Abstract
OBJECTIVES
The aim of this study was to determine the long-term results of mitral valve (MV) repair with anterior leaflet patch augmentation.
METHODS
Between 2012 and 2015, 45 patients underwent MV repair using the anterior leaflet patch augmentation technique at our institution. The mean age of the patients was 65.9 ± 13.0 years (16 males). We reviewed the MV pathology and the surgical techniques used and assessed the early and late results.
RESULTS
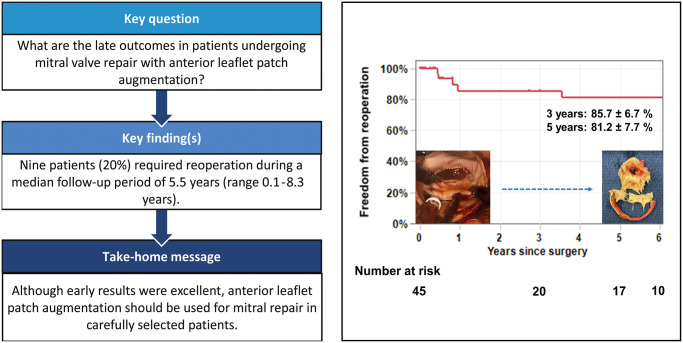
In terms of MV pathology, 43 patients (95.6%) had pure mitral regurgitation (MR) and 2 patients (4.4%) had mixed mitral stenosis and MR. Rheumatic changes were seen in 18 patients (40.0%). Postoperative echocardiography showed that 95.6% of patients had none to mild MR. During a median follow-up period of 5.5 years (range 0.1–8.3 years), there were 8 late deaths. Nine patients (20%) required reoperation. The mean interval between the initial operation and redo operation was 3.7 ± 3.1 years (range: 0.4–7.8 years). The causes of reoperation included patch dehiscence (n = 4), progression of mitral stenosis (n = 2), band dehiscence (n = 1), patch enlargement (n = 1) and unknown (n = 1). Eight patients underwent MV replacement and 1 underwent repeat MV repair. The freedom from reoperation at 3 and 5 years was 85.7 ± 6.7% and 81.2 ± 7.7%, respectively.
CONCLUSIONS
Anterior leaflet patch augmentation can provide excellent early results in the majority of the patients even in the presence of rheumatic pathology; however, we observed late reoperation in 20% of patients. Thus, this technique should be used with caution and careful follow-up with serial echocardiography is essential.
Keywords: Mitral valve repair, Anterior leaflet patch augmentation, Rheumatic heart disease, Functional mitral regurgitation
Mitral valve (MV) repair is a common procedure and the rule rather than exception in patients suffering from mitral regurgitation (MR) due to degenerative disease [1], with a repair rate of >90% by experienced centres [2, 3].
INTRODUCTION
Mitral valve (MV) repair is a common procedure and the rule rather than exception in patients suffering from mitral regurgitation (MR) due to degenerative disease [1], with a repair rate of >90% by experienced centres [2, 3]. It has been demonstrated that valve repair is superior to valve replacement in terms of both early and late outcomes when performed by skilled surgeons on adequately selected patients, and it has the benefits of preserving cardiac function and no need for long-term anticoagulation therapy [4]. In fact, David et al. [5] reported that, at 20 years postoperatively, the freedom from severe MR was 90.7% and the probability of reoperation was only 5.9%. Because of these excellent results, the most recent guidelines recommend early surgery even in the patients with asymptomatic MR [6]. However, MV repair can be challenging in patients with other pathophysiologies, such as rheumatic morphology (Carpentier classification type IIIa) and/or restricted leaflet motion (Carpentier classification type IIIb). In these circumstances, it is well known that MV repair is associated with increased risk of surgical failure [7]. To improve the outcomes, several innovative repair techniques have been proposed, including anterior leaflet augmentation. Acceptable short- and mid-term outcomes of this technique have been reported by some authors [8–12], but there have been only a few articles reporting long-term results. The aim of this study was to assess the early and late outcomes in patients who underwent MV repair with the anterior leaflet patch augmentation technique.
PATIENTS AND METHODS
Ethics statement
This study was approved by the Temple University Institutional Review Board (Protocol number: 28195, approved on 10 May 2021). Patient consent requirements were waived due to minimal risk of the study.
Between January 2012 and April 2015, 45 patients underwent MV repair using the anterior leaflet patch augmentation technique at our institution. We reviewed the medical charts and the operative records to identify the patient characteristics, aetiology of valve disease, operative techniques used and surgical results. Late outcomes were determined from clinic records when available or from written correspondence with patients’ physicians.
Indications and surgical techniques
The anterior leaflet patch augmentation technique was created to address decreased coaptation due to shortened leaflets and/or restrictive leaflet motion, particularly in patients with functional and/or restrictive aetiologies such as rheumatic changes. Our surgical technique has been described in detail elsewhere [13]. In short, patients underwent surgery via either a median sternotomy or minimally invasive right thoracotomy approach including the use of robotic technology. After the MV was inspected, the anterior leaflet was measured with an annuloplasty band or ring sizer based on the intertrigonal distance. An incision was made on the anterior leaflet at 3 mm from the annulus extending from one commissure to the other. Using the same ring sizer, the ovoid shape of the patch was created. Four types of patch materials were used: autologous pericardium, Peri-Guard (bovine pericardium, Baxter International, Deerfield, IL, USA), CorMatrix (extracellular matrix made from porcine intestinal submucosa, CorMatrix Cardiovascular, Roswell, GA, USA) and CardioCel (decellularized bovine pericardium, Admedus Regen, Malaga, WA, Australia). Autologous pericardium was fixed with 2% glutaraldehyde solution for 1–2 min. The patch was then sewn in running fashion with either 5–0 monofilament or 4–0 polytetrafluoroethylene sutures. Annuloplasty was added with a prosthetic ring or band. The size selection was based on the original sizing prior to patch augmentation.
Follow-up
Patients were followed up for 2–6 weeks by the surgeons at an outpatient clinic, and at 3, 6, and 12 months by the referring cardiologists. In terms of oral anticoagulation, all patients were given warfarin for 3–6 months postoperatively with a target prothrombin time international normalized ratio of 2–3, following the same protocol as conventional MV repair. Transthoracic echocardiography was performed annually during follow-up. MV lesions were graded based on the guidelines defined by the American Society of Echocardiography and the European Association of Echocardiography [14, 15]. Of note, it was common to observe elevated pressure gradient across the MV after a repair using anterior leaflet patch augmentation [8]; thus, the grade of mitral stenosis (MS) was carefully evaluated based on a comprehensive assessment considering the condition of the valve and MV area by planimetry.
Statistical analysis
Categorical variables are shown as counts (percentages). Continuous variables are shown as the mean ± standard deviation and/or median (interquartile range). The median follow-up time was calculated by the inverse Kaplan–Meier method. Overall survival, freedom from reoperation, freedom from moderate or more MR or MS, and freedom from composite end point were estimated by the Kaplan–Meier method. The composite end point was defined as death, reoperation, moderate or more MR or MS, and readmission for congestive heart failure. For the competing risk analysis, we also computed the cumulative incidence function for reoperation with death as a competing event. The associations of potential risk factors to composite end point were assessed with the Cox proportional hazards model. All statistical analyses were conducted with JMP Pro 15 software and SAS 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Patient characteristics
The baseline patient characteristics are shown in Table 1. Of the 45 patients studied, 29 (64.4%) were female, and the mean age was 65.9 ± 13.0 years. Twenty patients (44.4%) had history of either paroxysmal or persistent atrial fibrillation. In terms of preoperative functional status, 29 patients (64.4%) were in New York Heart Association functional class III or IV. There were 7 (15.6%) redo cases. Preoperative echocardiographic data are shown in Table 2. The mean left ventricular ejection fraction was 48 ± 13%, and there were 10 patients (22.2%) who had depressed ejection fraction of ≤ 35%. All the patients had moderate-to-severe MR, and 2 patients (4.4%) had mixed MS and MR. The mean Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) score and the European System for Cardiac Operative Risk Evaluation II score were 3.58 ± 3.64% and 5.37 ± 4.45%, respectively. There were 6 patients (13.3%) who had high STS-PROM scores of ≥8%. Of note, there were no patients who had active or healed infective endocarditis in this series.
Table 1:
Patient characteristics
| Variable | n = 45 |
|---|---|
| Age (years), mean ± SD | 65.9 ± 13.0 |
| Female, n (%) | 29 (64.4) |
| Body surface area (m2), mean ± SD | 1.9 ± 0.3 |
| Hypertension, n (%) | 38 (84.4) |
| Diabetes mellitus, n (%) | 15 (33.3) |
| Hyperlipidaemia, n (%) | 29 (64.4) |
| Creatinine (mg/dl), mean ± SD | 1.06 ± 0.39 |
| Haemodialysis | 0 |
| Liver cirrhosis, n (%) | 2 (4.4) |
| Coronary artery disease, n (%) | 19 (42.2) |
| Cerebrovascular disease, n (%) | 9 (20.0) |
| Atrial fibrillation, n (%) | 20 (44.4) |
| NYHA functional class, n (%) | |
| I | 0 |
| II | 16 (35.5) |
| III | 17 (37.8) |
| IV | 12 (26.7) |
| Previous cardiac surgery, n (%) | 7 (15.6) |
| STS-PROM score (%), mean ± SD | 3.58 ± 3.64 |
| >8%, n (%) | 6 (13.3) |
| EuroSCORE II (%), mean ± SD | 5.37 ± 4.45 |
EuroSCORE II: European System for Cardiac Operative Risk Evaluation II score; NYHA: New York Heart Association; SD: standard deviation; STS-PROM: Society of Thoracic Surgeons predicted risk of mortality.
Table 2:
Echocardiographic data
| Preoperative (n = 45) | Early postoperative (n = 45) | Late postoperative (n = 31) | |
|---|---|---|---|
| Mean interval (years), mean ± SD | 3.3 ± 2.6 | ||
| LVEDD (mm), mean ± SD | 53 ± 15 | 51 ± 8 | 49 ± 11 |
| LVESD (mm), mean ± SD | 39 ± 14 | 39 ± 11 | 38 ± 14 |
| EF (%), mean ± SD | 48 ± 13 | 43 ± 19 | 44 ± 16 |
| EF ≤ 35%, n (%) | 10 (22.2) | 12 (26.7) | 6 (22.6) |
| MR grade, n (%) | |||
| None or trivial | 0 | 34 (75.6) | 14 (45.2) |
| Mild | 0 | 9 (20.0) | 7 (22.6) |
| Moderate | 6 (13.3) | 2 (4.4) | 2 (6.5) |
| Moderately severe | 13 (28.9) | 0 | 2 (6.5) |
| Severe | 26 (57.8) | 0 | 6 (19.4) |
| MS grade, n (%) | |||
| Moderate | 2 (4.4) | 3 (6.7) | 6 (19.4) |
| Severe | 0 | 0 | 4 (12.9) |
EF: ejection fraction; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; MR: mitral regurgitation; MS: mitral stenosis; SD: standard deviation.
Intraoperative findings
Intraoperative findings are summarized in Table 3. With regard to MV pathology, 31 patients (68.9%) had organic changes, 11 patients (24.4%) had functional aetiology and 3 patients (6.7%) had mixed pathology. Of note, rheumatic changes were found in 18 patients (40.0%). These rheumatic changes included thickened or calcified leaflet/chordae/papillary muscle(s) and/or fusion of commissure(s). In terms of the patch materials utilized, CardioCel was used in 19 (42.2%) patients, CorMatrix in 17 (37.8%), autologous pericardium in 8 (17.8%) and Peri-Guard in 1 (2.2%). Concomitant annuloplasty was performed in 43 (95.6%) of the patients. Semi-rigid bands were mainly used (n = 36, 80%), followed by flexible bands (n = 6, 13.3%) and a semi-rigid ring (n = 1, 2.3%). The mean size of the ring or band was 28.4 ± 2.4 mm. Excision or division of secondary chordae was carried out in 10 (22.2%) and commissurotomy in 5 (11.1%) patients. Concomitant surgery included left atrial appendage closure (n = 17, 37.8%), atrial fibrillation ablation procedure (n = 6, 13.3%), atrial septal defect or patent foramen ovale closure (n = 5, 11.1%), tricuspid valve repair (n = 3, 6.7%) and coronary artery bypass grafting (n = 1, 2.2%). The mean cardiopulmonary bypass and aortic cross-clamp times were 130 ± 29 and 88 ± 14 min, respectively. No patients required a second aortic cross-clamping for a revision of original repairs.
Table 3:
Intraoperative findings
| Variable | n = 45 |
|---|---|
| Mitral valve pathology, n (%) | |
| Organic | 31 (68.9) |
| Rheumatic | 18 (40.0) |
| Functional | 11 (24.4) |
| Mixed | 3 (6.7) |
| Surgical approach, n (%) | |
| Right thoracotomy approach | 43 (95.6) |
| Median sternotomy | 2 (4.4) |
| Patch material, n (%) | |
| CardioCel | 19 (42.2) |
| CorMatrix | 17 (37.8) |
| Autologous pericardium | 8 (17.8) |
| Peri-Guard | 1 (2.2) |
| Mitral annuloplasty, n (%) | 43 (95.6) |
| Mean size of ring or band (mm), mean ± SD | 28.4 ± 2.4 |
| Semi-rigid band, n (%) | 36 (80.0) |
| Flexible band, n (%) | 6 (13.3) |
| Semi-rigid ring, n (%) | 1 (2.3) |
| Excision or division of chordae, n (%) | 10 (22.2) |
| Commissurotomy, n (%) | 5 (11.1) |
| Concomitant surgery, n (%) | |
| LAA closure | 17 (37.8) |
| AF ablation | 6 (13.3) |
| ASD or PFO closure | 5 (11.1) |
| TV repair | 3 (6.7) |
| CABG | 1 (2.2) |
| CPB time (min), mean ± SD | 130 ± 29 |
| Aortic cross-clamp time (min), mean ± SD | 88 ± 14 |
AF: atrial fibrillation; ASD: atrial septal defect; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; LAA: left atrial appendage; PFO: patent foramen ovale; TV: tricuspid valve; SD: standard deviation.
Early outcomes
There were 4 (8.9%) 30-day mortalities. Causes of 30-day mortality included low-output syndrome (n = 1), atrioventricular dissociation (n = 1), multiple ischaemic stroke (n = 1) and possible bowel ischaemia (n = 1). Early morbidity included re-exploration (n = 4), reintubation (n = 3) and/or tracheostomy (n = 2), ischaemic stroke (n = 2), new haemodialysis (n = 2) and permanent pacemaker implantation (n = 1).
Postoperative echocardiography (Table 2) showed that 43 patients (95.6%) had none to mild MR, while 2 patients (4.4%) had residual moderate MR. None had either severe MS or more than moderate MR.
Late outcomes
During a median follow-up of 5.5 years (0.5–7.1 years), there were 8 late deaths. The causes of death included congestive heart failure (n = 3), intracranial haemorrhage (n = 1), cancer (n = 1), sepsis (n = 1) and unknown (n = 2). Overall survival estimates at 3 and 5 years were 78.7 ± 6.9% and 71.4 ± 8.0%, respectively. No patient developed endocarditis during the follow-up period.
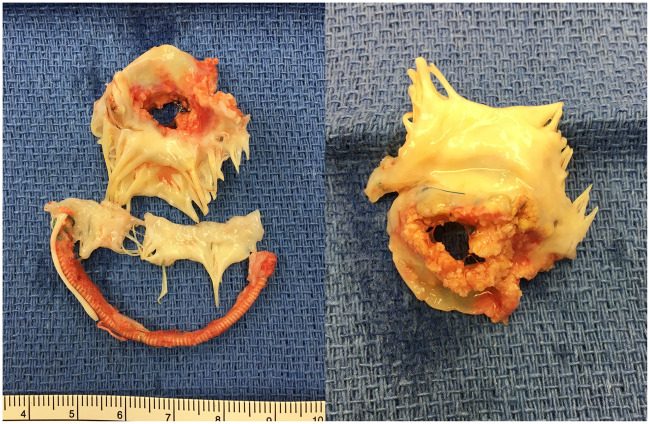
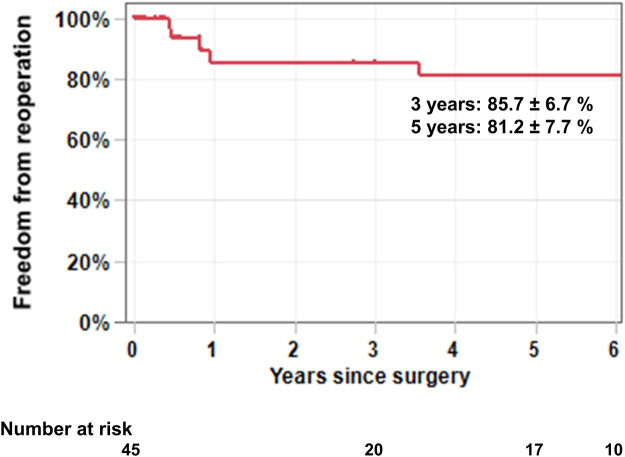
Nine patients (20%) required reoperation (Table 4). The median interval between the initial operation and redo operation was 3.5 years (0.8 ∼ 6.4 years). There were 5 patients who had had rheumatic changes. Patch materials implanted at the first operation were CorMatrix (n = 6), CardioCel (n = 2) and autologous pericardium (n = 1). The indications for reoperation were recurrent severe MR (n = 7) and severe MS (n = 2). The causes of recurrent MR included patch dehiscence (n = 4, Fig. 1 and Video 1), band dehiscence (n = 1), patch enlargement (n = 1) and unknown (n = 1). Of note, there was no calcification on the patch materials found in 4 reoperations done within 1 year of the initial operation. However, patches observed >3 years after the initial operation were found to be significantly calcified. There was no specific site to develop severe calcification on the patch materials. Eight patients underwent MV replacement and 1 underwent repeat MV repair. Of note, there were no operative mortalities related to reoperations. The freedom from reoperation at 3 and 5 years was 85.7 ± 6.7% and 81.2 ± 7.7%, respectively (Fig. 2). The cumulative incidence function for reoperation with death as a competing event at 3 and 5 years was 12.0 ± 5.8% and 16.0 ± 6.5%, respectively (Supplementary Material, Fig. S1).
Table 4:
Summary of reoperations
| Age (years)a | Interval (years) | Aetiology | Rheumatic change | Patch material | Indication | Cause of failure | Patch calcification | Procedure | Concomitant surgery | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | 0.9 | Organic | Yes | CorMatrix | MR | Patch dehiscence | None | Replacement | |
| 2 | 65 | 7.8 | Organic | No | CorMatrix | MR | Patch dehiscence | N/A | Replacement | CABG, AF ablation LAA closure |
| 3 | 56 | 0.5 | Functional | No | CorMatrix | MR | Band dehiscence | None | Re-repair | TV repair |
| 4 | 71 | 6.4 | Organic | Yes | CorMatrix | MR | N/A | N/A | Replacement | |
| 5 | 78 | 0.4 | Mixed | No | CorMatrix | MR | Patch enlargement | None | Replacement | |
| 6 | 54 | 3.5 | Organic | Yes | CorMatrix | MS | Progressive rheumatic disease | Calcified | Replacement | TV repair |
| 7 | 46 | 6.9 | Functional | No | Autologous pericardium | MR | Patch dehiscence | Calcified | Replacement | LAA closure |
| 8 | 58 | 6.4 | Organic | Yes | CardioCel | MR | Patch dehiscence | Calcified | Replacement | TV repair |
| 9 | 80 | 0.8 | Organic | Yes | CardioCel | MS | Progressive rheumatic disease | None | Replacement | |
| Mean ± SD | 60.2 ± 15.0 | 3.7 ± 3.1 |
At initial operation.
AF: atrial fibrillation; CABG: coronary artery bypass grafting; LAA: left atrium appendage; MR: mitral regurgitation; MS: mitral stenosis; N/A: not available; SD: standard deviation; TV: tricuspid valve.
Figure 1:
Gross findings of explanted mitral valve apparatus at redo surgery. The patient had undergone anterior leaflet patch augmentation with autologous pericardium 6.9 years prior to the redo surgery. The explanted anterior leaflet was heavily calcified and there was a large perforation due to patch dehiscence. MV: mitral valve.
Figure 2:
Freedom from reoperation after mitral valve repair using anterior leaflet patch augmentation. A total of 9 reoperations were required during the follow-up period. The freedom from reoperation at 3 and 5 years was 85.7 ± 6.7% and 81.2 ± 7.7%, respectively.
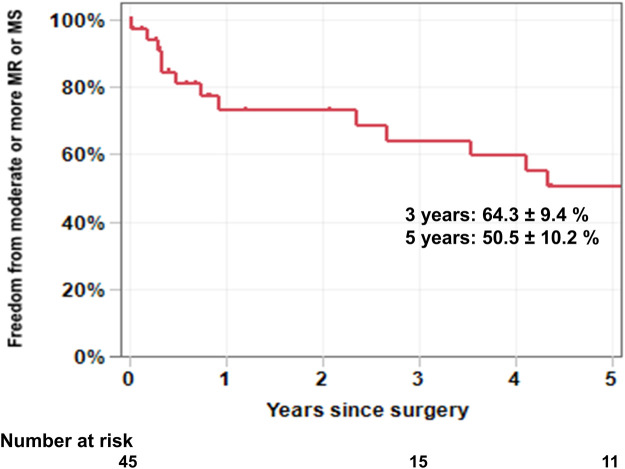
Follow-up echocardiographic data were also obtained in 31 patients (Table 2). The median interval between the initial operation and the most recent transthoracic echocardiography was 2.7 years (0.7–5.4 years). During follow-up, 6 patients (19.4%) developed moderate to severe MR, 6 patients (19.4%) developed moderate to severe MS, and 4 patients (12.9%) developed combined MR and MS. There were 3 patients in this series who developed severe MR and/or MS but have not undergone reoperation yet. One of these patients died of intracranial haemorrhage and the remaining 2 patients were being managed medically at the time of latest follow-up. The freedom from moderate or more MR or MS at 3 and 5 years was 64.3 ± 9.4% and 50.5 ± 10.2%, respectively (Fig. 3). Freedom from composite end point (death, reoperation, moderate or more MR or MS, and readmission for congestive heart failure) at 3 and 5 years was 58.5 ± 8.4% and 40.8 ± 8.9%, respectively (Supplementary Material, Fig. S2). On univariate analysis, preoperative and intraoperative variables associated with the composite end point included lower body mass index (P = 0.014), previous cardiac surgery (P = 0.019), higher STS-PROM score (P = 0.001) and longer CPB time (P = 0.006) (Table 5). A multivariate analysis was not performed because of the small sample size.
Figure 3:
Freedom from moderate or more mitral regurgitation or mitral stenosis. Six patients developed moderate to severe mitral regurgitation, 6 patients developed moderate to severe mitral stenosis, and 4 patients developed combined mitral regurgitation and mitral stenosis. The freedom from moderate or more mitral regurgitation or mitral stenosis at 3 and 5 years was 64.3 ± 9.4% and 50.5 ± 10.2%, respectively. MR: mitral regurgitation; MS: mitral stenosis.
Table 5:
Factors associated with composite end point
| HR (95% CI) | P-Value | |
|---|---|---|
| Age (years) | 1.03 (0.99–1.07) | 0.191 |
| Male | 1.83 (0.81–4.12) | 0.149 |
| Body mass index (kg/m2) | 0.09 (0.01–0.62) | 0.014 |
| Hypertension | 1.33 (0.39–4.56) | 0.636 |
| Diabetes mellitus | 1.36 (0.59–3.15) | 0.479 |
| Coronary artery disease | 1.29 (0.55–3.00) | 0.561 |
| Atrial fibrillation | 0.96 (0.42–2.18) | 0.915 |
| NYHA functional class | 1.29 (0.78–2.16) | 0.316 |
| Previous cardiac surgery | 3.55 (1.36–9.26) | 0.019 |
| STS-PROM score (%) | 1.19 (1.08–1.30) | 0.001 |
| EF | 0.99 (0.96–1.03) | 0.716 |
| MR grade | 0.94 (0.58–1.59) | 0.801 |
| Rheumatic change | 0.70 (0.30–1.62) | 0.699 |
| Right thoracotomy approach | 1.34 (0.18–10.18) | 0.769 |
| Patch material | ||
| Autologous pericardium | Reference | |
| CorMatrix | 0.99 (0.32–2.83) | 0.919 |
| CardioCel | 1.14 (0.36–3.63) | 0.826 |
| Mean size of ring or band (mm) | 1.08 (0.89–1.32) | 0.417 |
| CPB time (min) | 1.02 (1.01–1.04) | 0.006 |
| Aortic cross-clamp time (min) | 1.01 (0.98–1.05) | 0.502 |
CI: confidence interval; CPB: cardiopulmonary bypass; EF: ejection fraction; HR: hazard ratio; MR: mitral regurgitation; NYHA: New York Heart Association; STS-PROM: Society of Thoracic Surgeons predicted risk of mortality.
DISCUSSION
The aim of anterior leaflet patch augmentation is to increase coaptation by enlarging the surface area of the leaflet in functional physiology. This also contributes to mobilization of the leaflet in restrictive physiology. It has been reported that anterior leaflet augmentation can provide excellent early outcomes [8–12]. In the current study, we found that the vast majority of patients (95.6%) had none or only mild residual MR early after operation even in the presence of rheumatic pathology, which is certainly comparable to MV repair in patients with degenerative aetiologies [5].
Despite the excellent early outcomes reported, little is known about the late outcomes of this technique. There have been several studies reporting mid-term outcomes. The largest study, by Malhotra et al., reported that there was only a 5.4% reoperation rate among the 80 patients who underwent surgery with this technique, but the mean follow-up was limited to only 2 years [11]. Acar et al. reported that only 1 patient required reoperation out of 62 patients (reoperation rate: 2.5%) during 3.2 years of follow-up [8]. Romano et al. [9] reported the reoperation rate was 4.8% during a follow-up period of 2.2 years. Contrary to these low reoperation rates, in the current study, we observed late reoperations in 20% of patients during a median follow-up period of 5.5 years postoperatively. Fukunaga et al. [16] analysed 144 patients who underwent MV repair using glutaraldehyde-treated autologous pericardium. They reported that a total of 19 reoperations (reoperation rate: 13.2%) were necessary during the follow-up period (mean: 6.9; maximum: 21.1 years). Of note, calcification was recognized on the resected autologous pericardium in 5 cases at the time of reoperation. Although their study did not focus solely on anterior leaflet augmentation, the results were similar to ours in the sense that the patches were found to be calcified late after the operation. Perhaps our late results (reoperation rate of 20% at 5.5 years) may be the worst among the relevant reports. More importantly, follow-up echocardiography demonstrated even worse outcomes in terms of freedom from moderate or more MR or MS (50.5% at 5 years postoperatively). In the other words, almost 50% of the patients undergoing this technique had suffered failure of the repair at 5 years after operation.
We previously reported a high recurrence rate of severe MR for this technique when using CorMatrix (a porcine small intestinal submucosa extracellular matrix) [13]. Preclinical and early clinical studies supported the positive expectation that this extracellular matrix patch might fulfil several criteria for an ideal biological scaffold (strong, pliant and durable) [17]. We discontinued its use based on our own experiences described in our previous report [13]. Similar phenomena of early failure have also been reported by other authors [18, 19]. In the current study, patch failures were also observed in other materials. These patients were found to have progression of patch degeneration causing moderate to severe MS and eventually developed severe MR due to patch dehiscence. We then started to use the CardioCel, which is a decellularized bovine pericardium, as the next extracellular matrix patch. Although better long-term performance was expected [20], others had experienced early failures, including calcification proven by the histopathological analysis [21]. It is well known that glutaraldehyde-fixed autologous or xenograft pericardium may be associated with late calcification and stenosis [22]. No matter which patch is used, calcification and subsequent valve failure will often occur in the long term and this seems to be the critical limitation of this technique.
The optimal MV procedure in patients with rheumatic pathology remains controversial. MV replacement has been the most common surgical treatment worldwide [23]. There is a trend towards recognizing MV repair as a more favourable procedure in carefully selected patients [8, 9, 23, 24]. In addition to leaflet patch augmentation, surgical techniques may include a commissurotomy and various manoeuvres on the subvalvular apparatus including splitting the head of the papillary muscles and dividing fused chordal structures, with subsequent reconstruction using chordal transfer or artificial chordal implantation. Romano et al. [9] reported 2 reoperations for recurrent MR in patients with rheumatic pathology who had a heavily calcified subvalvular apparatus. They reported that those who have extended calcified subvalvular apparatus should have been initially considered for MV replacement. In addition to possible failure of the repair itself, we have to keep in mind that those patients with rheumatic aetiology may suffer further rheumatic degeneration of the original valve structure over time. In fact, Kuwaki et al. [25] reported that progression of rheumatic disease in the repaired MV was the reason for reoperation in almost all the patients after open commissurotomy. In this study, 2 patients required reoperation due to progressive rheumatic MS without patch failure. Moreover, the patients with rheumatic pathology tend to be younger, making implanted tissue denegation and calcification even faster than older patients.
We reported herein our series of anterior leaflet augmentation, while others apply this technique to the posterior leaflet as well [11, 24, 26–28]. Chauvaud et al. adapted pericardial patch augmentation mainly in the posterior leaflet (75%) and reported acceptable outcomes of freedom from reoperation at 10 years of 70% [24, 26]. In contrast to the relative better outcomes for posterior leaflet augmentation, anterior leaflet augmentation tends to fail due to the following possible mechanism: the anterior leaflet with patch materials will inevitably develop calcification eventually causing significant malfunction, while posterior patch augmentation may tolerate significant calcification because the calcification can function as a baffle for coaptation with the anterior leaflet. This is similar to the fact that anterior leaflet mobility is more important to MV function than posterior leaflet mobility. Thus, this is not a matter of the type of patch or the mitral pathology, but rather a limitation of this technique itself, augmenting the anterior leaflet with large patch that may eventually fail. Our results clearly suggest that anterior leaflet patch augmentation should be used with caution, and careful follow-up with serial echocardiography is essential.
Limitations
The present study has several limitations. First, this was a single-centre, retrospective observational study, which confers an inherent selection bias. The sample size was small, and some data were not available. Second, without a control group, comparison of the results was limited to historical outcomes of MV repair with or without this technique. Thus, our results are best interpreted as support for a cautious approach in using the anterior leaflet patch augmentation technique.
CONCLUSIONS
MV repair with anterior leaflet patch augmentation can provide excellent early results in the majority of patients even in the presence of rheumatic pathology. However, we observed late reoperation in 20% of patients during a median follow-up period of 5.5 years postoperatively. In addition, almost half of the patients had developed moderate or more MR or MS at 5 years postoperatively. Regardless of the type of patch used and mitral pathology, patch calcification and subsequent valvular dysfunction often occur late after surgery. Therefore, this technique should be used with caution and careful follow-up with serial echocardiography is essential.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
Data Availability Statement
All the co-authors have full access to the patient data. Also, the data gathered or analysed in this study are available upon reasonable request to the corresponding author.
Author contributions
Hiromu Kehara: Conceptualization; Data curation; Formal analysis; Investigation; Writing—original draft. Kenji Minakata: Conceptualization; Methodology; Writing—review & editing. James McCarthy: Conceptualization; Data curation. Gengo Sunagawa: Methodology; Validation. Chirantan Mangukia: Data curation; Validation. Stacey Brann: Conceptualization; Data curation; Validation. Huaqing Zhao: Data curation; Methodology. Robert Boova: Supervision; Validation. Yoshiya Toyoda: Supervision; Validation.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Amber Malhotra and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. T. Sloane Guy of the Department of Cardiac Surgery, Thomas Jefferson University, for initiating this project.
Glossary
ABBREVIATIONS
- MR
Mitral regurgitation
- MS
Mitral stenosis
- MV
Mitral valve
- STS-PROM
Society of Thoracic Surgeons predicted risk of mortality
Contributor Information
Hiromu Kehara, Division of Cardiovascular Surgery, Temple University and Lewis Katz School of Medicine, Philadelphia, PA, USA.
Kenji Minakata, Division of Cardiovascular Surgery, Temple University and Lewis Katz School of Medicine, Philadelphia, PA, USA.
James McCarthy, Division of Cardiovascular Surgery, Temple University and Lewis Katz School of Medicine, Philadelphia, PA, USA.
Gengo Sunagawa, Division of Cardiovascular Surgery, Temple University and Lewis Katz School of Medicine, Philadelphia, PA, USA.
Chirantan Mangukia, Division of Cardiovascular Surgery, Temple University and Lewis Katz School of Medicine, Philadelphia, PA, USA.
Stacey Brann, Division of Cardiovascular Surgery, Temple University and Lewis Katz School of Medicine, Philadelphia, PA, USA.
Huaqing Zhao, Center for Biostatistics and Epidemiology, Department of Biomedical Education and Data Science, Temple University and Lewis Katz School of Medicine, Philadelphia, PA, USA.
Robert Boova, Division of Cardiovascular Surgery, Temple University and Lewis Katz School of Medicine, Philadelphia, PA, USA.
Yoshiya Toyoda, Division of Cardiovascular Surgery, Temple University and Lewis Katz School of Medicine, Philadelphia, PA, USA.
REFERENCES
- 1.Bonis MD, Alfieri O, Dalrymple-Hay M, Del Forno B, Dulguerov F, Dreyfus G.. Mitral valve repair in degenerative mitral regurgitation: state of the art. Prog Cardiovasc Dis 2017;60:386–93. [DOI] [PubMed] [Google Scholar]
- 2.Hasan IS, Schaff HV, Daly RC, King KS, Stulak JM, Greason KL. et al. Does referral bias impact outcomes of surgery for degenerative mitral valve disease? Ann Thorac Surg 2020;110:1990–6. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy PM, Herborn J, Kruse J, Liu M, Andrei AC, Thomas JD.. A multiparameter algorithm to guide repair of degenerative mitral regurgitation. J Thorac Cardiovasc Surg 2020. 10; S0022-5223(20)32811-7. [DOI] [PubMed] [Google Scholar]
- 4.Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL.. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation 1995;91:1022–8. [DOI] [PubMed] [Google Scholar]
- 5.David TE, Armstrong S, McCrindle BW, Cedric M.. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation 2013;127:1485–92. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA. et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2017;70:252–89. [DOI] [PubMed] [Google Scholar]
- 7.Deloche A, Jebara VA, Relland JY, Chauvaud S, Fabiani JN, Perier P. et al. Valve repair with Carpentier techniques: the second decade. J Thorac Cardiovasc Surg 1990;99:990–1002. [PubMed] [Google Scholar]
- 8.Acar C, Saez de Ibarra J, Lansac E.. Anterior leaflet augmentation with autologous pericardium for mitral repair in rheumatic valve insufficiency. J Heart Valve Dis 2004;13:741–6. [PubMed] [Google Scholar]
- 9.Romano MA, Patel HJ, Pagani FD, Prager RL, Deeb GM, Bolling SF.. Anterior leaflet repair with patch augmentation for mitral regurgitation. Ann Thorac Surg 2005;79:1500–4. [DOI] [PubMed] [Google Scholar]
- 10.Mihos CG, Pineda AM, Capoulade R, Santana O.. A systematic review of mitral valve repair with autologous pericardial leaflet augmentation for rheumatic mitral regurgitation. Ann Thorac Surg 2016;102:1400–5. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra A, Majmudar S, Siddiqui S, Pandya H, Shah K, Sharma P. et al. Midterm results of mitral valve repair with pericardial leaflet augmentation: a single-center experience. Semin Thorac Cardiovasc Surg 2020;32:433–40. [DOI] [PubMed] [Google Scholar]
- 12.Kincaid EH, Riley RD, Hines MH, Hammon JW, Kon ND.. Anterior leaflet augmentation for Ischemic mitral regurgitation. Ann Thorac Surg 2004;78:564–8. [DOI] [PubMed] [Google Scholar]
- 13.Kelley TM Jr, Kashem M, Wang H, McCarthy J, Carroll ND, Moser GW. et al. Anterior leaflet augmentation with CorMatrix porcine extracellular matrix in twenty-five patients: unexpected patch failures and histologic analysis. Ann Thorac Surg 2017;103:114–21. [DOI] [PubMed] [Google Scholar]
- 14.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA. et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–71. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP. et al. ; European Association of Echocardiography. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1–23. [DOI] [PubMed] [Google Scholar]
- 16.Fukunaga N, Sakata R, Koyama T.. Reoperative analysis after mitral valve repair with glutaraldehyde-treated autologous pericardium. Interact CardioVasc Thorac Surg 2017;25:912–7. [DOI] [PubMed] [Google Scholar]
- 17.Buitrago D, Mulinari L.. Surgeons should be wise when choosing a material to repair heart defects. J Card Surg 2021;36:396–7. [DOI] [PubMed] [Google Scholar]
- 18.Nezhad ZM, Poncelet A, De Kerchove L, Fervaille C, Banse X, Bollen X. et al. CorMatrix valved conduit in a porcine model: long-term remodelling and biomechanical characterization. Interact CardioVasc Thorac Surg 2017;24:90–8. [DOI] [PubMed] [Google Scholar]
- 19.van Rijswijk JW, Talacua H, Mulder K, van Hout GPJ, Bouten CVC, Grundeman PF. et al. Failure of decellularized porcine small intestinal submucosa as a heart valved conduit. J Thorac Cardiovasc Surg 2020;160:e201–e215. [DOI] [PubMed] [Google Scholar]
- 20.Neethling WML, Puls K, Rea A.. Comparison of physical and biological properties of CardioCel with commonly used bioscaffolds. Interact CardioVasc Thorac Surg 2018;26:985–92. [DOI] [PubMed] [Google Scholar]
- 21.Deutsch O, Bruehl F, Cleuziou J, Prinzing A, Schlitter M, Krane M. et al. Histological examination of explanted tissue-engineered bovine pericardium following heart valve repair. Interact CardioVasc Thorac Surg 2020;30:64–73. [DOI] [PubMed] [Google Scholar]
- 22.Schoen FJ, Levy RJ.. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg 2005;79:1072–80. [DOI] [PubMed] [Google Scholar]
- 23.Vervoort D, Ouzounian M, Yanagawa B.. Mitral valve surgery for rheumatic heart disease: replace, repair, retrain? Curr Opin Cardiol 2021;36:179–85. [DOI] [PubMed] [Google Scholar]
- 24.Chauvaud S, Fuzellier JF, Berrebi A, Deloche A, Fabiani JN, Carpentier A.. Long-term (29 years) results of reconstructive surgery in rheumatic mitral valve insufficiency. Circulation 2001;104:I12–I15. [DOI] [PubMed] [Google Scholar]
- 25.Kuwaki K, Kawaharada N, Morishita K, Koyanagi T, Osawa H, Maeda T. et al. Mitral valve repair versus replacement in simultaneous mitral and aortic valve surgery for rheumatic disease. Ann Thorac Surg 2007;83:558–63. [DOI] [PubMed] [Google Scholar]
- 26.Chauvaud S, Jebara V, Chachques JC, el Asmar B, Mihaileanu S, Perier P. et al. Valve extension with glutaraldehyde-preserved autologous pericardium. Results in mitral valve repair. J Thorac Cardiovasc Surg 1991;102:171–8. [PubMed] [Google Scholar]
- 27.Ikeda N, Yamaguchi H, Takagaki M, Mitsuyama S, Ebato M, Tanno K. et al. Extended posterior leaflet augmentation for ischemic mitral regurgitation—augmented posterior leaflet snuggling up to anterior leaflet. Circ J 2019;83:567–75. [DOI] [PubMed] [Google Scholar]
- 28.Shibata T, Takahashi Y, Fujii H, Morisaki A, Abe Y.. Surgical considerations for atrial functional regurgitation of the mitral and tricuspid valves based on the etiological mechanism. Gen Thorac Cardiovasc Surg 2021;69:1041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the co-authors have full access to the patient data. Also, the data gathered or analysed in this study are available upon reasonable request to the corresponding author.