Abstract
Increasing temperature and thermal variability generate profound selection on populations. Given the fast rate of environmental change, understanding the role of plasticity and genetic adaptation in response to increasing temperatures is critical. This may be especially true for thermal effects on reproductive traits in which thermal fertility limits at high temperatures may be lower than for survival traits. Consequences of changing environments during development on adult phenotypes may be particularly problematic for core traits such as reproduction that begin early in development. Here we examine the consequences of developmental thermal plasticity on subsequent adult reproductive traits and its genetic basis.
We used a panel of Drosophila melanogaster (the Drosophila Genetic Reference Panel; DGRP) in which male fertility performance was previously defined as either showing relatively little (status = ‘high’‐performing lines) or substantial (‘low’‐performing lines) decline when exposed to increasing developmental temperatures. We used a thermal reaction norm approach to quantify variation in the consequences of developmental thermal plasticity on multiple adult reproductive traits, including sex‐specific responses, and to identify candidate genes underlying such variation.
Developmental thermal stress impacted the means and thermal reaction norms of all reproductive traits except offspring sex ratio. Mating success declined as temperature increased with no difference between high and low lines, whereas increasing temperature resulted in declines for both male and female fertility and productivity but depended on line status. Fertility and offspring number were positively correlated within and between the sexes across lines, but males were more affected than females.
We identified 933 SNPs with significant evolved genetic differentiation between high and low lines. In all, 54 of these lie within genomic windows of overall high differentiation, have significant effects of genotype on the male thermal reaction norm for productivity and are associated with 16 genes enriched for phenotypes affecting reproduction, stress responses and autophagy in Drosophila and other organisms.
Our results illustrate considerable plasticity in male thermal limits on several reproductive traits following development at high temperature, and we identify differentiated loci with relevant phenotypic effects that may contribute to this population variation. While our work is on a single population, phenotypic results align with an increasing number of studies demonstrating the potential for stronger selection of thermal stress on reproductive traits, particularly in males. Such large fitness costs may have both short‐ and long‐term consequences for the evolution of populations in response to a warming world.
Keywords: heat‐induced sterility, thermal reaction norm, reproduction, fertility, F st , sexual dimorphism
Exposing an insect population to increasing heat stress during development, the authors show significant variation in the population for consequences on some, but not all, subsequent adult reproductive traits. This variation is used to identify candidate genes that may underlie thermal fertility limits.
1. INTRODUCTION
Organisms’ responses to environmental change involve changes in behaviour such as migration, phenotypic plasticity (West‐Eberhard, 2003; Whitman & Agrawal, 2009) and genetic adaptation. Plasticity, which is broadly defined as environment‐dependent expression of a phenotype, may either inhibit or accelerate genetic adaptation (Ghalambor et al., 2007, 2015; Schmid & Guillame, 2017). Adaptation may also be limited, for example many studies have found reduced adaptive potential in thermal limits (the temperature at which physiological function fails; e.g. Bennett et al., 2021; Schou et al., 2014; van Heerwaarden et al., 2016). This latter finding is especially worrying given the fast rate of anthropogenic climate change (Arias et al., 2021; Candolin & Wong, 2012), increasing mean temperature, and duration and intensity of heat extremes (Arias et al., 2021). Thus, there is a premium on understanding the role of plasticity and genetic adaptation in response to the changing thermal environment, which is critical to successfully predict and mitigate risks that species face due to climate change (Bonamour et al., 2019; Lafuente & Beldade, 2019).
Stress experienced during development poses a particular challenge in terms of maintaining homeostasis and continuing normal development. Phenotypic changes induced during development affect adult phenotypes, including growth, behaviour, reproduction and metabolism (reviewed in Eyck et al., 2019). Developmental or juvenile stages of some organisms, for example, holometabolous insects, may be environmentally restricted to particular habitats. Such restrictions may prevent the ability to move away from unfavourable environmental conditions, increasing susceptibility to warming effects. Consequently, less mobile life stages may rely on plasticity to a greater extent to maintain homeostasis. However, the evolution of adaptive plastic responses requires high reliability of environmental cues (Moghadam et al., 2019; Uller et al., 2015), with evolutionary responses in the face of higher novelty and unpredictability of weather phenomena being less well understood (Bitter et al., 2021; Bonamour et al., 2019). Moreover, developmental plasticity that leads to changes in adult phenotypes are often irreversible, particularly for core processes (Hoffmann & Bridle, 2021; West‐Eberhard, 2003). With increasingly novel and variable thermal environments, there may be increasing risks of generating an inappropriate phenotype based on current sensitivity of genotypes to the environment. Thus, determining whether populations harbour phenotypic and genetic variation to maintain developmental homeostasis for core processes under increasing temperatures is of interest (West‐Eberhard, 2003).
The variation in response to thermal stress can differ not only between life cycle stages but also between the sexes. This sexual dimorphism is expected given vast differences in male and female reproductive physiologies (Baur et al., 2021; García‐Roa et al., 2020). In particular, spermatogenesis typically exhibits higher thermal sensitivity than oogenesis and this may result in male reproductive performance being more susceptible to thermal stress (e.g. David et al., 2005; Kurhanewicz et al., 2020; Porcelli et al., 2016; Rohmer et al., 2004). In many insects, spermatogenesis starts during development (Nijhout, 1998) and heat stress experienced by males during this time can manifest in the adult stage as sterility (e.g. see David et al., 2005; Green et al., 2019; Jørgensen et al., 2006; Pedersen et al., 2011; Porcelli et al., 2016; Rohmer et al., 2004; Sales et al., 2018; Sales et al., 2021; Saxon et al., 2018; Walsh et al., 2020; Zwoinska et al., 2020). However, in many sexually reproducing species, population growth is mainly dependent on female fertility (Caswell, 2006). Thus, understanding how both sexes are impacted by rapid climate change and how they interact is important for determining the short‐ and long‐term evolutionary consequences of thermally sensitive traits (Baur et al., 2021; Iossa, 2019; Walsh, Parratt, Atkinson, et al., 2019; Walsh, Parratt, Hoffmann, et al., 2019).
Plasticity is often modelled using a reaction norm, which represents the range of phenotypes expressed by a genotype across an environmental gradient (Stearns, 1992). Aspects of the reaction norm can define several biologically relevant parameters, such as both the cold and hot temperatures at which homeostasis fails (CTmin and CTmax, respectively), also called critical thermal limits, and optimum temperature (Topt). Trait performance can be quantified by a curve on this reaction norm, and the linear slope can be used to describe performance, with a steeper slope indicating a greater degree or amount of plasticity, whereas a shallower slope points to canalization of a phenotype (Stearns, 1992). There has been a recent emphasis on the application of this framework to define thermal fertility limits (the temperature threshold at which an organism is unable to reproduce, similar to critical thermal limits for survival traits; Walsh, Parratt, Atkinson, et al., 2019). In addition, recent comparative research on Drosophila species showed that thermal limits for male fertility are lower than limits for survival when individuals are stressed as adults and that such limits predict current species distributions better than survival limits (Parratt et al., 2021; van Heerwaarden & Sgrò, 2021). Examination of the thermal limits of more reproductive traits is required to get a broader perspective of the effect of increasing temperatures on reproductive processes.
Determining the genetic architecture of phenotypic traits is critical for understanding the potential of populations to respond to selection. Adaptation of complex quantitative traits, such as those influencing reproductive success, is predicted to occur through many loci of small effect, but several recent studies have identified large‐effect loci associated with rapid adaptation (Mallard et al., 2018; Messer et al., 2016). Loci contributing to inter‐genotype variation in plasticity may provide the raw material for evolution of plasticity, and artificial selection experiments have found that single allelic variants can alter trait sensitivity to the environment (reviewed in Lafuente & Beldade, 2019). Moreover, determining whether there is a distinct genetic basis between genes contributing to variation in trait plasticity with those contributing to variation in trait means allows determining the possibility of independent evolution of the two critical ways organisms can respond to climate change (e.g. Lafuente et al., 2018; Ørsted et al., 2019).
Here we focus on the benign to thermally stressful portion of the thermal response curve, to investigate how reproductive trait values may change via developmental thermal plasticity in response to increasing temperatures, while determining between‐sex correlation in responses, and identifying loci that contribute to changes in trait mean and plasticity. We do this by exposing a subset of inbred Drosophila melanogaster lines from the Drosophila Genetic Resource Panel (DGRP) to three different rearing temperatures (Huang et al., 2014; Mackay et al., 2012). This subset is determined from previous work across 127 DGRP lines in which we measured developmental thermal plasticity of male fertility (Zwoinska et al., 2020). From this work, we identified 20 lines that maintained fertility in the adult stage across all developmental temperatures (‘high’‐performing lines characterized by less steep negative reaction norm slopes across three developmental temperature treatments: 25°C, 27°C and 29°C) and 20 lines where fertility declined with increasing temperature (‘low’‐performing lines characterized by steeper negative reaction norm slopes; Zwoinska et al., 2020). We used these high and low lines and re‐measured male fertility to test repeatable responses, which suggests an underlying genetic control. We also measured additional reproductive traits that may be affected by thermal stress and impact population viability—mating success, offspring production and offspring sex ratio. We determined adult reproductive trait means following development at different temperatures, calculated the thermal reaction norms across these temperatures, and determined correlations between traits within sexes and between sexes across lines. We then identified loci contributing to intra‐population variation in plasticity and trait means. We first identified evolved genetic differences between low and high lines, and then tested for an effect of genotypes at these outlier loci on phenotypic variation between high and low lines in either the productivity thermal reaction norm or mean of productivity.
2. MATERIALS AND METHODS
2.1. Fly stocks
All flies used belong to isogenic lines of the Drosophila melanogaster Genetic Reference Panel (DGRP; Huang et al., 2014; Mackay et al., 2012). Given these are Drosophila, no ethical approval is required for the work. These lines originate from a single population collected in Raleigh, North Carolina, in 2003 and were subjected to 20 generations of full‐sib mating resulting in a panel of 205 inbred lines that was subsequently sequenced. Male thermal fertility limits have been described in 127 of these lines based on response to constant developmental temperatures of 25°C, 27°C and 29°C (Zwoinska et al., 2020). From these results, a subset representing lines exhibiting either little thermal effect on male fertility (‘high’ lines) or lines in which fertility declines steeply in response to increased temperature (‘low’ lines) were selected (Zwoinska et al., 2020 for more details; line performance category primarily was based on line response at 29°C and the slope of the reaction norm). A total of 20 high and 20 low lines was used to determine male performance. Female performance was measured at a separate time from the males and using a smaller subset of the DGRP lines (7 high lines and 8 low lines) that did not completely overlap with the male subset (NB: mean female fertility was previously reported in Zwoinska et al. (2020) but it was not decomposed by line status). We decomposed thermal effects of the sexes by mating focal DGRP individuals to wild‐type Canton Special (CS) strain raised at 25°C. All flies were maintained in standard culture vials using cornmeal medium (9 L water, 720 g cornmeal, 162 g dried yeast, 90 g soya flour, 720 g malt extract, 360 g molasses, 72 g agar, 36 ml propionic acid and 225 ml of 10% Nipagin) at 12‐h light:12‐h dark cycle.
2.2. Establishment of mating pairs
The protocol applied is described in detail in Zwoinska et al. (2020). Briefly, DGRP lines were reared at 25°C, adults transferred to egg laying media to oviposit and experimental vials established with 50 eggs/line/food vial. Vials were then randomly placed inside incubators set to 25°C, 27°C or 29°C. Focal DGRP virgins no older than 4 h from each line were subsequently collected under CO2 anaesthesia and transferred into individual vials and placed at 25°C. The following day a single focal individual was paired with an opposite sex CS individual. Control CS mating partners were generated following the same protocol except 100 eggs/vial were established, virgins were stored in sex‐specific groups of about 20, and were 3‐ to 6‐day old at the time of pairing. Pairs were kept at 25°C for 3 days, then discarded.
2.3. Reproductive trait measurements
Once mating pairs were established, the effect of developmental heat stress was measured for four different reproductive fitness traits of males: mating success in the first 8 hr of sexual interaction, fertility (measured as the presence/absence of larvae), offspring number and sex ratio. The experiment ran for two blocks, each consisting of all 40 DGRP lines with sample sizes for each trait around n = 10 focal males/block (see Table S1 for descriptive statistics). Mating observations started immediately after pairing in a climate room maintained at 25°C. Pairs were observed for mating for a maximum of 8 h after pairing, after which they were left unobserved. Thus, our measure of mating success is conservative because pairs could mate after 8 h, until being removed from the vial 3 days later. Fertility of the focal individual was assessed based on the presence of larvae, scored as presence/absence data 5 days after pairing (i.e. fertility; Zwoinska et al., 2020). Subsequent offspring numbers (i.e. productivity) and sex ratio for each focal individual were collected at two separate timepoints (12 days and 14–18 days after pairing) to ensure complete offspring counts, and the total summed for analysis. We repeated these experiments for focal DGRP females mated with CS males, with the exception that mating success was not measured. The female experiment was run in a single block with c. 30 focal individuals per line (see Table S1 for descriptive statistics). In all, 11 DGRP lines overlapped between the male and female experiments.
2.4. Statistical analyses of phenotypic data
Sample sizes and descriptive statistics of male and female responses for relevant traits at each temperature per line are found in Table S1. All analyses were carried out using the r statistical package (v. 3.0.3, R Foundation for Statistical Computing, 2020). Maximal models were simplified by sequentially eliminating non‐significant terms from the highest to the simplest order interaction, to establish a minimal model (Crawley, 2007; Table S2), and the significance of the dependent variables was established using type III Wald chi‐squared tests and Wald F tests, in the case of discrete distributions or continuous distributions, respectively (Anova, car package; Bolker, 2008; Table 1). A posteriori contrasts with Bonferroni corrections were done to interpret the significant effect of factors (pairs, emmeans package; Lenth, 2016; Table 2).
TABLE 1.
Generalized linear and linear mixed‐effect model output for reproductive traits of (a) males and (b) females. df indicates the degrees of freedom. χ 2 provides the chi‐square value obtained in each analysis. ‘temperature’, temperature in each treatment (25°C, 27°C or 29°C); ‘line status’, DGRP line classification based on male fertility performance (low or high lines); ‘block’, week in which the data were collected (1 or 2). Statistically significant effects are represented in bold, statistically marginally significant effects represented with *
| Var. of interest | Explanatory var. | df | χ 2 | p value |
|---|---|---|---|---|
| Male reproductive traits | ||||
| Mating success | Temperature × line status | 2 | 0.682 | 0.7111 |
| Temperature | 2 | 213.566 | <0.001 | |
| Line status | 1 | 0.001 | 0.978 | |
| Block | 1 | 12.891 | <0.001 | |
| Fertility | Temperature × line status | 2 | 43.522 | <0.001 |
| Temperature | 2 | 11.331 | 0.0036 | |
| Line status | 1 | 1.872 | 0.171 | |
| Block | 1 | 0.074 | 0.785 | |
| Productivity | Temperature × line status | 2 | 31.584 | <0.001 |
| Temperature | 2 | 5.376 | 0.068* | |
| Line status | 1 | 0.502 | 0.479 | |
| Block | 1 | 246.584 | <0.001 | |
| Sex ratio | Temperature × line status | 2 | 0.256 | 0.88 |
| Temperature | 2 | 0.291 | 0.865 | |
| Line status | 1 | 0.027 | 0.402 | |
| Block | 1 | 0.109 | 0.741 | |
| (b) Female reproductive traits | ||||
| Fertility | Temperature × line status | 2 | 7.297 | 0.026 |
| Temperature | 2 | 1.421 | 0.491 | |
| Line status | 1 | 0.013 | 0.91 | |
| Productivity | Temperature × line status | 2 | 3.008 | 0.222 |
| Temperature | 2 | 18.426 | <0.001 | |
| Line status | 1 | 1.018 | 0.313 | |
| Sex ratio | Temperature × line status | 2 | 1.362 | 0.836 |
| Temperature | 2 | 3.621 | 0.164 | |
| Line status | 1 | 1.371 | 0.242 | |
TABLE 2.
A posteriori contrasts of significant explanatory variables for reproductive traits of (a) males and (b) females. Contrasts with Bonferroni corrections were performed to interpret which conditions significantly differed from each other. ‘Temperature’, average temperature in each treatment (25°C, 27°C and 29°C; here represented as ‘25’, ‘27’ and ‘29’, respectively); ‘line status’, DGRP line classification based on male fertility performance (low or high lines; here represented as ‘Low’ and ‘High’, respectively; Zwoinska et al., 2020). Test statistics reported: z‐ratios for binary variables and t‐ratios for productivity. Statistically significant contrasts are represented in bold, statistically marginally significant contrasts are represented with *
| Var. of interest | Significant explanatory var. | Comparison | Estimate | SE | Test statistics | p value |
|---|---|---|---|---|---|---|
| (a) Male reproductive traits | ||||||
| Mating success | Temperature | 25 versus 27 | −0.07 | 0.140 | 0.14 | 1.000 |
| 25 versus 29 | 1.691 | 0.137 | 0.137 | <0.001 | ||
| 27 versus 29 | 1.961 | 0.138 | 0.138 | <0.001 | ||
| Fertility | Temperature × line status | 25 high versus 27 high | 0.248 | 0.484 | 0.248 | 1.000 |
| 25 high versus 29 high | 1.473 | 0.486 | 1.473 | 0.022 | ||
| 27 high versus 29 high | 1.225 | 0.451 | 2.715 | 0.060* | ||
| 25 low versus 27 low | −1.100 | 0.556 | −1.976 | 0.434 | ||
| 25 low versus 29 low | 4.445 | 0.471 | 9.428 | <0.001 | ||
| 27 low versus 29 low | 5.545 | 0.569 | 9.742 | <0.001 | ||
| 25 high versus 25 low | 0.568 | 0.415 | 1.368 | 1.000 | ||
| 27 high versus 27 low | −0.779 | 0.603 | −1.291 | 1.000 | ||
| 29 high versus 29 low | 3.540 | 0.547 | 6.469 | <0.001 | ||
| Productivity | Temperature × line status | 25 high versus 27 high | −0.024 | 0.052 | −0.462 | 1.000 |
| 25 high versus 29 high | 0.730 | 0.348 | 2.096 | 0.326 | ||
| 27 high versus 29 high | 0.754 | 0.34 | 2.221 | 0.238 | ||
| 25 low versus 27 low | 0.107 | 0.054 | 1.981 | 0.43 | ||
| 25 low versus 29 low | 3.653 | 0.390 | 9.359 | <0.001 | ||
| 27 low versus 29 low | 3.546 | 0.383 | 9.250 | <0.001 | ||
| 25 high versus 25 low | −0.031 | 0.043 | −0.709 | 1.000 | ||
| 27 high versus 27 low | 0.100 | 0.093 | 1.079 | 1.000 | ||
| 29 high versus 29 low | 2.893 | 0.533 | 5.429 | <0.001 | ||
| (b) Female reproductive traits | ||||||
| Fertility | Temperature × line status | 25 high versus 27 high | 0.135 | 0.471 | 0.286 | 1.000 |
| 25 high versus 29 high | −0.520 | 0.567 | −0.918 | 1.000 | ||
| 27 high versus 29 high | −0.655 | 0.557 | −1.175 | 1.000 | ||
| 25 low versus 27 low | 0.2137 | 0.498 | 0.429 | 1.000 | ||
| 25 low versus 29 low | 1.218 | 0.451 | 2.702 | 0.062* | ||
| 27 low versus 29 low | 1.004 | 0.436 | 2.305 | 1.000 | ||
| 25 high versus 25 low | −0.058 | 0.511 | −0.113 | 1.000 | ||
| 27 high versus 27 low | 0.021 | 0.486 | 0.044 | 1.000 | ||
| 29 high versus 29 low | 1.681 | 0.541 | 3.104 | 0.017 | ||
| Productivity | Temperature | 25 versus 27 | 0.077 | 0.104 | 0.743 | 1 |
| 25 versus 29 | 0.735 | 0.186 | 3.945 | <0.001 | ||
| 27 versus 29 | 0.658 | 0.233 | 2.882 | 0.015 | ||
For males, the effect of temperature (25°, 27° and 29°C), line status (high and low lines) and their interaction on the proportion of matings within the first 8 h (‘mating success’, hereafter) and the proportion of fertility (measured as the presence/absence of larvae) in each line, was determined using a mixed‐effect model with a logit link function and a binomial distribution (function glmer, lme4 package and function glmmtmb, glmmtmb package, respectively; Bates et al., 2015, Brooks et al., 2017). Productivity was analysed using a mixed‐effect model with a Quasi‐Poisson distribution, a log link function and the term ziformula = ~1, to account for overdispersion caused by zero inflation due to sterile males (glmmtmb, glmmtmb package; Brooks et al., 2017). Offspring sex ratio was analysed using a binomial distribution and a logit function. Models with temperature as a continuous variable more clearly test whether the effect of temperature is linear versus nonlinear. However, with three temperatures, as is the case here, any attempt to assess the shape of the effect of temperature will be very coarse. In turn, having temperature as a factor allows us to compare the response of high and low lines at each of the temperature treatments employed. Thus, in all cases, temperature, line status, their interaction and block were used as fixed factors, while line ID was used as a random factor. In any case, we have performed models with temperature as a continuous variable (using a linear, quadratic or negative exponential term; results not shown) and the results obtained are similar to the ones reported here. Models were run with random slopes (i.e. the random factor line ID was coded as (Temperature|DGRP line)). However, mating success and offspring sex‐ratio models did not converge, potentially due to overparameterization. This is likely due to the random‐effect variance of sex ratio being very close to zero and due to some categories in the mating success model containing proportions that are either mostly 0 or mostly 1 (at 29°C and 25°C, respectively). For these two traits, we therefore used random intercept models (i.e. the random factor line ID was coded as (1|DGRP line)).
For females, models with the same error structure and effect terms as the male dataset were run. A random slopes model was specified for productivity but this model failed to converge for offspring sex ratio, likely due to the same issue for that trait in the male dataset, so a random intercepts model was used. Model comparisons using AIC also showed that the fit for female fertility data was better with a random intercept model, which is what we report.
The significance of correlations between fertility and productivity in males and in females as well as the correlation between the slopes of productivity measures for males and females (see next section) in the overlapping DGRP lines was determined using the hmisc package (function rcorr; Harell Jr., 2019).
Developmental thermal reaction norms were estimated as the consequences on adult reproductive traits. Because the response to different temperatures is not necessarily linear, we divided the dataset in two, one including the two lowest temperatures (25°C and 27°C) and another including the two highest temperatures (27°C and 29°C). For each subset and all traits with a significant response to temperature, line‐specific slopes were extracted from random slopes models, where temperature, treated as a numeric variable, was added as a fixed factor as was block (for male data), and line ID was treated as random factor (Temperature|DGRP line). Models used to extract the random slopes for the proportion of mating (for males only) and fertility had a binary error distribution and a logit link function (glmmtmb package; Brooks et al., 2017), whereas those used to extract the random slopes for productivity presented a Poisson error distribution and log link function (glmmtmb package; Brooks et al., 2017). Model slopes were extracted with the coef command (Bates et al., 2015) and the average slope value for each line in males and females for all traits is found in Table S3. The model of the proportion of fertility in females failed to converge, so these slopes are not reported. We tested whether the slopes of the traits were significantly different from zero and determined whether lines categorized as high and low had dissimilar reaction norms. This was performed using a linear model with a normal error distribution and an identity link function with line status as a fixed explanatory factor and the line‐specific random slopes as the dependent variable (Table S2). The slope values of male fertility and productivity, as well as of female productivity, were transformed to meet normality requirements in the subset including the two lowest temperatures (25°C and 27°C; package MASS, function boxcox; Venables & Ripley, 2002). Raw datasets for each phenotypic trait measured are available online (Rodrigues et al., 2022).
2.5. Genomic analyses
Using the DGRP v. 2 SNP data (http://dgrp2.gnets.ncsu.edu/; Huang et al., 2014; Mackay et al., 2012), we extracted the genotype data for the high and low lines (N = 20 lines for each line status) using vcftools (v 0.1.17; Danecek et al., 2011). Note that due to missing data the total number of chromosomes varied substantially. We therefore filtered SNPs to exclude those with fewer than 10 chromosomes (<5 individuals) in either group of lines. We also applied an overall minor allele frequency filter of 0.05. We then used BayeScan (v.2.1; Foll & Gaggiotti, 2008) to search for F st outliers between high and low lines to identify potential evolved differences between these groups. BayeScan can identify regions of high genetic differentiation while accounting for correlated allele frequencies within and between groups due to shared ancestry (Foll & Gaggiotti, 2008). We used 5 pilot runs of 5,000 iterations each followed by a main chain of with 20,000 burn‐in iterations and 50,000 additional iterations keeping every 10th iteration. We confirmed the convergence of chains using Geweke’s and Heidelberg and Welch’s convergence diagnostics from the coda r package (0.19‐4; Plummer et al., 2006). To control for an inflated number of SNP outliers due to physical linkage of SNPs and the resulting correlation in allele frequencies (and therefore F st and q‐values), we summarized the signal by computing the mean q‐value for non‐overlapping windows of 5 kb (see Figure S1). Outlier windows were then called as those with a mean −log10(q‐value) ≥ 0.13 (the 99.9th percentile). Although the mean q‐value for each window is not completely independent of the SNP‐wise q‐values, we reason that this approach should identify windows containing a large number of SNPs with low q‐values; regions with true associations to the phenotype should have an effect at several linked SNPs, whereas spurious associations should affect only single SNPs. For each SNP identified as an F st outlier (q‐value < 0.05) within the outlier windows, we then collected genotype information for the phenotyped lines and performed an ANOVA to test for an effect of genotype on phenotype (slope of productivity from 27°C to 29°C and productivity at 29°C) using F‐tests. Our final set of candidate SNPs were those for which a significant effect of genotype on phenotype could be detected.
2.6. Functional annotation
We obtained the SNPeff (Cingolani et al., 2012) annotation for each SNP, also available as a DGRP resource (Huang et al., 2014; Mackay et al., 2012). We tested for enrichment of phenotypic terms with modPhEA (Weng & Liao, 2017) and gathered functional information for genes associated with SNPs from FlyBase (v. FB2021_04; Thurmond et al., 2019) and the literature and expression information from FlyAtlas (v.2; Leader et al., 2018).
3. RESULTS
3.1. Phenotypic responses to developmental thermal plasticity
Offspring sex ratio from either focal males or females was not affected by any factor tested (Table 1; Figure S2). However, temperature significantly negatively affected focal male mating success (χ 2 = 213.6, df = 2, p < 0.001; Table 1a; Figure 1a) which was independent of the line status (Table 1a). The change in mating success was observed at 29°C (Table 2a) with 0.77 ± 0.01 of the pairs mating within the first 8 h at 25°C and 27°C but only 0.47 ± 0.01 (high lines: 0.472 ± 0.03; low lines: 0.468 ± 0.03) at 29°C, corresponding to a decrease of c. 40%. Accordingly, mating performance (i.e. slope of the reaction norm) significantly decreased between 27°C and 29°C, but there was no difference between high and low lines (Table 3; Figures 2a,b).
FIGURE 1.
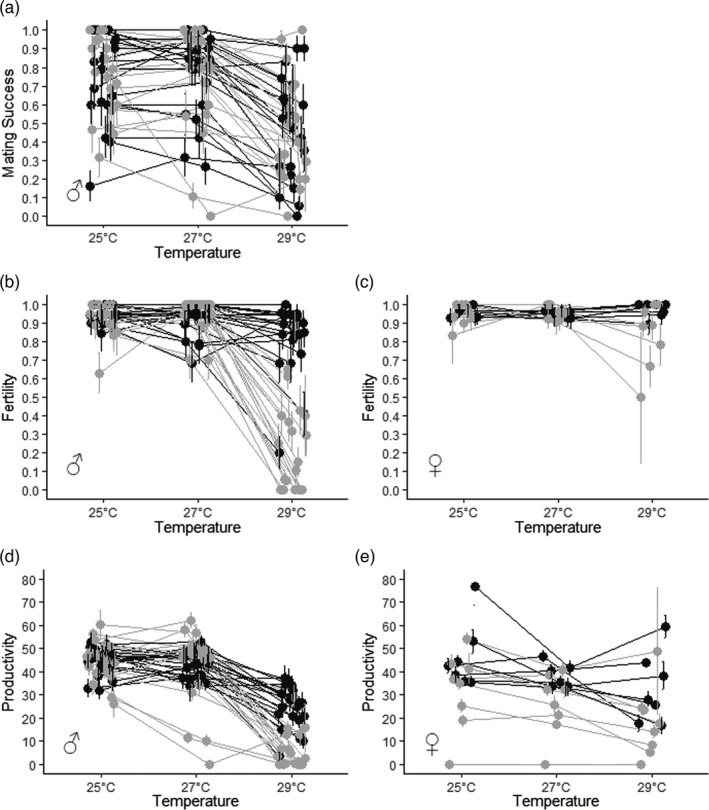
Phenotypic variation of reproductive traits in males and females exposed to three different temperatures during development. Flies developed at 25°C, 27°C or 29°C. In each of the 40 DGRP lines, we measured (a) the proportion of vials in which mating was observed within the first 8 hr (i.e. mating success), (b and c) the proportion of vials in which at least one larva emerged from eggs (i.e. fertility) and (d and e) the number of adult offspring (i.e. productivity) per vial. Colour tones represent different line status (‘high lines’, dark grey, ‘low lines’ light grey), each dot represents an individual line (±SE). (a, b and d) show the average trait values when DGRP males were exposed to the different temperatures; (c and e) show the average trait values when DGRP females were exposed to the different temperatures
TABLE 3.
Thermal reaction norm parameters for male and female reproductive performance. Random slopes extracted from random slope models examining the change in each response variable between either 25°C and 27°C or between 27°C and 29°C temperatures. Table reports two model outputs: ‘High lines (Intercept)’ which tests whether the slope of high lines differs from a slope of zero and ‘Low lines’ which tests whether the slope of high and low lines differ. df indicates the degrees of freedom. t value is the t test values obtained in each analysis. Statistically significant contrasts are represented in bold and marginal significance is marked with *
| Sex | Var. of interest | Reaction norm | Comparison | Estimate | SE | t value | p value |
|---|---|---|---|---|---|---|---|
| Male | Mating success | 25°C −27°C | High lines (Intercept) | 0.055 | 0.009 | 5.865 | <0.001 |
| Low lines | 0.003 | 0.013 | 0.236 | 0.815 | |||
| 27°C −29°C | High lines (Intercept) | −0.872 | 0.048 | −18.37 | <0.001 | ||
| Low lines | −0.037 | 0.067 | −0.557 | 0.581 | |||
| Fertility | 25°C −27°C1 | High lines (Intercept) | 0.065 | 0.079 | 0.817 | 0.419 | |
| Low lines | 0.23 | 0.112 | 2.046 | 0.048 | |||
| 27°C −29°C | High lines (Intercept) | −0.745 | 0.145 | −5.145 | <0.001 | ||
| Low lines | −1.707 | 0.205 | −8.335 | <0.001 | |||
| Productivity | 25°C −27°C1 | High lines (Intercept) | 0.001 | 0.022 | 0.060 | 0.952 | |
| Low lines | −0.015 | 0.031 | −0.477 | 0.636 | |||
| 27°C −29°C | High lines (Intercept) | −0.348 | 0.182 | −1.913 | 0.063* | ||
| Low lines | −1.39 | 0.258 | −5.396 | <0.001 | |||
| Female | Productivity | 25°C −27°C1 | High lines (Intercept) | −0.145 | 0.073 | −1.979 | 0.069* |
| Low lines | 0.075 | 0.100 | 0.747 | 0.468 | |||
| 27°C −29°C | High lines (Intercept) | 0.224 | 0.272 | −0.821 | 0.426 | ||
| Low lines | −0.597 | 0.3730 | −1.601 | 0.133 |
FIGURE 2.
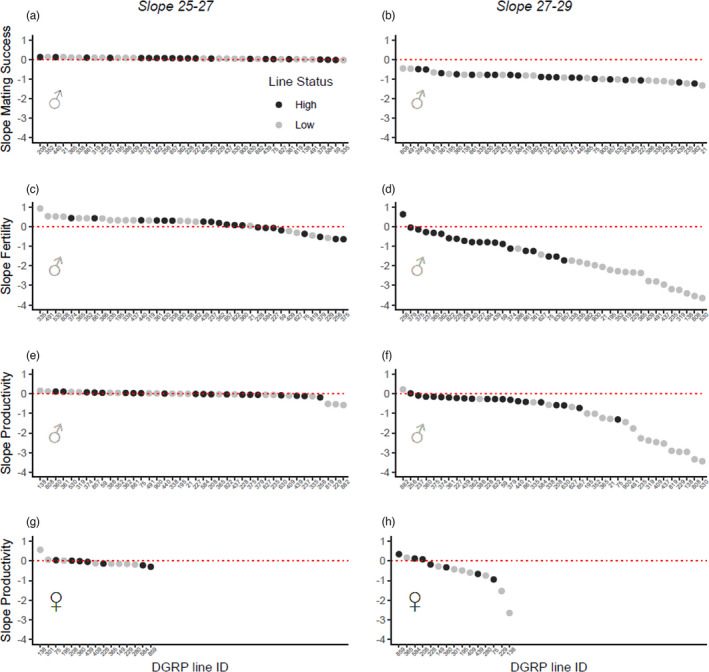
Reproductive performance of males and females following developmental heat stress. Flies developed at 25°C, 27°C or 29°C. Random slopes extracted from random slope models modelling the relationship between the different response variables and the two lowest (25°C and 27°C; panels a, c, e and g), or the two highest (27°C and 29°C; panels b, d, f and h), temperatures were obtained for each DGRP line. Response variables: (a and b) the proportion of vials with DGRP males in which at least one mating was observed within the first 8 h (i.e. mating success), (c and d) the proportion of vials with DGRP males in which at least one larva emerged from eggs (i.e. fertility), (e and f) the number of adult offspring (i.e. productivity) per vial with DGRP males and (g and h) the number of adult offspring (i.e. productivity) per vial with DGRP females. The DGRP lines are ordered according to their slope value, from the highest to the lowest. Colour tones represent different line status (‘high lines’, dark grey, ‘low lines’ light grey), each dot represents an individual line. Red dotted lines mark the intercept = 0
In contrast, focal male fertility was negatively affected by an interaction between temperature and line status (temperature × line status: χ 2 = 43.5, df = 2, p < 0.001; Table 1a). Assignment of line status based on fertility performance was consistent across studies, with 36 of the 40 lines previously categorized as having high or low performance at 29°C (Zwoinska et al., 2020) being replicated in this study. Fertility was close to 1 at 25°C and 27°C in both high and low lines (0.942 ± 0.005 and 0.937 ± 0.005, respectively) but fell to 0.81 ± 0.02 in the high lines and to 0.25 ± 0.02 in the low lines at 29°C. Thus, 29°C for low lines could be taken as close to their thermal fertility limit (Parratt et al., 2021). These reductions correspond to decreases of 15.1% and 73.4% in the fertility of high and low lines, respectively, relative to 25°C (Table 2a; Figure 1b). This pattern is mimicked in fertility performance (i.e. fertility reaction norm) which significantly declines between 27°C and 29°C in all lines, with low lines declining more than high lines (Table 3; Figure 2c,d). Focal female fertility also was affected by an interaction between temperature and line status (χ 2 = 7.3, df = 2, p = 0.026; Table 1b; Figure 1c), with high and low line females differing at 29°C (Table 2b), although this decrease was relatively marginal, being c. 9% relative to 25°C.
Focal male productivity also was negatively affected by an interaction between temperature and line status (χ 2 = 31.6, df = 2, p < 0.001, Table 1a), with offspring number reduced at 29°C in the low lines but no effect in high lines (Table 2a; Figure 1d). Reduction in the low lines was equivalent to a decrease in offspring production of c. 88% relative to 25°C, representing a thermal fertility limit (Table 2a). Productivity thermal reaction norms reflect the same pattern, with productivity substantially declining between 27°C and 29°C in low lines but only decreasing marginally in the high lines (Table 3; Figure 2e,f). In contrast, female focal productivity was negatively affected only by temperature (χ 2 = 18.4, df = 2, p < 0.001; Table 1; Figure 1e) with significant decreases at both 27°C and 29°C. Females decreased productivity at 27°C by c. 17% and at 29°C by c. 35% relative to 25°C (Table 2b). Neither of these represent the female thermal limit. Thus, female fertility but not productivity differed by line status. Female productivity thermal reaction norms also did not differ between high and low lines (Table 3; Figure 2g,h).
We found a strong positive correlation between fertility and productivity at 29°C for both males and females (males: correlation coefficient of 0.922, p < 0.001, Figure 3a; females: correlation coefficient of 0.646, p = 0.009, Figure 3b). In addition, there was a significant positive correlation between male and female productivity reaction norms across lines only at the highest temperatures (reaction norms between 25°C and 27°C, correlation coefficient: 0.499, p = 0.119; reaction norms between 27°C and 29°C, correlation coefficient: 0.757, p = 0.007; Figure 3c).
FIGURE 3.
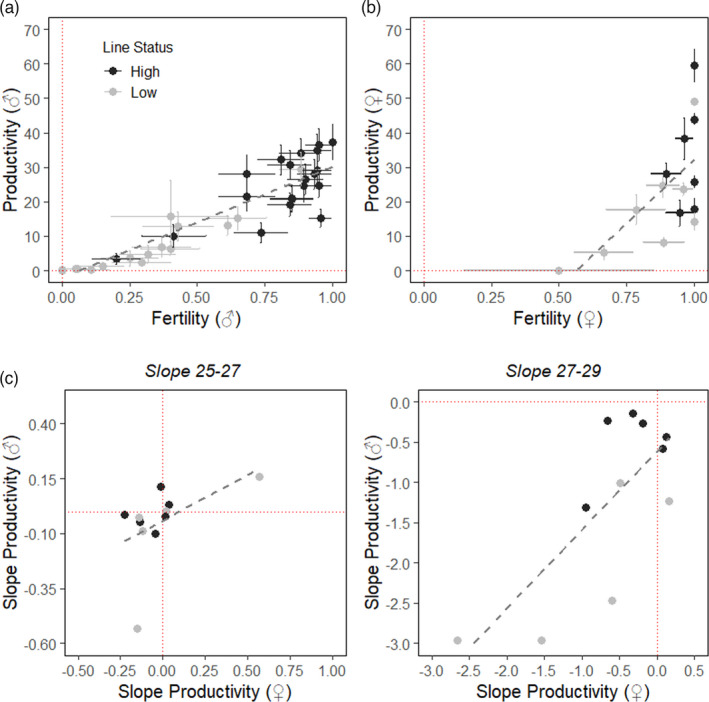
Phenotypic correlations between fertility and productivity. (a) Phenotypic correlation between fertility and productivity in males; (b) Phenotypic correlation between fertility and productivity in females; (c) Phenotypic correlation between the productivity thermal reaction norms (i.e. slopes) of males and females; random slopes were extracted from random slope models modelling the relationship between productivity of either sex and the two lowest (25°C and 27°C), or the two highest (27°C and 29°C) temperatures. Different colours represent different line status (‘high lines’, dark grey, ‘low lines’ light grey), each dot represents an individual line. Grey dashed lines represent the linear relationship between variables. Red dotted lines mark the intercept = 0 of both variables represented
3.2. Genomic patterns
In total, 1,922,228 SNPs were tested in BayeScan of which 933 (0.05%) were identified as being F st outliers (Figure 4). Using the mean q‐values of SNPs within 5 kb windows, we identified 24 windows with mean q‐values ≥ 0.13. Of the SNPs with BayeScan q‐values < 0.05, 89 (9.5%) were within these 24 windows. We found a significant effect of genotype on the slope of productivity between 27°C and 29°C for 54 of these SNPs (Table S4; Figure 4). Most of these 54 SNPs are annotated as ‘intronic’ (59.3%) and a large proportion are annotated as ‘intergenic’ (38.9%). A minority of 13% of SNPs are annotated as ‘synonymous coding’. The SNPs with coding annotations lie within two unique genes which are both expressed throughout adult and larval flies (Table S4). Overall, there are 16 genes within 4 kb of the 54 SNPs with significant effects of genotype on phenotype (2 kb upstream or downstream; Table S4). Using ModPhea, these genes are enriched only for a single phenotypic term after correcting for multiple testing, namely ‘embryonic/larval midgut’ (Table S4). These results were highly similar when we used productivity at 29°C as the phenotype instead of the slope of productivity between 27°C and 29°C, adding 10 SNPs with significant associations between genotype and phenotype (Table S4) and one additional gene. We queried the literature for studies on the 16 overlapping genes between traits and their potential relationship to the ability to produce offspring under stressful environments, which we note in the discussion.
FIGURE 4.
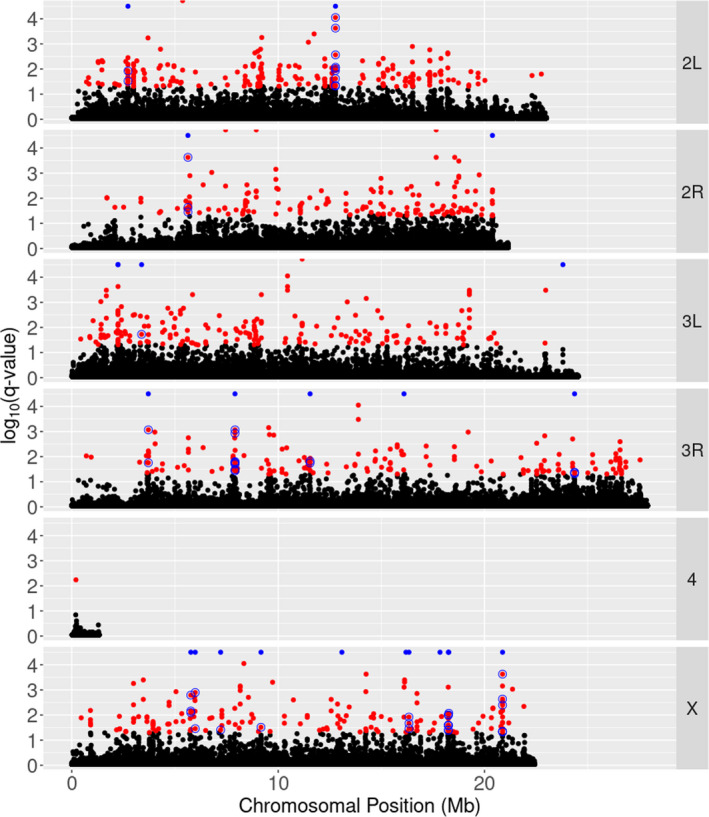
A Manhattan plot showing the log10(q‐value) from BayeScan for all tested SNPs. SNPs shown in red have q‐values < 0.05. Blue points along the top of each panel denote the locations of 5 kb windows that are in the 99.9th percentile of the mean −log10(q‐value) distribution (candidate windows). Note that although there appear to be only 22, there are in fact 24 blue points. The first point from the left on 3 L, and second point from the right on the X, are both groups of two distinct points that appear as overlapping on this scale. Red points circled in blue are those SNPs that lie within a candidate window that also have a significant effect of line genotype on phenotype (productivity). The distribution of the number of SNPs in each 5 kb window and the mean −log10(q‐value) for each window across the genome are given in Figure S1
4. DISCUSSION
Higher mean temperatures with a higher frequency and intensity of heat waves are hallmarks of ongoing climate change (Arias et al., 2021). To predict the impact of increasing temperatures and thermal variability on biodiversity, we need to understand the phenotypic and genetic variation in response to changing temperatures across life‐history stages as well as between the sexes. Using a subset of genotypes from the Drosophila Genetic Reference Panel (DGRP) with contrasting reaction norms for adult male fertility after experiencing different developmental temperatures (Zwoinska et al., 2020), we found concurrence with our previous study: the subset of genotypes examined here had indistinguishable male fertility when developing at 25°C and 27°C but development at 29°C generated two clusters of lines with either substantial (low performance) or little (high performance) decline in male fertility (Zwoinska et al., 2020). We found a similar pattern for offspring production with a decline by up to 90% when developing at 29°C in comparison to 25°C for low lines but no significant decline for high lines. The reduced fertility and productivity values represent a thermal reproductive limit for low lines (Parratt et al., 2021). Male mating success also decreased but was similar across lines and thus cannot explain line variation in either fertility or productivity. While female fertility and offspring production were also compromised the effect was smaller than in males. Thus, with the exception of sex ratio, all reproductive traits showed negative, but sex‐specific, reaction norm slopes at 29°C.
Our findings are consistent with previous work showing that higher developmental temperatures affect male reproductive performance to a larger degree than female reproductive performance (e.g. David et al., 2005; Kurhanewicz et al., 2020), at least with respect to sex‐specific responses within the same lines. The larger impact on male reproductive performance, including low line males reaching thermal limits, could negatively impact population size. A canalized response for female reproductive traits may be selected for, given that population growth is mainly dependent on female fertility in many sexually reproducing species (Caswell, 2006), but this cannot completely overcome the male‐biased reduction. However, for traits with a shared genetic basis and that are condition dependent, male‐biased thermal sensitivity may help purge deleterious mutations without negative effects on population fitness (Martinossi‐Allibert et al., 2019; Manning, 1984; Rowe & Houle, 1996). It is important to note, however, that males are not universally more sensitive to thermal stress (Walsh, Parratt, Hoffmann, et al., 2019) and that interactions between the sexes shape realized thermal sensitivity of reproduction (Baur et al., 2021). Further research is therefore needed to elucidate the consequences of sex‐specific responses and how interactions between the sexes shape species vulnerability to climate change (Iossa, 2019; Walsh, Parratt, Hoffmann, et al., 2019).
Our genomic analyses identified many SNPs with significant genetic differentiation between the high and low lines. Using a windows‐based approach, we conservatively identified loci that may affect the productivity performance phenotype, finding 54 SNPs where the genotype of a line has a significant effect on phenotype. Genes near these SNPs were enriched for effects in the embryonic/larval midgut. The larval midgut is especially vulnerable to brief heat shock during development (Krebs & Feder, 1998). We also examined evidence for function for the 16 genes individually near SNPs that were associated with the productivity thermal reaction norm. These genes appear to fall into three classes, with some having effects on male reproduction, some related to functions associated with mitigation of DNA damage and/or oxidative stress, and others functioning in autophagy.
Genes with effects on male reproduction include GAA1 (glycosylphosphatidylinositol anchor attachment 1), which impacts both animal and plant male fertility (Desnoyer & Palanivelu, 2020; Murata et al., 2012; Rikitake et al., 2020); Cfp1 (CXXC finger protein 1), which plays an important role in both fertility regulation and a repressive role in expression of heat shock (and salt) inducible genes in C. elegans (Pokhrel et al., 2019); and Papilin (Ppn), which is involved in morphological defects of D. melanogaster testes wherein high gene expression is hypothesized to compensate for these defects (Zhang et al., 2021).
Other genes associated with our outlier SNPs are Aladin and Rngo. Aladin is a component of the nuclear pore complex, and mutants in this gene are hypersensitive to oxidative stress with decreased ability for DNA repair (Preston et al., 2019). Rngo (rings lost), a homolog of human Ddi1 (DNA‐damage inducible protein 1), encodes a conserved ubiquitin receptor which plays a role in shuttling proteins for degradation. Null alleles of this gene result in defective ovary development in Drosophila (Morawe et al., 2011) and the protein is found in the D. melanogaster sperm proteome (Rettie & Dorus, 2012). Infertility following heat stress is often attributed to the inability to repair DNA damage and/or effectively mitigate reactive oxygen species (ROS; e.g. reviewed in Durairajanayagam et al., 2015). Oxidative stress damages DNA and oxidatively modified proteins are unstable and form cellular aggregates, negatively affecting cell function.
Our analysis also identified several genes that function in autophagic pathways with some genes being part of the TORC1 pathway which regulates growth, metabolism, stress responses, fecundity and lifespan (Nässel et al., 2015; e.g. Mipp2, raptor, Ids, Atg5, sqa). Autophagy is a ubiquitous, evolutionary conserved cellular process that degrades and recycles cellular products (Nagy et al., 2015; Levine & Kroemer, 2019). Autophagic activity increases under stress, including low nutrient levels, oxidative stress, and as part of the immune response, to provide resources recycled from non‐essential processes (Levine & Kroemer, 2019). Loss of autophagic activity impacts male fertility via lipid accumulation and loss of early sperm cyst cells while basal levels are required to maintain testis stem cells in Drosophila (Sênos Demarco et al., 2020). This set of candidate genes raises the hypothesis that developmental heat stress results in potential mis‐regulation of autophagy with substantial consequences on male fertility.
Overall, based on outlier loci and known functions, we hypothesize that there are consistent genetic differences between lines that may explain differences in consequences of developmental thermal plasticity on productivity observed across these lines. We emphasize that our results should be interpreted with the understanding that the DGRP lines lack power to detect small‐effect and low‐frequency loci, which may be the norm for loci underlying plasticity and we study 40 lines in total. However, the list of candidate loci generated from our study overlaps with genes shown to impact male fertility and stress responses in other organisms, suggesting a potential common underlying genetic basis for male fertility limits. Unlike other studies (Lafuente et al., 2018; Ørsted et al., 2019), we do not find differences in the genes underlying plasticity of the focal trait and the focal trait mean. Given that the mean of productivity for low lines represents a thermal fertility limit, our candidate genes may be more representative of thermal tolerance genes than for plasticity. Future work will need to manipulate these genes and test for effects on trait mean and the thermal reaction norm to directly link this variation with phenotypic response. Under the high temperature conditions here, low lines reach their thermal fertility limit whereas high lines do not. The ability of populations to shift thermal reaction norms to higher temperatures may have profound effects on responses to climate change; however, our study is unable to determine whether high lines have broader overall thermal performance curves or have shifted their performance to warmer temperatures. Our study can also not distinguish whether high lines are canalized (e.g. being insensitive to thermal stress; fertility) or whether high lines exhibit higher thermal reproductive limits because of more robust plastic responses facilitating reproductive homeostasis during development. Future work is required to answer these questions.
While we tentatively conclude that our approach has identified some potential large‐effect loci, we may be missing a number of other large‐effect loci, such as those on the Y chromosome. The Y chromosome is pivotal for male fertility (see references in Zhang et al., 2020), including for heat‐induced male sterility (Rohmer et al., 2004), but the Y chromosome is difficult to map and is not mapped in the DGRP. Additionally, the sexually dimorphic phenotypic response to developmental thermal plasticity points to a potential role in mediating phenotypes based on pervasive and genome‐wide sexually antagonistic genetic variation (Ruzicka et al., 2019) with recent work suggesting that the Y chromosome may be more important for sexually dimorphic phenotypes than previously assumed (Kaufmann et al., 2021). There will also be loci of small effect that we have not identified, as such loci that are difficult to detect even in large panels (Huang et al., 2014; Mackay et al., 2012). Moreover, the genotypes constituting the low performing lines may each carry different, unique variants that are otherwise neutral (Hermisson & Wagner, 2004; Paaby & Rockman, 2014) and only expressed under high temperatures, and thus will not be at sufficiently high frequency to be detected. Such stress‐induced plasticity could be due to cryptic genetic variation, which could provide a substrate for adaptation via selection on previously shielded phenotypes (Ledón‐Rettig et al., 2014; McGuigan & Sgrò, 2009; Paaby & Rockman, 2014). Our experiment asked about consequences during constant developmental temperatures but spatiotemporal variation in the thermal environment may mean that stabilizing selection leaves many thermal response genes polymorphic in the population, making small‐effect causal genes more difficult to detect. Finally, it is generally challenging to identify causal loci given that genetic architecture of fitness components may be environmentally specific (Lafuente et al., 2018; Ørsted et al., 2019).
The effects on fertility and offspring production observed in our study resulted from sustained high temperatures (29°C) for the 8 days needed for development. In Raleigh, North Carolina, where the DGRP founding population was collected, temperatures reach and stay at or above 29°C during most days in July, which is the hottest summer month. Nighttime temperatures typically remain slightly above 20°C in July (www.weather‐us.com). However, nights are warming faster than days both globally (Arias et al., 2021) and in North Carolina, where the increase in warm (minimum nighttime temperature of about 21°C or higher) and very warm (24°C or higher) nights was one of the strongest historical thermal trends (Kunkel et al., 2020). Further warming in North Carolina (Kunkel et al., 2020), coupled with asymmetry of daytime and nighttime warming, will narrow diurnal temperature amplitude, increasing the risk of sustained high stressful temperatures. Moreover, larvae are restricted to necrotic fruit during development. In an apple orchard in Indiana, USA sunlit necrotic fruits maintained temperatures greater than 35°C across the entire fruit and across the day (Feder, 1997), with eclosing adults having phenotypic abnormalities (Roberts & Feder, 1999). Thus, at least part of the population may be exposed to sustained high developmental temperatures during the day, subsequently compromising adult reproductive traits. In a warming world, such scenarios, including changes in thermal amplitude (Bitter et al., 2021; Rodrigues et al., 2021), will be experienced by an increasing number of organisms in their natural developmental environment.
In conclusion, we find inter‐genotype variation in developmental thermal plasticity on subsequent adult reproductive traits, underlain by genetic differentiation in the population. Our data support a growing body of literature demonstrating negative effects of increasing temperature on reproductive traits: in natural and laboratory settings, and in ectotherms and endotherms alike (e.g. Parratt et al., 2021; Schou et al., 2021; van Heerwaarden & Sgrò, 2021; Walsh, Parratt, Atkinson, et al., 2019; Walsh, Parratt, Hoffmann, et al., 2019; Zwoinska et al., 2020). Our candidate genes have been shown to impact male fertility or be associated with thermal stress responses in both ectotherms and endotherms, suggesting a potential shared genetic basis for thermal fertility limits. A recent experimental evolution study in which warming temperatures were applied across all life‐history stages found little evidence for adaptive responses of male fertility in six Drosophila species (van Heerwaarden & Sgrò, 2021). This result suggests limited adaptive potential for responses to thermal fertility limits, similar to studies on critical thermal limits of survival traits (e.g. Bennett et al., 2021; Schou et al., 2014; van Heerwaarden et al., 2016). Whether the intra‐population variation we find in the thermal reaction norm and thermal fertility limits arising from developmental plasticity in this population is sufficient for future adaptive responses to increasing temperatures remains to be determined.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHORS' CONTRIBUTIONS
R.R.S. conceived the ideas and received the funding; M.K.Z., L.R.R. and R.R.S. designed the methodology and collected the data; L.R.R. and R.A.W.W. analysed the data; R.R.S. led the writing of the manuscript but all authors contributed critically to writing the manuscript and all gave final approval for publication.
Supporting information
Appendix S1
Table S4
ACKNOWLEDGEMENTS
We would like to thank Allen Moore, Jon Slate, Chris Wheat and Andrew Beckerman for discussions about the work, Mike Ritchie for providing valuable comments on an earlier draft of the manuscript, comments from two anonymous reviewers, and Mirjam Amcoff for help with fly husbandry. We would also like to thank the organizers of the special issue for their patience during the submission process. Funding was partially provided by Carl Tryggers Stifelse (CTS 18:359) and the Swedish Research Council (Vetenskapsrådet; 2018‐04598) to R.R.S.
Rodrigues, L. R. , Zwoinska, M. K. , Wiberg, R. A., & Snook, R. R. (2022). The genetic basis and adult reproductive consequences of developmental thermal plasticity. Journal of Animal Ecology, 91, 1119–1134. 10.1111/1365-2656.13664
Handling Editor Matthew Barbour
Leonor R. Rodrigues, Martyna K. Zwoinska and R. Axel W. Wiberg joint first authors.
Contributor Information
Leonor R. Rodrigues, Email: leonor.rodrigues89@gmail.com.
Rhonda R. Snook, Email: rhonda.snook@zoologi.su.se.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Figshare at 10.17045/sthlmuni.14610381 (Rodrigues et al., 2022).
REFERENCES
- Arias, P.A. , Bellouin, N. , Coppola, E. , Jones, R. G. , Krinner, G. , Marotzke, J. , Naik, V. , Palmer, M.D. , Plattner, G.‐K. , Rogelj, J. , Rojas, M. , Sillmann, J. , Storelvmo, T. , Thorne, P. W. , Trewin, B. , Achuta Rao, K. , Adhikary, B. , Allan, R. P. , Armour, K. , … Zickfeld, K. (2021). Technical summary. In Masson‐Delmotte, V. , Zhai, P. , Pirani, A. , Connors, S. L. , Péan, C. , Berger, S. , Caud, N. , Chen, Y. , Goldfarb, L. , Gomis, M. I. , Huang, M. , Leitzell, K. , Lonnoy, E. , Matthews, J. B. R. , Maycock, T. K. , Waterfield, T. , Yelekçi, O. , Yu, R. , & Zhou, B. (Eds.), Climate change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press. Retrieved from https://www.ipcc.ch/report/ar6/wg1/ [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. M. , & Walker, S. C. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Baur, J. , Jagusch, D. , Michalak, P. , Koppik, M. , & Berger, D. (2022). The mating system affects the temperature sensitivity of male and female fertility. Functional Ecology, 36, 92–106. 10.1111/1365-2435.13952 [DOI] [Google Scholar]
- Bennett, J. M. , Sunday, J. , Calosi, P. , Villalobos, F. , Martínez, B. , Molina‐Venegas, R. , Araújo, M. B. , Algar, A. C. , Clusella‐Trullas, S. , Hawkins, B. A. , Keith, S. A. , Kühn, I. , Rahbek, C. , Rodríguez, L. , Singer, A. , Morales‐Castilla, I. , & Olalla‐Tárraga, M. A. (2021). The evolution of critical thermal limits of life on Earth. Nature Communications, 12, 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter, M. C. , Wong, J. M. , Dam, H. G. , Donelan, S. C. , Kenkel, C. D. , Komoroske, L. M. , Nickols, K. J. , Rivest, E. B. , Salinas, S. , Burgess, S. C. , & Lotterhos, K. E. (2021). Fluctuating selection and global change: A synthesis and review on disentangling the roles of climate amplitude, predictability and novelty. Proceedings of the Royal Society of London. Series B: Biological Sciences, 288, 20210727. 10.1098/rspb.2021.0727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolker, B. M. (2008). Ecological models and data in R. Princeton University Press. [Google Scholar]
- Bonamour, S. , Chevin, L.‐. M. , Charmantier, A. , & Teplitsky, C. (2019). Phenotypic plasticity in response to climate change: The importance of cue variation. Philosophical Transactions of the Royal Society B, 374, 20180178. 10.1098/rstb.2018.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, M. E. , Kristensen, K. , van Benthem, K. J. , Magnusson, A. , Berg, C. W. , Nielsen, A. , Skaug, H. J. , Mächler, M. , & Bolker, B. M. (2017). GlmmTMB balances speed and flexibility among packages for zero‐inflated generalized linear mixed modeling. The R Journal, 9, 378. [DOI] [Google Scholar]
- Candolin, U. , & Wong, B. B. M. (2012). Behavioural responses to a changing world: Mechanisms and consequences. Oxford University Press. [Google Scholar]
- Caswell, H. (2006). Matrix population models. In Encyclopedia of environmetrics. John Wiley & Sons, Ltd. 10.1002/9780470057339.vam006m [DOI] [Google Scholar]
- Cingolani, P. , Platts, A. , Wang, L. L. , Coon, M. , Nguyen, T. , Wang, L. , Land, S. J. , Lu, X. , & Ruden, D. M. (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso‐2; iso‐3. Fly, 6, 80–92. 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley, M. J. (2007). The R book. John Wiley & Sons, Ltd. 10.1002/9780470515075 [DOI]
- Danecek, P. , Auton, A. , Abecasis, G. , Albers, C. A. , Banks, E. , DePristo, M. A. , Handsaker, R. E. , Lunter, G. , Marth, G. T. , Sherry, S. T. , McVean, G. , Durbin, R. , & 1000 Genomes Project Analysis Group . (2011). The variant call format and VCFtools. Bioinformatics, 27, 2156–2158. 10.1093/bioinformatics/btr330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, J. R. , Araripe, L. O. , Chakir, M. , Legout, H. , Lemos, B. , Petavy, G. , Rohmer, C. , Joly, D. , & Moreteau, B. (2005). Male sterility at extreme temperatures: A significant but neglected phenomenon for understanding Drosophila climatic adaptations. Journal of Evolutionary Biology, 18, 838–846. [DOI] [PubMed] [Google Scholar]
- Desnoyer, N. , & Palanivelu, R. (2020). Bridging the GAPs in plant reproduction: A comparison of plant and animal GPI‐anchored proteins. Plant Reproduction, 33, 129–142. [DOI] [PubMed] [Google Scholar]
- Durairajanayagam, D. , Agarwal, A. , & Ong, C. (2015). Causes, effects and molecular mechanisms of testicular heat stress. Reproductive BioMedicine Online, 30, 14–27. 10.1016/j.rbmo.2014.09.018 [DOI] [PubMed] [Google Scholar]
- Eyck, H. J. F. , Buchanan, K. L. , Crino, O. L. , & Jessop, T. S. (2019). Effects of developmental stress on animal phenotype and performance: A quantitative review. Biological Reviews, 94, 1143–1160. 10.1111/brv.12496 [DOI] [PubMed] [Google Scholar]
- Feder, M. E. (1997). Necrotic fruit: A novel model system for thermal ecologists. Journal of Thermal Biology, 22, 1–9. [DOI] [Google Scholar]
- Foll, M. , & Gaggiotti, O. E. (2008). A genome‐scan method to identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics, 180, 977–993. 10.1534/genetics.108.092221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Roa, R. , Garcia‐Gonzalez, F. , Noble, D. W. A. , & Carazo, P. (2020). Temperature as a modulator of sexual selection. Biological Reviews, 95, 1607–1629. 10.1111/brv.12632 [DOI] [PubMed] [Google Scholar]
- Ghalambor, C. K. , Hoke, K. L. , Ruell, E. W. , Fischer, E. K. , Reznick, D. N. , & Hughes, K. A. (2015). Non‐adaptive plasticity potentiates rapid adaptive evolution of gene expression in nature. Nature, 525, 372–375. 10.1038/nature15256 [DOI] [PubMed] [Google Scholar]
- Ghalambor, C. K. , McKay, J. K. , Carroll, S. P. , & Reznick, D. N. (2007). Adaptive versus non‐adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology, 21, 394–407. [DOI] [Google Scholar]
- Green, K. C. , Moore, P. J. , & Sial, A. A. (2019). Impact of heat stress on development and fertility of Drosophila suzukii Matsumura (Diptera: Drosophilidae). Journal of insect physiology, 114, 45–52. 10.1016/j.jinsphys.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Harell Jr., F. E. (2019). Hmisc: Harrell miscellaneous. R package version 4.2‐0. Retrieved from https://CRAN.R‐project.org/package=Hmisc
- Hermisson, J. , & Wagner, G. P. (2004). The population genetic theory of hidden variation and genetic robustness. Genetics, 168(4), 2271–2284. 10.1534/genetics.104.029173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A. , & Bridle, J. (2021). The dangers of irreversibility in an age of increased uncertainty: Revisiting plasticity in invertebrates. Oikos. 10.1111/oik.08715 [DOI] [Google Scholar]
- Huang, W. , Massouras, A. , Inoue, Y. , Peiffer, J. , Ràmia, M. , Tarone, A. M. , Turlapati, L. , Zichner, T. , Zhu, D. , Lyman, R. F. , Magwire, M. M. , Blankenburg, K. , Carbone, M. A. , Chang, K. , Ellis, L. L. , Fernandez, S. , Han, Y. , Highnam, G. , Hjelmen, C. E. , … Mackay, T. F. C. (2014). Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Research, 24, 1193–1208. 10.1101/gr.171546.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossa, G. (2019). Sex‐specific differences in thermal fertility limits. Trends in Ecology & Evolution, 34(6), 490–492. 10.1016/j.tree.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Jørgensen, K. T. , Sørensen, J. G. , & Bundgaard, J. (2006). Heat tolerance and the effect of mild heat stress on reproductive characters in Drosophila buzzatii males. Journal of Thermal Biology, 31, 280–286. 10.1016/j.jtherbio.2005.11.026 [DOI] [Google Scholar]
- Kaufmann, P. , Wolak, M. E. , Husby, A. , & Immonnen, E. (2021). Rapid evolution of sexual size dimorphism facilitated by Y‐linked genetic variance. Nature Ecology & Evolution, 5, 1394–1402. [DOI] [PubMed] [Google Scholar]
- Krebs, R. A. , & Feder, M. E. (1998). Hsp70 and larval thermotolerance in Drosophila melanogaster: How much is enough and when is more too much? Journal of Insect Physiology, 44, 1091–1101. [DOI] [PubMed] [Google Scholar]
- Kunkel, K.E. , Easterling, D. R. , Ballinger, A. , Bililign, S. , Champion, S. M. , Corbett, D. R. , Dello, K. D. , Dissen, J. , Lackmann, G. M. , Luettich, R. A. , Perry Jr., L. B. , Robinson, W. A. , Stevens, L. E. , Stewart, B. C. , & Terando, A. J. (2020). North Carolina climate science report. North Carolina Institute for Climate Studies, 233, 236. Retrieved from https://ncics.org/nccsr [Google Scholar]
- Kurhanewicz, N. A. , Dinwiddie, D. , Bush, Z. D. , & Libuda, D. E. (2020). Elevated temperatures cause transposon‐associated DNA damage in C. elegans spermatocytes. Current Biology, 30, 5007–5017.e4. 10.1016/j.cub.2020.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente, E. , & Beldade, P. (2019). Genomics of developmental plasticity in animals. Frontiers in Genetics, 10, 720. 10.3389/fgene.2019.00720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente, E. , Duneau, D. , & Beldade, P. (2018). Genetic basis of thermal plasticity variation in Drosophila melanogaster body size. PLOS Genetics, 14, e1007686. 10.1371/journal.pgen.1007686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader, D. P. , Krause, S. A. , Pandit, A. , Davies, S. A. , & Dow, J. A. T. (2018). FlyAtlas 2: A new version of the Drosophila melanogaster expression atlas with RNA‐Seq, miRNA‐Seq and sex‐specific data. Nucleic Acids Research, 46, D809–D815. 10.1093/nar/gkx976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledón‐Rettig, C. C. , Pfennig, D. W. , Chunco, A. J. , & Dworkin, I. (2014). Cryptic genetic variation in natural populations: A predictive framework. Integrative and Comparative Biology, 54, 783–793. 10.1093/icb/icu077 [DOI] [PubMed] [Google Scholar]
- Lenth, R. V. (2016). Least‐squares means: The R package lsmeans. Journal of Statistical Software, 69, 1–33. 10.18637/jss.v069.i01 [DOI] [Google Scholar]
- Levine, B. , & Kroemer, G. (2019). Biological functions of autophagy genes: A disease perspective. Cell, 176, 11–42. 10.1016/j.cell.2018.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay, T. F. C. , Richards, S. , Stone, E. A. , Barbadilla, A. , Ayroles, J. F. , Zhu, D. , Casillas, S. , Han, Y. , Magwire, M. M. , Cridland, J. M. , Richardson, M. F. , Anholt, R. R. H. , Barrón, M. , Bess, C. , Blankenburg, K. P. , Carbone, M. A. , Castellano, D. , Chaboub, L. , Duncan, L. , … Gibbs, R. A. (2012). The Drosophila melanogaster genetic reference panel. Nature, 482, 173–178. 10.1038/nature10811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, F. , Nolte, V. , Tobler, R. , Kapun, M. , & Schlotterer, C. (2018). A simple genetic basis of adaptation to a novel thermal environment results in complex metabolic rewiring in Drosophila . Genome Biology, 19, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, J. T. (1984). Males and the advantage of sex. Journal of Theoretical Biology, 108, 215–220. [DOI] [PubMed] [Google Scholar]
- Martinossi‐Allibert, I. , Thilliez, E. , Arnqvist, G. , & Berger, D. (2019). Sexual selection, environmental robustness and evolutionary demography of maladapted populations: A test using experimental evolution in seed beetles. Evolutionary Applications, 12, 1371–1384. 10.1101/426056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan, K. , & Sgrò, C. M. (2009). Evolutionary consequences of cryptic genetic variation. Trends in Ecology & Evolution, 24, 305–311. 10.1016/j.tree.2009.02.001 [DOI] [PubMed] [Google Scholar]
- Messer, P. W. , Ellner, S. P. , & Hairston, N. G. (2016). Can population genetics adapt to rapid evolution? Trends in Genetics, 32, 408–418. 10.1016/j.tig.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Moghadam, N. N. , Ketola, T. , Pertoldi, C. , Bahrndorff, S. , & Kristensen, T. N. (2019). Heat hardening capacity in Drosophila melanogaster is life stage‐specific and juveniles show the highest plasticity. Biology Letters, 15, 20180628. 10.1098/rsbl.2018.0628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawe, T. , Honemann‐Capito, M. , von Stein, W. , & Wodarz, A. (2011). Loss of the extraproteasomal ubiquitin receptor Rings lost impairs ring canal growth in Drosophila oogenesis. Journal of Cell Biology, 193, 71–80. 10.1083/jcb.201009142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata, D. , Nomura, K. H. , Dejima, K. , Mizuguchi, S. , Kawasaki, N. , Matsuishi‐Nakajima, Y. , Ito, S. , Gengyo‐Ando, K. , Kage‐Nakadai, E. , Mitani, S. , & Nomura, K. (2012). GPI‐anchor synthesis is indispensable for the germline development of the nematode Caenorhabditis elegans . Molecular Biology of the Cell, 23, 982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, P. , Varga, A. , Kovács, A. L. , Takáts, S. , & Juhász, G. (2015). How and why to study autophagy in Drosophila: It’s more than just a garbage chute. Methods, 75, 151–116. 10.1016/j.ymeth.2014.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel, D. R. , Liu, Y. , & Luo, J. (2015). Insulin/IGF signaling and its regulation in Drosophila. General and Comparative Endocrinology, 221, 255–266. 10.1016/j.ygcen.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Nijhout, H. F. (1998). Insect hormones. Princeton University Press. 10.2307/j.ctv19fvz3n [DOI] [Google Scholar]
- Ørsted, M. , Hoffmann, A. A. , Rohde, P. D. , Sørensen, P. , & Kristensen, T. N. (2019). Strong impact of thermal environment on the quantitative genetic basis of a key stress tolerance trait. Heredity, 122, 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaby, A. B. , & Rockman, M. V. (2014). Cryptic genetic variation: Evolution’s hidden substrate. Nature Reviews, 15, 247–258. 10.1038/nrg3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parratt, S. R. , Walsh, B. S. , Metelmann, S. , White, N. , Manser, A. , Bretman, A. J. , Hoffmann, A. A. , Snook, R. R. , & Price, T. A. (2021). Temperatures that sterilise males better predict global species distributions than lethal temperatures. Nature Climate Change, 11, 481–484. [DOI] [Google Scholar]
- Pedersen, L. D. , Pedersen, A. R. , Bijlsma, R. , & Bundgaard, J. (2011). The effects of inbreeding and heat stress on male sterility in Drosophila melanogaster . Biological Journal of the Linnean Society, 104, 432–442. [DOI] [Google Scholar]
- Plummer, M. , Best, N. , Cowles, K. , & Vines K. (2006). CODA: Convergence diagnosis and output analysis for MCMC. R News, 6, 7‐11. Retrieved from https://cran.r‐project.org/doc/Rnews/Rnews_2006‐1.pdf#page=7 [Google Scholar]
- Pokhrel, B. , Chen, Y. , & Biro, J. J. (2019). Cfp‐1 interacts with HDAC1/2 complexes in C. elegans development. The FEBS Journal, 286, 2490–2504. 10.1111/febs.14833 [DOI] [PubMed] [Google Scholar]
- Porcelli, D. , Gaston, K. J. , Butlin, R. K. , & Snook, R. R. (2016). Local adaptation of reproductive performance during thermal stress. Journal of Evolutionary Biology, 30, 422–429. 10.1111/jeb.13018 [DOI] [PubMed] [Google Scholar]
- Preston, C. C. , Storm, E. C. , Leonard, R. J. , & Faustino, R. S. (2019). Emerging roles for nucleoporins in reproductive cellular physiology. Canadian journal of physiology and pharmacology, 97, 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Foundation for Statistical Computing . (2020) R: A language and environment for statistical computing . Retrieved from https://www.R‐project.org/
- Rettie, E. C. , & Dorus, S. (2012). Drosophila sperm proteome evolution: Insights from comparative genomic approaches. Spermatogenesis, 2, 213–223. 10.4161/spmg.21748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikitake, M. , Matsuda, A. , Murata, D. , Dejima, K. , Nomura, K. H. , Abbott, K. L. , Mitani, S. , & Nomura, K. (2020). Analysis of GPI‐anchored proteins involved in germline stem cell proliferation in the Caenorhabditis elegans germline stem cell niche. The Journal of Biochemistry, 168, 589–602. 10.1093/jb/mvaa075 [DOI] [PubMed] [Google Scholar]
- Roberts, S. P. , & Feder, M. E. (1999). Natural hyperthermia and expression of the heat shock protein Hsp70 affect developmental abnormalities in Drosophila melanogaster . Oecologia, 121, 323–329. 10.1007/s004420050935 [DOI] [PubMed] [Google Scholar]
- Rodrigues, L. , Zwoinska, M.K. , Wiberg, R.A.W. & Snook, R. R. (2022). Data from: The genetic basis and adult reproductive consequences of developmental thermal plasticity. Figshare, 10.17045/sthlmuni.13211204.v1 [DOI] [PMC free article] [PubMed]
- Rodrigues, L. R. , McDermott, H. A. , Villanueva, I. , Djukari, J. , Ruf, L. D. , Amcoff, M. , & Snook, R. R. (2021). Fluctuating heat stress during development exposes reproductive costs and putative benefits. Journal of Animal Ecology, 1–13. [DOI] [PubMed] [Google Scholar]
- Rohmer, C. , David, J. R. , Moreteau, B. , & Joly, D. (2004). Heat induced male sterility in Drosophila melanogaster: Adaptive genetic variations among geographic populations and role of the Y chromosome. Journal of Experimental Biology, 207, 2735–2743. 10.1242/jeb.01087 [DOI] [PubMed] [Google Scholar]
- Rowe, L. , & Houle, D. (1996). The lek paradox and the capture of genetic variance by condition dependent traits. Proceedings of the Royal Society of London. Series B: Biological Sciences, 263, 1415–1421. 10.1098/rspb.1996.0207 [DOI] [Google Scholar]
- Ruzicka, F. , Hill, M. S. , Pennell, T. M. , Flis, I. , Ingleby, F. C. , Mott, R. , Fowler, K. , Morrow, E. H. , & Reuter, M. (2019). Genome‐wide sexually antagonistic variants reveal long‐standing constraints on sexual dimorphism in fruit flies. PloS Biology, 17, e3000244. 10.1371/journal.pbio.3000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales, K. , Vasudeva, R. , Dickinson, M. E. , Godwin, J. L. , Lumley, A. J. , Michalczyk, Ł. , Hebberecht, L. , Thomas, P. , Franco, A. , & Gage, M. J. G. (2018). Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nature Communications, 9, 4771–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales, K. , Vasudeva, R. , & Gage, M. J. G. (2021). Fertility and mortality impacts of thermal stress from experimental heatwaves on different life stages and their recovery in a model insect. Royal Society Open Science, 8, 201717. 10.1098/rsos.201717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon, A. D. , O’Brien, E. K. , & Bridle, J. R. (2018). Temperature fluctuations during development reduce male fitness and may limit adaptive potential in tropical rainforest Drosophila . Journal of Evolutionary Biology, 31, 405–415. 10.1111/jeb.13231 [DOI] [PubMed] [Google Scholar]
- Schmid, M. , & Guillame, F. (2017). The role of phenotypic plasticity on population differentiation. Heredity, 119, 214–225. 10.1038/hdy.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou, M. F. , Bonato, M. , Engelbrecht, A. , Brand, Z. , Svensson, E. I. , Melgar, J. , Muvhali, P. T. , Cloete, S. W. P. , & Cornwallis, C. K. (2021). Extreme temperatures compromise male and female fertility in a large desert bird. Nature Communications, 12, 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou, M. F. , Kristensen, T. N. , Kellermann, V. , Schlötterer, C. , & Loeschcke, V. (2014). A Drosophila laboratory evolution experiment points to low evolutionary potential under increased temperatures likely to be experienced in the future. Journal of Evolutionary Biology, 27, 1859–1868. 10.1111/jeb.12436 [DOI] [PubMed] [Google Scholar]
- Sênos Demarco, R. , Uyemura, B. S. , & Jones, D. L. (2020). EGFR signaling stimulates autophagy to regulate stem cell maintenance and lipid homeostasis in the Drosophila testis . Cell Reproduction, 30, 1101–1116.e5. 10.1016/j.celrep.2019.12.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns, S. C. (1992). The evolution of life histories. Oxford University Press. [Google Scholar]
- Thurmond, J. , Goodman, J. L. , Strelets, V. B. , Attrill, H. , Gramates, L. S. , Marygold, S. J. , Matthews, B. B. , Millburn, G. , Antonazzo, G. , Trovisco, V. , Kaufman, T. C. , Calvi, B. R. , & FlyBase Consortium . (2019). FlyBase 2.0: The next generation. Nucleic Acids Research, 47, D759–D765. 10.1093/nar/gky1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uller, T. , Sinead, E. , & Pen, I. (2015). When is incomplete epigenetic resetting in germ cells favoured by natural selection? Proceedings of the Royal Society of London. Series B: Biological Sciences B, 282, 20150682. 10.1098/rspb.2015.0682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerwaarden, B. , Kellermann, V. , & Sgrò, C. M. (2016). Limited scope for plasticity to increase upper thermal limits. Functional Ecology, 30, 1947–1956. [DOI] [Google Scholar]
- van Heerwaarden, B. , & Sgrò, C. M. (2021). Male fertility thermal limits predict vulnerability to climate warming. Nature Communications, 12, 2214–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables, W. N. , & Ripley, B. D. (2002). Modern Applied Statistics with S (4th ed.). Springer. [Google Scholar]
- Walsh, B. S. , Mannion, N. L. M. , Price, T. A. R. , & Parratt, S. R. (2020). Sex‐specific sterility caused by extreme temperatures is likely to create cryptic changes to the operational sex ratio in Drosophila virilis . Current Zoology, 67, 341–343. 10.1093/cz/zoaa067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, B. S. , Parratt, S. R. , Atkinson, D. , Snook, R. R. , Bretman, A. , & Price, T. A. R. (2019). Integrated approaches to studying male and female thermal fertility limits. Trends in Ecology & Evolution, 34, 492–493. 10.1016/j.tree.2019.03.005 [DOI] [PubMed] [Google Scholar]
- Walsh, B. S. , Parratt, S. R. , Hoffmann, A. A. , Atkinson, D. , Snook, R. R. , Bretman, A. , & Price, T. A. R. (2019). The impact of climate change on fertility. Trends in Ecology & Evolution, 34, 249–259. 10.1016/j.tree.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Weather‐us.com (2021). Climate Raleigh, North Carolina, USA. Retrieved from https://www.weather‐us.com/en/north‐carolina‐usa/raleigh‐climate?c,mm,mb,km#temperature
- Weng, M.‐. P. , & Liao, B.‐. Y. (2017). ModPhEA: Model organism phenotype enrichment analysis of eukaryotic gene sets. Bioinformatics, 33, 3505–3507. 10.1093/bioinformatics/btx426 [DOI] [PubMed] [Google Scholar]
- West‐Eberhard, M. J. (2003). Developmental plasticity and evolution. Oxford University Press. [Google Scholar]
- Whitman, D. W. , & Agrawal, A. A. (2009). What is phenotypic plasticity and why is it important? In Whitman D. W. & Ananthakrishna T. N. (Eds.), Phenotypic plasticity of insects: Mechanisms and consequences (pp. 1–63). CRC Press. [Google Scholar]
- Zhang, J. , Luo, J. , Chen, J. , Dai, J. , & Montell, C. (2020). The role of Y chromosome genes in male fertility in Drosophila melanogaster . Genetics, 215, 623–633. 10.1534/genetics.120.303324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M.‐. J. , Shi, X.‐. X. , Wang, N. , Zhang, C. , Zhang, C. , Quais, M. K. , Ali, S. A. , Zhou, W. , Mao, C. , & Zhu, Z.‐. R. (2021). Transcriptional changes revealed genes and pathways involved in the deficient testis caused by the inhibition of alkaline ceramidase (Dacer) in Drosophila melanogaster . Archives of Insect Biochemistry and Physiology, 106, e21765. 10.1002/arch.2176 [DOI] [PubMed] [Google Scholar]
- Zwoinska, M. K. , Rodrigues, L. R. , Slate, J. , & Snook, R. R. (2020). Phenotypic responses to and genetic architecture of sterility following exposure to sub‐lethal temperature during development. Frontiers in Genetics, 11, 573–573. 10.3389/fgene.2020.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S4
Data Availability Statement
The data that support the findings of this study are openly available in Figshare at 10.17045/sthlmuni.14610381 (Rodrigues et al., 2022).


