Introduction
Inducing and maintaining clinical remission and mucosal healing is essential in the treatment of ulcerative colitis (UC), a non-specific chronic recurrent inflammatory disorder [1]. Mesalazine (5-ASA) is commonly prescribed as a first-line drug for UC. Despite the short-term efficacy of ciclosporin and infliximab, some patients may develop methylprednisolone resistance or dependence [2].
Thalidomide has been used to treat morning sickness, but was later withdrawn from the market due to its potential to cause congenital birth defects, like phocomelia or amelia. In recent years, thalidomide has been widely used to treat multiple myeloma, leprosy erythema nodosum, and rheumatoid arthritis, depending on its immunoregulatory, anti-angiogenic, anti-proliferative, or anti-apoptotic effects [3]. Thalidomide can resist the effects of TNF-α, NF-κB, and IL-12, suggesting that it may be effective for UC. However, the clinical application of thalidomide is limited by its adverse effects, such as peripheral nerve inflammation, lethargy, and constipation, which may be related to dose accumulation. Our previous study also found that thalidomide induced dose-related adverse drug reactions (ADRs), like lethargy and hand and foot numbness [4].
Patients and methods
We retrospectively selected adult patients with refractory UC treated with low-dose thalidomide combined with mesalazine in the Sixth Affiliated Hospital of Sun Yat-sen University from January 2018 to May 2020. The follow-up deadline was July 2020. The inclusion criteria were as follows: (i) the patient was diagnosed as UC based on the inflammatory bowel disease-classes criteria [5]; (ii) the patient with refractory UC was defined as having hormone inefficacy or hormone dependence; (iii) the patient was treated with low-dose thalidomide (25–50 mg/d) combined with oral administration of mesalazine (≥4 g/d) and retention enema (≥2 g/d); (iv) the patient provided informed consent to receive thalidomide. The exclusion criteria were as follows: (i) the patient failed to take medicine regularly during treatment; (ii) the patient was not followed up after treatment or missed some examinations during the follow-up. The research plan was approved by the Ethics Committee of the Hospital (2020ZSLYEC-241).
Severity was assessed as mild, moderate, or severe according to the modified Truelove and Witts severity index including defecation time, blood in the stool, temperature, pulse, hemoglobin, and erythrocyte sedimentation rate. The endoscopic severity assessment was carried out using the Mayo Endoscopic Score (MES) with an endoscopic score of 0–1 as mucosal healing. ADRs were assessed according to the symptoms and their durations during follow-up.
Results
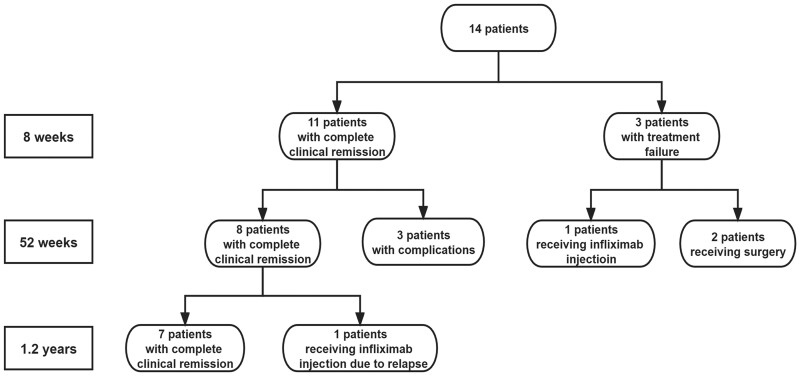
A total of 14 patients (9 males and 5 females) with UC were included. The median age was 45 years (range, 26–67 years). The median clinical response time was 3.5 weeks. At 8 weeks, complete clinical remission was achieved in 78.6% (11 of 14) of patients. Of the 14 patients, 3 (21.4%) experienced primary treatment failure, including one receiving infliximab injection and two receiving surgery later. Remission maintained in 57.1% (8 of 14) of the patients for 52 weeks. UC relapsed in 9.1% (1 of 11) of the patients 1.2 years later and was treated with infliximab injection. Medication discontinued in 27.3% (3 of 11) of the patients during remission maintenance for complications. Nerve injury occurred in 21.4% (3 of 14) of the patients, characterized by numbness in the lower extremities. The ADRs are gradually resolved after drug withdrawal and nerve treatment (Fig. 1 and Table 1).
Fig. 1.
The flowchart of low-dose thalidomide combined with mesalazine for adult patients with refractory ulcerative colitis
Table 1.
Clinical data of 14 patients with refractory ulcerative colitis
| No. | Gender | Age (years) | Course (years) | Thalidomide dose (mg) | Oral mesalazine dose (g) | Enema mesalazine dose (g) | Pretreatment MES | Severity | Duration (weeks) | Post-treatment MES | Ending |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 26 | 9 | 50 | 6 | 4 | 3 | Severe | 69 | – | Maintain remission |
| 2 | Male | 55 | 10 | 50 | 4 | 2 | 2 | Moderate | 69 | 0 | Maintain remission |
| 3 | Male | 28 | 5 | 50 | 6 | – | 3 | Severe | 116 | – | Maintain remission |
| 4 | Male | 64 | 12 | 50 | 4 | 4 | 1 | Severe | 52 | 1 | Maintain remission |
| 5 | Male | 67 | 18 | 50 | 4 | 4 | 1 | Severe | 43 | – | Maintain remission |
| 6 | Female | 46 | 4 | 50 | 4 | 2 | 3 | Severe | 69 | 2 | Maintain remission |
| 7 | Female | 63 | 4 | 50 | 4 | 4 | 1 | Severe | 65 | 0 | Maintain remission |
| 8 | Male | 47 | 7 | 50 | 4 | 1 | 2 | Severe | 26 | – | Nerve damage |
| 9 | Male | 43 | 20 | 50 | 4 | 2 | 1 | Moderate | 82 | 1 | Nerve damage |
| 10 | Male | 45 | 5 | 25 | 3 | 4 | 2 | Severe | 43 | 0 | Nerve damage |
| 11 | Female | 61 | 2 | 50 | 3 | Methylprednisolones | – | Severe | 5 | – | Operation |
| 12 | Female | 61 | 2 | 50 | 4 | – | – | Severe | 8 | – | Operation |
| 13 | Male | 64 | 5 | 50 | 4 | Methylprednisolones | 2 | Severe | 6 | 2 | Infliximab |
| 14 | Female | 33 | 4 | 50 | 3 | 3 | 1 | Severe | 66 | 2 | Infliximab |
MES, Mayo Endoscopic Score.
Discussion
Despite its good tolerance and few ADRs, 5-ASA does not induce a high remission rate in treating mild-to-moderate UC. Methylprednisolones and biological agents have limitations. Surgery is used for UC with strict indications, but also constrained by the risk of complications. Thalidomide can induce and maintain remission in the clinic [6]. In this study, low-dose thalidomide combined with mesalazine achieved a clinical remission rate of 78.6% (11 of 14) in UC patients, which is higher than those reported in the literature [7]. This suggests that this combination may have synergistic effects.
Lazzerini [8] reported that immunosuppressant therapy was effective in UC (1.5–2.5 mg/kg/d), with an 8-week remission induction rate of 78.3% (18 of 23). Considering its ADRs, we used low-dose thalidomide combined with mesalazine to treat adult patients with refractory UC, which achieved a 78.6% (11 of 14) clinical remission rate at 8 weeks. Previous reports suggested that the mucosal healing rate of thalidomide in refractory UC patients was 83.3% (10 of 12) at 52 weeks, suggesting the effectiveness of thalidomide in the long-term treatment of UC. Further research [9] found that the endoscopic mucosal healing rate of thalidomide for refractory inflammatory bowel disease (IBD) children was 41.4% (29 of 70) at 52 weeks. The long-term efficacy was comparable to that of infliximab and adalimumab. In this study, remission was maintained in 50% (7 of 14) of the patients during follow-up, with an average remission duration of 79 weeks.
The clinical application of thalidomide is restricted by its high incidence of ADRs (e.g. peripheral neuritis, lethargy, constipation) and high drug withdrawal rate. Lazzerini [8] reported that the cumulative rate of drug withdrawal due to ADRs, like peripheral neuritis and amenorrhea after remission induction, was 3.1/1,000 people/week; non-severe ADRs included neurological, cutaneous, and gastrointestinal reactions, with a cumulative incidence of 18.8 per 1,000 people per week. In our study, three patients developed nerve damage, which was resolved by the gradual reduction and eventual withdrawal of the drug. A low dose of thalidomide (50 mg/d) was used to reduce withdrawal due to ADRs and persist the treatment. Sehgal [10] reported an adverse reaction rate of 7.0% for mesalazine, which was mostly driven by dose-independent hypersensitivity. Our study found that after use of low-dose thalidomide combined with mesalazine, only nerve injury was observed, with an incidence of 21.4% (3 of 14) and an average drug maintenance of 1 year. However, this study is a single-center retrospective study with a small sample size and possible bias. The benefits of this combination should be validated with studies covering more centers.
In conclusion, low-dose thalidomide combined with mesalazine brings significant effect and limited ADRs in treating refractory UC, suggesting that thalidomide might be employed as a rescue therapy in severe UC cases refractory to conventional treatments.
Funding
This work was supported by the Sun Yat-sen University Clinical Research 5010 Program [grant number 2014008] and the Sixth Affiliated Hospital of Sun Yat-sen University of Horizontal Program [grant number H202101162024041054].
Conflict of Interest
None declared.
Contributor Information
Jun-Rong Chen, Department of General Practice, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Lei Mai, Department of Gastroenterology, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong, P. R. China.
Jia-Chen Sun, Department of Gastrointestinal Endoscopy, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Xiang Peng, Department of Gastroenterology, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Min Zhang, Department of Gastroenterology, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Min Zhi, Department of Gastroenterology, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
References
- 1. Ordás I, Eckmann L, Talamini M. et al. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 2. Singh S, Chowdhry M, Umar S. et al. Variations in the medical treatment of inflammatory bowel disease among gastroenterologists. Gastroenterol Rep (Oxf) 2018;6:61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Puzik A, Thiel A, Faust K. et al. Thalidomide has anti-inflammatory properties in neonatal immune cells. Innate Immun 2013;19:42–52. [DOI] [PubMed] [Google Scholar]
- 4. Peng X, Zhi M, Wei M. et al. Thalidomide results in diminished ovarian reserve in reproductive age female IBD patients. Medicine (Baltimore) 2017;96:e6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner D, Ruemmele FM, Orlanski-Meyer E. et al. Management of paediatric ulcerative colitis, part 1: ambulatory care-an evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;67:257–91. [DOI] [PubMed] [Google Scholar]
- 6. Millrine D, Kishimoto T.. A brighter side to thalidomide: its potential use in immunological disorders. Trends Mol Med 2017;23:348–61. [DOI] [PubMed] [Google Scholar]
- 7. Tian C, Huang Y, Wu X. et al. The efficacy and safety of mesalamine and probiotics in mild-to-moderate ulcerative colitis: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2020;2020:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lazzerini M, Martelossi S, Magazzù G. et al. Effect of thalidomide on clinical remission in children and adolescents with ulcerative colitis refractory to other immunosuppressives: pilot randomized clinical trial. Inflamm Bowel Dis 2015;21:1739–49. [DOI] [PubMed] [Google Scholar]
- 9. Lazzerini M, Villanacci V, Pellegrin MC. et al. Endoscopic and histologic healing in children with inflammatory bowel diseases treated with thalidomide. Clin Gastroenterol Hepatol 2017;15:1382–9.e1. [DOI] [PubMed] [Google Scholar]
- 10. Sehgal P, Colombel JF, Aboubakr A. et al. Systematic review: safety of mesalazine in ulcerative colitis. Aliment Pharmacol Ther 2018;47:1597–609. [DOI] [PubMed] [Google Scholar]