE secondary to other viruses like COVID-19.8
ANE pathogenesis remains unclear, with one of the most accepted hypotheses being hypercytokinemia or cytokine storm, described as an exaggerated immune response to viral infection producing a systemic inflammatory response syndrome.9 Typically, neurological manifestations are preceded by prodromal respiratory and systemic symptoms. Diagnostic criteria include sudden coma, convulsion or altered mental status, and a bilateral symmetrical, multifocal, hemorrhagic lesion of the thalami without significant pleocytosis in CSF.5 CSF analysis often shows elevated proteins without pleocytosis,2 and influenza infection is commonly documented through respiratory tests and rarely identified in CSF.4,10
Apparent diffusion coefficient (ADC) shows images with higher-than-normal ADC values at the center of the lesion due to hemorrhage. These lesions are surrounded by low ADC values at the peripheral region, suggesting cytotoxic edema. The gradient echo imaging shows blooming hemorrhagic areas in the center of the lesions with surrounding hyperintensity of swollen thalami.11 Additional MRI changes may be present in the medial temporal lobes, external capsules, claustrum, insular cortices, hippocampi, amygdala, and mammillary and spinal cord, and some present without thalamic involvement.2,12 The incidence of involvement of white matter and cerebellum and parenchymal hemorrhages seems to be higher in adults than in children.13
Vaccination efficacy to prevent influenza infection is around 59% to 83%; however, no specific trial has evaluated how preventable neurological complications are in vaccinated patients.2 Despite no strong evidence supporting treatment for ANE, glucocorticoids are commonly used, and plasmapheresis, intravenous immunoglobulin, and antivirals (oseltamivir) have been used as first-line treatment.14 This case demonstrates that, while rare, postviral ANE can occur in both adults and children, and early diagnosis relies mainly on clinical and neuroimaging findings.
References
- 1.Ziemele D, Ķauķe G, Skrējāne K, Jaunozoliņa L, Karelis G.. A fatal case of COVID-19-associated acute necrotizing encephalopathy. Eur J Neurol. 2021;28(11):3870–3872. doi: 10.1111/ene.14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu X, Wu W, Pan W, Wu L, Liu K, Zhang HL.. Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediators Inflamm. 2015;2015:792578. doi: 10.1155/2015/792578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito Y, Ichiyama T, Kimura H, et al. Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol. 1999;58(4):420–425. doi:. [DOI] [PubMed] [Google Scholar]
- 4.Popescu CP, Florescu SA, Lupulescu E, et al. Neurologic complications of influenza B virus infection in adults, Romania. Emerg Infect Dis. 2017;23(4):574–581. doi: 10.3201/eid2304.161317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welk A, Schmeh I, Knuf M, et al. Acute encephalopathy in children associated with influenza A: a retrospective case series. Klin Padiatr. 2016;228(5):280–281. doi: 10.1055/s-0042-111686. [DOI] [PubMed] [Google Scholar]
- 6.Chen L-W, Teng C-K, Tsai Y-S, et al. Influenza-associated neurological complications during 2014-2017 in Taiwan. Brain Dev. 2018;40(9):799–806. doi: 10.1016/j.braindev.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Meijer WJ, Linn FHH, Wensing AMJ, et al. Acute influenza virus-associated encephalitis and encephalopathy in adults: a challenging diagnosis. JMM Case Rep. 2016;3(6):e005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odagiri A, Yamaoka A, Miyata K, et al. Elderly-onset acute necrotizing encephalopathy mimicking severe heat stroke: a case report and review of the literature. Acute Med Surg. 2019;6(3):316–320. doi: 10.1002/ams2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuguchi M, Abe J, Mikkaichi K, et al. Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. 1995;58(5):555–561. doi: 10.1136/jnnp.58.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piet E, Tattevin P, Mailles A, Stahl JP. . Influenza B meningoencephalitis. Med Mal Infect. 2017;47(6):435–436. doi: 10.1016/j.medmal.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Albayram S, Bilgi Z, Selcuk H, et al. Diffusion-weighted MR imaging findings of acute necrotizing encephalopathy. AJNR Am J Neuroradiol. 2004;25(5):792–797. [PMC free article] [PubMed] [Google Scholar]
- 12.Singh RR, Sedani S, Lim M, Wassmer E, Absoud M.. RANBP2 mutation and acute necrotizing encephalopathy: 2 cases and a literature review of the expanding clinico-radiological phenotype. Eur J Paediatr Neurol. 2015;19(2):106–113 doi: 10.1016/j.ejpn.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Vanjare HA, Selvi BT, Karuppusami R, et al. Clinical and radiologic findings of acute necrotizing encephalopathy in young adults. AJNR Am J Neuroradiol. 2020;41(12):2250–2254. doi: 10.3174/ajnr.A6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song Y, Li S, Xiao W, et al. Influenza-associated encephalopathy and acute necrotizing encephalopathy in children: a retrospective single-center study. Med Sci Monit. 2020;26:e928374. doi: 10.12659/MSM.928374. [DOI] [PMC free article] [PubMed] [Google Scholar]