Abstract
Background
Metabolic dysfunction-associated fatty liver disease (MAFLD) is now the term used for hepatic steatosis in patients who are overweight or obese, have type 2 diabetes mellitus (T2DM), or evidence of metabolic dysregulation. The prevalence of MAFLD among morbidly obese subjects is 65–93%. Hepatic dendritic cells (hDCs) are antigen-presenting cells that induce T cell-mediated immunity. MAFLD pathogenesis involves numerous immune cell-mediated inflammatory processes, while the particular role of hDCs is yet to be well defined. This study aimed to identify hDCs in liver biopsies from 128 patients with MAFLD associated with obesity.
Material/Methods
In this cross-sectional study, 128 liver biopsies from 128 patients with MAFLD (diagnosed as presence of hepatic steatosis, plus T2DM, metabolic dysregulation or overweight/obesity) were collected and assessed for CD11c+ immunoreactivity degree (CD11c as dendritic cell biomarker), through antigen retrieval, reaction with CD11c antibodies (primary), and marking with diaminobenzidine chromogen.
Results
Among the 128 patients with MAFLD, 64 (50%) had MAFLD and fibrosis and 72 (56.2%) positively expressed hDCs (CD11c+). Among morbidly obese patients, 49 (64.5%) positively expressed hDCs (CD11c+) in liver tissue; from patients with obesity grade I- grade II (GI–II), 18 (54.5%) positively expressed hDCs (CD11c+) in liver tissue; and from non-obese patients with MAFLD, 5 (26.3%) positively expressed hDCs (CD11c+) in liver tissue.
Conclusions
hDC expression increases significantly in morbidly obese patients with MAFLD compared with non-obese patients, independent of the degree of fibrosis, suggesting the role of adaptive changes within hDCs in the perpetuation of inflammatory insults in chronic liver diseases.
Keywords: CD11c Antigen; Dendritic Cells; Non-Alcoholic Fatty Liver Disease; Obesity, Morbid
Background
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a major health problem, and its global prevalence was estimated to be 25.2% [1], with an estimated projected prevalence of 33.5% by 2030 [2]. MAFLD is defined as the presence of liver steatosis, presenting with any of the following additional elements: overweight or obesity, type 2 diabetes mellitus (T2DM), or metabolic dysfunction. The increase in MAFLD prevalence is fueled by the global epidemic of obesity, with over 650 million (13%) of adults worldwide being obese [3]. In morbidly obese patients (defined as body mass index [BMI] ≥40 kg/m2), the prevalence of MAFLD is 65–93% [4–7].
Although MAFLD pathophysiology is extraordinarily complex and not yet completely understood, several factors contributing to MAFLD development and progression have been suggested, including age, sex, ethnicity, and genetic background [8]. In addition, MAFLD involves various immune cell-mediated inflammatory processes. In the ordinary state, the liver preserves a tolerogenic environment despite the diverse harmful stimuli originating from the enterohepatic circulation, and has the capacity of recovering from acute liver injury [9]. In contrast, the hepatic accumulation of lipids derived from overnutrition and dysfunctional or insulin-resistant adipocytes induce liver lipotoxicity which overwhelms the organ’s repair capacity and promotes inflammatory pathways, cellular dysfunction, and lipoapoptosis, with consequent development of a fibro-inflammatory process [9]. This fibro-inflammatory process is promoted and maintained by the complex and dynamic interaction among immune cells (including T, B cells, Kupffer Cells, and hepatic dendritic cells [hDCs] [10]) as well as with other hepatic cells, such as hepatic stellate cells (hSC). In the liver, hDCs are bone marrow-derived cells found principally in the periportal and pericentral space [11]. They represent <1% of the non-parenchymal hepatic cells and are a diversified population of hepatic antigen-presenting cells (APCs) linked to innate and adaptive immunity and considered as key modulator of the hepatic immune system [11]. The HDCs express high levels of MHC-Class II molecules (eg, HLA-DR) and CD45+, but are negative for other hematopoietic lineage markers [11]. There are 3 described subsets in different experimental models of HDCs (CD19−, CD11c+): (1) lymphoid or cDC1 (CD8α+, CD103+, B220−, and CD11b− in mice and CD1c+ in humans); (2) myeloid (CD8α−, B220−, and CD11b+); and (3) plasmacytoid (B220+, CD11b−) [11]. Emerging evidence suggests a pivotal role for hDCs in the pathogenesis of MAFLD [12–15], although it is still not well characterized, particularly when compared to other immune cell subsets. Furthermore, these cells play a role in hepatic lipid storage, thus providing a crucial nexus between inflammation and lipid metabolism [11].
hDCs account for 20–27% of the nonparenchymal cells during fibrosis progression, which were identified through CD11c-positivity. This is an important base for the identification of hDCs by using this biomarker, as experimental evidencce showed that these cells also express other markers such as CD40 and MHCII (proving their antigen-presenting capability), corresponding to the dendritic cell immunophenotype [14].
Dual Role of hDCs
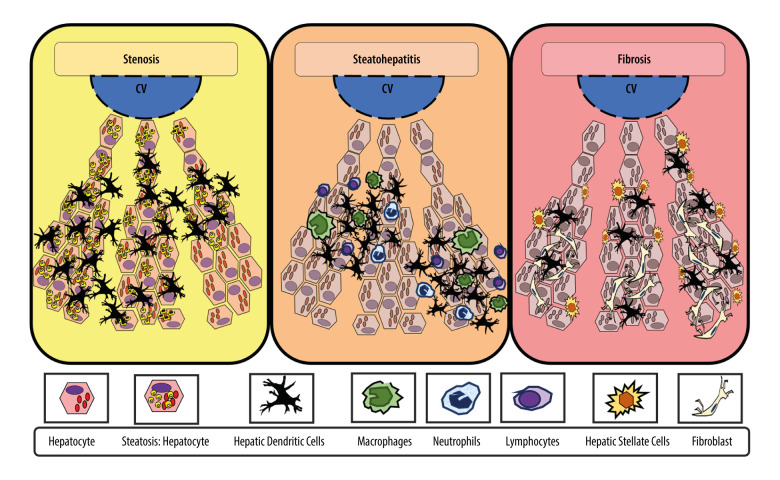
Broadly speaking, DCs have migratory and adaptive features (when changing from a tolerant to a reactive state); they are also important in antigen capture, processing, and presentation. However, hDCs are derived from bone marrow and tend to remain tolerant in the healthy liver [16]. A dual role of hDCs in the development and progression of MAFLD has been described; the reported interaction between the role of hDCs in obesity, MAFLD, and steatohepatitis in experimental models and in humans can be understood as depicted in Figure 1.
Figure 1. Adaptive changes of hepatic dendritic cells (CD11c+, hDCs) along the metabolic dysfunction-associated fatty liver disease (MAFLD) spectrum of disease.
hDCs have an important role in capturing, processing, and presenting antigens. Initially, during MAFLD, when steatosis is stablished, hDCs tend to promote lipid hepatocytes storage with promotion of a tolerant and protective environment against fibro-inflammatory process. In contrast, during inflammation, the hDCs pool changes (eg, reduced lymphocytoid and plasmacytoid cells), with further expansion of matured hDCs and other innate immune cells (eg, neutrophils, macrophages) that promote and maintain fibro-inflammatory liver damage in advanced disease. In inflammation, fibrosis, and severe obesity, hDCs (CD11c+) appeared to be reduced or focally expressed, thus representing the tolerogenic and protective phenotype. Finally, during fibrosis, the hDCs (CD11c+) increased cytokine production induced a proinflammatory environment and proliferative responses of hepatic stellate cells (HSCs). Thus, focal expression of hDCs may stimulate HSCs, while diffuse expression of hDCs (CD11c+) in early stages might be characteristic of protective and tolerogenic cells (CD11c+).
It has been proposed that in homeostatic conditions, classical hDCs (Cor+, CD11c+) aim to maintain quiescence and tolerance of HSCs and KCs by secreting specific cytokines (eg, IL-10), thereby ameliorating the magnitude of TLR response to bacterial components, as well as by clearing cellular debris [11]. Thus, initially, hDCs (CD40+ expressing CD11c+ cells) promote lipid storage in hepatocytes and suppress inflammasome and T cell activation in the healthy liver, and they induce a Treg response against obesity-induced ectopic lipid storage and metabolic dysfunction, acting as a protective factor in acute conditions [17]. In contrast, during steatohepatitis, hDCs (CD11c+ MHCII+) expand and mature, thereby assuming an activated immune phenotype which involves the expression of costimulatory molecules, production of cytokines, and increased CD4 T cell activation; furthermore, CD40+ on hDCs promotes liver inflammation and leads to higher hepatic and plasmatic cholesterol levels. Therefore, adaptive changes of hDCs contribute to the promotion and perpetuation of liver fibro-inflammatory insults in advanced chronic disease [11,18].
Interestingly, the relationship between CD11c+ cells and fibrosis might be influenced by increased cytokine production and proliferative responses of hepatic stellate cells. Thus, significant dynamic changes of CD11c+ cells during fibrosis progression might impact the inflammatory environment of the liver, but effects on fibrosis progression have not been assessed [14].
hDCs in Steatohepatitis and Fibrosis
A study carried out in 2013 by Henning and colleagues showed that the depletion of hDCs in steatohepatitis models leads to the promotion and maintenance of the intrahepatic fibro-inflammatory processes by “uncontrolled” innate immune cell interactions, thereby stimulating apoptosis and accelerating fibrosis instauration [12]. Additionally, during fibro-inflammatory injury recovery, the absence of hDCs delayed the resolution of damage [12,13]. Of note, HSC cytokine production and proliferative responses might be influenced by the presence of hDCs (CD11c+) [14].
As a factor on its own, inflammation promotes massive expansion and maturation of hDCs (CD11c+/MHCIIhigh/CD103−/CD11b+) by diverse stimuli, such as CD80 stimulation [18]. As human studies progress, hDCs’ role in the development and progression of MAFLD has continuously yielded intriguing results, establishing it as a current topic of interest [19].
In this study we assessed the presence hDCs using CD11c+ as a broad biomarker of hDC expression in liver tissue from patients with MAFLD and stratified its differential distribution according to patients’ body weight and presence of liver fibrosis. Therefore, this study aimed to identify hepatic dendritic cells in liver biopsy samples from 128 patients with MAFLD associated with obesity.
Material and Methods
Ethics Statement
The study was approved by the local Ethics Committee in Medica Sur Foundation and Clinic, with the identification number 2020-EXT-449. All participants in this study signed an informed consent acknowledging the purpose of the liver biopsies taken during their surgical procedure, establishing clearly the anonymity of their personal data and the aims of the study in which they accepted to be enrolled.
Participants’ Selection
In this observational cross-sectional study, 128 liver biopsies from adult patients with prior Non-Alcoholic Fatty Liver Disease (NAFLD) diagnosis (ie, exclusion diagnosis criteria were used to determine NAFLD in these patients) were initially compiled from the Pathology Department of the Medica Sur Clinic & Foundation (2012–2020), with the institution’s research and ethics committee approval. Medical records from the included patients were checked for clinical data registers (eg, sex, BMI, elements of metabolic syndrome). The MAFLD diagnosis was made based on histological evidence of steatosis plus either obesity, type 2 diabetes mellitus (T2DM), or metabolic dysfunction [20].
BMI, T2DM, Metabolic Dysfunction, and Fibrosis: Operational Definitions
According to the objectives of the study, participants were classified using the World Health Organization’s stratification of BMI into those with normal weight and overweight (BMI: 18–24.9 kg/m2 and 25–29.9 km/m2, respectively), obesity grade I and II (GI–II) (BMI: >30–34.9), and morbid obesity (BMI: >40 kg/m2) [21]. Non-obese MAFLD patients included those who had other MAFLD criteria exempting obesity, such as prior T2DM diagnosis or metabolic dysfunction in medical records (based on clinical and laboratory data). Metabolic dysfunction was defined as the presence of at least 2 risk factor abnormalities (hypertriglyceridemia, low high-density lipoprotein cholesterol levels, hypertension, high fasting glucose, and/or prediabetes) [20]. Liver fibrosis categorical determination (presence vs absence) was made blindly by 1 expert pathologist using trichrome Masson staining of each liver sample.
Immunohistochemistry Methodology
All 128 samples of liver tissue were included for immunohistochemical determination of the expression of CD11c+ cells (considered in this study as hDCs, given the fact that they are the most widely used marker for hDC identification). The samples underwent the steps that are described below in the order in which these took place.
Initial Sample Processing
Accordingly, the 3-μm-thick formalin buffered-fixed, paraffin-embedded liver tissue slices from Tru-Cut biopsies, laparoscopic biopsies, and partial hepatectomies were deparaffinated and rehydrated.
Control (Immunohistochemistry Method)
Human tonsil tissue was used as control for the immunoohistochemical marking, as suggested by BioSV (provider of the antibodies) in the catalog. Other tissues suggested to be used as control were: bone marrow, spleen, colon, and hairy cell leukemia. Human tonsil tissue samples (obtained from tonsillectomies carried out in the surgical department and preserved in the pathology department in Medica Sur Hospital) were cut 3-μm-thick and placed in each of the slides where the liver biopsies were placed for staining. The same IHC process carried out in the liver biopsies was done simultaneously on the tonsil control tissue.
Method of Antigen Retrieval
The antigenic recovery process was carried out using pressure-boiled Declere 1: 20 (Cell Marque, Hot Springs, AR) in a Microwave Tender Cooker (2.5 Quarts, NordicWare®); subsequently, we carried out the unspecified-site blockade (3% H2O2 for 5 min). Having finished this process, the material was cooled at room temperature for 30 min and water-washed.
Immunohistochemical Markers (Primary Antibody)
Next, after buffering with TBS (DakoCytomation, Carpinteria, CA), the samples were treated for 1 h with the antibody (Rabbit Monoclonal Anti-CD11c IgG, BSB 6445, clone: EP 157 by BioSB®), at a dilution of 1: 100. Previously, the antibody was titrated on the fabricant’s recommended tissue (tonsil tissue). Diaminobenzidine (Catalog number BSB 0005, BioSB®) was employed as chromogen. No secondary antibodies were used for this process.
Immunostaining Evaluation
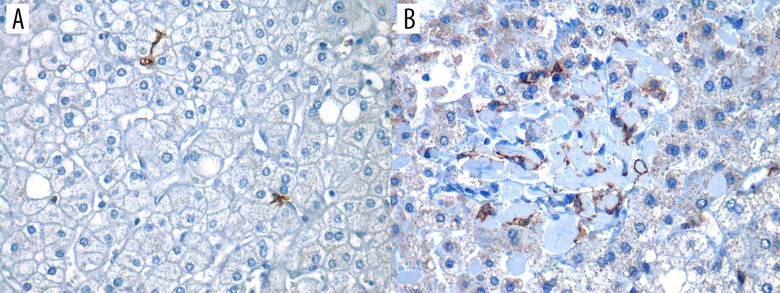
Lastly, the slices were presented on permanent resin and protected with coverslips for microscopic analysis by pathologists. For evaluation of immune marking, light microscopy was used (Olympus Nikon E100®). As assessed by the expert pathologist, any brown membrane staining on any field within the biopsy was considered as “positive” staining, as shown in Figure 2 (both A and B would represent positive staining).
Figure 2. Positive expression of hepatic dendritic cells (CD11c+, hDCs) in liver tissue (40×).
Photomicrograph showing CD11c+ immunostained cells in 2 different liver biopsies, visualized in 40× objective. (A) Focal expression: low number of hDCs (CD11c+) in less than 3 fields/40×. (B) Diffuse expression: high number of hDCs (CD11c+) are found in more than 3 fields/40×.
Sub-Analysis Methodology: hDCs’ Expression Across Distinct Dtages of MAFLD
We carried out a sub-analysis in 35 random liver biopsies to perform this sub-analysis based on routine hematoxylin and eosin, as well as trichrome Masson stains, to determine liver steatosis, inflammation, ballooning degeneration, and fibrosis. The operational definitions for these histopathological terms were based on standardized pathology outlines and are shown in Table 1. Furthermore, all samples were blindly reviewed by 1 expert pathologist, who semi-quantitatively stratified the expression of hDCs (CD11c+) as focal (sparse number of hDCs in less than 3 fields/40× [Figure 2A]) or diffuse (high number of hDCs were observed in more than 3/fields/40× [Figure 2B]).
Table 1.
Operational definitions for histopathological diagnosis of liver injury:
The histological diagnoses established by the expert pathologist are presented below. These are written-out here for reference to the terms used within the text.
| Histological diagnoses | Operational definitions |
|---|---|
| Ballooning degeneration | Hepatocytes greater in size compared to adjacent hepatocytes, with rounded shape (instead of regular polygonal shape), vacuolization, and cytoplasmic clearing. Finding of Mallory hyaline bodies supports this diagnosis |
| Simple steatosis | In this case, the expert pathologist considered the definition of “macrovesicular steatosis” rather than “microvesicular steatosis” given that it’s the most common form seen overall and is the variant present in steatohepatitis, obesity, and diabetes, among others. Macrovesicular steatosis: “Large” fat droplets that are greater than 20 μm, cells with peripherally pushed nucleus, usually a single lipid droplet inside the cell |
| Inflammation | Presence of leukocyte infiltrate within tissues, consisting of neutrophils and macrophages in acute inflammatory processes and lymphocytes in chronic stages |
| Fibrosis | Collagen deposition which may or may not have architectural distortion within hepatic tissue; collagen fibers are seen as blue colored when evaluated with Masson stain. * The degree of fibrosis was classified based on the Kleiner classification for liver steatosis |
Statistical Analysis
Baseline characteristics of participants according to BMI are presented as number and percentage for categorical variables. The uncorrected χ2 test for categorical variables was used for statistical inference. We considered significant differences as those with a 2-sided P value of <0.05. All statistical analyses were made on Epi Info v5.5.5 app for iOS from the Centers for Disease Control.
Results
Overall Analysis of 128 Patients, Independent of MAFLD Stage
The most relevant characteristics of the 128 included patients with MAFLD are summarized in Table 2.
Table 2.
Participant information database-main characteristics:
The main characteristics of the patients enrolled in the study are presented below, with most being composed of women, morbidly obese individuals, and variable CD11c+ cell expression.
| Patients with MAFLD (n=128) Mean age: 44.9 (±12.5) |
(N) | (%) |
|---|---|---|
| Men | 48 | 37.50 |
| Women | 80 | 62.50 |
| Non-obese (<30 kg/m2+either T2DM or metabolic dysfunction) | 19 | 14.84 |
| Obesity grade I–II (≥30–39.9 kg/m2) | 33 | 25.78 |
| Morbid obesity (≥40 kg/m2) | 76 | 59.38 |
| Fibrosis | 64 | 50.00 |
| Non-fibrosis | 64 | 50.00 |
| Hepatic dendritic cells’ expression (CD11c+) | 72 | 56.25 |
| No expression of Hepatic dendritic cells (CD11c+) | 56 | 43.75 |
MAFLD – metabolic (dysfunction)-associated fatty liver disease; T2DM – type 2 diabetes mellitus.
The main characteristics of the gathered data include 49 (64.5%) patients who positively expressed hDCs (CD11c+) in liver tissue.
Of the 33 patients with obesity GI–II, 18 (54.5%) positively expressed hDCs (CD11c+) in liver tissue, while from the 19 non-obese patients, a total of 5 (26.3%) positively expressed hDCs (Table 3).
Table 3.
Hepatic dendritic cells’ (CD11c + ) expression in MAFLD according to patients’ BMI and either presence or absence of fibrosis:
The findings of liver fibrosis among different weight groups was variable, finding that the most marked difference is dependent on the number of patients in each group (eg, non-obese patients constitute a lower percentage of the population, for which they constitute a lower percentage of patients with fibrosis, as well as without fibrosis).
| Patients with MAFLD (n=128) | Hepatic dendritic cells (CD11c+) N (%) 72 |
|---|---|
| Morbidly obese patients with no fibrosis (n=44) | 29 (65.91) |
| Morbidly obese patients with fibrosis (n=32) | 20 (62.50) |
| Patients with obesity grade I–II and no fibrosis (n=13) | 8 (61.54) |
| Patients with obesity grade I–II and fibrosis (n=20) | 10 (50.00) |
| Non-obese patients with no fibrosis (n=7) | 2 (28.57) |
| Non-obese patients with fibrosis (n=12) | 3 (25.00) |
BMI – body mass index; MAFLD – metabolic (dysfunction)-associated fatty liver disease.
Importantly, from the 72 liver biopsies positively expressing hDCs (CD11c+), 49 (68.1%) were from morbidly obese individuals, 18 (25.0%) from patients with obesity GI–II, and 5 (6.9%) from non-obese individuals; out of these, 33 liver samples (45.8%) had fibrosis (Table 3). Furthermore, 20 (27.8%) were morbidly obese with fibrosis, 10 (13.9%) were patients with obesity GI–II and fibrosis, and 3 (4.2%) were non-obese with fibrosis (Table 3).
Given the gathered data, it was found that the odds ratio (OR) for hDCs (CD11c+) expression in liver tissue was 1.51 (0.65–3.49; χ2=0.957; P=0.327) for morbid obese patients compared with patients with obesity GI–II, and it was 5.08 (1.65–15.63; χ2=9.021; P=0.0027) when comparing the morbidly obese with non-obese patients, showing a significant difference in dendritic cell expression, especially among morbidly obese individuals and non-obese patients. The difference was lower when comparing patients with obesity GI–II and non-obese patients, with OR for hDCs (CD11c+) expression in liver tissue of 3.36 (0.98–11.49; χ2=3.895; P=0.048) (Table 4).
Table 4.
Hepatic dendritic cells’ expression in MAFLD according to patients’ BMI and either the presence or absence of fibrosis:
The data show the difference in dendritic cell expression among different weight groups; the most outstanding is the difference between CD11c+ cell expression in morbidly obese individuals compared to lower degrees in obese and non-obese individuals.
| Study variables Patients with MAFLD (n=128) |
CD11c+ cells Yes (n=72) N (%) |
CD11c+ cells No (n=56) N (%) |
Odds ratio (OR) | Statistical test (chi square or χ2) significance level (P value*) |
|---|---|---|---|---|
|
| ||||
| Morbid obesity | 49 (68.06) | 27 (48.21) | 1.51 (0.65–3.49) | χ2=0.9576 |
| Obesity GI–II | 18 (25) | 15 (26.79) | p=0.327 | |
|
| ||||
| Morbid obesity | 49 (68.06) | 27 (48.21) | 5.08 (1.65–15.6) | χ2=9.0216 |
| Non-obese | 5 (6.94) | 14 (25) | P=0.0027 | |
|
| ||||
| Obesity GI–II | 18 (25) | 15 (26.79) | 3.36 (0.98–11.49) | χ2=3.895 |
| Non-obese | 5 (6.94) | 14 (25) | p=0.048 | |
|
| ||||
| Fibrosis | 33 (45.83) | 31 (55.36) | 0.68 (0.33–1.37) | χ2=1.1429 |
| No fibrosis | 39 (54.17) | 25 (44.64) | P=0.285 | |
|
| ||||
| Morbid obese with fibrosis | 20 (27.78) | 12 (21.43) | 1.66 (0.53–5.16) | χ2=0.7879 |
| Obese grade I–II with fibrosis | 10 (13.88) | 10 (17.86) | P=0.3747 | |
|
| ||||
| Morbid obese with fibrosis | 20 (27.78) | 12 (21.43) | 5 (1.12–22.18) | χ2=4.919 |
| Non-obese with fibrosis | 3 (4.17) | 9 (16.07) | P=0.0266 | |
|
| ||||
| Obese grade I–II with fibrosis | 10 (13.88) | 10 (17.86) | 3 (0.62–14.46) | χ2=1.943 |
| Non-obese with fibrosis | 3 (4.17) | 9 (16.07) | P=0.1633 | |
BMI – body mass index; MAFLD – metabolic (dysfunction)-associated fatty liver disease.
We considered significant differences as those with a 2-sided P value of <0.05.
hDCs’ Expression in Liver Tissue with Different Stages of MAFLD (Sub-Analysis)
Subgroup Participants’ Characteristics
From the 35 patients with MAFLD included in this sub-analysis, 18 (51.4%) were men and 17 (48.6%) were women; 2 (5.7%) were morbidly obese, 14 (40%) had obesity GI–II, and 19 (54.3%) were non-obese; additionally, 30 (85.7%) focally expressed hDCs (CD11c+) and 5 (14.3%) diffusely expressed hDCs (CD11c+) (Table 5).
Table 5.
Sub-analysis of focal and diffuse expression of hepatic dendritic cells (CD11c+) in MAFLD according to patients’ BMI and histologic stage of disease. Based on the data that the table presents, we can see how the totality of patients who present CD11c+ cell expression or any degree of liver damage also present with focal expression of these cells.
| Patients with MAFLD (n=35) | N (%) | Focal expression of hDCs (CD11c+) 30 (85.71%) N (%) |
Diffuse expression of hDCs (CD11c+) 5 (14.29%) N (%) |
|---|---|---|---|
| Men | 18 (51.43) | 16 (88.89) | 2 (11.11) |
| Women | 17 (48.57) | 14 (82.35) | 3 (17.65) |
| Non-obese | 19 (54.29) | 14 (73.68) | 5 (26.32) |
| Obese grade I–II | 14 (40.00) | 14 (100.00) | 0 (0.00) |
| Morbidly obese | 2 (5.71) | 2 (100.00) | 0 (0.00) |
| Simple steatosis | 5 (14.28) | 4 (80.00) | 1 (20.00) |
| Inflammation | 27 (77.14) | 23 (85.19) | 4 (14.81) |
| Ballooning degeneration | 13 (37.14) | 11 (84.62) | 2 (15.38) |
| Fibrosis | 24 (68.57) | 21 (87.5) | 3 (12.50) |
BMI – body mass index; hDCs – hepatic dendritic cells; MAFLD – metabolic (dysfunction)-associated fatty liver disease.
Stage of MAFLD According to Patients’ BMI (Figure 3)
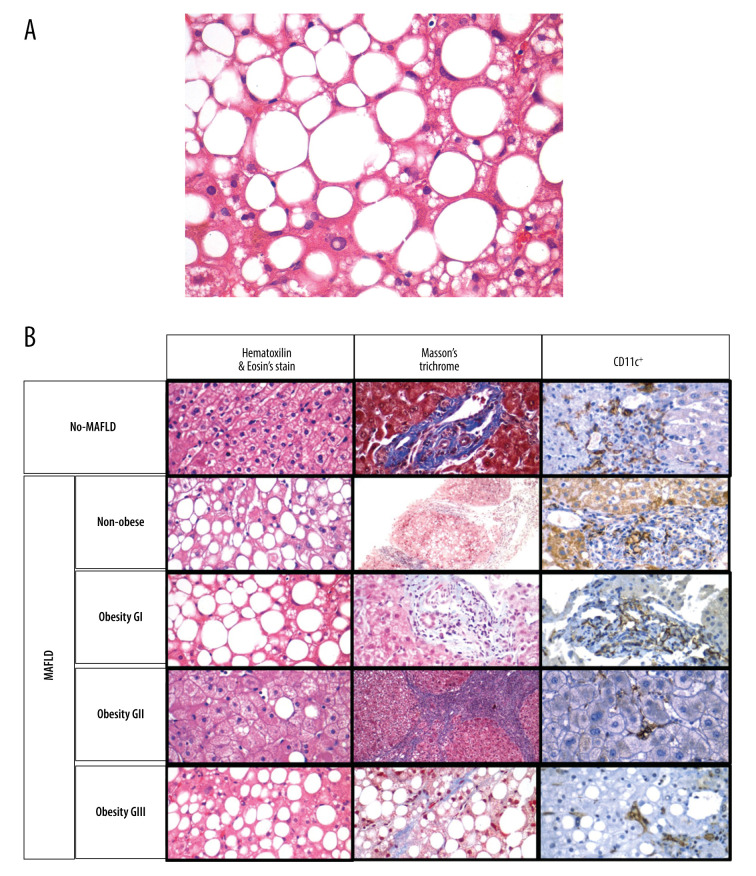
Figure 3. (A) A photomicrograph of the liver biopsy from a 54-year-old woman with metabolic dysfunction-associated fatty liver disease (MAFLD).
The histology of the liver biopsy shows steatosis with round vacuoles in the liver cells where lipid has been removed during tissue processing. Note the lack of inflammation or fibrosis in this biopsy, although there may be areas of increased mononuclear cells. No necrosis is seen. Hematoxylin and eosin (H&E). Magnification ×40. (B) Comparison of liver biopsy (stained by hematoxylin and eosin, and Masson’s trichrome stain, as well as CD11c+ expression) from individuals with no metabolic dysfunction-associated fatty liver disease (MAFLD) and from patients with MAFLD in different categories according to body mass index (BMI). Based on different stains, it is possible to see the expression of dendritic cells (last row, as brown colored) varies in number and in distribution. Furthermore, the histological differences on the degree of lipid droplet saturation as well as fibrosis (with both hematoxylin and eosin, as with Masson’s stain) can be observed.
The 2 morbidly obese patients (100%) in the 35-sample analysis showed inflammation and fibrosis, but only 1 (50%) had ballooning degeneration. From the 14 (40%) patients with obesity GI–II, 12 (85.7%) had inflammation, 10 (71.4%) had fibrosis, and 5 (35.7%) had ballooning degeneration. From the 19 (54.3%) non-obese patients, 13 (68.4%) had inflammation, 12 (63.2%) had fibrosis, and 7 (36.8%) had ballooning degeneration; all 5 (26.3%) patients who diffusely expressed hDCs (CD11c+) in the liver were non-obese.
Diffuse hDC Expression According to MAFLD Stage
From the 5 patients with simple steatosis (14.3%), 1 (20%) diffusely expressed hDCs (CD11c+) on liver tissue. In addition, out of the 27 patients with inflammation (77.1%) and the 13 with ballooning degeneration (37.1%), 4 (14.8%) and 2 (15.4%) patients diffusely expressed hDCs (CD11c+) on liver tissue, respectively. Finally, among the patients with fibrosis (24, 68.6%), 3 (12.5%) of them diffusely expressed hDCs (CD11c+) on liver tissue (Table 5).
Focal hDC Expression According to MAFLD Stage
Out of the same number of patients with simple steatosis, inflammation, ballooning degeneration, and fibrosis, focal expression of dendritic cells was present in 4 (80%), 23 (85.2%), 11 (84.6%), 13 (37.1%), and 21 (87.5%), respectively.
Discussion
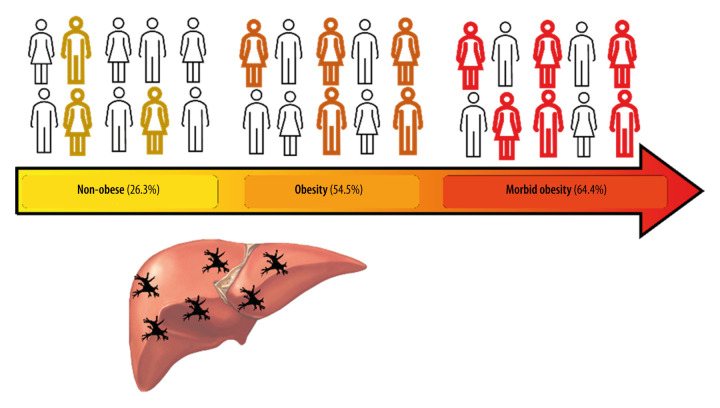
In this study, we showed that MAFLD patients with concomitant morbid obesity expressed hDCs (CD11c+) on liver tissue more frequently compared to patients with less severe or no obesity. A significant finding was that hDC (CD11c+) expression was not altered depending on the degree of fibrosis (Figure 4). As mentioned in the Background section, previous studies suggested a relationship between hDC expression and fibrosis, which was not found here. In agreement with past studies, hDC expression varied according to presence of liver disease, in this case, MAFLD.
Figure 4. Increased hepatic dendritic cells (CD11c+, hDCs) expression in liver tissue according to patient’s body mass index (BMI).
A graphic/schematic distribution of the liver expression of hDCs (CD11c+) according to patients’ BMI. Hematoxylin and Eosin (H), and Masson trichrome stains in liver biopsy form patients with different BMIs. Liver expression of hDCs (CD11c+) according to patients’ BMI.
The use of different cell surface markers (eg, lin−, HLA−DR+, CD1c+, CD163+) to characterize the differentiation of hDCs from other innate immune cells has been described thoroughly. In this study we employed a rabbit monoclonal anti-CD11c to characterize hDCs in liver tissue from MAFLD patients as previously described; however, it has been suggested that the CD11c+ molecule might not be specific to hDCs in humans (as it is in murine models) and that other immune cell subsets (eg, DCs, neutrophils, inflammatory monocytes, and natural killer, T, or B cells, and macrophages) regularly express CD11c+. Another limitation is having a single expert pathologist evaluating the immune-stained liver biopsies, whereas blind analysis by multiple pathologists would have increased the study’s impact.
Analyzing CD11c+ cells in patients of various BMIs is based on the finding of infiltration of these cells in specific tissues under different conditions. Studies in obese individuals showed that adipose tissue (AT) infiltration by innate immune cells (eg, CD11c+CD163+) was positively associated with increases in BMI, oxidative stress, and insulin resistance (IR) due to their functions in lipid metabolism and their role in initiating adaptive immune responses [22,23]. Furthermore, the increased levels of inflammatory interleukins (eg, IL-2) were positively correlated with CD11c+ cell expression in AT and also with weight and metabolic dysfunction [24]. Thus, the DCs (CD45+CD64−CD11c+) in AT might be independent contributors to obesity-induced chronic low-grade inflammatory or dysmetabolic state [25,26]. Furthermore, an association between fatty liver state and hDC function (tolerogenic vs proinflammatory) can be inferred due to differences in nomenclature of HDCs based on lipid content, with high-lipid hDCs inducing robust T cell activation and cytokine secretion, whereas low-lipid hDCs promote immune tolerance [11].
In a recent human in vivo and ex vivo study, the CD11c+ cells were defined as anti-inflammatory AT macrophages which additionally expressed CD206+ and CD9+ (crown-like structure macrophages) [27], similar to results in obese patients with steatohepatitis who underwent bariatric surgery (CD11c+ CD206+) [13]. The authors found increased classical DCs in obese patients, but no difference was found when patients with MAFLD were compared with those who were non-MAFLD; additionally, IR was related to a change in immune cell populations to proinflammatory phenotypes [25]. Thus, the increase of CD11c+ cell expression in AT during obesity and MAFLD is suggested to be related to the increases in BMI, IR, and inflammatory state and with steatohepatitis by initiating and promoting a proinflammatory state and metabolic dysfunction.
Bringing the conveyed information together, there’s a wide range of implications appertaining to our findings. To start with, even when hDCs have been found to have opposite functions in liver diseases depending on their temporality (acute or chronic), the fact that MAFLD in obese individuals showed increased expression of hDCs suggests a possible role in disease progression. However, as mentioned, obesity independently increases the transcription of CD11c, as well as AT infiltration by innate immune cells, which also express CD11c, such as macrophages. Furthermore, experimental murine studies consistently showed an increased infiltration of CD11c+ cells in the liver in obese states, raising the probability of finding hDCs during fibrosis progression [10,28]. The increased hDCs found in obese individuals with MAFLD in this study might be a result of the hypothesized function of hDCs in chronic inflammatory states as perpetuators, without overlooking the possibility that the causal agent might be the increased BMI per se, and not only the presence of MAFLD. Another important point is the fact that HSC proliferation is stimulated by the hDCs’ adaptive response, which makes the lack correlation between hepatic fibrosis and dendritic cell expression intriguing. However, it was also found that some of the dendritic cell features that have been described in fibrosis progression resemble “TNF-α/inducible nitric oxide synthase (iNOS)- producing DCs” (Tip-DCs), which are Ly6C+/Gr1+ monocyte-derived macrophages commonly found in inflamed tissue.
Finally, it is of utmost importance to highlight the fact that based on this and the preceding studies on the topic, hDCs’ role as possibly perpetuating inflammatory responses and potential fibrosis inducing capability (through HSC stimulation) cannot be inferred yet. This is based on the fact that while the process of NK cell activation by DCs is a well-defined process, there is also clear evidence that NK cell activity is protective during fibrosis progression [13]. This makes the possibility of hDCs’ role in liver disease as a modulator much more likely rather than it being completely injurious or protective.
Strengths and Limitations
An important limitation of this study is that it was not a longitudinal design and, thus, results might not represent high-strength evidence for causality. Secondly, flow cytometry was not available for this study; therefore, single-cell analysis was not included. Thirdly, even though the use of different cell surface markers (eg, lin−, HLA−DR+, CD1c+, CD163+) to characterize the differentiation of hDCs from other innate immune cells has been thoroughly described, we used only the most commonly expressed marker (CD11c) in an attempt to describe hDCs. Given the lack of information on the role of these cells on MAFLD pathogenesis, the use of this wide and sensitive cell surface marker gives us a wider perspective on the role of these cells and opens assumptions regarding involvement of other immune cells which have not been described yet, questioning whether it is an actual limitation or not. Regarding methodology and analysis, one of the limitations consisted in having a single expert pathologist evaluating the immune-stained liver biopsies. In this case, blind analysis by multiple pathologists would have increased the study’s impact.
The main strength of this study is that it is the first to assess MAFLD variation among different phenotypes (according to BMI), including patients with different grades of obesity (n=109) and lean/overweight patients (n=19). It is also the first study in humans describing the expression of hDCs (CD11c+) in the liver of patients with MAFLD, with the previously mentioned stratification according to their BMI. Importantly, showing the 3-variable relations among the expression of hDCs, BMI, and MAFLD opens up discussion regarding a potential role of the innate immune system in humans, leading to future studies analyzing specific molecular mechanisms and possible variations according to BMI.
Conclusions
In this study, hDCs (CD11c+ cells) were found to be higher in liver tissues from MAFLD patients with higher BMI, especially those with morbid obesity. However, diagnosis of fibrosis in MAFLD patients did not increase the probability of expressing hDCs (CD11c+ cells). Despite the fact that hDC expression in MAFLD patients varies depending on BMI, we showed that hDC expression (CD11c+) was also increased in liver tissue from obese MAFLD patients with fibrosis. Thus, obesity might be directly associated with the increased expression of hDCs (CD11c+) and this association remains unchanged despite the presence of fibrosis. Future research for characterization of this relationship, including additional and specific hDCs markers, morbid obesity-specific histological assessment scores, determination of the source of immune effector cells, and standardized clinical measurements, is encouraged. In conclusion, hDC expression is significantly higher in morbidly obese patients with MAFLD compared with non-obese patients, independent of the degree of fibrosis, suggesting the role of adaptive changes within hDCs in the perpetuation of inflammatory insults in chronic liver diseases.
Acknowledgments
We especially thank Prof. Mohammed Eslam who critically reviewed this manuscript.
Footnotes
This study was partially presented as a poster in the International Liver Congress from the European Association for Study of the Liver (EASL) in Paris, France (Poster Presentations: Experimental and Pathophysiology. 2018; 68(1): S363–S364
Conflict of interest: None declared
Ethics Statement
The study was conducted according to the guidelines of the Declaration of Helsinki (1975) and approved by the Institutional Ethics Committee of Medica Sur Clinic Foundation (CONBIOÉTI-CA-09-CEI-018-20160729).
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This study was partially financed by Medica Sur Clinic & Foundation with the active participation of the Pathology Department of Medica Sur Hospital. The principal author (B. B.F.) received a scholarship from Programa Universitario de Investigación en Salud (PUIS), UNAM
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease – meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Cesare M, Bentham J, Stevens GA, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Praveenraj P, Gomes RM, Kumar S, et al. Prevalence and predictors of non-alcoholic fatty liver disease in morbidly obese South Indian patients undergoing bariatric surgery. Obes Surg. 2015;25(11):2078–87. doi: 10.1007/s11695-015-1655-1. [DOI] [PubMed] [Google Scholar]
- 5.Ong JP, Elariny H, Collantes R, et al. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15(3):310–15. doi: 10.1381/0960892053576820. [DOI] [PubMed] [Google Scholar]
- 6.Morita S, Neto DDS, Morita FHA, et al. Prevalence of non-alcoholic fatty liver disease and steatohepatitis risk factors in patients undergoing bariatric surgery. Obes Surg. 2015;25(12):2335–43. doi: 10.1007/s11695-015-1696-5. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi K, Abrams GA. Prevalence of biopsy-proven non-alcoholic fatty liver disease in severely obese subjects without metabolic syndrome. Clin Obes. 2016;6(2):117–23. doi: 10.1111/cob.12132. [DOI] [PubMed] [Google Scholar]
- 8.Lonardo A, Bellentani S, Argo CK, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig Liver Dis. 2015;47(12):997–1006. doi: 10.1016/j.dld.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Mendez-Sanchez N, Cruz-Ramon VC, Ramirez-Perez OL, et al. New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int J Mol Sci. 2018;19(7):2034. doi: 10.3390/ijms19072034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen MTA, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 11.Méndez-Sánchez N, Córdova-Gallardo J, Barranco-Fragoso B, Eslam M. Hepatic dendritic cells in the development and progression of metabolic steatohepatitis. Front Immunol. 2021;12(March):1–11. doi: 10.3389/fimmu.2021.641240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henning JR, Graffeo CS, Rehman A, et al. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology. 2013;58(2):589–602. doi: 10.1002/hep.26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao J, Sastre D, Fiel MI, et al. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology. 2012;55(1):244–55. doi: 10.1002/hep.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aloman C, Tacke F. Dendritic cells in liver fibrosis: Conductor of the inflammatory orchestra? Hepatology. 2010;5(1):705–11. doi: 10.1002/hep.23542. [DOI] [PubMed] [Google Scholar]
- 15.Deczkowska A, David E, Ramadori P, et al. XCR1+ type 1 conventional dendritic cells drive liver pathology in non-alcoholic steatohepatitis. Nat Med. 2021;27(6):1043–54. doi: 10.1038/s41591-021-01344-3. [DOI] [PubMed] [Google Scholar]
- 16.Heier EC, Meier A, Julich-Haertel H, et al. Murine CD103+ dendritic cells protect against steatosis progression towards steatohepatitis. J Hepatol. 2017;66(6):1241–50. doi: 10.1016/j.jhep.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Aarts S, Reiche M, den Toom M, et al. Depletion of CD40 on CD11c+ cells worsens the metabolic syndrome and ameliorates hepatic inflammation during NASH. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-50976-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukacs-Kornek V, Schuppan D. Dendritic cells in liver injury and fibrosis: Shortcomings and promises. J Hepatol. 2013;59(5):1124–26. doi: 10.1016/j.jhep.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Bernsmeier C, Albano E. Liver dendritic cells and NAFLD evolution: A remaining open issue. J Hepatol. 2017;66(6):1120–22. doi: 10.1016/j.jhep.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73(1):202–9. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Flynn MAT, McNeil DA, Maloff B, et al. Reducing obesity and related chronic disease risk in children and youth: A synthesis of evidence with “best practice” recommendations. Obses Rev. 2006;7(Suppl 1):7–66. doi: 10.1111/j.1467-789X.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima S, Koh V, Kua L-F, et al. Accumulation of CD11c + CD163 + adipose tissue macrophages through upregulation of intracellular 11β-HSD1 in human obesity. J Immunol. 2016;197(9):3735–45. doi: 10.4049/jimmunol.1600895. [DOI] [PubMed] [Google Scholar]
- 23.Wentworth JM, Naselli G, Brown WA, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–56. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochumon S, Al Madhoun A, Al-Rashed F, et al. Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-73347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engin A. Human protein kinases and obesity. Adv Exp Med Biol. 2017;960:111–34. doi: 10.1007/978-3-319-48382-5_5. [DOI] [PubMed] [Google Scholar]
- 26.Cho KW, Zamarron BF, Muir LA, et al. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. J Immunol. 2016;197(9):3650–61. doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs A, Samovski D, Smith GI, et al. Associations among adipose tissue immunology, inflammation, exosomes and insulin sensitivity in people with obesity and nonalcoholic fatty liver disease. Gastroenterology. 2021;161(3):968–81. doi: 10.1053/j.gastro.2021.05.008. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefanovic-Racic M, Yang X, Turner MS, et al. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61(9):2330–39. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]