Abstract
Stressful experiences evoke, among others, a rapid increase in brain (nor)epinephrine (NE) levels and a slower increase in glucocorticoid hormones (GCs) in the brain. Microglia are key regulators of neuronal function and contain receptors for NE and GCs. These brain cells may therefore potentially be involved in modulating stress effects on neuronal function and learning and memory. In this review, we discuss that stress induces (1) an increase in microglial numbers as well as (2) a shift toward a pro‐inflammatory profile. These microglia have (3) impaired crosstalk with neurons and (4) disrupted glutamate signaling. Moreover, microglial immune responses after stress (5) alter the kynurenine pathway through metabolites that impair glutamatergic transmission. All these effects could be involved in the impairments in memory and in synaptic plasticity caused by (prolonged) stress, implicating microglia as a potential novel target in stress‐related memory impairments.
Keywords: corticosterone, glucocorticoids, learning, norepinephrine, synapses
Abbreviations
- ACTH
adrenocorticotrophic hormone
- AMPAR
α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor
- AVP
arginine vasopressin
- CD
cluster of differentiation
- CNS
central nervous system
- CRH
corticotropin releasing hormone
- DG
dentate gyrus
- DTR
diphtheria toxin receptor
- FACS
Fluorescence Activated Cell Sorting
- GCs
glucocorticoids
- GR
glucocorticoid receptor
- GRE
glucocorticoid response element
- HMGB1
High Mobility Group Box 1
- HPA
hypothalamic–pituitary–adrenal
- IDO
indoleamine 2,3‐dioxygenase
- IL
interleukin
- KO
knockout
- LC
Locus coeruleus
- LPS
lipopolysaccharide
- LTP
long‐term potentiation
- mEPSC
miniature excitatory postsynaptic current
- MR
mineralocorticoid receptor
- NE
norepinephrine
- NLRP3
NOD‐like receptor protein 3
- NMDAR
N‐methyl‐d‐aspartate receptor
- PND
postnatal day
- PTSD
post‐traumatic stress disorder
- PVN
paraventricular nucleus
- TNF
Tumor necrosis factor
1. STRESS, NOREPINEPHRINE, AND GLUCOCORTICOID HORMONES
In daily life, people are regularly exposed to experiences that are perceived as arousing and stressful. Exposure to such events triggers the activation of brain systems that allow coping with potentially threatening situations (De Kloet et al., 2005; Joëls & Baram, 2009; Lupien et al., 2007). One of the first reactions in response to stress is the activation of the locus coeruleus (LC) which triggers the release of norepinephrine (NE) in various brain areas such as amygdala, hippocampus, and prefrontal cortex (Figure 1) (reviewed in Benarroch, 2018; Joëls et al., 2006). In the brain, NE acts via α and β‐adrenergic receptors (Molinoff, 1984) and modulates attention, and learning and memory (Roozendaal et al., 2009; Sara, 2009). In parallel, sympathetic nervous system activation results in an increase in peripheral NE levels, which (indirectly) act to further enhance central NE transmission (De Kloet et al., 2005; Joëls & Baram, 2009).
FIGURE 1.
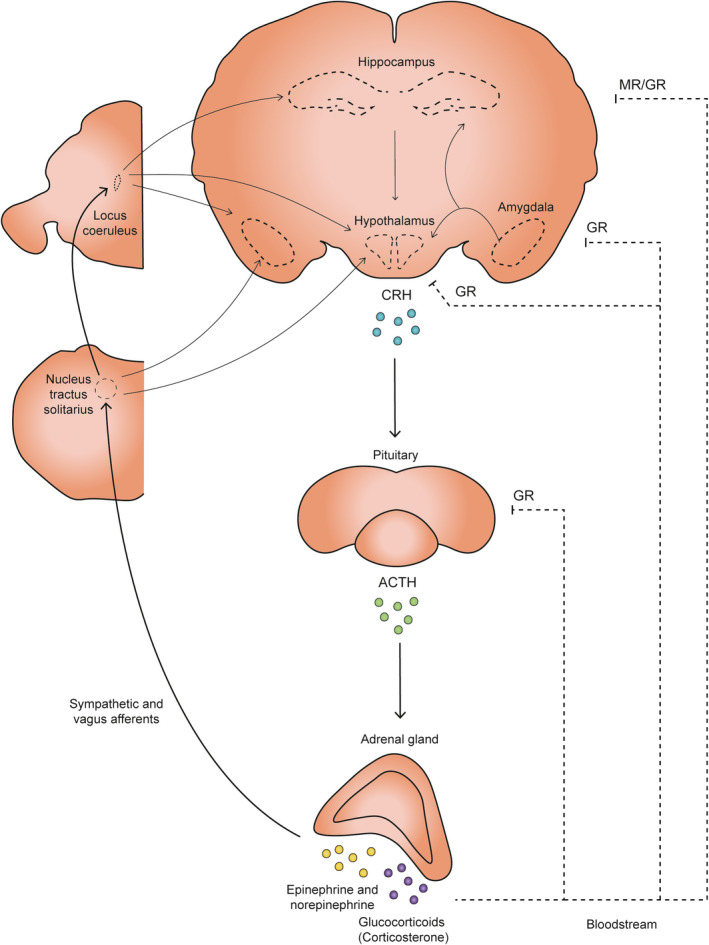
Schematic diagram depicting major afferent and efferent connections of the autonomic nervous and hypothalamic–pituitary–adrenal (HPA) axis. ACTH: adrenocorticotropic hormone, CRH: Corticotropin release hormone, MR: mineralocorticoid receptor, GR: glucocorticoid receptor
In addition, stressful experiences activate the hypothalamic‐pituitary‐adrenal (HPA) axis via the parvocellular neurons of the hypothalamus (PVN). This triggers the release of corticotropin‐releasing hormone (CRH) and arginine vasopressin (AVP) into the pituitary portal circulation (Joëls & Baram, 2009; Sapolsky et al., 2000; Whitnall, 1993). These two peptides promote the release of adrenocorticotrophic hormone (ACTH), which in turn triggers the secretion of glucocorticoid hormones (GCs): corticosterone in rats and mice, and mainly, cortisol in humans and other mammals (Figure 1) (De Kloet et al., 2005). Due to their lipophilic nature, GCs readily enter the brain and bind to high‐affinity mineralocorticoid receptors (MR) and lower‐affinity glucocorticoid receptors (GR) (De Kloet & Meijer, 2019; Reul & De Kloet, 1985). MRs and GRs can be present both in the cytosol and at the cellular membrane of neurons and glial cells (De Kloet et al., 2005, 2018; Karst et al., 2005). Together, these actions of GCs and NE mediate behavioral adaptation to stressful encounters, which includes learning and memory (Bahtiyar et al., 2020; Joëls et al., 2012; Joëls et al., 2018), as discussed in brief below.
2. STRESS HORMONES AND MEMORY
2.1. Norepinephrine
Various lines of evidence report that systemic injections of NE enhance long‐term retention of memory when administered shortly after training (Colucci et al., 2019; Costa‐Miserachs et al., 1994; Gold & van Buskirk, 1975, 1978; Liang et al., 1986; McGaugh & Cahill, 1997; Roozendaal et al., 2009). Similar effects were found in object recognition using yohimbine, a selective presynaptic α2‐adrenoceptor antagonist, that enhances NE outflow (Roozendaal et al., 2006). Consistently, pharmacological depletion of NE impairs memory consolidation in the two‐way shuttle box avoidance task (Archer et al., 1984), spatial water maze (Cahill et al., 1994), and inhibitory avoidance discrimination test (Atucha & Roozendaal, 2015; Cahill et al., 1994).
Mechanistically, NE enhances long‐term potentiation (LTP)—one of the major cellular mechanisms that may underlie learning and memory processes (Bliss & Collingridge, 1993; Cooke & Bliss, 2006; Kessels & Malinow, 2009; Nabavi et al., 2014)—in both hippocampus (Huang & Kandel, 1996; Jȩdrzejewska‐Szmek et al., 2017) and amygdala (Tully et al., 2007). Hu et al. (2007) reported that NE enhances phosphorylation of GluA1 and lowers the threshold for LTP and memory formation. Moreover, NE primes synapses for future changes in long‐lasting plasticity (Maity et al., 2015).
2.2. Glucocorticoid hormones
GCs, via activation of MRs, are involved in appraisal and response selection in novel contexts (Oitzl & de Kloet, 1992; Sandi & Rose, 1994). Moreover, activation of MRs mediates effects of stress on memory systems, promotes habitual processes both in rodents and humans (Arp et al., 2014; Schwabe et al., 2010, 2013; Vogel et al., 2015, 2017), and is involved in spatial and fear‐related memory processes (Berger et al., 2006; Joëls et al., 2018; Oitzl & De Kloet, 1992; Zhou et al., 2010). Activation of GRs is critical for an enhanced memory consolidation that is observed with elevated GC levels (Chen et al., 2012; Donley et al., 2005; Hui et al., 2004; Krugers et al., 2011; Oitzl et al., 2001; Pugh et al., 1997; Revest et al., 2005, 2010, 2014; Roozendaal & McGaugh, 1997; Sandi & Rose, 1994; Xiong & Krugers, 2015; Zhou et al., 2010). At the cellular level, activation of MRs, within minutes, enhances the frequency of hippocampal mEPSCs, enhances the mobility of GluA1/2 AMPARs, and retention of GluN2B containing N‐methyl‐D‐aspartate receptors (NMDARs) (Groc et al., 2008; Karst & Joëls, 2005; Mikasova et al., 2017). At a longer time‐scale, GCs, acting through GRs, enhance the lateral diffusion of AMPARS, hippocampal synaptic retention of AMPARs (Groc et al., 2008; Martin et al., 2009; Xiong & Krugers, 2015), and enhance the amplitude of miniature excitatory postsynaptic currents (mEPSCs) (Karst & Joëls, 2005; Xiong & Krugers, 2015; Xiong et al., 2016). Prolonged exposure to stress and GCs hampers learning and memory (Conrad et al., 1996; Krugers et al., 1997; Wei et al., 2014), synaptic plasticity (e.g. Krugers et al., 2006; Wei et al., 2014), and reduces prefrontal synaptic transmission by reducing neuronal glutamate receptor expression (Yuen et al., 2012). Together, the effects of glucocorticoids on synapses ‐ at least in part ‐ mediate the regulation of stress on learning and memory, although the exact molecular trajectories between receptor activation and functional outcome remain largely unknown (Liu et al., 2010; Wei et al., 2014; Yuen et al., 2009, 2011).
2.3. (Nor)epinephrine–glucocorticoid interactions
NE and GCs also in synergy, modulate neuronal function and behavior (Hermans et al., 2014; Joëls et al., 2011). For example, metyrapone (a GC synthesis inhibitor) attenuates the memory‐enhancing effects of NE (Roozendaall et al., 1996), while β‐adrenergic receptor activation is required for GCs to modulate memory storage processes (McReynolds et al., 2010). Similarly, corticosterone enhances object recognition memory, an effect that is also impeded by β‐adrenoceptor antagonist treatment (Okuda et al., 2004). These findings strongly suggest that adrenergic activation is essential for enabling GC enhancement of memory consolidation.
At the cellular level, NE and GCs interact to modify AMPAR function (Zhou et al., 2012). Corticosterone, at a dose that activates both MRs and GRs, rapidly increases AMPAR surface expression ‐ a postsynaptic index of glutamatergic transmission –particularly, when combined with moderate doses of the β‐adrenergic receptor agonist isoproterenol. Moreover, the combination of both hormones enhances mEPSC frequency, which reflects enhanced glutamatergic transmission. Accordingly, co‐application of GCs with isoproterenol led to a significant enhancement of the early, but not the later LTP phase (Pu et al., 2009). On the contrary, brief administration of GCs several hours before application of isoproterenol markedly suppressed the efficacy of isoproterenol to potentiate the later phase of LTP (Pu et al., 2009). While the studies mentioned here only reflect a summary of the extensive literature, there is substantial evidence that activation of the autonomic nervous system and HPA axis, alone as well as together, modify neuronal function and behavioral adaptation to stressful experiences: NEs and GCs promote behavioral adaptation to stressors by enhancing learning and memory processes, while prolonged (chronic) exposure to stress and stress hormones hampers learning and memory, presumably by altering synaptic function.
3. A ROLE FOR MICROGLIA IN STRESS‐EFFECTS ON MEMORY?
Microglia contribute to many physiological synaptic‐related processes, such as developmentally regulated neuronal apoptosis (Wakselman et al., 2008), hippocampal neurogenesis (Diaz‐Aparicio et al., 2020), synaptic pruning (Gunner et al., 2019; Hong et al., 2016; Kettenmann et al., 2013; Schafer et al., 2012; Sellgren et al., 2017, 2019; Stephan et al., 2012), synaptic plasticity (Wu et al., 2015), and learning and memory (Morris et al., 2013). Changes in neuronal communication are critical for learning and memory processes (Kessels & Malinow, 2009; Nabavi et al., 2014) and several lines of evidence indicate that neuronal function and synaptic communication are modified by glial cells, among others, microglia (Ikegami et al., 2019; Wake et al., 2013). This warrants the question of whether and how microglia might play a role in stress‐effects on neuronal function and memory formation.
Microglia cells are commonly defined as resident phagocytes of the innate immune system and are present throughout the central nervous system (CNS; Umpierre & Wu, 2020). They account for approximately 5%–12% of the total number of brain cells and represent a heterogeneous cell population with an uneven distribution in number, and likely also different properties (Doorn et al., 2015), between different subregions of the CNS. Microglia are prominently present in the olfactory telencephalon, basal ganglia, substantia nigra, and the hippocampus (Lawson et al., 1990; Sierra et al., 2019).
Microglia contribute to synaptic plasticity by synaptic pruning both in physiological (Paolicelli et al., 2011) and pathophysiological (Sellgren et al., 2019) conditions. They do this by acting as “synaptic sensors” (Boxes 1 and 2), responding to changes in neural activity and neurotransmitter release, suggesting that microglia play a role in regulating experience‐dependent synaptic plasticity (Kettenmann et al., 2013; Morris et al., 2013; Tremblay et al., 2011). In line with this, microglial processes are often in close proximity to neuronal somas and dendritic spines, and the dynamics of microglial processes are regulated by sensory experience and/or neuronal activity (Kim, Cho, et al., 2017; Tremblay et al., 2010; Weinhard et al., 2018). Due to their roles in synaptic development and function, microglia are currently regarded as the fourth component of the “quad‐partite synapse” (Paolicelli & Gross, 2011; Schafer et al., 2013), in addition to the pre‐ and postsynaptic terminals and the astrocytes.
BOX 1. Ligand and receptors involved in microglia–neuron communication.
Microglia communicate with other cells in the CNS by sensome receptors (Kettenmann et al., 2013). These receptors contribute to the direct communication between neurons and microglia and allow microglia to adapt to changing environments. This communication with other cells allows microglia to shape the CNS and to play a role in different developmental relevant processes such as blood vessel formation, myelination, and neurogenesis (Frost & Schafer, 2016).
CX3CL1‐CX3CR1: Fractalkine, also known as CX3CL1, is a chemokine that in the brain is primarily expressed by neurons (Tarozzo et al., 2003). CX3CL1 is detected both as a neuronal membrane‐bound protein, and as a soluble protein in the extracellular space. Fractalkine receptor, CX3CR1, is predominantly found on microglia (Harrison et al., 1998; Meucci et al., 2000; Hatori et al., 2002; Paolicelli et al., 2014). Fractalkine signaling is critical in microglial‐dependent memory as it regulates processes such as synapse development and synaptic plasticity (For a review, Paolicelli et al., 2014). Specifically, it has been implicated in microglial pruning of dendritic spines.
CD200‐CD200R: CD200 is a glycoprotein widely found on the cell membranes of neurons, (Barclay et al., 2002; Koning et al., 2009). The target receptor of CD200, CD200R, is located selectively on myeloid cells (in the central nervous system this is predominantly the microglia) (Hernangomez et al., 2012). CD200 downregulates the activity of microglial cells by ligation of the CD200 receptor (CD200R), keeping the microglia in resting state (Hoek et al., 2000; Biber et al., 2007)
DAP12‐TREM2: DNAX‐activating protein of 12 kDa DAP12 is a transmembrane protein that is exclusively expressed by microglia in the brain that serves as an adaptor to several myeloid receptors, like the triggering receptor expressed on myeloid cells transmembrane TREM2 (for a review of DAP12‐TREM2 signaling, see Konishi & Kiyama 2018). In humans, recessive genetic mutations in DAP12 are responsible for the microgliopathy Nasu‐Hakola disease (NHD), a rare inherited neuropsychiatric condition characterized by early dementia (Xing et al., 2015).
Complement: Complement signaling is involved in microglial pruning of developing neuronal synapses. The complements C1q and C3 localize to synapses during physiological development and mediate synapse pruning by phagocytic microglia (Stevens et al. 2007, Schafer et al. 2012).
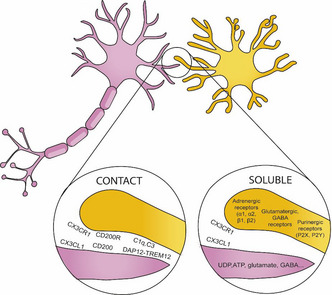
BOX 2. Microglia–neuronal communication in the context of synapses.
Microglia can engulf small portions of axons, a process named trogocytosis, and contribute to limit axonal outgrowth and to eliminate presynaptic regions (Weinhard et al., 2018). Microglia have been also proposed to phagocytose dendritic spines (Filipello et al., 2018; Kim, Cho, et al., 2017; Kim, Mun, et al., 2017; Paolicelli et al., 2011).
Microglia may modulate neuronal connectivity by physically interposing their cell bodies and processes between post‐ and presynaptic regions (Chen et al., 2014). Microglial–neuron interaction has been associated with the generation of filopodia from the dendritic shaft and the head of dendritic spines, contributing to the formation and relocation of dendritic spines, respectively (Miyamoto et al., 2016; Weinhard et al., 2018). This is supported by the observation that the majority of filopodia induced by microglia originate from mature spine heads (Weinhard et al., 2018).
Microglia establish close contacts with the axon initial segment (Baalman et al., 2015; Clark et al., 2016), suggesting a yet unexplored mechanism to affect neuronal connectivity. Therefore, through the establishment of close contacts with neurites and synaptic elements, microglia participates in the elimination, interruption, and formation of neuronal connections.
Microglia have been shown to regulate neurite growth through the release of different soluble factors: plasminogen and thrombospondin, which in vitro induce neurite outgrowth (Chamak et al., 1994, 1995; Nagata et al., 1993), insulin growth factor‐1 (IGF‐1), and TNF‐α (Batchelor et al., 1999, 2002; Guthrie et al., 1995; Liu et al., 2017) or interleukin‐10 (IL‐10), which increases the number of pre‐ and postsynaptic terminals in neuronal cell cultures from rat hippocampus, an effect that is antagonized by interleukin‐1β (IL‐1β) (Lim et al., 2013).
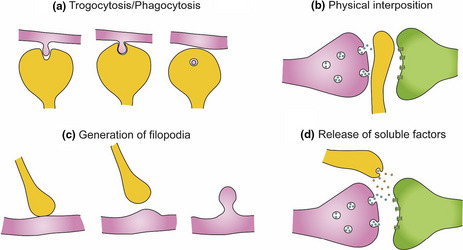
3.1. Microglia express stress‐hormones receptors
Tanaka et al. (1997) demonstrated the expression of GRs and MRs in microglial cells by immunoblotting. Sierra et al. (2008) confirmed the presence of these receptors by real‐time PCR in isolated hippocampal microglia using Fluorescence Activated Cell Sorting (FACS). These studies suggest that GRs are more abundant than MRs in microglia at both mRNA and protein level. In functional terms, the deletion of GR in microglia reduces microglial motility, increases amoeboid morphology, and elicits greater neuronal damage and demyelination after intraparenchymal injection of lipopolysaccharide (LPS) (Carrillo‐de Sauvage et al., 2013). In contrast, activation of GRs in a model of Alzheimer's disease increased the pro‐inflammatory marker (cluster of differentiation) CD68, microglial density per se, as well as activation in the hippocampal CA1 area (Pedrazzoli et al., 2019). Interestingly, other conditions with increased GCs levels, such as aging, have been associated with a decrease in microglial complexity (van Olst et al., 2018). Specifically, enhancing GCs levels in young mice enhanced microglial ramifications, while the specific GR knockdown in old mice aggravated age‐associated microglial amoebification in the hippocampus. This suggests that microglial GR regulates essential microglial properties during inflammation, and have a key role in neuronal survival and function.
Receptors for NE are also present in microglial cells. Mori et al. (2002) demonstrated, via real time‐PCR, that microglial cells express mRNAs Fα1A, α2A, β1, and β2 adrenergic receptors. However, its expression depends on its activity state. Analysis of adrenergic receptor expression with quantitative PCR indicated that resting microglia primarily express β2 receptors but switch expression to α2A receptors under pro‐inflammatory conditions modeled by LPS (Gyoneva & Traynelis, 2013). In both conditions, treatment with NE caused a retraction of microglial processes by blocking the ATP‐induced process extension and migration in vitro (Gyoneva & Traynelis, 2013). In addition, selective stimulation of adrenergic receptors reduced the release of pro‐inflammatory mediators such as nitric oxide, IL‐6, and TNF‐ α (Färber et al., 2005). Similarly, β2‐adrenergic stimulation after stroke caused an enlarged morphology of microglia, impaired microglial proliferation, and reduced expression of TNFα (Lechtenberg et al., 2019). Hence, microglia may be kept quiescent and in a non‐inflammatory state via NE release. This is key as deficiency of NE under pathological conditions may then facilitate an inflammatory state that can impair normal learning and memory processing and even prevent microglial phagocytosis of amyloid plaques (Heneka et al., 2010).
3.2. Microglia and memory
A role for microglia in learning and memory processes is suggested by different lines of evidence. Torres et al. (2016) depleted microglia (a) locally by injecting clodronate in the hippocampus or (b) systemically using a Csf1R inhibitor (thus, blocking the cytokine Csf—which involved in microglial growth and differentiation). The depletion resulted in an impaired performance in the Barnes maze that was rescued after microglial repopulation 20 days after clodronate treatment. Chaaya et al. (2019) reported an increase in microglial numbers and a change in microglial morphology after fear conditioning. Examination of microglial morphology within the DG revealed altered branching, with processes with significantly shorter and less complex extensions. Microglia also play a role in the maintenance of dendritic spine densities in the adult brain, which is relevant for learning and memory (Hayashi‐Takagi et al., 2015). Depletion of microglia via diphtheria toxin showed a decrease in learning‐induced synaptic remodeling (formation and elimination of hippocampal dendritic spines) related to deficits in multiple learning tasks (Parkhurst et al., 2013). Recent evidence suggests that microglia are also involved in forgetting. Wang et al. (2020) used CD11b‐DTR mice, in which diphtheria toxin receptor (DTR) is specifically expressed in CD11b‐expressing myeloid cells, including microglia (Prinz et al., 2011). By administering diphtheria toxin (DT), they depleted the microglia in the hippocampus and showed that these mice showed significantly higher freezing levels at the retrieval phase (35 days after training) and confirmed this by targeting microglia with a Csf1R inhibitor. These results show that microglia are required to mediate the forgetting of remote memories. Finally, several lines of evidence in humans (using, e.g., PET imaging) suggest that activation of microglia is related to cognitive alterations (Cagnin et al., 2001; Eckenweber et al., 2020; Fan et al., 2017; Hamelin et al., 2016).
3.3. Microglia and synapses
During development and learning experiences, morphological maturation of microglial cells takes place in close proximity to the period of spine formation, which may suggest that microglia are actively involved in the synaptogenesis of neuronal circuits (Dalmau et al., 1998; Matcovitch‐Natan et al., 2016; Reemst et al., 2016). Also, microglia are canonically associated with synaptic pruning in physiological (Schafer et al., 2012) and pathological (Sellgren et al., 2017; Stephan et al., 2012) conditions. Moreover, microglia have been shown to mediate synaptic loss in Alzheimer models (Hong et al., 2016) and to show distinct patterns of activation in response to amyloid and tau in Alzheimer's disease (Gerrits et al., 2021). Moreover, microglia may participate in functional synapse maturation through the modulation of excitatory synaptic transmission. In vitro studies have demonstrated that conditioned media from cultured microglia potentiated the amplitude of NMDA‐induced currents and could enhance LTP in synapses of the Schaffer collateral pathway (Hayashi et al., 2006). Consistent with this, LTP was reduced in organotypic hippocampal slices prepared from Cx3cr1KO mice—which have impaired proper neuron–microglial communication (Rogers et al., 2011). Using the same animal model (Parkhurst et al., 2013), further demonstrated a decline in the frequency of NMDAR‐ and AMPA‐mediated miniature excitatory postsynaptic currents (mEPSCs). Moreover, administering minocycline (a specific inhibitor of the pro‐inflammatory response in microglia—Garrido‐Mesa et al., 2013) could partly restore the decreased induction of LTP in hippocampal pyramidal cells after stress, which indicates a role of pro‐inflammatory microglia in the impairment of spatial learning and memory in the Morris water maze (Liu et al., 2015). Finally, targeting microglia with a mutation of KARAP/DAP12—selectively expressed in microglia in the brain—leads to an altered synaptic phenotype in hippocampus that involves reduced synaptic recruitment of GluR2 that triggers an NMDAR‐independent form of LTP (Roumier et al., 2004).
4. EVIDENCE OF STRESS‐MEDIATED EFFECTS IN MICROGLIA IN THE CONTEXT OF MEMORY AND MEMORY SYNAPSES
4.1. Number and morphology of microglia
Tables 1 and 2 show the effects of acute (Table 1) and chronic stressors (Table 2) on microglia number and morphology. As shown in Table 1, acute stress, in general, does not alter microglia number or morphology in the hippocampus, although Sugama et al. (2007) report an increase in the microglial surface area after acute stress exposure. Chronic stress exposure, on the other hand, increases the number and complexity of microglia. This has consistently been shown using different microglial markers such as ionized calcium‐binding adapter molecule 1 (Iba‐1), also known as allograft inflammatory factor 1 (AIF‐1) (Ito et al., 1998), CD68 (Walker & Lue, 2015), CD11b, and CD45 Greter et al., 2015), ED‐1 Graeber & Streit, 2010), and translocator protein (TSPO) (Beckers et al., 2018). It is important to mention though that some of these markers are also expressed by peripheral immune cells, and results have to be carefully interpreted in conditions when the blood–brain barrier is disrupted (Grassivaro et al., 2020), which may occur after chronic stress (Lehmann et al., 2018). In addition, chronic stress alters the morphology of microglia. In general, chronic stress induces microglia to enter a reactive phase characterized by enlarged somas, and retraction and thickening of processes, with an excess of short and thick hyper‐ramified processes which give them a bushy appearance. These hypertrophic microglia are functional cells that can exert increased response to pro‐inflammatory stimuli (Perry & Holmes, 2014), by increasing their pro‐inflammatory cytokines expression. Moreover, the increased number of distal processes is often associated with exacerbated interactions with neurons and synapses (Šišková & Tremblay, 2013).
TABLE 1.
Effects of acute stressors on microglial number and morphology. F: female, M: male
| Stress | Age | Specie | Sex | Marker | Effect | Reference |
|---|---|---|---|---|---|---|
| Water immersion‐resistant stress (2 hr) | 8 weeks old | Mice | M | Iba‐1 |
|
Ohgidani et al. (2016) |
| Restraint combined with head water immersion (2 hr) | 9/10 weeks old | Rats | M | Cd11b and Ed‐1 |
|
Sugama et al. (2007) |
| Restrain stress (3 hr) | Rats | F/M | Iba‐1 |
|
Bollinger et al. (2017) |
TABLE 2.
Effects of chronic stressors on microglial number and morphology. F: female, M: male
| Stressor | Age | Specie | Sex | Marker | Effect | Reference |
|---|---|---|---|---|---|---|
| Maternal sleep deprivation (72 hr) | E4‐E12 | Rats | M | Iba‐1 |
|
Zhao et al. (2014) |
| Maternal sleep deprivation (72 hr) | E4‐E12 | Rats | M | Iba‐1 |
|
Zhao et al. (2015) |
| Maternal restrain stress (Daily, 3 times/day for 45 min) | E12–delivery | Mice | F/M | Iba‐1 |
|
Diz‐Chaves et al. (2012) |
| Maternal exposition to bright light (3 sessions for 45 min) | E12–delivery | Mice | F/M | Iba‐1 |
|
Diz‐Chaves et al. (2013) |
| Adverse early life environment | E13 to P21 | Mice | F/M | AIF‐1 |
|
Cohen et al. (2016) |
| Maternal deprivation (3 hr per day) | PND 1–10 | Rats | M | Iba‐1 |
|
Réus et al. (2019) |
| Maternal deprivation (3 hr per day) | PND 1–14 | Mice | F/M | Iba‐1 |
|
Han et al. (2019) |
| Maternal deprivation (3 hr per day) | PND1‐PND14 | Rats | M | Iba‐1 |
|
Roque et al. (2016) |
| Brief daily maternal separation (40 min per day) | PND1‐PND21 | Mice | M | Iba‐1 |
|
Delpech et al. (2016) |
| Limiting bedding and nesting | PND2 ‐ PND9 | Mice | M | Iba‐1 and CD68 |
|
Hoeijmakers et al. (2017) |
| Limiting bedding and nesting | PND2 ‐ PND9 | Mice | M | CD68 |
|
Yam et al. (2019) |
| Social isolation (30 min per day) | PND14 ‐ PND21 | Mice | F/M | Iba‐1 and Cd11b |
|
Gong et al. (2018) |
| Social Isolation Protocol (6 weeks) | PND 21 | Rats | M | Iba‐1 |
|
Wang et al. (2017) |
| Unpredictable restraint stress (2 weeks) | PND 85 | Rats | M | Iba‐1 |
|
Tynan et al. (2010) |
| Chronic mild stress (different stressors) (Daily for 12 weeks) | 6/7 weeks old | Rats | M | TSPO marked by [18F] DPA‐714 |
|
Wang et al. (2018) |
| Repeated social defeat (2 hr for 6 nights) | 6/8 weeks old | Mice | M | Iba‐1 and CD45 |
|
McKim et al. (2016) |
| Social disruption stress (2 hr for 6 nights) | 7/8 weeks old | Mice | M | Iba‐1 |
|
Wohleb et al. (2011) |
| Social defeat stress (14 days) | 8/10 weeks. | Mice | M | CD68 |
|
Lehmann et al. (2016) |
| Chronic unpredictable stress (40 days) | 3/4 months old | Mice | Iba‐1 |
|
Bian et al. (2012) | |
| Chronic restrain stress (3 hr per day for 10 days) | Rats | F/M | Iba‐1 |
|
Bollinger et al. (2017) |
Mechanistically, there is evidence that these changes in microglial number might be mediated via stress‐induced elevation in stress hormones. Nair and Bonneau (2006) demonstrated that blocking GC synthesis using metyrapone reversed the increase in hippocampal microglia number found on the fourth day of a chronic restrain stress paradigm. In the same way, animals treated with the GR antagonist RU486 prior to the stressor did not exhibit the same increase in microglia number when restrained. Accordingly, NE could be also involved, as Sugama et al. (2019) probed that the increase in microglial number and change in morphology (enlarged with shorter processes and enlarged soma) after restraint stress were significantly inhibited by pre‐treatment with the β‐blocker propranolol. This was confirmed in a double knockout for β‐1 and β‐2 adrenergic receptors. However, the specific mechanisms underlying these effects remain unknown. Chronic stress not only increases the microglial population in the hippocampus and promotes a shift toward a more inflammatory phenotype, but it also promotes peripheral recruitment and migration of peripheral macrophages and/or bone marrow–derived monocytes into the brain. Chronic stress exposure, e.g., potentiates the influx of bone marrow–derived cells by extravasation into the brain parenchyma of the ventral hippocampus and differentiation into microglia (Brevet et al., 2010). Finally, in contrast to chronic stress in adulthood, stress during the early postnatal period affects microglia in a different manner when measured in early stages. Réus et al. (2019) demonstrated that maternal deprivation decreases the level of Iba‐1 immunolabeling in the hippocampus at PND10, which then increases at PND20 and remains elevated from PND 30 onwards. In agreement, Hoeijmakers et al. (2017) showed reduced coverage of Iba1+ cells in the dentate gyrus at P9 in a model of fragmented maternal care, with specifically reduced complexity of the most distal branching of microglia of the stressed exposed offspring. At 4 months of age, increased Iba1 density and CD68 immunoreactivity were found (Catale et al., 2020; Hoeijmakers et al., 2017; Reemst et al., 2016). Together, these studies show that microglial sensitivity to chronic stress differs in its responses at different ages of exposure.
4.2. Gene expression
Table 3 (acute stress) and Table 4 (chronic stress) summarize the changes in microglia‐related gene expression after stress. It is important to note that in some cases, such changes in, e.g., cytokine expression, might be mediated by other cell types, and are likely not always exclusively related to microglia. Several studies have further used minocycline (Han et al., 2019; Wadhwa et al., 2017; Wang et al., 2017; Zhao et al., 2015), and reported a reversal in specific gene expression changes when microglia could not be activated, thereby identifying microglia‐specific effects.
TABLE 3.
Effects of acute stressors on microglia‐related gene expression. F: female, M: male
| Stressor | Age | Specie | Sex | Effect | Reference |
|---|---|---|---|---|---|
| Water immersion resistant stress (2 hr) | 8 weeks old | Mice | M |
|
Ohgidani et al. (2016) |
| Inescapable tail shock (100 trials of 5 s/trial) | 60/90 days | Rats | M |
|
Weber et al. (2015) |
| Inescapable tail shock (100 trials of 5 s/trial) | 60/90 days | Rats | M |
|
Frank et al. (2018) |
| Acute restraint stress (1 hr) | Rats | M |
|
Iwata et al. (2016) |
TABLE 4.
Effects of chronic stressors on microglia‐related gene expression. F: female, M: male
| Stressor | Age | Specie | Sex | Effect | Reference |
|---|---|---|---|---|---|
| Maternal sleep deprivation (72 hr) | E4‐E12 | Rats | M |
Minocycline treatment revert the effect. |
Zhao et al. (2015) |
| Maternal restraint stress (daily, 3 times/day for 45 min) | E12 ‐ delivery | Mice | F/M |
|
Diz‐Chaves et al. (2012) |
| Maternal exposition to bright light (3 sessions for 45 min) | E12‐ delivery | Mice | F/M |
|
Diz‐Chaves et al. (2013) |
| Maternal environmental stress + perinatal Western diet | E13‐PND21 | Mice | F/M |
|
Cohen et al. (2016) |
| Maternal deprivation (Daily, 3 hr per day) | PND1 ‐PND10 | Rats | M |
|
Giridharan et al. (2019) |
| Maternal deprivation (3 hr per day) | PND1 ‐ PND14 | Mice | F/M |
|
Han et al. (2019) |
| Maternal deprivation (3 hr per day) | PND1 – PND14 | Rats | M |
|
Roque et al. (2016) |
| Brief daily maternal separation (40 min per day) | PND1 ‐ PND21 | Mice | M |
|
Delpech et al. (2016) |
| Limiting bedding and nesting | PND2 ‐ PND9 | Mice | M |
|
Hoeijmakers et al. (2017) |
| Social isolation (30 min per day) | PND14 ‐PND21 | Mice | F/M |
|
Gong et al. (2018) |
| Social Isolation Protocol (6 weeks) | PND 21 | Rats | M |
|
Wang et al. (2017) |
| Sleep deprivation (48 hr) | 6/7 weeks old | Rats | M |
|
Wadhwa et al. (2017) |
| Chronic mild stress (different stressors) (Daily for 12 weeks) | 6/7 weeks old | Rats | M |
|
Wang et al. (2018) |
| Restraint stress (6 hr for 28 days) | 6/8 weeks old | Mice | M |
|
Voorhees et al. (2013) |
| Social disruption stress (2 hr for 6 nights) | 7/8 weeks old | Mice | M |
|
Wohleb et al. (2011) |
| Chronic unpredictable stress (duration depending on the stressor, 3 times a day for 3 weeks) | 7/8 weeks old | Rats | M |
Minocycline treatment reversed these effects. |
Liu et al. (2015) |
| Restraint stress (2 hr per day for 30 days) | 8 weeks old | Mice | M |
|
Alcocer‐Gómez et al. (2016) |
| Chronic unpredictable mild stress (4 weeks) | 8 weeks old | Mice | M |
|
Wang et al. (2018) |
| Chronic unpredictable stress (40 days) | 3/4 months old | Mice | M |
|
Bian et al. (2012) |
4.2.1. Cytokines
With specific exceptions, an increase in the expression of cytokines in the hippocampus is found after stress exposure, regardless of the kind of stressor (psychological/physical), the duration (acute/chronic), or the period of the stressor exposure (from prenatal stress to adulthood) (see Tables 3 and 4). The data collected for chronic stressors (Table 4) has been addressed in both males and females but not in the case of acute stressors (Table 3) as there was a lack of studies using females.
The stress‐induced changes in cytokines and chemokines can both modulate molecular and cellular mechanisms sub‐serving learning, memory, and cognition (for more detailed information Donzis & Tronson, 2014; Rizzo et al., 2018).
Interleukin (IL)‐1 family: IL‐1s are a group of 11 cytokines with a pro‐inflammatory profile. Some of the IL‐1s are consistently expressed after various stressors (Tables 3 and 4). However, it is important to note that, not only microglia but also neurons and astrocytes release IL‐1 α/β after a stressor and may also be involved in the modulatory role of IL‐1 in learning and memory (Augusto‐Oliveira et al., 2019). Several lines of evidence indicate that IL‐1s are involved in learning and memory. For example, IL‐1b is released by microglia in the hippocampus in vivo during learning tasks such as fear conditioning (Williamson et al., 2011). Moreover, mice receiving a peripheral injection of IL‐1b on the second day of spatial water maze training showed impaired spatial learning (Oitzl et al., 1993). In addition, when injected immediately following fear conditioning, IL‐1b impaired contextual fear memory (Barrientos et al., 2004; Huang & Sheng, 2010). IL‐1 has been reported to regulate contextual memory in an inverted U‐shaped pattern, with physiological levels of IL‐1 promoting memory formation, whereas any deviation from the physiological range, either by excess elevation in IL‐1 levels or by blockade of IL‐1 signaling, results in impaired memory (Goshen et al., 2007). Along the same line, Ross et al. (2003) demonstrated a dual role of IL‐1 in LTP in hippocampal slices ex vivo. Endogenous IL‐1 is required for LTP, whereas high concentrations of exogenous IL‐1, which correspond to pathological levels, inhibit LTP. Similar results were found by Avital et al. (2003). Furthermore, IL‐1 signaling during development is relevant for later hippocampal‐dependent learning and memory, as prenatal exposure to an IL‐1 antagonist, impaired memory performance in the fear‐conditioning paradigm (Goshen et al., 2007). This may be related to IL‐1 playing a role in neuronal differentiation and the number of progenitor cells converting into neurons (Potter et al., 1999). Finally, IL‐33 (also known as IL‐1F11) is a relatively newly described member of the IL‐1 family that is expressed by adult hippocampal neurons in an experience‐dependent manner, and whose receptor is expressed in glial cells (including microglia) (Yasuoka et al., 2011). While no literature is available on stress and IL‐33, IL‐33 is involved in learning and memory. Loss of IL‐33 its receptor has been linked to impaired spine plasticity (via altered synaptic engulfment), reduced newborn neuron integration, and a diminished precision of remote fear memories (Nguyen et al., 2020; Vainchtein et al., 2018).
IL‐6: Most of the studies reported in Tables 3 and 4 show increased IL‐6 levels—a pro‐inflammatory cytokine expressed by both glial and neuronal cells—after exposure to stressors. Trangenic overexpression (Heyser et al., 1997) or application of IL‐6 (Li et al., 1997; Tancredi et al., 2000) causes broad memory impairments and diminished LTP, respectively. Nevertheless, genetic deletion of IL‐6 fails to disrupt learning and memory (Braida et al., 2004), suggesting a more subtle modulatory role. Hippocampal IL‐6 levels are increased after learning (del Rey et al., 2013) and after LTP induction (Balschun et al., 2004; Jankowsky et al., 2000). IL‐6 application results in a decrease in LTP maintenance, suggesting that expression of IL‐6 after learning may be an endogenous mechanism for limiting plasticity (Balschun et al., 2004).
IL‐10s: IL‐10 is an anti‐inflammatory cytokine expressed by glial cells that is either not modified or reduced after stress (Tables 3 and 4). IL‐10 protects against memory deficits (Richwine et al., 2009). Therefore, its reduction after stress exposure supports an overall memory impairment response after stress. Conversely, IL‐10 ameliorates the performance in the radial arm water maze in mice models of Alzheimer's disease (Kiyota et al., 2011). In addition, microglial IL‐10 increases the number of pre‐ and postsynaptic terminals in neuronal cell cultures from rat hippocampus (Lim et al., 2013).
Tumor necrosis factor (TNF)‐α: TNF‐α deserves special attention, as it is a pro‐inflammatory cytokine that has been argued to be exclusively expressed by microglia in the CNS (Barres, 2008; Welser‐Alves & Milner, 2013). Ohgidani et al. (2016) reported that microglia (through TNF‐α) play a pivotal role in the disruption in working memory due to acute stress. Immersion stress impaired memory, which was recovered by the TNF‐α inhibitor etanercept. Moreover, reducing TNF‐α expression pharmacologically normalizes spatial recognition memory impairment altered by chronic neuroinflammation (Belarbi et al., 2012). In the same way, as discussed for IL‐1, it has been suggested that basal concentrations of TNF‐α are necessary for synaptic efficacy, while high concentrations of TNF‐α could be neurotoxic and low concentrations will impair synaptic strength (Rizzo et al., 2018). At the synaptic level, Furukawa and Mattson (1998) showed that TNF‐α alters glutamatergic transmission and neuronal excitability. TNF‐α increases surface expression of AMPA receptors (Beattie et al., 2002) while decreasing surface GABA (Stellwagen et al., 2005), thereby enhancing synaptic transmission, while impairing hippocampal LTP (Cunningham et al., 1996; Tancredi et al., 1992). Finally, TNF‐α affects neuronal branching in vitro (Bernardino et al., 2008; Keohane et al., 2010). TNF‐α receptor knockout mice express decreased arborization of the apical dendrites of the CA1 and CA3 regions and accelerated dentate gyrus development (Golan et al., 2004).
Taken together, literature suggests that stress induces a pro‐inflammatory state in microglia, enhances expression of IL‐1, IL‐6, and TNF‐α, and decreases the anti‐inflammatory cytokine IL‐10, which may elicit memory impairments and related LTP deficits. This stress‐induced inflammatory response might be also mediated by stress hormones, as Sugama et al. (2019) show that propranolol significantly suppressed the increase in IL‐1β expression after restrain stress. Similar results were found by Johnson et al. (2005), after tail shock exposure and were confirmed by selective lesions of the LC with DSP4, pointing to central NE in this reduction effect. In vitro studies show that NE agonism—via isoproterenol—enhanced IL‐1β and IL‐6, but not TNF‐α production following LPS stimulation (Johnson et al., 2013). In addition, there could also be effects mediated by GCs, as the increase in IL‐1β in hippocampal microglia after inescapable shock was completely blocked by GR antagonism via RU486 (Frank et al., 2012).
4.2.2. Other molecules
Sensome receptors: Tables 3 and 4 show that acute (Frank et al., 2018) and chronic (Frank et al., 2018; Han et al., 2019; Wang et al.,; 2017) stress reduces either ligands or receptors involved in neuronal–microglial crosstalk, in particular, CD200‐CD200R. This is important as (a) CD200R is exclusively expressed by microglia in the brain (Hernangómez et al., 2012) and (b) there is evidence that corticosterone decreases CD200R expression (Fonken et al., 2016). CD200‐CD200R signaling is believed to inhibit microglial inflammatory function in order to maintain homeostasis (Manich et al., 2019), and its dysfunction might lead to cognitive impairments. In particular, deficiency in the microglia‐expressed CD200 receptor for the CD200 ligand produced by neurons has been seen to result in markedly impaired LTP in Schaffer collateral–CA1 synapses (Costello et al., 2011). In addition, CD200‐deficient mice exhibited poorer performance in Morris water maze and novel object recognition tests, in combination with a decreased frequency of mEPSC in the hippocampus (Feng et al., 2019). Regarding morphology, these KO mice expressed reduced dendritic density as well as mushroom spines on both the primary and secondary branches.
HMGB‐1 and NLRP3 protein: Inflammasomes are multi‐protein signaling complexes that trigger the activation of inflammatory caspases and cytokines. Among various inflammasome complexes, the NOD‐like receptor protein 3 (NLRP3) inflammasome is best characterized (Swanson et al., 2019). This inflammasome can be activated by several molecules, among others, the alarmin high mobility group box 1 (HMGB1) (Andersson et al., 2018). HMGB1 is an endogenous danger signal or alarmin that mediates activation of the innate immune response after stress exposure (Frank et al., 2015). Increased levels of the inflammasome have been reported after either acute (Iwata et al., 2016; Weber et al., 2015) or chronic (Alcocer‐Gómez et al., 2016; Wang et al., 2018) exposure to stressors in adulthood. This increase might be driven via GC signaling, as corticosterone induces a concentration‐dependent increase in NLRP3 expression in hippocampal microglia (Feng, Zhao, et al., 2019; Frank et al., 2014). The importance of this particular signaling pathway may be that NLRP3 inflammasome activation is (a) limited to the microglia in the mouse brain (Gustin et al., 2015) and (b) its involvement in learning and memory. As an example, Heneka et al. (2013) showed in APP/PS1 mice that NLRP3 or caspase‐1 deficiency completely prevented mice from a spatial memory impairment in the Morris water maze test and in the object recognition memory test. In the same way, disruption of its signaling suppressed LTP in hippocampal slices and induced a small, but statistically significant reduction of spine density in the pyramidal neurons of APP/PS1 mice, which was prevented by NLRP3 or caspase‐1 deficiency. Moreover, Wang et al. (2018) showed poor learning and memory in the Morris water maze in relation to an increased expression of NLRP3 and IL‐1b, in the hippocampus. An NLRP3–caspase‐1 pathway inhibitor attenuated the cognitive impairment highlighting the role of this pathway in memory. Therefore, stress‐induced activation of the NLRP3 inflammasome could underlie cognitive impairments not only by itself but also disrupting IL‐1β production.
Taken together, stress modifies gene expression toward an activated pro‐inflammatory state and impaired neuronal communication, which could explain stress‐induced deficits in memory processing.
4.3. Microglial–neuronal crosstalk
The crosstalk between microglia and neurons is important for neuronal function as discussed before (Boxes 1 and 2). Evidence now suggests that this interaction, and in particular Cx3CL1‐Cx3Cr1 signaling, may be affected by stress. Firstly, NE output via reboxetine (a norepinephrine reuptake inhibitor) stimulates the production of CX3CR1 (González‐Prieto et al., 2020). Secondly, deletion of Cx3cr1 prevented the stress‐effects on microglial morphology and phagocytosis of cellular elements (Milior et al., 2016), In addition, Cx3cr1 deficiency has profound defects in synapse elimination following sensory lesioning (Gunner et al., 2019). This leads to deficits in glutamatergic transmission, as well as short‐ and long‐term neuronal plasticity, highlighting the importance of this crosstalk for memory processing. In particular, CA1 synapses of Cx3cr1 KO show a decrease in AMPA/NMDA ratio (pointing to immature synapses) and a defective AMPA receptor EPSCs (Basilico et al., 2019). Cx3cr1‐deficient mice also exhibit increased fear acquisition as well as retrieval (Schubert et al., 2018) and increased freezing during the reinstatement, suggesting that the retrieval of fear was potentiated in these mice. This may be particularly relevant considering recent findings on the role of microglia in the process of forgetting (Wang et al., 2020). In summary, neuron–microglia signaling through CX3CR1 is necessary for synaptic function and prides a potential connection among microglia, stress, and memory.
4.4. Synaptic transmission
As discussed before, in particular, chronic stress alters microglial function and this may mediate stress‐effects on learning and memory. Microglial cells may also mediate stress‐effects on synapses. Liu et al. (2015) demonstrated that chronic unpredictable stress in adult rats leads to a pro‐inflammatory phenotype in hippocampal microglia in relation to the attenuated phosphorylation of glutamate receptor GluA1, an effect that is inhibited by minocycline treatment. In addition, minocycline prevented stress‐effects on LTP induction (Liu et al., 2015). Similarly, rats that underwent social isolation during adolescence showed a downregulation of the expression of hippocampal NMDAR subunit NR1 and AMPAR subunits GluA1 and GluA2, which were restored after minocycline treatment (Wang et al., 2017). These data suggest a role of microglia in synaptic plasticity through regulation of AMPA/NMDA signaling after stress exposure. Interestingly, the relation between microglia and NMDA receptors is bidirectional. Nair and Bonneau (2006) demonstrated that chronic stress induces microglial proliferation which was mediated by GC regulation of NMDARs. Recently, studies have shown that NE is involved in the role of microglia in modifying synaptic contacts, by increasing the microglia–dendrite contact area and contact duration (Liu et al., 2019; Stowell et al., 2019). Taken together, there is evidence that prolonged stress might alter synaptic mechanisms via changes in microglia.
4.5. Kynurenine pathway
In the brain, tryptophan is converted into several bioactive molecules, the best known of which is serotonin. However, only a small percentage of tryptophan is metabolized into serotonin. More than 95% of tryptophan is converted into kynurenine in a process known as kynurenine pathway. This pathway is present in many different tissues, notably in the liver and brain. In the brain, microglia play a critical role in producing the enzyme indoleamine 2,3‐dioxygenase (IDO), an enzyme that catalyzes the conversion of tryptophan into kynurenine (Savitz, 2020). Kynurenine is further metabolized to, among others, quinolinic acid (Schwarcz et al., 2012). The role of kynurenine metabolites in memory is mainly (either direct or indirect) via the regulation of neurotransmitter systems. For example, kynurenine and quinolinic acid have opposing effects on NMDAR function (Perkins & Stone, 1985). Quinolinic acid contributes to the activation of NMDAR itself and by inhibiting glutamate re‐uptake, resulting in excessive extracellular glutamate, inducing further NMDAR agonism (Maddison & Giorgini, 2015). Also, quinolinic acid has been shown to stimulate the release of glutamate from neurons, inhibiting its re‐uptake by astrocytes (Jo et al., 2015). Overall, Rahman et al. (2018) have demonstrated that intraventricular quinolinic acid infusion results in significant impairment of learning in adolescent animals.
There is growing evidence for kynurenine pathway hyperfunction in stress‐related disorders (Savitz et al., 2015). Brooks et al. (2016) showed that GCs induce amplified the ability of interferon‐γ to increase Ido1 expression—and, in consequence, shifting the route toward more kynurenine production—in organotypic hippocampal slice cultures. Also, both GR (using the agonist dexamethasone) and MR (using the agonist aldosterone) are involved in this increase. Vecchiarelli et al. (2016) have demonstrated that acute restraint stress increases Ido1 expression in the amygdala, promoting the production of excitotoxic NMDAR agonist quinolinic acid, and thus potentially increase excitability. Similar findings were found after chronic stress exposure. Fuertig et al. (2016) demonstrated that chronic social defeat results in higher kynurenine levels in both blood and brain (specifically in memory‐related structures such as amygdala and hippocampus), related to an increase in freezing behavior during fear conditioning. On the other hand, IDO inhibition did reduce chronic social defeat‐induced fear memory such that the extent of fear expression got close to that of non‐stressed animals. In concordance, Réus et al. (2019) demonstrated that maternal deprivation causes a decrease in mRNA Ido expression in the hippocampus at PND10. In conclusion, kynurenine metabolites—for whose production microglia is key—are overexpressed after stress exposure and might affect memory processing via altered glutamate transmission.
5. CONCLUDING REMARKS AND OUTSTANDING QUESTIONS
In the 90s, a novel concept was proposed: the tripartite synapse (Araque et al., 1999), in which, in addition to the information flow between the pre‐ and postsynaptic neurons, astrocytes regulate synaptic transmission between both elements, sensing the extracellular space surrounding synapses and releasing gliotransmitters or recycling neurotransmitters in response to synaptic activity (González‐Arias & Perea, 2019; Perea et al., 2009). In 2013, Schafer and collaborators (Schafer et al., 2013) increased the complexity of this concept for the synapse by adding a new player in this game: microglia. The 'quad‐partite synapse', as it was called, highlights the importance of microglia in the brain and their close interaction with synapses. In this review, we have summarized how microglia (with a focus on hippocampal microglia) are affected by stress, how they regulate synapses and memory formation, and whether/how they may play a role in stress‐effects on learning and memory. It is important to mention that GC and NE secretion upon stress may also directly affect astrocytes in the context of memory (Pearson‐Leary et al., 2016) and that microglia might also be influencing neuronal function, and thus learning and memory, in an indirect manner by first affecting astrocyte function. This crosstalk may be mediated via the release of growth factors, neurotransmitters and gliotransmitters, cytokines, and chemokines (Jha et al., 2019; Matejuk & Ransohoff, 2020). Some of those, selectively expressed by microglia following stress, can indeed modulate astrocyte function, thereby impairing synaptic activity and neuronal function (Bezzi et al., 2001; Liddelow et al., 2017).
In summary, literature suggests that stress induces (1) an increase in microglial numbers as well as (2) a shift toward a pro‐inflammatory profile. These microglia have (3) an impaired crosstalk with neurons, and (4) show a downregulation of their glutamate signaling. Moreover, microglial immune responses after stress (5) alter the kynurenine pathway through metabolites that again alter glutamatergic transmission (Figure 2). Yet, a number of outstanding questions remain to be addressed.
FIGURE 2.
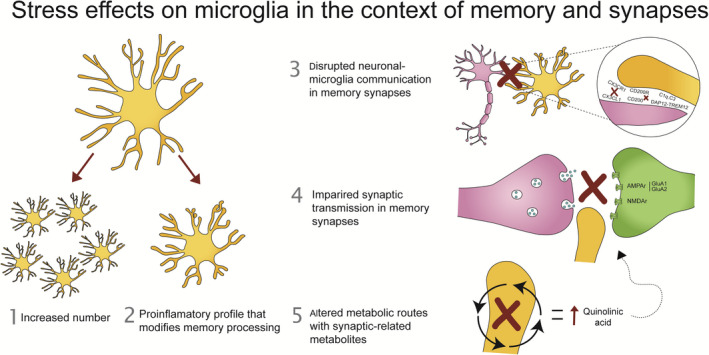
Association between stress, memory and microglia. Stressful situations can alter the microglia, leading to an (1) increase in number and a (2) shift towards a more proinflammatory profile, that is related to disruptions in memory processing. These microglia are (2) not properly communicating with neurons, which underlie memory malfunction/deficits, and could be mediated by (3) synaptic transmission, and (4) metabolic routes with synaptic‐related metabolites
5.1. Do stress hormones alter learning and memory via microglia?
The information collected in this review points toward the possibility that microglia might be playing a determinant role in stressful memories, although the underlying mechanisms are still unknown. As microglia are known to express receptors of both main stress hormones ‐ GCs and NE (Mori et al., 2002; Sierra et al., 2008; Tanaka et al., 1997) ‐ animal models lacking these receptors specifically in microglia (Carrillo‐de Sauvage et al., 2013) will be critical to understanding how these hormones/transmitters affect synaptic structure and function, and memory processes such as consolidation, retrieval, and extinction, through modulation of microglial function. As stress‐effects on both microglia and memory seem to depend ‐ among others ‐ on the duration of exposure, it will be important to understand whether/how microglia are involved in effects of acute versus chronic stress exposure on memory formation.
5.2. Are microglia involved in sex differences in stress‐effects on learning and memory?
Effects of (acute and chronic) stressors on memory are often found to be sex dependent (Bowman et al., 2009; Foy et al., 1999; Vouimba et al., 2000; Warren et al., 1995; Woolley & McEwen, 1992, 1993). A potential role for microglia will be particularly relevant given that (a) several microglial processes, such as maturation, have been reported to be sex dependent (Thion et al., 2018), (b) microglia are affected by stress in a sex‐specific manner (Bollinger et al., 2017), specifically in corticolimbic regions, and (c) microglia express receptors not only for stress hormones—which in the case of GR is more abundant in females (Sierra et al., 2008)—but also for estrogen and progesterone (Sierra et al., 2008),. Therefore, sex steroids may exert a modulatory effect on stress‐induced microglial cell activation and memory. In fact, some of the molecular properties of microglia in the hippocampus are also differentially modified by sex including gene expression of several neuronal crosstalk markers and cytokines that are also crucial for memory (Bollinger et al., 2017). This might be crucial to understand the sex differential vulnerability to some memory‐related stress conditions, such as post‐traumatic stress disorder (PTSD; Shansky, 2015).
5.3. Role of microglia in effects of early life stress on learning and memory
Stress early in life is widely known to affect memory and synapses (e.g. Brunson et al., 2005; Lesuis et al., 2019; Liu et al., 2000; Naninck et al., 2015; Oomen et al., 2010). Evidence suggests that these effects in this specific time window may as well be mediated by microglia. Shortly after early life adversity, coverage of microglia appears to be reduced, while later in life the coverage is enhanced (Hoeijmakers et al., 2017; Réus et al., 2019, but see also Delpech et al., 2016 and Roque et al., 2016). As microglia are involved in synapse pruning (Paolicelli et al., 2011) and developmental apoptosis (Wakselman et al., 2008) in the hippocampus during the neonatal period, while also contributing to neurogenesis and neuroprotection in the developing brain (Cowan & Petri, 2018; Rodríguez‐Iglesias et al., 2019; Sierra et al., 2010), alterations in microglia function may have long‐lasting consequences for brain structure, neuronal function and memory over the lifespan. In line with this, it has been reported that transient depletion of microglia from PND0 to PND14 is accompanied by later working memory deficits and an altered expression of fear, anxiety‐like and risk‐assessment behaviors (VanRyzin et al., 2016). It will therefore be important to investigate in detail the relationship among early life experiences, microglia, and the sensitivity to stress and memory later in life (Desplats et al., 2019; Weber et al., 2019).
6. CONCLUSION
Microglia are highly sensitive to stress and stress hormones. Here, we review that alterations in microglia due to stressors might be affecting synapses, thereby causing impairments in learning and memory. In the upcoming years, microglia might become a pharmacologically relevant cellular target to prevent stress‐related memory disorders but more in‐depth research in the mechanisms involved is required.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
JSG, JCB, OA, CPF, PJL, BJLE, SLL, OCM, and HJK drafted the paper.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15188.
ACKNOWLEDGMENTS
This research was supported by a personal grant from Fundación Mutua Madrileña to JSG.
Sanguino‐Gómez J, Buurstede JC, Abiega O, et al. An emerging role for microglia in stress‐effects on memory. Eur J Neurosci. 2022;55(9):2491–2518. 10.1111/ejn.15188
Edited by: Mathias Schmidt
Contributor Information
Jeniffer Sanguino‐Gómez, Email: j.sanguinogomez@uva.nl.
Harm J. Krugers, Email: h.krugers@uva.nl.
DATA AVAILABILITY STATEMENT
This review does not contain data.
REFERENCES
- Alcocer‐Gómez, E. , Ulecia‐Morón, C. , Marín‐Aguilar, F. , Rybkina, T. , Casas‐Barquero, N. , Ruiz‐Cabello, J. , Ryffel, B. , Apetoh, L. , Ghiringhelli, F. , Bullón, P. , Sánchez‐Alcazar, J. A. , Carrión, A. M. , & Cordero, M. D. (2016). Stress‐induced depressive behaviors require a functional NLRP3 inflammasome. Molecular Neurobiology, 53(7), 4874–4882. 10.1007/s12035-015-9408-7 [DOI] [PubMed] [Google Scholar]
- Andersson, U. , Yang, H. , & Harris, H. (2018). High‐mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Seminars in Immunology, 38, 40–48. 10.1016/j.smim.2018.02.011 [DOI] [PubMed] [Google Scholar]
- Araque, A. , Parpura, V. , Sanzgiri, R. P. , & Haydon, P. G. (1999). Tripartite synapses: Glia, the unacknowledged partner. Trends in Neurosciences, 22(5), 208–215. 10.1016/S0166-2236(98)01349-6 [DOI] [PubMed] [Google Scholar]
- Archer, T. , Söderberg, U. , Ross, S. B. , & Jonsson, G. (1984). Role of olfactory bulbectomy and DSP4 treatment in avoidance learning in the rat. Behavioral Neuroscience, 98(3), 496. 10.1037/0735-7044.98.3.496 [DOI] [PubMed] [Google Scholar]
- Arp, J. M. , ter Horst, J. P. , Kanatsou, S. , Fernández, G. , Joëls, M. , Krugers, H. J. , & Oitzl, M. S. (2014). Mineralocorticoid receptors guide spatial and stimulus‐response learning in mice. PLoS One, 9(1), e86236.– 10.1371/journal.pone.0086236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atucha, E. , & Roozendaal, B. (2015). The inhibitory avoidance discrimination task to investigate accuracy of memory. Frontiers in Behavioral Neuroscience, 9, 60. 10.3389/fnbeh.2015.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusto‐Oliveira, M. , Arrifano, G. P. , Malva, J. O. , & Crespo‐Lopez, M. E. (2019). Adult hippocampal neurogenesis in different taxonomic groups: Possible functional similarities and striking controversies. Cells, 8(2), 125. 10.3390/cells8020125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital, A. , Goshen, I. , Kamsler, A. , Segal, M. , Iverfeldt, K. , Richter‐Levin, G. , & Yirmiya, R. (2003). Impaired interleukin‐1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus, 13(7), 826–834. 10.1002/hipo.10135 [DOI] [PubMed] [Google Scholar]
- Baalman, K. , Marin, M. A. , Ho, T. S. Y. , Godoy, M. , Cherian, L. , Robertson, C. , & Rasband, M. N. (2015). Axon initial segment–associated microglia. Journal of Neuroscience, 35(5), 2283–2292. 10.1523/JNEUROSCI.3751-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahtiyar, S. , Karaca, K. G. , & Henckens, M. J. A. G. (2020). Norepinephrine and glucocorticoid effects on the brain mechanisms underlying memory accuracy and generalization. Roozendaal B.Mol Cell Neurosci, 108. 103537 [DOI] [PubMed] [Google Scholar]
- Balschun, D. , Wetzel, W. , Del Rey, A. , Pitossi, F. , Schneider, H. , Zuschratter, W. , & Besedovsky, H. O. (2004). Interleukin‐6: A cytokine to forget. The FASEB Journal, 18(14), 1788–1790. 10.1096/fj.04-1625fje [DOI] [PubMed] [Google Scholar]
- Barclay, A. N. , Wright, G. J. , Brooke, G. , & Brown, M. H. (2002). CD200 and membrane protein interactions in the control of myeloid cells. Trends in Immunology, 23(6), 285–290. 10.1016/S1471-4906(02)02223-8 [DOI] [PubMed] [Google Scholar]
- Barres, B. A. (2008). The mystery and magic of glia: A perspective on their roles in health and disease. Neuron, 60, 430–440. 10.1016/j.neuron.2008.10.013 [DOI] [PubMed] [Google Scholar]
- Barrientos, R. M. , Sprunger, D. B. , Campeau, S. , Watkins, L. R. , Rudy, J. W. , & Maier, S. F. (2004). BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL‐1β administration. Journal of Neuroimmunology, 155(1–2), 119–126. 10.1016/j.jneuroim.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Basilico, B. , Pagani, F. , Grimaldi, A. , Cortese, B. , Di Angelantonio, S. , Weinhard, L. , Gross, C. , Limatola, C. , Maggi, L. , & Ragozzino, D. (2019). Microglia shape presynaptic properties at developing glutamatergic synapses. Glia, 67(1), 53–67. 10.1002/glia.23508 [DOI] [PubMed] [Google Scholar]
- Batchelor, P. E. , Liberatore, G. T. , Wong, J. Y. , Porritt, M. J. , Frerichs, F. , Donnan, G. A. , & Howells, D. W. (1999). Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain‐derived neurotrophic factor and glial cell line‐derived neurotrophic factor. Journal of Neuroscience, 19(5), 1708–1716. 10.1523/JNEUROSCI.19-05-01708.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor, P. E. , Porritt, M. J. , Martinello, P. , Parish, C. L. , Liberatore, G. T. , Donnan, G. A. , & Howells, D. W. (2002). Macrophages and microglia produce local trophic gradients that stimulate axonal sprouting toward but not beyond the wound edge. Molecular and Cellular Neuroscience, 21(3), 436–453. 10.1006/mcne.2002.1185 [DOI] [PubMed] [Google Scholar]
- Beattie, E. C. , Stellwagen, D. , Morishita, W. , Bresnahan, J. C. , Ha, B. K. , Von Zastrow, M. , & Malenka, R. C. (2002). Control of synaptic strength by glial TNFα. Science, 295(5563), 2282–2285. [DOI] [PubMed] [Google Scholar]
- Beckers, L. , Ory, D. , Geric, I. , Declercq, L. , Koole, M. , Kassiou, M. , Bormans, G. , & Baes, M. (2018). Increased expression of translocator protein (TSPO) marks pro‐inflammatory microglia but does not predict neurodegeneration. Molecular Imaging and Biology, 20(1), 94–102. 10.1007/s11307-017-1099-1 [DOI] [PubMed] [Google Scholar]
- Belarbi, K. , Jopson, T. , Tweedie, D. , Arellano, C. , Luo, W. , Greig, N. H. , & Rosi, S. (2012). TNF‐α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. Journal of Neuroinflammation, 9(1), 23. 10.1186/1742-2094-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch, E. E. (2018). Locus coeruleus. Cell and Tissue Research, 373(1), 221–232. 10.1007/s00441-017-2649-1 [DOI] [PubMed] [Google Scholar]
- Berger, S. , Wolfer, D. P. , Selbach, O. , Alter, H. , Erdmann, G. , Reichardt, H. M. , Chepkova, A. N. , Welzl, H. , Haas, H. L. , Lipp, H.‐P. , & Schutz, G. (2006). Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proceedings of the National Academy of Sciences of the United States of America, 103(1), 195–200. 10.1073/pnas.0503878102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino, L. , Agasse, F. , Silva, B. , Ferreira, R. , Grade, S. , & Malva, J. O. (2008). Tumor necrosis factor‐α modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells, 26(9), 2361–2371. 10.1634/stemcells.2007-0914 [DOI] [PubMed] [Google Scholar]
- Bezzi, P. , Domercq, M. , Brambilla, L. , Galli, R. , Schols, D. , De Clercq, E. , Vescovi, A. , Bagetta, G. , Kollias, G. , Meldolesi, J. , & Volterra, A. (2001). CXCR4‐activated astrocyte glutamate release via TNFα: Amplification by microglia triggers neurotoxicity. Nature Neuroscience, 4(7), 702–710. 10.1038/89490 [DOI] [PubMed] [Google Scholar]
- Bian, Y. , Pan, Z. , Hou, Z. , Huang, C. , Li, W. , & Zhao, B. (2012). Learning, memory, and glial cell changes following recovery from chronic unpredictable stress. Brain Research Bulletin, 88(5), 471–476. 10.1016/j.brainresbull.2012.04.008 [DOI] [PubMed] [Google Scholar]
- Biber, K. , Neumann, H. , Inoue, K. , & Boddeke, H. W. (2007). Neuronal ‘On’ and ‘Off’ signals control microglia. Trends in Neurosciences, 30(11), 596–602. 10.1016/j.tins.2007.08.007 [DOI] [PubMed] [Google Scholar]
- Bliss, T. V. , & Collingridge, G. L. (1993). A synaptic model of memory: Long‐term potentiation in the hippocampus. Nature, 361(6407), 31–39. [DOI] [PubMed] [Google Scholar]
- Bollinger, J. L. , Collins, K. E. , Patel, R. , & Wellman, C. L. (2017). Behavioral stress alters corticolimbic microglia in a sex‐and brain region‐specific manner. PLoS One, 12(12), e0187631. 10.1371/journal.pone.0187631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, R. E. , Micik, R. , Gautreaux, C. , Fernandez, L. , & Luine, V. N. (2009). Sex‐dependent changes in anxiety, memory, and monoamines following one week of stress. Physiology & Behavior, 97(1), 21–29. 10.1016/j.physbeh.2009.01.012 [DOI] [PubMed] [Google Scholar]
- Braida, D. , Sacerdote, P. , Panerai, A. E. , Bianchi, M. , Aloisi, A. M. , Iosuè, S. , & Sala, M. (2004). Cognitive function in young and adult IL (interleukin)‐6 deficient mice. Behavioral Brain Research, 153(2), 423–429. 10.1016/j.bbr.2003.12.018 [DOI] [PubMed] [Google Scholar]
- Brevet, M. , Kojima, H. , Asakawa, A. , Atsuchi, K. , Ushikai, M. , Ataka, K. , Inui, A. , Kimura, H. , Sevestre, H. , & Fujimiya, M. (2010). Chronic foot‐shock stress potentiates the influx of bone marrow‐derived microglia into hippocampus. Journal of Neuroscience Research, 88(9), 1890–1897. 10.1002/jnr.22362 [DOI] [PubMed] [Google Scholar]
- Brooks, A. K. , Lawson, M. A. , Smith, R. A. , Janda, T. M. , Kelley, K. W. , & McCusker, R. H. (2016). Interactions between inflammatory mediators and corticosteroids regulate transcription of genes within the Kynurenine Pathway in the mouse hippocampus. Journal of Neuroinflammation, 13(1), 98. 10.1186/s12974-016-0563-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson, K. L. , Kramár, E. , Lin, B. , Chen, Y. , Colgin, L. L. , Yanagihara, T. K. , & Baram, T. Z. (2005). Mechanisms of late‐onset cognitive decline after early‐life stress. Journal of Neuroscience, 25(41), 9328–9338. 10.1523/JNEUROSCI.2281-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin, A. , Brooks, D. J. , Kennedy, A. M. , Gunn, R. N. , Myers, R. , Turkheimer, F. E. , & Banati, R. B. (2001). In‐vivo measurement of activated microglia in dementia. The Lancet, 358(9280), 461–467. [DOI] [PubMed] [Google Scholar]
- Cahill, L. , Prins, B. , Weber, M. , & McGaugh, J. L. (1994). β‐Adrenergic activation and memory for emotional events. Nature, 371(6499), 702–704. [DOI] [PubMed] [Google Scholar]
- Carrillo‐de Sauvage, M. á. , Maatouk, L. , Arnoux, I. , Pasco, M. , Sanz Diez, A. , Delahaye, M. , Herrero, M. T. , Newman, T. A. , Calvo, C. F. , Audinat, E. , Tronche, F. , & Vyas, S. (2013). Potent and multiple regulatory actions of microglial glucocorticoid receptors during CNS inflammation. Cell Death & Differentiation, 20(11), 1546–1557. 10.1038/cdd.2013.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catale, C. , Gironda, S. , Iacono, L. L. , & Carola, V. (2020). Microglial function in the effects of early‐life stress on brain and behavioral development. Journal of Clinical Medicine, 9(2), 468. 10.3390/jcm9020468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaya, N. , Jacques, A. , Belmer, A. , Beecher, K. , Ali, S. A. , Chehrehasa, F. , Battle, A. R. , Johnson, L. R. , & Bartlett, S. E. (2019). Contextual fear conditioning alter microglia number and morphology in the rat dorsal hippocampus. Frontiers in Cellular Neuroscience, 13, 214. 10.3389/fncel.2019.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamak, B. , Dobbertin, A. , & Mallat, M. (1995). Immunohistochemical detection of thrombospondin in microglia in the developing rat brain. Neuroscience, 69(1), 177–187. 10.1016/0306-4522(95)00236-C [DOI] [PubMed] [Google Scholar]
- Chamak, B. , Morandi, V. , & Mallat, M. (1994). Brain macrophages stimulate neurite growth and regeneration by secreting thrombospondin. Journal of Neuroscience Research, 38(2), 221–233. 10.1002/jnr.490380213 [DOI] [PubMed] [Google Scholar]
- Chen, D. Y. , Bambah‐Mukku, D. , Pollonini, G. , & Alberini, C. M. (2012). Glucocorticoid receptors recruit the CaMKIIa‐BDNF‐CREB pathways to mediate memory consolidation. Nature Neuroscience, 15, 1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Jalabi, W. , Hu, W. , Park, H.‐J. , Gale, J. T. , Kidd, G. J. , Bernatowicz, R. , Gossman, Z. C. , Chen, J. T. , Dutta, R. , & Trapp, B. D. (2014). Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nature Communications, 5(1), 1–12. 10.1038/ncomms5486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, K. C. , Josephson, A. , Benusa, S. D. , Hartley, R. K. , Baer, M. , Thummala, S. , Joslyn, M. , Sword, B. A. , Elford, H. , Oh, U. , Dilsizoglu‐Senol, A. , Lubetzki, C. , Davenne, M. , DeVries, G. H. , & Dupree, J. L. (2016). Compromised axon initial segment integrity in EAE is preceded by microglial reactivity and contact. Glia, 64(7), 1190–1209. 10.1002/glia.22991 [DOI] [PubMed] [Google Scholar]
- Cohen, S. , Ke, X. , Liu, Q. , Fu, Q. , Majnik, A. , & Lane, R. (2016). Adverse early life environment increases hippocampal microglia abundance in conjunction with decreased neural stem cells in juvenile mice. International Journal of Developmental Neuroscience, 55, 56–65. 10.1016/j.ijdevneu.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Colucci, P. , Mancini, G. F. , Santori, A. , Zwergel, C. , Mai, A. , Trezza, V. , Roozendaal, B. , & Campolongo, P. (2019). Amphetamine and the smart drug 3, 4‐methylenedioxypyrovalerone (MDPV) induce generalization of fear memory in rats. Frontiers in Molecular Neuroscience, 12, 292. 10.3389/fnmol.2019.00292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, C. D. , Galea, L. A. , Kuroda, Y. , & McEwen, B. S. (1996). Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine treatment. Behavioral Neuroscience, 110(6), 1321. 10.1037/0735-7044.110.6.1321 [DOI] [PubMed] [Google Scholar]
- Cooke, S. F. , & Bliss, T. V. P. (2006). Plasticity in the human central nervous system. Brain, 129(7), 1659–1673. 10.1093/brain/awl082 [DOI] [PubMed] [Google Scholar]
- Costa‐Miserachs, D. , Portell‐Cortés, I. , Aldavert‐Vera, L. , Torras‐García, M. , & Morgado‐Bernal, I. (1994). Long‐term memory facilitation in rats by posttraining epinephrine. Behavioral Neuroscience, 108(3), 469. 10.1037/0735-7044.108.3.469 [DOI] [PubMed] [Google Scholar]
- Costello, D. A. , Lyons, A. , Denieffe, S. , Browne, T. C. , Cox, F. F. , & Lynch, M. A. (2011). Long term potentiation is impaired in membrane glycoprotein CD200‐deficient mice a role for toll‐like receptor activation. Journal of Biological Chemistry, 286(40), 34722–34732. 10.1074/jbc.M111.280826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan, M. , & Petri, W. A. Jr (2018). Microglia: Immune regulators of neurodevelopment. Frontiers in Immunology, 9, 2576. 10.3389/fimmu.2018.02576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, A. J. , Murray, C. A. , O'Neill, L. , Lynch, M. A. , & O'Connor, J. J. (1996). Interleukin‐1β (IL‐1β) and tumour necrosis factor (TNF) inhibit long‐term potentiation in the rat dentate gyrus in vitro. Neuroscience Letters, 203(1), 17–20. 10.1016/0304-3940(95)12252-4 [DOI] [PubMed] [Google Scholar]
- Dalmau, I. , Finsen, B. , Zimmer, J. , González, B. , & Castellano, B. (1998). Development of microglia in the postnatal rat hippocampus. Hippocampus, 8(5), 458–474. [DOI] [PubMed] [Google Scholar]
- De Kloet, E. R. , Joëls, M. , & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6(6), 463–475. 10.1038/nrn1683 [DOI] [PubMed] [Google Scholar]
- De Kloet, E. R. , & Meijer, O. C. (2019). MR/GR signaling in the brain during the stress response. Aldosterone‐mineralocorticoid receptor‐cell biology to translational medicine. IntechOpen. [Google Scholar]
- De Kloet, E. R. , Meijer, O. C. , de Nicola, A. F. , de Rijk, R. H. , & Joëls, M. (2018). Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro‐inflammation. Frontiers in Neuroendocrinology, 49, 124–145. 10.1016/j.yfrne.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Del Rey, A. , Balschun, D. , Wetzel, W. , Randolf, A. , & Besedovsky, H. O. (2013). A cytokine network involving brain‐borne IL‐1β, IL‐1ra, IL‐18, IL‐6, and TNFα operates during long‐term potentiation and learning. Brain, Behavior, and Immunity, 33, 15–23. 10.1016/j.bbi.2013.05.011 [DOI] [PubMed] [Google Scholar]
- Delpech, J. C. , Wei, L. , Hao, J. , Yu, X. , Madore, C. , Butovsky, O. , & Kaffman, A. (2016). Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain, Behavior, and Immunity, 57, 79–93. 10.1016/j.bbi.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats, P. , Gutierrez, A. M. , Antonelli, M. C. , & Frasch, M. G. (2019). Microglial memory of early life stress and inflammation: Susceptibility to neurodegeneration in adulthood. Neuroscience & Biobehavioral Reviews, 117, 232–242. 10.1016/j.neubiorev.2019.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz‐Aparicio, I. , Paris, I. , Sierra‐Torre, V. , Plaza‐Zabala, A. , Rodríguez‐Iglesias, N. , Márquez‐Ropero, M. , Beccari, S. , Huguet, P. , Abiega, O. , Alberdi, E. , Matute, C. , Bernales, I. , Schulz, A. , Otrokocsi, L. , Sperlagh, B. , Happonen, K. E. , Lemke, G. , Maletic‐Savatic, M. , Valero, J. , & Sierra, A. (2020). Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. Journal of Neuroscience, 40(7), 1453–1482. 10.1523/JNEUROSCI.0993-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz‐Chaves, Y. , Astiz, M. , Bellini, M. J. , & Garcia‐Segura, L. M. (2013). Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain, Behavior, and Immunity, 28, 196–206. 10.1016/j.bbi.2012.11.013 [DOI] [PubMed] [Google Scholar]
- Diz‐Chaves, Y. , Pernía, O. , Carrero, P. , & Garcia‐Segura, L. M. (2012). Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. Journal of Neuroinflammation, 9(1), 71. 10.1186/1742-2094-9-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donley, M. P. , Schulkin, J. , & Rosen, J. B. (2005). Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long‐term memory of contextual fear. Behavioral Brain Research, 164(2), 197–205. 10.1016/j.bbr.2005.06.020 [DOI] [PubMed] [Google Scholar]
- Donzis, E. J. , & Tronson, N. C. (2014). Modulation of learning and memory by cytokines: Signaling mechanisms and long term consequences. Neurobiology of Learning and Memory, 115, 68–77. 10.1016/j.nlm.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorn, K. J. , Brevé, J. J. , Drukarch, B. , Boddeke, H. W. , Huitinga, I. , Lucassen, P. J. , & van Dam, A. M. (2015). Brain region‐specific gene expression profiles in freshly isolated rat microglia. Frontiers in Cellular Neuroscience, 9, 84. 10.3389/fncel.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenweber, F. , Medina‐Luque, J. , Blume, T. , Sacher, C. , Biechele, G. , Wind, K. , Deussing, M. , Briel, N. , Lindner, S. , Boening, G. , von Ungern‐Sternberg, B. , Unterrainer, M. , Albert, N. L. , Zwergal, A. , Levin, J. , Bartenstein, P. , Cumming, P. , Rominger, A. , Höglinger, G. U. , … Brendel, M. (2020). Longitudinal TSPO expression in tau transgenic P301S mice predicts increased tau accumulation and deteriorated spatial learning. Journal of Neuroinflammation, 17(1), 1–12. 10.1186/s12974-020-01883-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Z. , Brooks, D. J. , Okello, A. , & Edison, P. (2017). An early and late peak in microglial activation in Alzheimer's disease trajectory. Brain, 140(3), 792–803. 10.1093/brain/aww349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Färber, K. , Pannasch, U. , & Kettenmann, H. (2005). Dopamine and noradrenaline control distinct functions in rodent microglial cells. Molecular and Cellular Neuroscience, 29(1), 128–138. 10.1016/j.mcn.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Feng, D. , Huang, A. , Yan, W. , & Chen, D. (2019). CD200 dysfunction in neuron contributes to synaptic deficits and cognitive impairment. Biochemical and Biophysical Research Communications, 516(4), 1053–1059. 10.1016/j.bbrc.2019.06.134 [DOI] [PubMed] [Google Scholar]
- Feng, X. , Zhao, Y. , Yang, T. , Song, M. , Wang, C. , Yao, Y. , & Fan, H. (2019). Glucocorticoid‐driven NLRP3 inflammasome activation in hippocampal microglia mediates chronic stress‐induced depressive‐like behaviors. Frontiers in Molecular Neuroscience, 12, 210. 10.3389/fnmol.2019.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipello, F. , Morini, R. , Corradini, I. , Zerbi, V. , Canzi, A. , Michalski, B. , Erreni, M. , Markicevic, M. , Starvaggi‐Cucuzza, C. , Otero, K. , Piccio, L. , Cignarella, F. , Perrucci, F. , Tamborini, M. , Genua, M. , Rajendran, L. , Menna, E. , Vetrano, S. , Fahnestock, M. , … Matteoli, M. (2018). The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity, 48(5), 979–991. 10.1016/j.immuni.2018.04.016 [DOI] [PubMed] [Google Scholar]
- Fonken, L. K. , Weber, M. D. , Daut, R. A. , Kitt, M. M. , Frank, M. G. , Watkins, L. R. , & Maier, S. F. (2016). Stress‐induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology, 66, 82–90. 10.1016/j.psyneuen.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy, M. R. , Xu, J. , Xie, X. , Brinton, R. D. , Thompson, R. F. , & Berger, T. W. (1999). 17β‐estradiol enhances NMDA receptor‐mediated EPSPs and long‐term potentiation. Journal of Neurophysiology, 81(2), 925–929. 10.1152/jn.1999.81.2.925 [DOI] [PubMed] [Google Scholar]
- Frank, M. G. , Fonken, L. K. , Annis, J. L. , Watkins, L. R. , & Maier, S. F. (2018). Stress disinhibits microglia via down‐regulation of CD200R: A mechanism of neuroinflammatory priming. Brain, Behavior, and Immunity, 69, 62–73. 10.1016/j.bbi.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, M. G. , Hershman, S. A. , Weber, M. D. , Watkins, L. R. , & Maier, S. F. (2014). Chronic exposure to exogenous glucocorticoids primes microglia to pro‐inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology, 40, 191–200. 10.1016/j.psyneuen.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, M. G. , Thompson, B. M. , Watkins, L. R. , & Maier, S. F. (2012). Glucocorticoids mediate stress‐induced priming of microglial pro‐inflammatory responses. Brain, Behavior, and Immunity, 26(2), 337–345. 10.1016/j.bbi.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, M. G. , Weber, M. D. , Watkins, L. R. , & Maier, S. F. (2015). Stress sounds the alarmin: The role of the danger‐associated molecular pattern HMGB1 in stress‐induced neuroinflammatory priming. Brain, Behavior, and Immunity, 48, 1–7. 10.1016/j.bbi.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, J. L. , & Schafer, D. P. (2016). Microglia: Architects of the developing nervous system. Trends in Cell Biology, 26(8), 587–597. 10.1016/j.tcb.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertig, R. , Azzinnari, D. , Bergamini, G. , Cathomas, F. , Sigrist, H. , Seifritz, E. , & Pryce, C. R. (2016). Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behavior: Both effects are reversed by inhibition of indoleamine 2, 3‐dioxygenase. Brain, Behavior, and Immunity, 54, 59–72. [DOI] [PubMed] [Google Scholar]
- Furukawa, K. , & Mattson, M. P. (1998). The transcription factor NF‐κB mediates increases in calcium currents and decreases in NMDA‐and AMPA/kainate‐induced currents induced by tumor necrosis factor‐α in hippocampal neurons. Journal of Neurochemistry, 70(5), 1876–1886. 10.1046/j.1471-4159.1998.70051876.x [DOI] [PubMed] [Google Scholar]
- Garrido‐Mesa, N. , Zarzuelo, A. , & Gálvez, J. (2013). Minocycline: Far beyond an antibiotic. British Journal of Pharmacology, 169(2), 337–352. 10.1111/bph.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits E., Brouwer N., Kooistra S. M., Woodbury M. E., Vermeiren Y., Lambourne M., Mulder J., Kummer M., Möller T., Biber K., den Dunnen W. F. A., De Deyn P. P., Eggen B. J. L., & Boddeke E. W. G. M. (2021). Distinct amyloid‐β and tau‐associated microglia profiles in Alzheimer’s disease. Acta Neuropathologica, 10.1007/s00401-021-02263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giridharan, V. V. , Réus, G. Z. , Selvaraj, S. , Scaini, G. , Barichello, T. , & Quevedo, J. (2019). Maternal deprivation increases microglial activation and neuroinflammatory markers in the prefrontal cortex and hippocampus of infant rats. Journal of Psychiatric Research, 115, 13–20. 10.1016/j.jpsychires.2019.05.001 [DOI] [PubMed] [Google Scholar]
- Golan, H. , Levav, T. , Mendelsohn, A. , & Huleihel, M. (2004). Involvement of tumor necrosis factor alpha in hippocampal development and function. Cerebral Cortex, 14(1), 97–105. 10.1093/cercor/bhg108 [DOI] [PubMed] [Google Scholar]
- Gold, P. E. , & Van Buskirk, R. B. (1975). Facilitation of time‐dependent memory processes with posttrial epinephrine injections. Behavioral Biology, 13(2), 145–153. 10.1016/S0091-6773(75)91784-8 [DOI] [PubMed] [Google Scholar]
- Gold, P. E. , & Van Buskirk, R. (1978). Effects of α‐and β‐adrenergic receptor antagonists on post‐trial epinephrine modulation of memory: Relationship to post‐training brain (nor)epinephrine concentrations. Behavioral Biology, 24(2), 168–184. 10.1016/S0091-6773(78)93045-6 [DOI] [PubMed] [Google Scholar]
- Gong, Y. U. , Tong, L. , Yang, R. , Hu, W. , Xu, X. , Wang, W. , Wang, P. , Lu, X. U. , Gao, M. , Wu, Y. , Xu, X. , Zhang, Y. , Chen, Z. , & Huang, C. (2018). Dynamic changes in hippocampal microglia contribute to depressive‐like behavior induced by early social isolation. Neuropharmacology, 135, 223–233. 10.1016/j.neuropharm.2018.03.023 [DOI] [PubMed] [Google Scholar]
- González‐Arias, C. , & Perea, G. (2019). Gliotransmission at tripartite synapses. Computational Glioscience, 213–226. [Google Scholar]
- González‐Prieto, M. , Gutiérrez, I. L. , García‐Bueno, B. , Caso, J. R. , Leza, J. C. , Ortega‐Hernández, A. , & Madrigal, J. L. (2020). Microglial CX3CR1 production increases in Alzheimer's disease and is regulated by noradrenaline. Glia, 69(1):73‐90. [DOI] [PubMed] [Google Scholar]
- Goshen, I. , Kreisel, T. , Ounallah‐Saad, H. , Renbaum, P. , Zalzstein, Y. , Ben‐Hur, T. , Levy‐Lahad, E. , & Yirmiya, R. (2007). A dual role for interleukin‐1 in hippocampal‐dependent memory processes. Psychoneuroendocrinology, 32(8–10), 1106–1115. 10.1016/j.psyneuen.2007.09.004 [DOI] [PubMed] [Google Scholar]
- Graeber, M. B. , & Streit, W. J. (2010). Microglia: Biology and pathology. Acta Neuropathologica, 119(1), 89–105. 10.1007/s00401-009-0622-0 [DOI] [PubMed] [Google Scholar]
- Grassivaro, F. , Menon, R. , Acquaviva, M. , Ottoboni, L. , Ruffini, F. , Bergamaschi, A. , Muzio, L. , Farina, C. , & Martino, G. (2020). Convergence between microglia and peripheral macrophages phenotype during development and neuroinflammation. Journal of Neuroscience, 40(4), 784–795. 10.1523/JNEUROSCI.1523-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter, M. , Lelios, I. , & Croxford, A. L. (2015). Microglia versus myeloid cell nomenclature during brain inflammation. Frontiers in Immunology, 6, 249. 10.3389/fimmu.2015.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc, L. , Choquet, D. , & Chaouloff, F. (2008). The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nature Neuroscience, 11(8), 868–870. [DOI] [PubMed] [Google Scholar]
- Gunner, G. , Cheadle, L. , Johnson, K. M. , Ayata, P. , Badimon, A. , Mondo, E. , Nagy, M. A. , Liu, L. , Bemiller, S. M. , Kim, K.‐W. , Lira, S. A. , Lamb, B. T. , Tapper, A. R. , Ransohoff, R. M. , Greenberg, M. E. , Schaefer, A. , & Schafer, D. P. (2019). Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nature Neuroscience, 22(7), 1075. 10.1038/s41593-019-0419-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin, A. , Kirchmeyer, M. , Koncina, E. , Felten, P. , Losciuto, S. , Heurtaux, T. , Tardivel, A. , Heuschling, P. , & Dostert, C. (2015). NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS One, 10(6), e0130624. 10.1371/journal.pone.0130624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, K. M. , Nguyen, T. , & Gall, C. M. (1995). Insulin‐like growth factor‐1 mRNA is increased in deafferented hippocampus: Spatiotemporal correspondence of a trophic event with axon sprouting. Journal of Comparative Neurology, 352(1), 147–160. 10.1002/cne.903520111 [DOI] [PubMed] [Google Scholar]
- Gyoneva, S. , & Traynelis, S. F. (2013). Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. Journal of Biological Chemistry, 288(21), 15291–15302. 10.1074/jbc.M113.458901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin, L. , Lagarde, J. , Dorothée, G. , Leroy, C. , Labit, M. , & Comley, R. A. ; Clinical IMABio3 team . (2016). Early and protective microglial activation in Alzheimer's disease: A prospective study using 18 F‐DPA‐714 PET imaging. Brain, 139(4), 1252–1264. [DOI] [PubMed] [Google Scholar]
- Han, Y. , Zhang, L. , Wang, Q. , Zhang, D. , Zhao, Q. , Zhang, J. , Xie, L. , Liu, G. , & You, Z. (2019). Minocycline inhibits microglial activation and alleviates depressive‐like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology, 107, 37–45. 10.1016/j.psyneuen.2019.04.021 [DOI] [PubMed] [Google Scholar]
- Harrison, J. K. , Jiang, Y. , Chen, S. , Xia, Y. , Maciejewski, D. , McNamara, R. K. , Streit, W. J. , Salafranca, M. N. , Adhikari, S. , Thompson, D. A. , Botti, P. , Bacon, K. B. , & Feng, L. (1998). Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1‐expressing microglia. Proceedings of the National Academy of Sciences of the United States of America, 95(18), 10896–10901. 10.1073/pnas.95.18.10896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori, K. , Nagai, A. , Heisel, R. , Ryu, J. K. , & Kim, S. U. (2002). Fractalkine and fractalkine receptors in human neurons and glial cells. Journal of Neuroscience Research, 69(3), 418–426. 10.1002/jnr.10304 [DOI] [PubMed] [Google Scholar]
- Hayashi, Y. , Ishibashi, H. , Hashimoto, K. , & Nakanishi, H. (2006). Potentiation of the NMDA receptor‐mediated responses through the activation of the glycine site by microglia secreting soluble factors. Glia, 53(6), 660–668. 10.1002/glia.20322 [DOI] [PubMed] [Google Scholar]
- Hayashi‐Takagi, A. , Yagishita, S. , Nakamura, M. , Shirai, F. , Wu, Y. I. , Loshbaugh, A. L. , & Kasai, H. (2015). Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature, 525(7569), 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka, M. T. , Kummer, M. P. , Stutz, A. , Delekate, A. , Schwartz, S. , Vieira‐Saecker, A. , & Gelpi, E. (2013). NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature, 493(7434), 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka, M. T. , Nadrigny, F. , Regen, T. , Martinez‐Hernandez, A. , Dumitrescu‐Ozimek, L. , Terwel, D. , Jardanhazi‐Kurutz, D. , Walter, J. , Kirchhoff, F. , Hanisch, U.‐K. , & Kummer, M. P. (2010). Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine. Proceedings of the National Academy of Sciences of the United States of America, 107(13), 6058–6063. 10.1073/pnas.0909586107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans, E. J. , Henckens, M. J. , Joëls, M. , & Fernández, G. (2014). Dynamic adaptation of large‐scale brain networks in response to acute stressors. Trends in Neurosciences, 37(6), 304–314. 10.1016/j.tins.2014.03.006 [DOI] [PubMed] [Google Scholar]
- Hernangómez, M. , Mestre, L. , Correa, F. G. , Loría, F. , Mecha, M. , Iñigo, P. M. , Docagne, F. , Williams, R. O. , Borrell, J. , & Guaza, C. (2012). CD200‐CD200R1 interaction contributes to neuroprotective effects of anandamide on experimentally induced inflammation. Glia, 60(9), 1437–1450. 10.1002/glia.22366 [DOI] [PubMed] [Google Scholar]
- Heyser, C. J. , Masliah, E. , Samimi, A. , Campbell, I. L. , & Gold, L. H. (1997). Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proceedings of the National Academy of Sciences of the United States of America, 94(4), 1500–1505. 10.1073/pnas.94.4.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers, L. , Ruigrok, S. R. , Amelianchik, A. , Ivan, D. , van Dam, A. M. , Lucassen, P. J. , & Korosi, A. (2017). Early‐life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer's disease mouse model. Brain, Behavior, and Immunity, 63, 160–175. 10.1016/j.bbi.2016.12.023 [DOI] [PubMed] [Google Scholar]
- Hoek, R. M. , Ruuls, S. R. , Murphy, C. A. , Wright, G. J. , Goddard, R. , Zurawski, S. M. , & Barclay, A. N. (2000). Down‐regulation of the macrophage lineage through interaction with OX2 (CD200). Science, 290(5497), 1768–1771. [DOI] [PubMed] [Google Scholar]
- Hong, S. , Beja‐Glasser, V. F. , Nfonoyim, B. M. , Frouin, A. , Li, S. , Ramakrishnan, S. , & Lemere, C. A. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science, 352(6286), 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, H. , Real, E. , Takamiya, K. , Kang, M. G. , Ledoux, J. , Huganir, R. L. , & Malinow, R. (2007). Emotion enhances learning via (nor)epinephrine regulation of AMPA‐receptor trafficking. Cell, 131(1), 160–173. 10.1016/j.cell.2007.09.017 [DOI] [PubMed] [Google Scholar]
- Huang, Y. Y. , & Kandel, E. R. (1996). Modulation of both the early and the late phase of mossy fiber LTP by the activation of β‐adrenergic receptors. Neuron, 16(3), 611–617. 10.1016/S0896-6273(00)80080-X [DOI] [PubMed] [Google Scholar]
- Huang, Z. B. , & Sheng, G. Q. (2010). Interleukin‐1β with learning and memory. Neuroscience Bulletin, 26(6), 455–468. 10.1007/s12264-010-6023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui, G. K. , Figueroa, I. R. , Poytress, B. S. , Roozendaal, B. , McGaugh, J. L. , & Weinberger, N. M. (2004). Memory enhancement of classical fear conditioning by post‐training injections of corticosterone in rats. Neurobiology of Learning and Memory, 81(1), 67–74. 10.1016/j.nlm.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Ikegami, A. , Haruwaka, K. , & Wake, H. (2019). Microglia: Lifelong modulator of neural circuits. Neuropathology, 39(3), 173–180. 10.1111/neup.12560 [DOI] [PubMed] [Google Scholar]
- Ito, D. , Imai, Y. , Ohsawa, K. , Nakajima, K. , Fukuuchi, Y. , & Kohsaka, S. (1998). Microglia‐specific localisation of a novel calcium binding protein, Iba1. Molecular Brain Research, 57(1), 1–9. 10.1016/S0169-328X(98)00040-0 [DOI] [PubMed] [Google Scholar]
- Iwata, M. , Ota, K. T. , Li, X.‐Y. , Sakaue, F. , Li, N. , Dutheil, S. , Banasr, M. , Duric, V. , Yamanashi, T. , Kaneko, K. , Rasmussen, K. , Glasebrook, A. , Koester, A. , Song, D. , Jones, K. A. , Zorn, S. , Smagin, G. , & Duman, R. S. (2016). Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biological Psychiatry, 80(1), 12–22. 10.1016/j.biopsych.2015.11.026 [DOI] [PubMed] [Google Scholar]
- Jankowsky, J. L. , Derrick, B. E. , & Patterson, P. H. (2000). Cytokine responses to LTP induction in the rat hippocampus: A comparison of in vitro and in vivo techniques. Learning & Memory, 7(6), 400–412. 10.1101/lm.32600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jȩdrzejewska‐Szmek, J. , Luczak, V. , Abel, T. , & Blackwell, K. T. (2017). β‐adrenergic signaling broadly contributes to LTP induction. PLoS Computational Biology, 13(7), e1005657. 10.1371/journal.pcbi.1005657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha, M. K. , Jo, M. , Kim, J. H. , & Suk, K. (2019). Microglia‐astrocyte crosstalk: An intimate molecular conversation. The Neuroscientist, 25(3), 227–240. 10.1177/1073858418783959 [DOI] [PubMed] [Google Scholar]
- Jo, W. K. , Zhang, Y. , Emrich, H. M. , & Dietrich, D. E. (2015). Glia in the cytokine‐mediated onset of depression: Fine tuning the immune response. Frontiers in Cellular Neuroscience, 9, 268. 10.3389/fncel.2015.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls, M. , & Baram, T. Z. (2009). The neuro‐symphony of stress. Nature Reviews Neuroscience, 10(6), 459–466. 10.1038/nrn2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls, M. , Fernandez, G. , & Roozendaal, B. (2011). Stress and emotional memory: A matter of timing. Trends in Cognitive Sciences, 15(6), 280–288. 10.1016/j.tics.2011.04.004 [DOI] [PubMed] [Google Scholar]
- Joëls, M. , Pu, Z. , Wiegert, O. , Oitzl, M. S. , & Krugers, H. J. (2006). Learning under stress: How does it work? Trends in Cognitive Sciences, 10(4), 152–158. 10.1016/j.tics.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Joëls, M. , Sarabdjitsingh, R. A. , & Karst, H. (2012). Unraveling the time domains of corticosteroid hormone influences on brain activity: Rapid, slow, and chronic modes. Pharmacological Reviews, 64(4), 901–938. 10.1124/pr.112.005892 [DOI] [PubMed] [Google Scholar]
- Joëls, M. , Karst, H. , & Sarabdjitsingh, R. A. (2018). The stressed brain of humans and rodents. Acta Physiol (Oxf), 223(2).e13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, J. D. , Campisi, J. , Sharkey, C. M. , Kennedy, S. L. , Nickerson, M. , Greenwood, B. N. , & Fleshner, M. (2005). Catecholamines mediate stress‐induced increases in peripheral and central inflammatory cytokines. Neuroscience, 135(4), 1295–1307. 10.1016/j.neuroscience.2005.06.090 [DOI] [PubMed] [Google Scholar]
- Johnson, J. D. , Zimomra, Z. R. , & Stewart, L. T. (2013). Beta‐adrenergic receptor activation primes microglia cytokine production. Journal of Neuroimmunology, 254(1–2), 161–164. 10.1016/j.jneuroim.2012.08.007 [DOI] [PubMed] [Google Scholar]
- Karst, H. , Berger, S. , Turiault, M. , Tronche, F. , Schütz, G. , & Joëls, M. (2005). Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proceedings of the National Academy of Sciences of the United States of America, 102(52), 19204–19207. 10.1073/pnas.0507572102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst, H. , & Joëls, M. (2005). Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. Journal of Neurophysiology, 94(5), 3479–3486. 10.1152/jn.00143.2005 [DOI] [PubMed] [Google Scholar]
- Keohane, A. , Ryan, S. , Maloney, E. , Sullivan, A. M. , & Nolan, Y. M. (2010). Tumour necrosis factor‐α impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: Role of Hes1. Molecular and Cellular Neuroscience, 43(1), 127–135. 10.1016/j.mcn.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Kessels, H. W. , & Malinow, R. (2009). Synaptic AMPA receptor plasticity and behavior. Neuron, 61(3), 340–350. 10.1016/j.neuron.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann, H. , Kirchhoff, F. , & Verkhratsky, A. (2013). Microglia: New roles for the synaptic stripper. Neuron, 77(1), 10–18. 10.1016/j.neuron.2012.12.023 [DOI] [PubMed] [Google Scholar]
- Kim, H. J. , Cho, M. H. , Shim, W. H. , Kim, J. K. , Jeon, E. Y. , Kim, D. H. , & Yoon, S. Y. (2017). Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Molecular Psychiatry, 22(11), 1576–1584. 10.1038/mp.2016.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.‐M. , Mun, B.‐R. , Lee, S.‐J. , Joh, Y. , Lee, H.‐Y. , Ji, K.‐Y. , Choi, H.‐R. , Lee, E.‐H. , Kim, E.‐M. , Jang, J.‐H. , Song, H.‐W. , Mook‐Jung, I. , Choi, W.‐S. , & Kang, H.‐S. (2017). TREM2 promotes Aβ phagocytosis by upregulating C/EBPα‐dependent CD36 expression in microglia. Scientific Reports, 7(1), 1–12. 10.1038/s41598-017-11634-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota, T. , Ingraham, K. L. , Swan, R. J. , Jacobsen, M. T. , Andrews, S. J. , & Ikezu, T. (2011). AAV serotype 2/1‐mediated gene delivery of anti‐inflammatory interleukin‐10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Therapy, 19(7), 724–733. 10.1038/gt.2011.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning, N. , Swaab, D. F. , Hoek, R. M. , & Huitinga, I. (2009). Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron‐glia and glia‐glia interactions. Journal of Neuropathology & Experimental Neurology, 68(2), 159–167. 10.1097/NEN.0b013e3181964113 [DOI] [PubMed] [Google Scholar]
- Konishi, H. , & Kiyama, H. (2018). Microglial TREM2/DAP12 signaling: A double‐edged sword in neural diseases. Frontiers in Cellular Neuroscience, 12, 206. 10.3389/fncel.2018.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugers, H. J. , Douma, B. R. , Andringa, G. , Bohus, B. , Korf, J. , & Luiten, P. G. (1997). Exposure to chronic psychosocial stress and corticosterone in the rat: Effects on spatial discrimination learning and hippocampal protein kinase Cγ immunoreactivity. Hippocampus, 7(4), 427–436. [DOI] [PubMed] [Google Scholar]
- Krugers, H. J. , Goltstein, P. M. , Van Der Linden, S. , & Joels, M. (2006). Blockade of glucocorticoid receptors rapidly restores hippocampal CA1 synaptic plasticity after exposure to chronic stress. European Journal of Neuroscience, 23(11), 3051–3055. 10.1111/j.1460-9568.2006.04842.x [DOI] [PubMed] [Google Scholar]
- Krugers, H. J. , Zhou, M. , Joëls, M. , & Kindt, M. (2011). Regulation of excitatory synapses and fearful memories by stress hormones. Frontiers in Behavioral Neuroscience, 5, 62. 10.3389/fnbeh.2011.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, L. J. , Perry, V. H. , Dri, P. , & Gordon, S. (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience, 39(1), 151–170. 10.1016/0306-4522(90)90229-W [DOI] [PubMed] [Google Scholar]
- Lechtenberg, K. J. , Meyer, S. T. , Doyle, J. B. , Peterson, T. C. , & Buckwalter, M. S. (2019). Augmented β2‐adrenergic signaling dampens the neuroinflammatory response following ischemic stroke and increases stroke size. Journal of Neuroinflammation, 16(1), 112. 10.1186/s12974-019-1506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, M. L. , Cooper, H. A. , Maric, D. , & Herkenham, M. (2016). Social defeat induces depressive‐like states and microglial activation without involvement of peripheral macrophages. Journal of Neuroinflammation, 13(1), 224. 10.1186/s12974-016-0672-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, M. L. , Weigel, T. K. , Cooper, H. A. , Elkahloun, A. G. , Kigar, S. L. , & Herkenham, M. (2018). Decoding microglia responses to psychosocial stress reveals blood‐brain barrier breakdown that may drive stress susceptibility. Scientific Reports, 8(1), 1–19. 10.1038/s41598-018-28737-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuis, S. L. , Lucassen, P. J. , & Krugers, H. J. (2019). Early life stress impairs fear memory and synaptic plasticity; a potential role for GluN2B. Neuropharmacology, 149, 195–203. 10.1016/j.neuropharm.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Lesuis, S. L. , Timmermans, W. , Lucassen, P. J. , Hoogenraad, C. C. , & Krugers, H. J. (2020). Glucocorticoid and β‐adrenergic regulation of hippocampal dendritic spines. Journal of Neuroendocrinology, 32(1), e12811. 10.1111/jne.12811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, A. J. , Katafuchi, T. , Oda, S. , Hori, T. , & Oomura, Y. (1997). Interleukin‐6 inhibits long‐term potentiation in rat hippocampal slices. Brain Research, 748(1–2), 30–38. 10.1016/S0006-8993(96)01283-8 [DOI] [PubMed] [Google Scholar]
- Liang, K. C. , Juler, R. G. , & McGaugh, J. L. (1986). Modulating effects of posttraining epinephrine on memory: Involvement of the amygdala noradrenergic system. Brain Research, 368(1), 125–133. 10.1016/0006-8993(86)91049-8 [DOI] [PubMed] [Google Scholar]
- Liddelow, S. A. , Guttenplan, K. A. , Clarke, L. E. , Bennett, F. C. , Bohlen, C. J. , Schirmer, L. , & Barres, B. A. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S.‐H. , Park, E. , You, B. , Jung, Y. , Park, A.‐R. , Park, S. G. , & Lee, J.‐R. (2013). Neuronal synapse formation induced by microglia and interleukin 10. PLoS One, 8(11), e81218. 10.1371/journal.pone.0081218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. , Diorio, J. , Day, J. C. , Francis, D. D. , & Meaney, M. J. (2000). Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience, 3(8), 799–806. 10.1038/77702 [DOI] [PubMed] [Google Scholar]
- Liu, M. , Li, J. , Dai, P. , Zhao, F. , Zheng, G. , Jing, J. , Wang, J. , Luo, W. , & Chen, J. (2015). Microglia activation regulates GluR1 phosphorylation in chronic unpredictable stress‐induced cognitive dysfunction. Stress, 18(1), 96–106. 10.3109/10253890.2014.995085 [DOI] [PubMed] [Google Scholar]
- Liu, W. , Yuen, E. Y. , & Yan, Z. (2010). The stress hormone corticosterone increases synaptic α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors via serum‐and glucocorticoid‐inducible kinase (SGK) regulation of the GDI‐Rab4 complex. Journal of Biological Chemistry, 285(9), 6101–6108. 10.1074/jbc.M109.050229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. U. , Ying, Y. , Li, Y. , Eyo, U. B. , Chen, T. , Zheng, J. , & Wu, L. J. (2019). Neuronal network activity controls microglial process surveillance in awake mice via (nor)epinephrine signaling. Nature Neuroscience, 22, 1771–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Zhou, L.‐J. , Wang, J. , Li, D. , Ren, W.‐J. , Peng, J. , Wei, X. , Xu, T. , Xin, W.‐J. , Pang, R.‐P. , Li, Y.‐Y. , Qin, Z.‐H. , Murugan, M. , Mattson, M. P. , Wu, L.‐J. , & Liu, X.‐G. (2017). TNF‐α differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia‐dependent mechanisms after peripheral nerve injury. Journal of Neuroscience, 37(4), 871–881. 10.1523/JNEUROSCI.2235-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien, S. J. , Maheu, F. , Tu, M. , Fiocco, A. , & Schramek, T. E. (2007). The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition, 65(3), 209–237. 10.1016/j.bandc.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Maddison, D. C. , & Giorgini, F. (2015). The kynurenine pathway and neurodegenerative disease. Seminars in Cell & Developmental Biology, 40, 134–141. 10.1016/j.semcdb.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Maity, S. , Rah, S. , Sonenberg, N. , Gkogkas, C. G. , & Nguyen, P. V. (2015). (nor)epinephrine triggers metaplasticity of LTP by increasing translation of specific mRNAs. Learning & Memory, 22(10), 499–508. 10.1101/lm.039222.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manich, G. , Recasens, M. , Valente, T. , Almolda, B. , González, B. , & Castellano, B. (2019). Role of the CD200‐CD200R axis during homeostasis and neuroinflammation. Neuroscience, 405, 118–136. 10.1016/j.neuroscience.2018.10.030 [DOI] [PubMed] [Google Scholar]
- Martin, S. , Henley, J. M. , Holman, D. , Zhou, M. , Wiegert, O. , van Spronsen, M. , Joëls, M. , Hoogenraad, C. C. , & Krugers, H. J. (2009). Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS One, 4(3), e4714. 10.1371/journal.pone.0004714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcovitch‐Natan, O. , Winter, D. R. , Giladi, A. , Vargas Aguilar, S. , Spinrad, A. , Sarrazin, S. , Ben‐Yehuda, H. , David, E. , Zelada Gonzalez, F. , Perrin, P. , Keren‐Shaul, H. , Gury, M. , Lara‐Astaiso, D. , Thaiss, C. A. , Cohen, M. , Bahar Halpern, K. , Baruch, K. , Deczkowska, A. , Lorenzo‐Vivas, E. , … Amit, I. (2016). Microglia development follows a stepwise program to regulate brain homeostasis. Science, 353(6301), aad8670. 10.1126/science.aad8670 [DOI] [PubMed] [Google Scholar]
- Matejuk, A. , & Ransohoff, R. M. (2020). Crosstalk between astrocytes and microglia: An overview. Frontiers in Immunology, 11, 1416. 10.3389/fimmu.2020.01416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh, J. L. , & Cahill, L. (1997). Interaction of neuromodulatory systems in modulating memory storage. Behavioral Brain Research, 83(1–2), 31–38. 10.1016/S0166-4328(97)86042-1 [DOI] [PubMed] [Google Scholar]
- McKim, D. B. , Niraula, A. , Tarr, A. J. , Wohleb, E. S. , Sheridan, J. F. , & Godbout, J. P. (2016). Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. Journal of Neuroscience, 36(9), 2590–2604. 10.1523/JNEUROSCI.2394-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds, J. R. , Donowho, K. , Abdi, A. , McGaugh, J. L. , Roozendaal, B. , & McIntyre, C. K. (2010). Memory‐enhancing corticosterone treatment increases amygdala (nor)epinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiology of Learning and Memory, 93(3), 312–321. 10.1016/j.nlm.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci, O. , Fatatis, A. , Simen, A. A. , & Miller, R. J. (2000). Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proceedings of the National Academy of Sciences of the United States of America, 97(14), 8075–8080. 10.1073/pnas.090017497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikasova, L. , Xiong, H. , Kerkhofs, A. , Bouchet, D. , Krugers, H. J. , & Groc, L. (2017). Stress hormone rapidly tunes synaptic NMDA receptor through membrane dynamics and mineralocorticoid signaling. Scientific Reports, 7(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milior, G. , Lecours, C. , Samson, L. , Bisht, K. , Poggini, S. , Pagani, F. , Deflorio, C. , Lauro, C. , Alboni, S. , Limatola, C. , Branchi, I. , Tremblay, M.‐E. , & Maggi, L. (2016). Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain, Behavior, and Immunity, 55, 114–125. 10.1016/j.bbi.2015.07.024 [DOI] [PubMed] [Google Scholar]
- Miyamoto, A. , Wake, H. , Ishikawa, A. W. , Eto, K. , Shibata, K. , Murakoshi, H. , Koizumi, S. , Moorhouse, A. J. , Yoshimura, Y. , & Nabekura, J. (2016). Microglia contact induces synapse formation in developing somatosensory cortex. Nature Communications, 7(1), 1–12. 10.1038/ncomms12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinoff, P. B. (1984). a‐ and b adrenergic receptor subtypes properties. Distribution and Regulation. Drugs, 28(Supplement 2), 1–15. [DOI] [PubMed] [Google Scholar]
- Mori, K. , Ozaki, E. , Zhang, B. O. , Yang, L. , Yokoyama, A. , Takeda, I. , Maeda, N. , Sakanaka, M. , & Tanaka, J. (2002). Effects of (nor)epinephrine on rat cultured microglial cells that express α1, α2, β1 and β2 adrenergic receptors. Neuropharmacology, 43(6), 1026–1034. 10.1016/S0028-3908(02)00211-3 [DOI] [PubMed] [Google Scholar]
- Morris, G. P. , Clark, I. A. , Zinn, R. , & Vissel, B. (2013). Microglia: A new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiology of Learning and Memory, 105, 40–53. 10.1016/j.nlm.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Nabavi, S. , Fox, R. , Proulx, C. D. , Lin, J. Y. , Tsien, R. Y. , & Malinow, R. (2014). Engineering a memory with LTD and LTP. Nature, 511(7509), 348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata, K. , Nakajima, K. , Takemoto, N. , Saito, H. , & Kohsaka, S. (1993). Microglia‐derived plasminogen enhances neurite outgrowth from explant cultures of rat brain. International Journal of Developmental Neuroscience, 11(2), 227–237. 10.1016/0736-5748(93)90081-N [DOI] [PubMed] [Google Scholar]
- Nair, A. , & Bonneau, R. H. (2006). Stress‐induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. Journal of Neuroimmunology, 171(1–2), 72–85. 10.1016/j.jneuroim.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Naninck, E. F. , Hoeijmakers, L. , Kakava‐Georgiadou, N. , Meesters, A. , Lazic, S. E. , Lucassen, P. J. , & Korosi, A. (2015). Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus, 25(3), 309–328. 10.1002/hipo.22374 [DOI] [PubMed] [Google Scholar]
- Nguyen, P. T. , Dorman, L. C. , Pan, S. , Vainchtein, I. D. , Han, R. T. , Nakao‐Inoue, H. , Taloma, S. E. , Barron, J. J. , Molofsky, A. B. , Kheirbek, M. A. , & Molofsky, A. V. (2020). Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell, 182(2), 388–403. 10.1016/j.cell.2020.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgidani, M. , Kato, T. A. , Sagata, N. , Hayakawa, K. , Shimokawa, N. , Sato‐Kasai, M. , & Kanba, S. (2016). TNF‐α from hippocampal microglia induces working memory deficits by acute stress in mice. Brain, Behavior, and Immunity, 55, 17–24. 10.1016/j.bbi.2015.08.022 [DOI] [PubMed] [Google Scholar]
- Oitzl, M. S. , & De Kloet, E. R. (1992). Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behavioral Neuroscience, 106(1), 62. 10.1037/0735-7044.106.1.62 [DOI] [PubMed] [Google Scholar]
- Oitzl, M. S. , Reichardt, H. M. , Joëls, M. , & de Kloet, E. R. (2001). Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proceedings of the National Academy of Sciences of the United States of America, 98(22), 12790–12795. 10.1073/pnas.231313998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl, M. S. , Van Oers, H. , Schöbitz, B. , & de Kloet, E. R. (1993). Interleukin‐1β, but not interleukin‐6, impairs spatial navigation learning. Brain Research, 613(1), 160–163. 10.1016/0006-8993(93)90468-3 [DOI] [PubMed] [Google Scholar]
- Okuda, S. , Roozendaal, B. , & McGaugh, J. L. (2004). Glucocorticoid effects on object recognition memory require training‐associated emotional arousal. Proceedings of the National Academy of Sciences of the United States of America, 101(3), 853–858. 10.1073/pnas.0307803100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen, C. A. , Soeters, H. , Audureau, N. , Vermunt, L. , van Hasselt, F. N. , Manders, E. M. M. , Joels, M. , Lucassen, P. J. , & Krugers, H. (2010). Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high‐stress conditions in adulthood. Journal of Neuroscience, 30(19), 6635–6645. 10.1523/JNEUROSCI.0247-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli, R. C. , Bisht, K. , & Tremblay, M. È. (2014). Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Frontiers in Cellular Neuroscience, 8, 129. 10.3389/fncel.2014.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli, R. C. , Bolasco, G. , Pagani, F. , Maggi, L. , Scianni, M. , Panzanelli, P. , & Ragozzino, D. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science, 333(6048), 1456–1458. [DOI] [PubMed] [Google Scholar]
- Paolicelli, R. C. , & Gross, C. T. (2011). Microglia in development: Linking brain wiring to brain environment. Neuron Glia Biology, 7(1), 77–83. 10.1017/S1740925X12000105 [DOI] [PubMed] [Google Scholar]
- Parkhurst, C. N. , Yang, G. , Ninan, I. , Savas, J. N. , Yates, J. R. , Lafaille, J. J. , Hempstead, B. L. , Littman, D. R. , & Gan, W.‐B. (2013). Microglia promote learning‐dependent synapse formation through brain‐derived neurotrophic factor. Cell, 155(7), 1596–1609. 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson‐Leary, J. , Osborne, D. M. , & McNay, E. C. (2016). Role of glia in stress‐induced enhancement and impairment of memory. Frontiers in integrative neuroscience, 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzoli, M. , Losurdo, M. , Paolone, G. , Medelin, M. , Jaupaj, L. , Cisterna, B. , Slanzi, A. , Malatesta, M. , Coco, S. , & Buffelli, M. (2019). Glucocorticoid receptors modulate dendritic spine plasticity and microglia activity in an animal model of Alzheimer's disease. Neurobiology of Disease, 132, 104568. 10.1016/j.nbd.2019.104568 [DOI] [PubMed] [Google Scholar]
- Perea, G. , Navarrete, M. , & Araque, A. (2009). Tripartite synapses: Astrocytes process and control synaptic information. Trends in Neurosciences, 32(8), 421–431. 10.1016/j.tins.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Perkins, M. N. , & Stone, T. W. (1985). Actions of kynurenic acid and quinolinic acid in the rat hippocampus in vivo. Experimental Neurology, 88(3), 570–579. 10.1016/0014-4886(85)90072-X [DOI] [PubMed] [Google Scholar]
- Perry, V. H. , & Holmes, C. (2014). Microglial priming in neurodegenerative disease. Nature Reviews Neurology, 10(4), 217–224. 10.1038/nrneurol.2014.38 [DOI] [PubMed] [Google Scholar]
- Potter, E. D. , Ling, Z. D. , & Carvey, P. M. (1999). Cytokine‐induced conversion of mesencephalic‐derived progenitor cells into dopamine neurons. Cell and Tissue Research, 296(2), 235–246. 10.1007/s004410051285 [DOI] [PubMed] [Google Scholar]
- Prinz, M. , Priller, J. , Sisodia, S. S. , & Ransohoff, R. M. (2011). Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nature Neuroscience, 14(10), 1227. 10.1038/nn.2923 [DOI] [PubMed] [Google Scholar]
- Pu, Z. , Krugers, H. J. , & Joëls, M. (2009). β‐adrenergic facilitation of synaptic plasticity in the rat basolateral amygdala in vitro is gradually reversed by corticosterone. Learning & Memory, 16(2), 155–160. 10.1101/lm.1272409 [DOI] [PubMed] [Google Scholar]
- Pugh, C. R. , Tremblay, D. , Fleshner, M. , & Rudy, J. W. (1997). A selective role for corticosterone in contextual‐fear conditioning. Behavioral Neuroscience, 111(3), 503. 10.1037/0735-7044.111.3.503 [DOI] [PubMed] [Google Scholar]
- Rahman, A. , Rao, M. S. , & Khan, K. M. (2018). Intraventricular infusion of quinolinic acid impairs spatial learning and memory in young rats: A novel mechanism of lead‐induced neurotoxicity. Journal of Neuroinflammation, 15(1), 1–15. 10.1186/s12974-018-1306-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reemst, K. , Noctor, S. C. , Lucassen, P. J. , & Hol, E. M. (2016). The indispensable roles of microglia and astrocytes during brain development. Frontiers in Human Neuroscience, 10, 566. 10.3389/fnhum.2016.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul, J. M. H. M. , & De Kloet, E. R. (1985). Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology, 117(6), 2505–2511. 10.1210/endo-117-6-2505 [DOI] [PubMed] [Google Scholar]
- Réus, G. Z. , Silva, R. H. , de Moura, A. B. , Presa, J. F. , Abelaira, H. M. , Abatti, M. , Vieira, A. , Pescador, B. , Michels, M. , Ignácio, Z. M. , Dal‐Pizzol, F. , & Quevedo, J. (2019). Early maternal deprivation induces microglial activation, alters glial fibrillary acidic protein immunoreactivity and indoleamine 2,3‐dioxygenase during the development of offspring rats. Molecular Neurobiology, 56(2), 1096–1108. 10.1007/s12035-018-1161-2 [DOI] [PubMed] [Google Scholar]
- Revest, J.‐M. , Di Blasi, F. , Kitchener, P. , Rougé‐Pont, F. , Desmedt, A. , Turiault, M. , Tronche, F. , & Piazza, P. V. (2005). The MAPK pathway and Egr‐1 mediate stress‐related behavioral effects of glucocorticoids. Nature Neuroscience, 8(5), 664–672. 10.1038/nn1441 [DOI] [PubMed] [Google Scholar]
- Revest, J.‐M. , Kaouane, N. , Mondin, M. , Le Roux, A. , Rougé‐Pont, F. , Vallée, M. , Barik, J. , Tronche, F. , Desmedt, A. , & Piazza, P. V. (2010). The enhancement of stress‐related memory by glucocorticoids depends on synapsin‐Ia/Ib. Molecular Psychiatry, 15(12), 1140–1151. 10.1038/mp.2010.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest, J.‐M. , Le Roux, A. , Roullot‐Lacarrière, V. , Kaouane, N. , Vallée, M. , Kasanetz, F. , Rougé‐Pont, F. , Tronche, F. , Desmedt, A. , & Piazza, P. V. (2014). BDNF‐TrkB signaling through Erk1/2 MAPK phosphorylation mediates the enhancement of fear memory induced by glucocorticoids. Molecular Psychiatry, 19(9), 1001–1009. 10.1038/mp.2013.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richwine, A. F. , Sparkman, N. L. , Dilger, R. N. , Buchanan, J. B. , & Johnson, R. W. (2009). Cognitive deficits in interleukin‐10‐deficient mice after peripheral injection of lipopolysaccharide. Brain, Behavior, and Immunity, 23(6), 794–802. 10.1016/j.bbi.2009.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, F. R. , Musella, A. , De Vito, F. , Fresegna, D. , Bullitta, S. , Vanni, V. , Centonze, D. (2018). Tumor necrosis factor and interleukin‐1β modulate synaptic plasticity during neuroinflammation. Neural Plasticity, 2018, 8430123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Iglesias, N. , Sierra, A. , & Valero, J. (2019). Rewiring of memory circuits: Connecting adult newborn neurons with the help of microglia. Frontiers in Cell and Developmental Biology, 7, 24. 10.3389/fcell.2019.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, J. T. , Morganti, J. M. , Bachstetter, A. D. , Hudson, C. E. , Peters, M. M. , Grimmig, B. A. , Weeber, E. J. , Bickford, P. C. , & Gemma, C. (2011). CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. Journal of Neuroscience, 31(45), 16241–16250. 10.1523/JNEUROSCI.3667-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal, B. , McEwen, B. S. , & Chattarji, S. (2009). Stress, memory and the amygdala. Nature Reviews Neuroscience, 10(6), 423–433. 10.1038/nrn2651 [DOI] [PubMed] [Google Scholar]
- Roozendaal, B. , & McGaugh, J. L. (1997). Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiology of Learning and Memory, 67(2), 176–179. 10.1006/nlme.1996.3765 [DOI] [PubMed] [Google Scholar]
- Roozendaal, B. , Okuda, S. , Van der Zee, E. A. , & McGaugh, J. L. (2006). Glucocorticoid enhancement of memory requires arousal‐induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America, 103(17), 6741–6746. 10.1073/pnas.0601874103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal, B. , Bohus, B. , & McGaugh, J. L. (1996). Dose‐dependent suppression of adrenocortical activity with metyrapone: Effects on emotion and memory. Psychoneuroendocrinology, 21(8), 681–693. 10.1016/S0306-4530(96)00028-5 [DOI] [PubMed] [Google Scholar]
- Roque, A. , Ochoa‐Zarzosa, A. , & Torner, L. (2016). Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain, Behavior, and Immunity, 55, 39–48. 10.1016/j.bbi.2015.09.017 [DOI] [PubMed] [Google Scholar]
- Ross, F. M. , Allan, S. M. , Rothwell, N. J. , & Verkhratsky, A. (2003). A dual role for interleukin‐1 in LTP in mouse hippocampal slices. Journal of Neuroimmunology, 144(1–2), 61–67. 10.1016/j.jneuroim.2003.08.030 [DOI] [PubMed] [Google Scholar]
- Roumier, A. , Béchade, C. , Poncer, J. C. , Smalla, K. H. , Tomasello, E. , Vivier, E. , & Bessis, A. (2004). Impaired synaptic function in the microglial KARAP/DAP12‐deficient mouse. Journal of Neuroscience, 24(50), 11421–11428. 10.1523/JNEUROSCI.2251-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi, C. , & Rose, S. P. (1994). Corticosteroid receptor antagonists are amnestic for passive avoidance learning in day‐old chicks. European Journal of Neuroscience, 6(8), 1292–1297. 10.1111/j.1460-9568.1994.tb00319.x [DOI] [PubMed] [Google Scholar]
- Sapolsky, R. M. , Romero, L. M. , & Munck, U. (2000). How do glucocorticoids influence stress responses? Preparative actions. Endocrine Reviews, 21(April), 55–89. [DOI] [PubMed] [Google Scholar]
- Sara, S. J. (2009). The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews Neuroscience, 10(3), 211–223. 10.1038/nrn2573 [DOI] [PubMed] [Google Scholar]
- Savitz, J. (2020). The kynurenine pathway: A finger in every pie. Molecular Psychiatry, 25(1), 131–147. 10.1038/s41380-019-0414-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz, J. , Dantzer, R. , Meier, T. B. , Wurfel, B. E. , Victor, T. A. , McIntosh, S. A. , Ford, B. N. , Morris, H. M. , Bodurka, J. , Teague, T. K. , & Drevets, W. C. (2015). Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology, 62, 54–58. 10.1016/j.psyneuen.2015.07.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D. P. , Lehrman, E. K. , Kautzman, A. G. , Koyama, R. , Mardinly, A. R. , Yamasaki, R. , Ransohoff, R. M. , Greenberg, M. E. , Barres, B. A. , & Stevens, B. (2012). Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron, 74(4), 691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D. P. , Lehrman, E. K. , & Stevens, B. (2013). The “quad‐partite” synapse: Microglia‐synapse interactions in the developing and mature CNS. Glia, 61(1), 24–36. 10.1002/glia.22389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, I. , Ahlbrand, R. , Winter, A. , Vollmer, L. , Lewkowich, I. , & Sah, R. (2018). Enhanced fear and altered neuronal activation in forebrain limbic regions of CX3CR1‐deficient mice. Brain, Behavior, and Immunity, 68, 34–43. 10.1016/j.bbi.2017.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe, L. , Tegenthoff, M. , Höffken, O. , & Wolf, O. T. (2013). Mineralocorticoid receptor blockade prevents stress‐induced modulation of multiple memory systems in the human brain. Biological Psychiatry, 74(11), 801–808S. 10.1016/j.biopsych.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Schwabe, L. , Wolf, O. T. , & Oitzl, M. S. (2010). Memory formation under stress: Quantity and quality. Neuroscience & Biobehavioral Reviews, 34(4), 584–591. 10.1016/j.neubiorev.2009.11.015 [DOI] [PubMed] [Google Scholar]
- Schwarcz, R. , Bruno, J. P. , Muchowski, P. J. , & Wu, H. Q. (2012). Kynurenines in the mammalian brain: When physiology meets pathology. Nature Reviews Neuroscience, 13(7), 465–477. 10.1038/nrn3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren, C. M. , Gracias, J. , Watmuff, B. , Biag, J. D. , Thanos, J. M. , Whittredge, P. B. , Fu, T. , Worringer, K. , Brown, H. E. , Wang, J. , Kaykas, A. , Karmacharya, R. , Goold, C. P. , Sheridan, S. D. , & Perlis, R. H. (2019). Increased synapse elimination by microglia in schizophrenia patient‐derived models of synaptic pruning. Nature Neuroscience, 22(3), 374–385. 10.1038/s41593-018-0334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren, C. M. , Sheridan, S. D. , Gracias, J. , Xuan, D. , Fu, T. , & Perlis, R. H. (2017). Patient‐specific models of microglia‐mediated engulfment of synapses and neural progenitors. Molecular Psychiatry, 22(2), 170–177. 10.1038/mp.2016.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky, R. M. (2015). Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiology of Stress, 1, 60–65. 10.1016/j.ynstr.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra, A. , Encinas, J. M. , Deudero, J. J. , Chancey, J. H. , Enikolopov, G. , Overstreet‐Wadiche, L. S. , Tsirka, S. E. , & Maletic‐Savatic, M. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis‐coupled phagocytosis. Cell Stem Cell, 7, 483–495. 10.1016/j.stem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra, A. , Gottfried‐Blackmore, A. , Milner, T. A. , McEwen, B. S. , & Bulloch, K. (2008). Steroid hormone receptor expression and function in microglia. Glia, 56(6), 659–674. 10.1002/glia.20644 [DOI] [PubMed] [Google Scholar]
- Sierra, A. , Paolicelli, R. C. , & Kettenmann, H. (2019). Cien años de microglía: Milestones in a century of microglial research. Trends in Neurosciences, 42(11), 778–792. 10.1016/j.tins.2019.09.004 [DOI] [PubMed] [Google Scholar]
- Šišková, Z. , & Tremblay, M.‐È. (2013). Microglia and synapse: Interactions in health and neurodegeneration. Neural Plasticity, 2013, 1–10. 10.1155/2013/425845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen, D. , Beattie, E. C. , Seo, J. Y. , & Malenka, R. C. (2005). Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor‐α. Journal of Neuroscience, 25(12), 3219–3228. 10.1523/JNEUROSCI.4486-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, A. H. , Barres, B. A. , & Stevens, B. (2012). The complement system: An unexpected role in synaptic pruning during development and disease. Annual Review of Neuroscience, 35, 369–389. 10.1146/annurev-neuro-061010-113810 [DOI] [PubMed] [Google Scholar]
- Stevens, B. , Allen, N. J. , Vazquez, L. E. , Howell, G. R. , Christopherson, K. S. , Nouri, N. , Micheva, K. D. , Mehalow, A. K. , Huberman, A. D. , Stafford, B. , Sher, A. , Litke, A. M. , Lambris, J. D. , Smith, S. J. , John, S. W. M. , & Barres, B. A. (2007). The classical complement cascade mediates CNS synapse elimination. Cell, 131(6), 1164–1178. 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- Stowell, R. D. , Sipe, G. O. , Dawes, R. P. , Batchelor, H. N. , Lordy, K. A. , Whitelaw, B. S. , Stoessel, M. B. , Bidlack, J. M. , Brown, E. , Sur, M. , & Majewska, A. K. (2019). Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nature Neuroscience, 22(11), 1782–1792. 10.1038/s41593-019-0514-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama, S. , Fujita, M. , Hashimoto, M. , & Conti, B. (2007). Stress induced morphological microglial activation in the rodent brain: Involvement of interleukin‐18. Neuroscience, 146(3), 1388–1399. 10.1016/j.neuroscience.2007.02.043 [DOI] [PubMed] [Google Scholar]
- Sugama, S. , Takenouchi, T. , Hashimoto, M. , Ohata, H. , Takenaka, Y. , & Kakinuma, Y. (2019). Stress‐induced microglial activation occurs through β‐adrenergic receptor: noradrenaline as a key neurotransmitter in microglial activation. Journal of neuroinflammation, 16(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, K. V. , Deng, M. , & Ting, J. P. Y. (2019). The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nature Reviews Immunology, 19(8), 477–489. 10.1038/s41577-019-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, J. , Fujita, H. , Matsuda, S. , Toku, K. , Sakanaka, M. , & Maeda, N. (1997). Glucocorticoid‐and mineralocorticoid receptors in microglial cells: The two receptors mediate differential effects of corticosteroids. Glia, 20(1), 23–37. [DOI] [PubMed] [Google Scholar]
- Tancredi, V. , D'Antuono, M. , Cafè, C. , Giovedì, S. , Buè, M. C. , D'Arcangelo, G. , Onofri, F. , & Benfenati, F. (2000). The inhibitory effects of interleukin‐6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen‐activated protein kinase ERK. Journal of Neurochemistry, 75(2), 634–643. 10.1046/j.1471-4159.2000.0750634.x [DOI] [PubMed] [Google Scholar]
- Tancredi, V. , D'Arcangelo, G. , Grassi, F. , Tarroni, P. , Palmieri, G. , Santoni, A. , & Eusebi, F. (1992). Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neuroscience Letters, 146(2), 176–178. 10.1016/0304-3940(92)90071-E [DOI] [PubMed] [Google Scholar]
- Tarozzo, G. , Bortolazzi, S. , Crochemore, C. , Chen, S. C. , Lira, A. S. , Abrams, J. S. , & Beltramo, M. (2003). Fractalkine protein localization and gene expression in mouse brain. Journal of Neuroscience Research, 73(1), 81–88. 10.1002/jnr.10645 [DOI] [PubMed] [Google Scholar]
- Thion, M. S. , Low, D. , Silvin, A. , Chen, J. , Grisel, P. , Schulte‐Schrepping, J. , Blecher, R. , Ulas, T. , Squarzoni, P. , Hoeffel, G. , Coulpier, F. , Siopi, E. , David, F. S. , Scholz, C. , Shihui, F. , Lum, J. , Amoyo, A. A. , Larbi, A. , Poidinger, M. , … Garel, S. (2018). Microbiome influences prenatal and adult microglia in a sex‐specific manner. Cell, 172(3), 500–516. 10.1016/j.cell.2017.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, L. , Danver, J. , Ji, K. , Miyauchi, J. T. , Chen, D. , Anderson, M. E. , West, B. L. , Robinson, J. K. , & Tsirka, S. E. (2016). Dynamic microglial modulation of spatial learning and social behavior. Brain, Behavior, and Immunity, 55, 6–16. 10.1016/j.bbi.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, M. È. , Lowery, R. L. , & Majewska, A. K. (2010). Microglial interactions with synapses are modulated by visual experience. PLoS Biology, 8(11), e1000527. 10.1371/journal.pbio.1000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, M. È. , Stevens, B. , Sierra, A. , Wake, H. , Bessis, A. , & Nimmerjahn, A. (2011). The role of microglia in the healthy brain. Journal of Neuroscience, 31(45), 16064–16069. 10.1523/JNEUROSCI.4158-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully, K. , Li, Y. , Tsvetkov, E. , & Bolshakov, V. Y. (2007). (nor)epinephrine enables the induction of associative long‐term potentiation at thalamo‐amygdala synapses. Proceedings of the National Academy of Sciences of the United States of America, 104(35), 14146–14150. 10.1073/pnas.0704621104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan, R. J. , Naicker, S. , Hinwood, M. , Nalivaiko, E. , Buller, K. M. , Pow, D. V. , Day, T. A. , & Walker, F. R. (2010). Chronic stress alters the density and morphology of microglia in a subset of stress‐responsive brain regions. Brain, Behavior, and Immunity, 24(7), 1058–1068. 10.1016/j.bbi.2010.02.001 [DOI] [PubMed] [Google Scholar]
- Umpierre, A. D. , & Wu, L. J. (2020). Microglia research in the 100th year since its discovery. Neuroscience Bulletin, 36(3), 303–306. 10.1007/s12264-020-00477-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein, I. D. , Chin, G. , Cho, F. S. , Kelley, K. W. , Miller, J. G. , Chien, E. C. , Liddelow, S. A. , Nguyen, P. T. , Nakao‐Inoue, H. , Dorman, L. C. , Akil, O. , Joshita, S. , Barres, B. A. , Paz, J. T. , Molofsky, A. B. , & Molofsky, A. V. (2018). Astrocyte‐derived interleukin‐33 promotes microglial synapse engulfment and neural circuit development. Science, 359, 1269–1273. 10.1126/science.aal3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Olst, L. , Bielefeld, P. , Fitzsimons, C. P. , de Vries, H. E. , & Schouten, M. (2018). Glucocorticoid‐mediated modulation of morphological changes associated with aging in microglia. Aging Cell, 17, e12790. 10.1111/acel.12790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin, J. W. , Stacey, J. Y. , Perez‐Pouchoulen, M. , & McCarthy, M. M. (2016). Temporary depletion of microglia during the early postnatal period induces lasting sex‐dependent and sex‐independent effects on behavior in rats. Eneuro, 3(6). 10.1523/ENEURO.0297-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli, H. A. , Gandhi, C. P. , & Hill, M. N. (2016). Acute psychological stress modulates the expression of enzymes involved in the kynurenine pathway throughout corticolimbic circuits in adult male rats. Neural Plasticity, 2016, 1–12. 10.1155/2016/7215684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, S. , Klumpers, F. , Krugers, H. J. , Fang, Z. , Oplaat, K. T. , Oitzl, M. S. , Joëls, M. , & Fernández, G. (2015). Blocking the mineralocorticoid receptor in humans prevents the stress‐induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology, 40(4), 947–956. 10.1038/npp.2014.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, S. , Klumpers, F. , Schröder, T. N. , Oplaat, K. T. , Krugers, H. J. , Oitzl, M. S. , Joëls, M. , Doeller, C. F. , & Fernández, G. (2017). Stress induces a shift towards striatum‐dependent stimulus‐response learning via the mineralocorticoid receptor. Neuropsychopharmacology, 42(6), 1262–1271. 10.1038/npp.2016.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees, J. L. , Tarr, A. J. , Wohleb, E. S. , Godbout, J. P. , Mo, X. , Sheridan, J. F. , Eubank, T. D. , & Marsh, C. B. (2013). Prolonged restraint stress increases IL‐6, reduces IL‐10, and causes persistent depressive‐like behavior that is reversed by recombinant IL‐10. PLoS One, 8(3), e58488. 10.1371/journal.pone.0058488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouimba, R. M. , Foy, M. R. , Foy, J. G. , & Thompson, R. F. (2000). 17β‐estradiol suppresses expression of long‐term depression in aged rats. Brain Research Bulletin, 53(6), 783–787. 10.1016/S0361-9230(00)00377-4 [DOI] [PubMed] [Google Scholar]
- Wadhwa, M. , Prabhakar, A. , Ray, K. , Roy, K. , Kumari, P. , Jha, P. K. , & Panjwani, U. (2017). Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. Journal of Neuroinflammation, 14(1), 222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wake, H. , Moorhouse, A. J. , Miyamoto, A. , & Nabekura, J. (2013). Microglia: Actively surveying and shaping neuronal circuit structure and function. Trends in Neurosciences, 36(4), 209–217. 10.1016/j.tins.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Wakselman, S. , Béchade, C. , Roumier, A. , Bernard, D. , Triller, A. , & Bessis, A. (2008). Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. Journal of Neuroscience, 28(32), 8138–8143. 10.1523/JNEUROSCI.1006-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, D. G. , & Lue, L. F. (2015). Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimer's Research & Therapy, 7(1), 56. 10.1186/s13195-015-0139-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Yue, H. , Hu, Z. , Shen, Y. , Ma, J. , Li, J. , & Wang, L. (2020). Microglia mediate forgetting via complement‐dependent synaptic elimination. Science, 367(6478), 688–694. [DOI] [PubMed] [Google Scholar]
- Wang, H.‐T. , Huang, F.‐L. , Hu, Z.‐L. , Zhang, W.‐J. , Qiao, X.‐Q. , Huang, Y.‐Q. , Dai, R.‐P. , Li, F. , & Li, C.‐Q. (2017). Early‐life social isolation‐induced depressive‐like behavior in rats results in microglial activation and neuronal histone methylation that are mitigated by minocycline. Neurotoxicity Research, 31(4), 505–520. 10.1007/s12640-016-9696-3 [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Meng, S. , Cao, L. , Chen, Y. , Zuo, Z. , & Peng, S. (2018). Critical role of NLRP3‐caspase‐1 pathway in age‐dependent isoflurane‐induced microglial inflammatory response and cognitive impairment. Journal of Neuroinflammation, 15(1), 109. 10.1186/s12974-018-1137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, S. G. , Humphreys, A. G. , Juraska, J. M. , & Greenough, W. T. (1995). LTP varies across the estrous cycle: Enhanced synaptic plasticity in proestrus rats. Brain Research, 703(1–2), 26–30. 10.1016/0006-8993(95)01059-9 [DOI] [PubMed] [Google Scholar]
- Weber, M. D. , Frank, M. G. , Tracey, K. J. , Watkins, L. R. , & Maier, S. F. (2015). Stress induces the danger‐associated molecular pattern HMGB‐1 in the hippocampus of male Sprague Dawley rats: A priming stimulus of microglia and the NLRP3 inflammasome. Journal of Neuroscience, 35(1), 316–324. 10.1523/JNEUROSCI.3561-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, M. D. , McKim, D. B. , Niraula, A. , Witcher, K. G. , Yin, W. , Sobol, C. G. , Wang, Y. , Sawicki, C. M. , Sheridan, J. F. , & Godbout, J. P. (2019). The influence of microglial elimination and repopulation on stress sensitization induced by repeated social defeat. Biological psychiatry, 85(8), 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, J. , Yuen, E. Y. , Liu, W. , Li, X. , Zhong, P. , Karatsoreos, I. N. , McEwen, B. S. , & Yan, Z. (2014). Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Molecular Psychiatry, 19(5), 588–598. 10.1038/mp.2013.83 [DOI] [PubMed] [Google Scholar]
- Weinhard, L. , di Bartolomei, G. , Bolasco, G. , Machado, P. , Schieber, N. L. , Neniskyte, U. , Exiga, M. , Vadisiute, A. , Raggioli, A. , Schertel, A. , Schwab, Y. , & Gross, C. T. (2018). Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nature Communications, 9(1), 1228. 10.1038/s41467-018-03566-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welser‐Alves, J. V. , & Milner, R. (2013). Microglia are the major source of TNF‐α and TGF‐β1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochemistry International, 63(1), 47–53. 10.1016/j.neuint.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnall, M. H. (1993). Regulation of the hypothalamic corticotropin‐releasing hormone neurosecretory system. Progress in Neurobiology, 40(5), 573–629. 10.1016/0301-0082(93)90035-Q [DOI] [PubMed] [Google Scholar]
- Williamson, L. L. , Sholar, P. W. , Mistry, R. S. , Smith, S. H. , & Bilbo, S. D. (2011). Microglia and memory: Modulation by early‐life infection. Journal of Neuroscience, 31(43), 15511–15521. 10.1523/JNEUROSCI.3688-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb, E. S. , Hanke, M. L. , Corona, A. W. , Powell, N. D. , Stiner, L. M. , Bailey, M. T. , Nelson, R. J. , Godbout, J. P. , & Sheridan, J. F. (2011). β‐Adrenergic receptor antagonism prevents anxiety‐like behavior and microglial reactivity induced by repeated social defeat. Journal of Neuroscience, 31(17), 6277–6288. 10.1523/JNEUROSCI.0450-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley, C. S. , & McEwen, B. S. (1992). Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. Journal of Neuroscience, 12(7), 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley, C. S. , & McEwen, B. S. (1993). Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. Journal of Comparative Neurology, 336(2), 293–306. 10.1002/cne.903360210 [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Dissing‐Olesen, L. , MacVicar, B. A. , & Stevens, B. (2015). Microglia: Dynamic mediators of synapse development and plasticity. Trends in Immunology, 36(10), 605–613. 10.1016/j.it.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, J. , Titus, A. R. , & Humphrey, M. B. (2015). The TREM2‐DAP12 signaling pathway in Nasu‐Hakola disease: A molecular genetics perspective. Research and Reports in Biochemistry, 5, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, H. , & Krugers, H. J. (2015). Tuning hippocampal synapses by stress‐hormones: Relevance for emotional memory formation. Brain Research, 1621, 114–120. 10.1016/j.brainres.2015.04.010 [DOI] [PubMed] [Google Scholar]
- Xiong, H. , Cassé, F. , Zhou, M. , Xiong, Z. Q. , Joels, M. , Martin, S. , & Krugers, H. J. (2016). Interactions between N‐Ethylmaleimide‐sensitive factor and GluA2 contribute to effects of glucocorticoid hormones on AMPA receptor function in the rodent hippocampus. Hippocampus, 26(7), 848–856. [DOI] [PubMed] [Google Scholar]
- Yam, K.‐Y. , Schipper, L. , Reemst, K. , Ruigrok, S. R. , Abbink, M. R. , Hoeijmakers, L. , Naninck, E. F. G. , Zarekiani, P. , Oosting, A. , Van Der Beek, E. M. , Lucassen, P. J. , & Korosi, A. (2019). Increasing availability of ω‐3 fatty acid in the early‐life diet prevents the early‐life stress‐induced cognitive impairments without affecting metabolic alterations. The FASEB Journal, 33(4), 5729–5740. 10.1096/fj.201802297R [DOI] [PubMed] [Google Scholar]
- Yasuoka, S. , Kawanokuchi, J. , Parajuli, B. , Jin, S. , Doi, Y. , Noda, M. , Sonobe, Y. , Takeuchi, H. , Mizuno, T. , & Suzumura, A. (2011). Production and functions of IL‐33 in the central nervous system. Brain Research, 1385, 8–17. 10.1016/j.brainres.2011.02.045 [DOI] [PubMed] [Google Scholar]
- Yuen, E. Y. , Liu, W. , Karatsoreos, I. N. , Feng, J. , McEwen, B. S. , & Yan, Z. (2009). Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America, 106(33), 14075–14079. 10.1073/pnas.0906791106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen, E. Y. , Liu, W. , Karatsoreos, I. N. , Ren, Y. , Feng, J. , McEwen, B. S. , & Yan, Z. (2011). Mechanisms for acute stress‐induced enhancement of glutamatergic transmission and working memory. Molecular Psychiatry, 16(2), 156–170. 10.1038/mp.2010.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen, E. Y. , Wei, J. , Liu, W. , Zhong, P. , Li, X. , & Yan, Z. (2012). Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron, 73, 962–977. 10.1016/j.neuron.2011.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. , Peng, C. , Wu, X. , Chen, Y. , Wang, C. , & You, Z. (2014). Maternal sleep deprivation inhibits hippocampal neurogenesis associated with inflammatory response in young offspring rats. Neurobiology of Disease, 68, 57–65. 10.1016/j.nbd.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Xie, X. , Fan, Y. , Zhang, J. , Jiang, W. , Wu, X. , Yan, S. , Chen, Y. , Peng, C. , & You, Z. (2015). Phenotypic dysregulation of microglial activation in young offspring rats with maternal sleep deprivation‐induced cognitive impairment. Scientific Reports, 5, 9513. 10.1038/srep09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M. , Bakker, E. H. , Velzing, E. H. , Berger, S. , Oitzl, M. , Joëls, M. , & Krugers, H. J. (2010). Both mineralocorticoid and glucocorticoid receptors regulate emotional memory in mice. Neurobiology of Learning and Memory, 94(4), 530–537. 10.1016/j.nlm.2010.09.005 [DOI] [PubMed] [Google Scholar]
- Zhou, M. , Hoogenraad, C. C. , Joëls, M. , & Krugers, H. J. (2012). Combined β‐adrenergic and corticosteroid receptor activation regulates AMPA receptor function in hippocampal neurons. Journal of Psychopharmacology, 26(4), 516–524. 10.1177/0269881111424930 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review does not contain data.


