Abstract
Neurological manifestations of COVID-19 in the pediatric population are not as well described as those in the adult population. We describe a case of myelin oligodendrocyte glycoprotein immunoglobulin G (MOG-IgG)–associated disorder in a 9-year-old girl, who experienced complete recovery. This rare disorder is a demyelinating disease that often relapses and has the potential to cause severe morbidity. The case highlights the need for early recognition of asymptomatic and subacute presentations of demyelinating disorders and testing for MOG-IgG antibodies, as the management of presumed monophasic demyelinating disorders vs MOG-IgG–positive demyelinating disorder is different.
Keywords: Acquired neuropathy, acute disseminating encephalomyelitis, chronic inflammatory demyelinating polyneuropathy, COVID-19, Guillain-Barré syndrome, MOG-IgG antibody, SARS-CoV2
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an autoimmune disease characterized by sensory dysfunction, progressive or recurrent symmetric proximal and distal weakness, and absent or reduced tendon reflexes of all extremities, developing over at least 2 months.1,2 Approximately 50% of patients will experience a recurrent demyelinating attack.3,4 We present a case of CIDP in a previously healthy child. She tested positive for COVID but was asymptomatic.
CASE DESCRIPTION
A previously healthy 9-year-old, right-hand–dominant girl presented with an 8-week history of progressive weakness of the bilateral lower extremities with resultant falls. At the time of presentation, she was unable to walk without frequent tripping and falling. She walked with a high-step and “slapping gait.” There was no known history of sick contacts and no recent travel prior to presentation. There was no history of diarrhea, fever, headache, mental status changes, cough, congestion, or bowel and bladder habit changes. Eight weeks after symptom onset, she presented to our facility. Her bilateral hip flexion was 4+/5; bilateral quadriceps and hamstring strength, 4+/5; bilateral ankle flexion and ankle extension, 2/5; ankle inversion and eversion, 2/5; bilateral knee flexion strength, 4/5; bilateral finger flexion, extension, and handgrip, 4-/5; and bilateral deltoids, biceps, and triceps, 5/5. Mentation was normal and there was no cranial nerve involvement or neck and truncal muscle weakness. Reflexes at the bilateral biceps and brachioradialis were diminished at 1+. Reflexes at the ankles and knees were absent bilaterally. Sensation was intact to light touch throughout. Romberg’s sign was positive. Vibration sensation was decreased, and there was a discrepancy with toe proprioception. She was ataxic, had a bilateral foot drop, could not walk steadily without falling, was unable to walk on her toes and her heels, and required a gait training belt with maximum standby assistance. Her blood pressure, heart rate, and negative inspiratory pressure were normal.
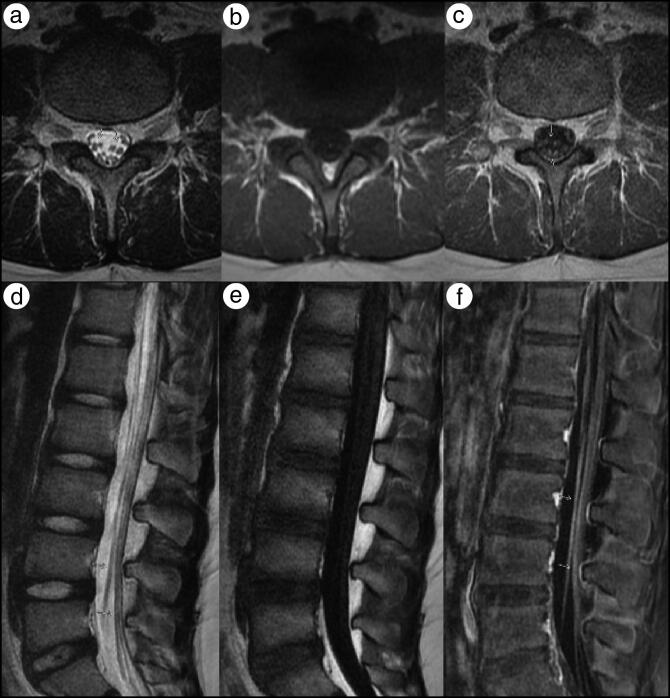
Results were normal for a complete blood count, complete metabolic panel, creatine kinase, vitamin B12, folate, methylmalonic acid, copper, and vitamin E. SARS-CoV-2 spike protein IgG was positive (signal/cut-off ratio of 3.1, where normal is <1.4). Magnetic resonance imaging (MRI) of the lumbar spine showed nerve root enhancement of the cauda equina nerve roots consistent with an infectious, postinfectious, and/or postinflammatory disorder (Figure 1). MRI of the brain with and without contrast was reported as normal.
Figure 1.
MRI of the lumbar spine. (a) Axial T2, (b) precontrast T1, and (c) postcontrast T1-weighted MR images and (d) sagittal T2, (e) precontrast T1, and (f) postcontrast fat saturation T1-weighted images demonstrate thickening and scattered enhancement of the anterior and to a lesser extent posterior cauda equina nerve roots (yellow arrows).
Cerebrospinal fluid analysis showed normal glucose and cell count with an elevated protein of 143.2 mg/dL and albumin-cytological disassociation (cell count 3/mm3), consistent with a demyelinating disorder; immunoglobulin G (14.8 mg/dL) and albumin (79 mg/dL) were also elevated, and oligoclonal bands were negative (Table 1). The electromyogram and nerve conduction studies were compatible with a predominantly demyelinating polyneuropathy with significant slowing of motor conduction velocities and motor conduction block with absent F waves, as well as prolongation of distal motor latencies (Table 2). Myelin oligodendrocyte glycoprotein immunoglobulin G (MOG-IgG) antibodies were positive. MOG titers were elevated to 1:100 (normal < 1:20) (Mayo Clinic Laboratories, Rochester, MN). Ganglioside antibodies, including asialo-GM1, GM1, GM2, GD1a, GD1b, and GQ1b IgG/IgM, were negative (ARUP Laboratories, Salt Lake City, UT).
Table 1.
Cerebrospinal fluid analysis on admission
| Component | Value | Reference range |
|---|---|---|
| Protein (mg/dL) | 143.2 | 12–60 |
| Glucose (mg/dL) | 50 | 40–70 |
| Cell count (mm3) | 3 | 0–5 |
| Lymphocytes (%) | 76% | |
| Red blood cells (mm3) | 0 | 0 |
| Albumin (mg/dL) | 79 | <79 |
| Oligoclonal bands | 0 | 0 |
| Immunoglobulin G (mg/dL) | 14.8 | 8.1 |
Table 2.
Nerve conduction study*
| Nerve/sites | Muscle | Latency (ms) | Amp (mV) | Rel amp (%) | Duration (ms) | Segments | Distance | Lat diff (ms) | Velocity (ms) |
|---|---|---|---|---|---|---|---|---|---|
| Right peroneal–extensor digitorum brevis (EDB) | |||||||||
| Ankle | EDB | NR | NR | NR | NR | Ankle–EDB | 6 | ||
| Right peroneal–tibialis anterior (TA) | |||||||||
| Fibular head | TA | 2.92 | 3.4 | 100 | 10.83 | Fibular head–TA | |||
| Popliteal fossa | TA | 4.84 | 3.1 | 91.1 | 7.81 | Popliteal fossa–Fibular head | 6 | 1.93 | 31 |
| Right tibial–abductor hallucis (AH) | |||||||||
| Ankle | AH | 5.26 | 0.2 | 100 | 5.10 | Ankle–AH | 8 | ||
| Popliteal fossa | AH | Popliteal fossa–Ankle | |||||||
| Right median–abductor pollicis brevis (APB) | |||||||||
| Wrist | APB | 5.16 | 5.2 | 100 | 5.83 | Wrist–APB | 6 | ||
| Elbow | APB | 10.16 | 0.2 | 4.71 | 5.00 | Elbow–Wrist | 12 | 5.00 | 24 |
F wave was 4.8 for R Median–APB, M Lat.
She received intravenous immunoglobulin during the admission. After discharge, the patient received inpatient rehabilitation for 4 weeks at an outside facility. Two months after discharge and inpatient rehabilitation, her strength had improved. Bilateral knee extension and flexion was normal. Bilateral ankle dorsiflexion was 4/5; ankle plantar flexion, 4+/5; bilateral ankle eversion, 3/5; and bilateral ankle inversion, 3/5. Reflexes were normal at the upper limbs and were absent at the knee and ankles. Eight months from the initial presentation, she was continuing to receive intravenous immunoglobulin every 6 to 8 weeks and showed significant clinical improvement. She was able to walk on her toes and heels.
DISCUSSION
CIDP is a clinically heterogeneous, sensory-motor immune-mediated neuropathy typically characterized by symmetrical involvement.1 The definitive diagnosis of CIDP requires progressive, stepwise, recurrent proximal or distal weakness, sensory dysfunction that develops over a period of 2 months, and areflexia or hyporeflexia.5 Electrodiagnostic findings of CIDP can include slowing of conduction velocities, prolongation of distal motor latencies, temporal dispersion, conduction block, and prolongation of F-waves.5 A viral infection can lead to direct damage due to cross-immunity, neuritis or myositis, or part of a systemic inflammatory response syndrome.
MOG-IgG–associated disorder (MOGAD) is a rare and recently described central nervous system demyelinating disease that often relapses and has the potential to cause severe morbidity.3 Other studies have suggested an increased risk of relapse in patients with persistence of MOG-IgG seropositivity during follow-up. The prevalence of MOG-IgG antibodies can be seen in up to 30% of pediatric patients with acquired demyelinating syndromes.4
COVID-19 can cause general central nervous system manifestations including encephalopathy, seizures, and worsening of autoimmune disease. Peripheral nervous system involvement has been reported in patients with COVID-19 infection.6 The incidence of neurological complications such as acquired demyelinating neuropathy has increased during the SARS-CoV-2 pandemic,7 but the data are limited in the pediatric population. COVID-19 and associated acquired demyelinating and autoimmune neuropathy such as acute inflammatory demyelinating polyneuropathy is documented in adults but has been rarely reported in the pediatric population.8–13 The potential association between SARS-CoV-2 infection and exacerbations of neuroimmunological disorders remains speculative, but is certainly probable.
References
- 1.Kieseier BC, Mathey EK, Sommer C, Hartung HP.. Immune-mediated neuropathies. Nat Rev Dis Primers. 2018;4(1):31. doi: 10.1038/s41572-018-0027-2. [DOI] [PubMed] [Google Scholar]
- 2.Joint Task Force of the EFNS and the PNS . European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15(4):295–301. doi: 10.1111/j.1529-8027.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- 3.Md Noh MSF. Myelin oligodendrocyte glycoprotein-antibody (MOG-IgG) associated disease with centrally located long spinal cord lesion in a 14-month old child. J Cent Nerv Syst Dis. 2020;12:1179573520955008. doi: 10.1177/1179573520955008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters P, Fadda G, Woodhall M, et al. ; Canadian Pediatric Demyelinating Disease Network . Serial anti-myelin oligodendrocyte glycoprotein antibody analyses and outcomes in children with demyelinating syndromes. JAMA Neurol. 2020;77(1):82–93. doi: 10.1001/jamaneurol.2019.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van den Bergh PYK, van Doorn PA, Hadden RDM, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force—second revision. J Peripher Nerv Syst. 2021;26(3):242–268. doi: 10.1111/jns.12455. [DOI] [PubMed] [Google Scholar]
- 6.Luigetti M, Iorio R, Bentivoglio AR, et al. ; Gemelli Against COVID-19 Group.. Assessment of neurological manifestations in hospitalized patients with COVID-19. Eur J Neurol. 2020;27(11):2322–2328. doi: 10.1111/ene.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panda PK, Sharawat IK, Panda P, Natarajan V, Bhakat R, Dawman L.. Neurological complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis. J Trop Pediatr. 2021;67(3) fmaa070. doi: 10.1093/tropej/fmaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalifa M, Zakaria F, Ragab Y, et al. Guillain-Barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J Pediatric Infect Dis Soc. 2020;9(4):510–513. doi: 10.1093/jpids/piaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manji HK, George U, Mkopi NP, Manji KP.. Guillain-Barré syndrome associated with COVID-19 infection. Pan Afr Med J. 2020;35(Suppl 2):118. doi: 10.11604/pamj.supp.2020.35.2.25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naseri M, Ashrafzadeh F, Khademi G, Imannezhad S, Sorouri S, Sezavar M.. COVID-19–associated acute transverse myelitis in children: a case report and review of literature. J Pediatr Rev. 2022;10:433–438. [Google Scholar]
- 11.Curtis M, Bhumbra S, Felker MV, et al. Guillain-Barré syndrome in a child with COVID-19 infection. Pediatrics. 2021;147(4):e2020015115. doi: 10.1542/peds.2020-015115. [DOI] [PubMed] [Google Scholar]
- 12.Araújo NM, Ferreira LC, Dantas DP, et al. First report of SARS-CoV-2 detection in cerebrospinal fluid in a child with Guillain-Barré syndrome. Pediatr Infect Dis J. 2021;40(7):e274–e276. doi: 10.1097/INF.0000000000003146. [DOI] [PubMed] [Google Scholar]
- 13.Khera D, Didel S, Panda S, Tiwari S, Singh K.. Concurrent longitudinally extensive transverse myelitis and Guillain-Barré syndrome in a child secondary to COVID-19 infection: a severe neuroimmunologic complication of COVID-19. Pediatr Infect Dis J. 2021;40(6):e236–e239. doi: 10.1097/INF.0000000000003124. [DOI] [PubMed] [Google Scholar]