Abstract
Background
Understanding the hierarchy of functional impairment in older adults has helped illuminate mechanisms of impairment and inform interventions, but little is known about whether hierarchies vary by age. We compared the pattern of new-onset impairments in activities of daily living (ADLs) and instrumental ADLs (IADLs) from middle age through older age.
Methods
We conducted a cohort study using nationally representative data from 32 486 individuals enrolled in the Health and Retirement Study. The outcomes were new-onset impairment in each ADL and IADL, defined as self-reported difficulty performing each task, assessed yearly for 9 years. We used multistate models and competing risks survival analysis to estimate the cumulative incidence of impairment in each task by age group (ages 50–64, 65–74, 75–84, and 85 or older).
Results
The pattern of incident ADL impairments differed by age group. Among individuals ages 50–64 and 65–74 who were independent at baseline, over 9 years’ follow-up, difficulties dressing and transferring were the most common impairments to develop. In individuals ages 75–84 and 85 or older who were independent at baseline, difficulties bathing, dressing, and walking were most common. For IADLs, the pattern of impairments was similar across age groups; difficulty shopping was most common followed by difficulty managing money and preparing meals. Complementary analyses demonstrated a similar pattern.
Conclusions
These findings suggest that the hierarchy of ADL impairment differs by age. These findings have implications for the development of age-specific interventions to prevent or delay functional impairment.
Keywords: Activities of daily living, Functional impairment, Middle-aged
Difficulty performing basic activities of daily living (ADLs) such as bathing and dressing is common in older adults, affecting nearly 25% of people aged 65 or older (1). People who develop these difficulties, often called “functional impairment,” have poorer quality of life and a higher risk of acute care utilization, nursing home admission, caregiving needs, and death (2–4). Prior research shows that when older adults develop difficulty performing ADLs, these difficulties develop in a predictable order, called a “hierarchy of disability” (5). The first tasks affected are those that require strength, balance, and coordination, including bathing and dressing, while tasks that require manual dexterity, such as eating, are affected later (5–10). Understanding this hierarchy has illuminated mechanisms of functional impairment and informed interventions to prevent or delay impairment among older adults (11–13).
This research has focused almost entirely on older adults. However, nearly 15% of middle-aged people have functional impairment, and this proportion is increasing (14,15). Furthermore, when middle-aged people develop functional impairment, these impairments are associated with adverse outcomes similar to those seen in older adults (16,17). Despite the increasing prevalence of functional impairment in middle-aged people and its implications for quality of life, health outcomes, and costs (14), little is known about the hierarchy of functional impairment in middle age. Several studies suggest that in contrast to older adults who typically initially develop difficulty bathing, in middle-aged people, transferring and walking across a room are the most common ADL impairments (18,19). However, previous studies in middle-aged people assessed prevalent rather than incident functional impairment, and therefore did not distinguish between long-standing impairments due to congenital conditions or trauma versus impairments that develop in middle age and may have different clinical implications (14,15,18–21). Moreover, previous studies had relatively small sample sizes (18) and infrequent assessment intervals, up to 10 years apart (20).
Understanding the hierarchy of functional impairment in middle age and how this hierarchy changes with age may help illuminate mechanisms of functional impairment, inform interventions, and identify people in the early stages of impairment who could benefit from interventions to prevent further decline and promote recovery (7,20). We used nationally representative, longitudinal data with 2-year assessment intervals to compare the cumulative incidence of functional impairments in middle age through older age; examine how risk factors may contribute to differences in the pattern of impairments by age; and determine the order in which impairments develop.
Method
Setting and Participants
We used longitudinal data from the Health and Retirement Study (HRS) (22). The HRS is a panel study of a representative sample of Americans aged 50 or older. The first participants were enrolled in 1992 and additional participants are enrolled every 6 years, such that the sample remains representative of the population aged 50 or older. Participants are interviewed every 2 years, primarily by telephone; face-to-face interviews are conducted for those unable to access a telephone or too ill to participate by telephone.
Sample
We created a nationally representative cohort of individuals enrolled in the 1992, 1993, 1998, 2004, or 2010 study waves. Individuals enrolled in 1993 were part of the Asset and Health Dynamics Among the Oldest Old (AHEAD) study, a cohort of individuals aged 70 or older. Of 35 600 individuals in these waves, we excluded 2 853 who did not complete any follow-up interviews, 244 who were in a nursing home at their first interview, and 17 who had missing data for all ADLs and instrumental ADLs (IADLs) at their first interview, for a final cohort of 32 486 participants. We followed these participants at approximately 2-year intervals through 2016. The institutional review boards of the University of Pennsylvania, the University of California, San Francisco, and the San Francisco Veterans Affairs Medical Center approved the study.
Measures
Outcomes
To determine the hierarchy of functional impairment by age, we examined 2 sets of outcomes: the cumulative incidence of the first episode of impairment in each of 6 ADLs, and the cumulative incidence of the first episode of impairment in each of 5 IADLs. We also examined the order in which ADL impairments developed. To determine cumulative incidence, we examined the proportion of participants who developed a first episode of impairment in each ADL and IADL by age group, accounting for death as a competing risk. Age groups included 50–64 years (middle age), 65–75 years (young older age), 75–84 years (older age), and 85 or older (oldest age). To define ADL and IADL impairments, we used self-reported data. At baseline (date of study enrollment) and each biennial study wave, participants reported if they had difficulty performing each ADL (bathing, dressing, transferring, toileting, eating, walking across a room) and each IADL (managing money, managing medication, shopping for groceries, preparing meals, making telephone calls). We defined a first episode of impairment in each ADL or IADL as reporting difficulty performing that activity, even if that impairment was only reported at a single study wave. We chose this definition because an initial episode of functional impairment has been shown to be a sentinel event that predicts subsequent adverse outcomes, even if that impairment subsequently resolves (23–25).
Other measures
Other measures included sociodemographics and health status variables that predict functional impairment among older adults (26). Sociodemographics were assessed at study entry and included self-reported age, sex, race/ethnicity, marital status, and educational attainment. We also included year of study enrollment (1992, 1993, 1994, 1998, 2004, or 2010). Measures of health status included self-reported medical conditions (hypertension, stroke, diabetes, cardiac disease, chronic lung disease, cancer, arthritis). We also included visual impairment, defined as self-reported fair or poor eyesight despite best correction, and hearing impairment, defined as self-reported fair or poor hearing or use of a hearing aid. We assessed cognitive impairment using a modified version of the Telephone Interview for Cognitive Status (range, 0–27; dementia defined as a score of 0–6, cognitive impairment without dementia defined as a score of 7–11, normal cognition defined as a score of 12–27) (27,28) and depression using the 8-item Center for Epidemiological Studies-Depression scale (range, 0–8; clinically significant depressive symptoms defined as a score ≥3) (29). We calculated body mass index using self-reported weight and height. We assessed health-related behaviors including self-reported alcohol use, smoking status, and physical inactivity, defined as participating in activity once weekly or less (30).
Statistical Analysis
We used descriptive statistics to examine baseline participant characteristics. Analyses incorporated survey weights, strata, and clusters to account for the complex HRS survey design.
We used complementary methods to determine the cumulative incidence of each ADL and IADL impairment by age group; to determine how risk factors may explain differences in the pattern of impairments by age; and to examine the order in which impairments develop. First, we used multistate survival modeling as our primary approach to determine the cumulative incidence of the first episode of each ADL and IADL impairment by age group (31). Multistate models estimate the probability that individuals transition between 3 or more states and can be used to characterize longitudinal trajectories in data sets in which participants enroll at different ages and are followed for different time periods. We used multistate modeling as our primary approach rather than direct estimates of cumulative incidence because the study focuses on new-onset functional impairment; multistate models allowed us to statistically mimic a synthetic cohort of individuals who were aged 50 and difficulty-free at enrollment through age 100. This approach addresses the bias inherent in a direct analysis of the combined prevalent and incident cohort, in which individuals who are older at enrollment are more likely to have prevalent impairment and unobserved impairment before enrollment. We used a 3-state model based on first-order Markov assumptions to calculate the probability of transitioning between states for each ADL and IADL at each age, using the “msm” package for R (Supplementary Methods 1) (32). The 3 states included (i) independently performs that task; (ii) has difficulty performing that task; and (iii) death. We included current age and gender as covariates in the models.
We then used these transition probabilities to simulate functional outcomes for each ADL and IADL every year through age 100 for a large number of microsimulated participants (1 million male and 1 million female) who were independent at study entry at age 50. The large number of microsimulations ensures negligible Monte Carlo error is introduced into the point estimates; uncertainty in the estimate is determined by bootstrapping the entire microsimulation process as described below, and is thus determined only by the actual sample size of the data.
Next, we used the data generated through multistate modeling to determine the cumulative incidence of developing new impairment in each ADL and IADL for each age group. To do so, we used competing risks survival analysis to account for the competing risk of death (33–35). For individuals ages 50–64, we defined the baseline as age 50 and the event time as the years to onset of impairment before age 65. We used a similar approach for the other age groups. We censored participants who ended their observation period without the event and did not die. We performed survey-weighted bootstrapping to calculate 95% confidence intervals (95% CIs). We used 100 bootstrap samples and generated 100 000 microsimulations for each sample (50 000 male and 50 000 female; Supplementary Methods 1).
In sensitivity analyses, we calculated confidence intervals without weights. We also fit a multistate model with gender and 4 restricted cubic splines of age as time-variant covariates and 5 knots estimated using default quantiles. We compared the model with splines to the linear model using a likelihood ratio test. Although the model containing splines fit significantly better, the predictions were similar to those for the linear model (Supplementary Table 1; Supplementary Figures 1–11). Thus, since we used only the predicted transition probabilities for the microsimulations, we chose the more parsimonious linear model (36,37). The maximum follow-up time varied across age groups (ie, 14 years for ages 50–64, 9 years for 65–74 and 75–84, and 15 years for 85 or older). To have a uniform follow-up time across age groups, we reported the cumulative incidence at 9 years.
Next, we performed sensitivity analyses in which we directly estimated the cumulative incidence of each ADL and IADL impairment using a survival analysis framework. The purpose of these models was twofold: first, to provide a comparison, using real data, for the multistate models; and second, to use these models to determine how risk factors may explain differences in the pattern of impairments by age group. In these analyses, we began with the same overall cohort of 32 486 individuals used in the multistate models. To maximize the sample size in the oldest age groups, we created individual cohorts for each ADL and IADL, rather than replicating the multistate models in which participants were independent in all ADLs and IADLs at baseline. We excluded individuals with impairment in the ADL or IADL of interest at enrollment, but included individuals with other ADL or IADL impairments (Supplementary Methods 2). We defined the baseline as age 50 and the event time as the age of onset of impairment. Because HRS assessments are biennial, the date of onset of functional impairment cannot be observed exactly. We estimated the event time to be halfway between the date when impairment was first reported and the date of the previous assessment. We censored participants who ended their observation period or were lost to follow-up; we retained those who missed the first follow-up but had a subsequent assessment. In unadjusted models, we estimated the cumulative incidence of each ADL and IADL in the presence of death (Supplementary Methods 2) (35).
To examine how risk factors may contribute to patterns of ADL and IADL impairment by age group, we used the same competing risks survival analysis framework, now adjusting for potential risk factors. We used these models to calculate adjusted subdistribution hazard ratios for the association of risk factors with each ADL and IADL impairment in the presence of death as a competing risk (38). These models included probability weights and a derived survey cluster variable to adjust the standard errors for the complex sampling design. We used the same risk factors in each model (see Table 1), selected based on their importance for predicting functional impairment among older adults (26) and assessed at baseline. To account for cohort effects, we included year of enrollment. We tested interaction terms between age and several variables that we hypothesized may interact with age (gender, married/partnered, educational attainment, body mass index, smoking, cognition). All of these terms were significant and thus we ran 2 sets of adjusted models, one with and one without interactions (Supplementary Methods 2).
Table 1.
Characteristics of 32 486 Community-Dwelling Adults Ages 50 or Older
| Characteristics | Participants, No. (weighted %) (N = 32 486) |
|---|---|
| Age group at baseline | |
| 50–64 years | 21 965 (74.1) |
| 65–74 years | 6 439 (16.5) |
| 75–84 years | 3 285 (7.6) |
| 85 years or older | 797 (1.8) |
| Enrollment year and years of birth | |
| 1992 (born 1931–1941) | 9 841 (19.7) |
| 1993 (born <1924) | 6 529 (15.5) |
| 1994 (born 1924–1930) | 3 611 (9.5) |
| 1998 (born 1942–1947) | 3 364 (14.9) |
| 2004 (born 1948–1953) | 4 457 (20.7) |
| 2010 (born 1954–1959) | 4 684 (19.8) |
| Female | 18 216 (53.5) |
| Race/ethnicity | |
| White | 22 401 (78.0) |
| Black | 5 668 (10.7) |
| Hispanic/Latino | 3 513 (8.1) |
| Other | 861 (3.2) |
| Married/partnered | 22 724 (70.7) |
| Educational attainment | |
| Less than high school | 9 961 (24.6) |
| High school | 9 445 (28.4) |
| Some college | 7 013 (23.8) |
| College or higher | 6 061 (23.3) |
| Chronic medical conditions | |
| Hypertension | 12 864(37.3) |
| Stroke | 1 475 (4.0) |
| Diabetes | 3 997 (11.4) |
| Heart disease | 4 772 (13.6) |
| Chronic lung disease | 1 765 (5.3) |
| Cancer (other than nonmelanoma skin cancer) | 2 317 (6.9) |
| Arthritis | 10 866 (32.7) |
| Depression* | 5 067 (21.8) |
| Visual impairment | 6 505 (18.1) |
| Hearing impairment | 5 479 (16.0) |
| Body mass index | |
| <18.5 (underweight) | 466 (1.3) |
| 18.5–24.9 (normal weight) | 10 541 (32.2) |
| 25–29.9 (overweight) | 12 582 (39.2) |
| >30 (obese) | 8 450 (27.3) |
| Number of drinks per day >3 | 2 643 (10.1) |
| Currently smokes | 6 747(20.8) |
| Infrequent physical activity* | 17 897 (66.2) |
| Cognitive status based on Telephone Interview for Cognitive Impairment score | |
| Normal cognition | 24 827 (83.7) |
| Cognitive impairment without dementia | 4 854 (12.5) |
| Dementia | 1 663 (3.8) |
Notes: Analyses incorporated survey weights, strata, and clusters to account for the complex Health and Retirement Study (HRS) survey design.
*Variables excluded from multivariable competing risks regression models due to high percentage of missing data.
We used these models to calculate adjusted cumulative incidences of each ADL and IADL and each age group at the means of the predictors in the model, using the Stata command “stcurve.” These analyses do not include confidence intervals, as “stcurve” does not currently compute standard errors or confidence intervals for cumulative incidence functions.
Last, we examined the order in which ADL impairments developed at the level of the participant. We focused on ADLs with the most pronounced difference in relative cumulative incidence by age group, namely bathing, dressing, and transferring. To conduct these analyses, we created a cohort without missing data for these ADLs at baseline (Supplementary Methods 3). We then examined the proportion of participants with each of 26 outcomes in the following 6 categories: (i) did not develop difficulty in bathing, dressing, or transferring over follow-up; (ii) developed difficulty with 1 of the 3 ADLs; (iii) developed difficulty with 2 of the 3 ADLs in the same wave; (iv) developed difficulty with all 3 ADLs in the same wave; (v) developed difficulty with 2 ADLs in different waves; or (vi) developed difficulty with 3 ADLs in different waves.
We performed analyses using SAS/STAT 15.1 (SAS Institute Inc., Cary, NC), Stata 16.1 (StataCorp LLC, College Station, TX), and R version 4.0.4 (2021 The R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant Characteristics
Of the 32 486 participants, 53.5% were women, 78.0% were White, 10.7% Black, and 8.1% Latino (Table 1). The majority were ages 50–64 at study enrollment (74.1%), with smaller percentages ages 65–74 (16.5%), 75–84 (7.6%), or 85 or older (1.8%). One-quarter (24.6%) had less than a high school education. The most common chronic condition was hypertension (37.3%), followed by arthritis (32.7%), heart disease (13.6%), and diabetes (11.4%). More than one-fifth (21.8%) had clinically significant depressive symptoms. Sensory impairments were common, with 18.1% reporting visual impairment and 16.0% hearing impairment. More than one-third (39.2%) were overweight and 27.3% were obese. The majority were physically inactive (66.2%).
Cumulative Incidence of ADL Impairments by Age Group
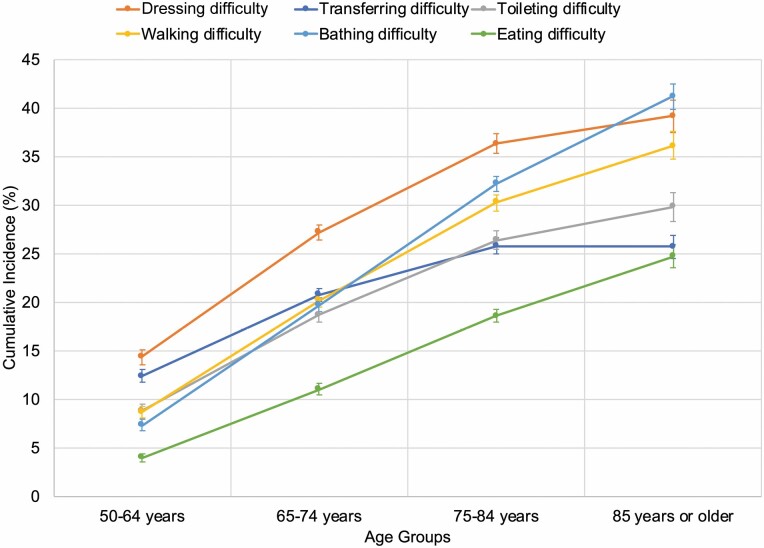
In analyses based on multistate models, the cumulative incidence of each ADL increased with age with the exception of transferring, for which the cumulative incidence was similar in the 2 oldest age groups (Figure 1). The pattern of incident ADL impairments differed between the younger and older age groups. In individuals ages 50–64 years, difficulty dressing developed most commonly over 9 years’ follow-up (14.4%; 95% CI, 13.6%–15.1%), followed by difficulty transferring (12.4%; 95% CI, 11.7%–13.1%), toileting and walking (with similar incidences of 8.8% [95% CI, 8.1%–9.5%] and 8.7% [95% CI, 8.1%–9.3%], respectively), bathing (7.3%; 95% CI, 6.7%–7.9%), and eating (4.0%; 95% CI, 3.5%–4.4%). The pattern of impairments was similar in participants ages 65–74; difficulty dressing developed most commonly (27.2%; 95% CI, 26.4%–28.0%), followed by transferring, walking, bathing, and toileting (with similar incidences of 20.8% [95% CI, 20.1%–21.4%]; 20.2% [95% CI, 19.5%–20.8%]; 19.7% [95% CI, 19.0%–20.5%]; and 18.7% [95% CI, 17.9%–19.5%], respectively). Difficulty eating developed least commonly (11.0%, 95% CI, 10.4%–11.6%).
Figure 1.
Cumulative incidence of ADL impairments by age group. ADL indicates activities of daily living. The figure shows the cumulative incidence of new ADL impairments at 9 years with 95% confidence intervals for each age group (ie, 50–64 years, 65–74 years, 75–84 years, and 85 years or older). Cumulative incidences were determined using multistate modeling and competing risk survival analysis to account for the competing risk of death. Analyses incorporated survey weights, strata, and clusters to account for the complex Health and Retirement Study (HRS) survey design.
For participants ages 75–84, the pattern of ADL impairments shifted, with bathing and walking impairment becoming more common and transferring becoming less common. While difficulty dressing still developed most commonly (36.3%; 95% CI, 35.3%–37.3%), followed by difficulty bathing (32.2%; 95% CI, 31.4%–33.0%), walking (30.3%; 95% CI, 29.4%–31.1%), toileting (26.4%, 95% CI, 25.5%–27.3%), transferring (25.6%; 95% CI, 25.0%–26.6%), and eating (18.6%; 95% CI, 18.0%–19.3%). For participants ages 85 or older, difficulty bathing, dressing, and walking had similar cumulative incidences (41.2% [95% CI, 39.8%–42.5%]; 39.2% [95% CI, 37.5%–40.8%]; 36.1% [95% CI, 34.7%–37.6%], respectively), followed by difficulty toileting (29.8%; 95% CI, 28.3%–31.3%), transferring (25.7%; 95% CI, 24.5%–26.9%), and eating (24.7%; 95% CI, 23.6%–25.9%). In sensitivity analyses using unweighted data, point estimates and confidence intervals were similar.
In sensitivity analyses using direct cumulative incidence estimates, the pattern of ADL impairments by age group was generally similar to that based on the multistate model (Supplementary Figure 12). However, transferring difficulty developed less commonly in participants ages 65–74, and bathing difficulty developed most commonly in participants ages 75–84 and 85 or older, compared to just in the oldest age group in the multistate model. Additionally, the cumulative incidence of each ADL impairment in the oldest age group was higher than those based on the multistate model, and the width of the corresponding confidence intervals were considerably wider than those for estimates derived using the multistate model.
Cumulative Incidence of IADL Impairments by Age Group
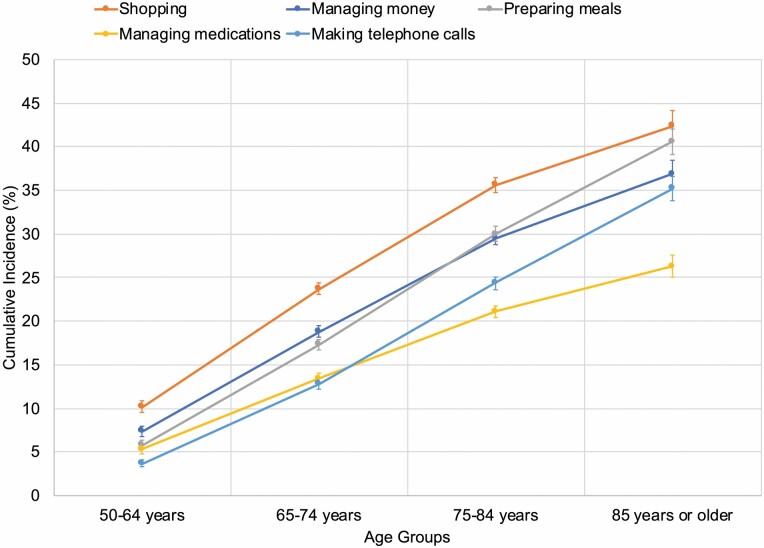
In analyses based on multistate models, the cumulative incidence of IADLs increased with age, as for ADLs (Figure 2). However, the pattern of impairments was more similar by age for IADLs than ADLs. For participants ages 50–64, shopping difficulty developed most commonly (10.2%; 95% CI, 9.5%–10.9%), followed by difficulty managing money (7.4%; 95% CI, 6.8%–7.9%), preparing meals and managing medications (with similar incidences of 5.8%; 95% CI, 5.3%–6.3% and 5.3%; 95% CI, 4.8%–5.8%), and making telephone calls (3.7%; 95% CI, 3.3%–4.1%). Impairments showed the same pattern for participants ages 65–74. For participants ages 75–84 and 85 or older, the pattern of impairments differed slightly. Difficulty shopping still developed most commonly, but difficulty preparing meals was more common than difficulty managing money, and difficulty making telephone calls was more common than difficulty managing medications.
Figure 2.
Cumulative incidence of IADL impairments by age group. IADL indicates instrumental activities of daily living. The figure shows the cumulative incidence of new IADL impairments at 9 years with 95% confidence intervals for each age group (ie, 50–64 years, 65–74 years, 75–84 years, and 85 years or older). Cumulative incidences were determined using multistate modeling and competing risk survival analysis to account for the competing risk of death. Analyses incorporated survey weights, strata, and clusters to account for the complex Health and Retirement Study (HRS) survey design.
In sensitivity analyses using direct cumulative incidence estimates, the pattern of IADL impairments was generally similar (Supplementary Figure 13). However, among participants ages 65–74, difficulty making telephone calls developed more commonly than difficulty managing medications, and in the oldest age group, the cumulative incidences were similar for difficulties shopping and managing money. Also, the cumulative incidence of each IADL impairment in the oldest age group was higher compared to those based on the multistate model, and the width of the corresponding confidence intervals were wider than those based on the multistate model.
Adjusted Cumulative Incidences of ADL and IADL Impairments by Age Group
In sensitivity analyses using direct estimates of cumulative incidence adjusted for potential risk factors and cohort year, the patterns of ADL and IADL impairments were similar to the other analyses (Supplementary Results 1–2; Supplementary Tables 2–4; Supplementary Figures 14 and 15). However, in the 3 oldest age groups, the cumulative incidences for all ADLs and IADLs were substantially lower than in the unadjusted analyses.
In adjusted cumulative incidence analyses including interaction terms, the patterns of ADL and IADL impairments in the 3 youngest age groups were again similar to the other analyses (Supplementary Results 1–2; Supplementary Tables 5 and 6; Supplementary Figures 16 and 17). However, the cumulative incidences of several ADLs and IADLs in individuals ages 85 or older were lower than in individuals ages 75–84. The pattern of IADL impairments also differed in individuals ages 85 or older, with difficulty managing money more common than difficulty shopping.
Order in Which ADL Impairments Developed by Age Group
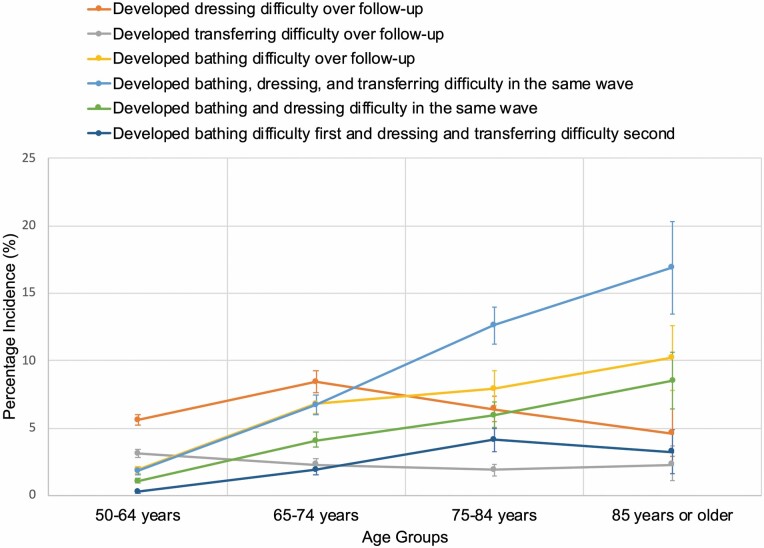
In analyses examining the order in which impairments in bathing, dressing, and transferring developed, the findings were consistent with the cumulative incidence analyses, while revealing additional patterns in the development of combinations of ADL impairments (Figure 3; Supplementary Table 7).
Figure 3.
Order in which incident difficulty dressing, transferring, and bathing developed by age group. The figure shows the order in which incidence difficulty dressing, transferring, and bathing developed by age group (ie, 50–64 years, 65–74 years, 75–84 years, and 85 years or older) among individuals who were independent in these activities of daily living (ADLs) at baseline. The figure shows the proportion of individuals with each of the listed outcomes, which were the most common incident impairments observed in the cohort. Proportions were determined by counting new-onset ADL impairments over follow-up. Analyses incorporated survey weights, strata, and clusters to account for the complex Health and Retirement Study (HRS) survey design.
First, consistent with the cumulative incidence analyses, the pattern of affected ADLs differed by age. Dressing and transferring difficulty developed more commonly in younger age groups, whereas bathing difficulty developed more commonly in older age groups. Specifically, among participants ages 50–64, dressing difficulty was most common (5.6%; 95% CI, 5.3%–6.0%), followed by transferring (3.1%; 95% CI, 2.8%–3.4%) and bathing (1.9%; 95% CI, 1.7%–2.2%). This pattern shifted in the older age groups. In participants ages 65–74, dressing difficulty was most common (8.4%, 95% CI, 7.6%–9.2%), followed by bathing (6.8%; 95% CI, 6.1%–7.4%). In persons ages 75–84, bathing and dressing difficulties had similar incidences (7.9%; 95% CI, 6.7%–9.2% and 6.4%; 95% CI, 5.5%–7.4%, respectively). In persons aged 85 or older, bathing difficulty alone and bathing and dressing difficulty in the same wave had similar incidences (10.2%; 95% CI, 7.8%–12.6% and 8.5%; 95% CI, 6.4%–10.7%) and were more common than dressing difficulty alone (4.6%; 95% CI, 2.9%–6.4%).
Second, younger individuals were less likely than older individuals to develop impairments in multiple ADLs in a single wave. For example, in individuals ages 50–64 and 65–74, developing impairments in all 3 ADLs in a single wave was the third-most common pattern (1.8%; 95% CI, 1.6%–2.0% and 6.7%; 95% CI, 6.0%–7.4%, respectively). In individuals ages 75–84 and 85 or older, it was the most common pattern (ages 75–84, 12.6%; 95% CI, 11.2%–13.9%; ages 85 or older, 16.9%; 95% CI, 13.5%–20.3%).
Discussion
In this nationally representative study, we found that patterns of incident ADL impairment differed in younger versus older age groups. In individuals ages 50–64 and 65–74, difficulties dressing and transferring developed more frequently than difficulties toileting, walking, bathing, and eating, whereas in individuals ages 75–84 and 85 or older, difficulties bathing, dressing, and walking were most common. In contrast, patterns of IADL impairment were similar across age groups, with difficulty shopping developing more frequently than difficulty managing money or preparing meals. The patterns of ADL and IADL impairment were similar across differing modeling approaches and after multivariable adjustment. Analyses of the order in which ADL impairments developed were consistent with the cumulative incidence analyses and also showed that older individuals were more likely to develop multiple ADL impairments in a single study wave. These findings suggest that a hierarchy of ADL impairment exists in middle-aged as well as older adults and that this hierarchy differs by age. These findings have implications for the development of age-specific interventions to prevent or delay ADL impairment.
Consistent with prior research, we found that among adults aged 75 or older, impairments in bathing, dressing, and walking were more common than impairments in transferring, toileting, and eating (5–10). This ordering is thought to reflect a hierarchy in which tasks requiring strength, balance, and coordination are affected first, while tasks requiring manual dexterity, such as eating, are affected later (5–10). To our knowledge, prior research has not examined the hierarchy of incident ADL and IADL impairment in middle-aged adults, although studies have examined the hierarchy of higher-level physical functions such as running and lifting weights (7). However, cross-sectional research shows that the most common ADL impairments in middle-aged people are transferring and walking (18,39).
Our study extends this work by using longitudinal data to compare the patterns of incident ADL impairments in middle-aged versus older adults. Consistent with prior cross-sectional studies, we found that dressing and transferring difficulties were most common in adults ages 50–64 and 65–74, while bathing and walking difficulties were less common. This pattern persisted after multivariable adjustment. Analyses of the order in which ADL impairments developed were also consistent with this pattern, showing that younger participants were more likely to develop dressing and transferring difficulty, whereas older participants were more likely to develop bathing difficulty. Younger participants were also less likely than older participants to develop multiple ADL impairments in a single wave.
It is not yet clear why dressing and transferring difficulties develop more commonly than bathing and walking difficulties in middle-aged adults. These tasks require similar abilities, including upper and lower extremity strength and mobility (9,40). However, studies suggest that in older adults, difficulty bathing is prevalent because it is a complex activity: bathing includes multiple subtasks and impairment results from the interplay of risk factors including balance difficulty, arthritis, and fear of falling (40). Compared to older adults, middle-aged adults have a lower prevalence of balance difficulties and falls (41). On the other hand, transferring is affected by conditions which are common in middle-aged people, including arthritis, obesity, back pain, and neck pain (14). While we adjusted for arthritis and body mass index in our models, we were unable to adjust for balance difficulty, physical performance, or fear of falling, as these variables were not available for all age groups. Thus, these factors may contribute to the observed differences in patterns of ADLs by age. The finding that younger participants were less likely to develop multiple ADL impairments in a single study wave contrasts with prior hypotheses that ADL impairment in middle versus older age is more likely to be “catastrophic,” meaning that a discrete event such as an accident or stroke simultaneously affects an individuals’ ability to perform multiple tasks (5,42). Study assessments in the HRS are biennial, and thus we cannot determine if impairments reported in a single wave occurred simultaneously. However, our findings show that developing individual ADL impairments—namely dressing and transferring—is the most common pattern in middle-aged adults, whereas older adults are more likely to develop impairment in multiple ADLs.
While patterns of ADL impairment differed by age, patterns of IADL impairments were similar. Difficulty shopping was the most common impairment in all age groups and there were only minor differences in the ordering of other IADLs. These patterns persisted after adjustment for sociodemographics, health status, and cognition. However, the pattern of impairments in the oldest age group changed after adjusting for these characteristics plus interactions between age and potential risk factors, with difficulty managing money and making telephone calls becoming more common. Moreover, the adjusted cumulative incidences of these IADLs in participants ages 85 or older were lower than in those ages 74–85. These results suggest that in the 3 younger age groups, these risk factors had a relatively similar effect on risk of IADL impairment. However, in the oldest age group, the impact of these risk factors differed. Additional study is needed to understand why some risk factors may affect risk of IADL impairment differently among the oldest old.
These findings have clinical implications. Current approaches to addressing ADL impairment in middle age are often reactive, such as treatments for arthritis or chronic pain that are delivered after functional impairment develops. Our findings suggest that like in older adults, ADL impairment in middle age is common and has a unique hierarchy of development. Thus, a proactive approach to preventing ADL impairment in this age group is needed. Among older adults, understanding the hierarchy of functional impairment has informed interventions to prevent or delay ADL impairment (11–13). Similar interventions may hold promise for middle-aged adults. However, interventions will need to be adapted to focus on preventing the ADLs that develop most frequently in middle age, namely difficulty dressing and transferring. Studying the components of these activities among middle-aged adults, similar to studies of bathing in older adults (10,40), may help inform preventive interventions for middle-aged people.
This study has several limitations. Measures of ADLs and IADLs were self-reported. However, self-reported functional status is an important patient-centered measure (45) that strongly predicts adverse outcomes (2–4,45). The multistate models were adjusted only for age and gender, and thus the cumulative incidences represent gross population estimates. However, the multistate cumulative incidence estimates were similar to the direct estimates after multivariable adjustment. To conduct direct estimates of cumulative incidence, we included individuals enrolled at age 70 or older. These participants are more likely to have unobserved impairment before baseline compared to participants who enrolled at younger ages. Thus, the direct cumulative incidence estimates for older age groups are more likely to include individuals with prior functional impairment. In analyses of the order of ADL impairments, we focused on ADLs with the most pronounced differences in relative cumulative incidence, rather than all 6; examining 3 ADLs resulted in 26 possible combinations, many with low incidence. Including all 6 ADLs would generate a very large number of combinations and make it more difficult to draw meaningful inferences. Due to high levels of missingness for depression and physical inactivity, we were unable to adjust for these variables in the multivariable analyses.
Conclusions
We found that the hierarchy of ADL impairment differs by age. In people ages 50–64 and 65–74, difficulty dressing and transferring developed most commonly, whereas in adults ages 75 or older, difficulty with bathing, dressing, and walking were most common. In contrast, the pattern of IADL impairments was relatively similar by age. These findings were similar across differing modeling approaches and after multivariable adjustment. Analyses of the order in which ADL impairments developed were consistent with the cumulative incidence analyses. Our findings suggest that interventions to prevent or delay ADL impairment in middle age need to be tailored to meet the specific functional needs of this age group, which differ from those of older adults.
Funding
This work was supported by the Research Evaluation & Allocation Committee (REAC), School of Medicine, University of California, San Francisco (no grant number to R.T.B.); by the National Institute on Aging at the National Institutes of Health (grant numbers K23AG045290, K76AG057016 to R.T.B.; grant number P30AG044281 to R.T.B., L.G.D.-R., W.J.B., S.J.L., M.A.S., Kenneth Covinsky, principal investigator; grant number K24AG049057 to M.A.S.; grant number R01AG0478897 to S.J.L.); by the American Federation for Aging Research (grant number K76AG057016 to R.T.B.); and by the Veterans Affairs Health Services Research and Development Service (grant number IIR 15-434 to S.J.L.).
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors thank Joshua Seeherman for assistance with preparing figures for the manuscript.
Contributor Information
Rebecca T Brown, Division of Geriatric Medicine, Perelman School of Medicine of the University of Pennsylvania, Philadelphia, USA; Geriatrics and Extended Care Program, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, Pennsylvania, USA; Center for Health Equity Research and Promotion, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, Pennsylvania, USA.
L Grisell Diaz-Ramirez, Division of Geriatrics, Department of Medicine, University of California, San Francisco, USA; Geriatrics, Palliative, and Extended Care Service Line, San Francisco Veterans Affairs Medical Center, California, USA.
W John Boscardin, Division of Geriatrics, Department of Medicine, University of California, San Francisco, USA; Department of Epidemiology & Biostatistics, University of California, San Francisco, USA.
Anne R Cappola, Division of Endocrinology, Diabetes, and Metabolism, Perelman School of Medicine of the University of Pennsylvania, Philadelphia, USA.
Sei J Lee, Division of Geriatrics, Department of Medicine, University of California, San Francisco, USA; Geriatrics, Palliative, and Extended Care Service Line, San Francisco Veterans Affairs Medical Center, California, USA.
Michael A Steinman, Division of Geriatrics, Department of Medicine, University of California, San Francisco, USA; Geriatrics, Palliative, and Extended Care Service Line, San Francisco Veterans Affairs Medical Center, California, USA.
Author Contributions
Drs. R.T.B. and W.J.B. and Ms. L.G.D.-R. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: R.T.B., L.G.D.-R., W.J.B., and M.A.S. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: R.T.B. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: L.G.D.-R. and W.J.B. Obtained funding: R.T.B. Administrative, technical, or material support: R.T.B. Supervision: R.T.B., W.J.B., and M.A.S.
References
- 1. Freedman VA, Spillman BC, Andreski PM, et al. Trends in late-life activity limitations in the United States: an update from five national surveys. Demography. 2013;50(2):661–671. doi: 10.1007/s13524-012-0167-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fried TR, Bradley EH, Williams CS, Tinetti ME. Functional disability and health care expenditures for older persons. Arch Intern Med. 2001;161(21):2602–2607. doi: 10.1001/archinte.161.21.2602 [DOI] [PubMed] [Google Scholar]
- 3. Gaugler JE, Duval S, Anderson KA, Kane RL. Predicting nursing home admission in the U.S: a meta-analysis. BMC Geriatr. 2007;7:13. doi: 10.1186/1471-2318-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193. doi: 10.1001/jama.279.15.1187 [DOI] [PubMed] [Google Scholar]
- 5. Ferrucci L, Guralnik JM, Cecchi F, et al. Constant hierarchic patterns of physical functioning across seven populations in five countries. Gerontologist. 1998;38(3):286–294. doi: 10.1093/geront/38.3.286 [DOI] [PubMed] [Google Scholar]
- 6. Dunlop DD, Hughes SL, Manheim LM. Disability in activities of daily living: patterns of change and a hierarchy of disability. Am J Public Health. 1997;87(3):378–383. doi: 10.2105/ajph.87.3.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeh CJ, Wang CY, Tang PF, Lee MC, Lin HS, Chen HY. Hierarchy of higher-level physical functions: a longitudinal investigation on a nationally representative population of community-dwelling middle-aged and elderly persons. Disabil Rehabil. 2012;34(15):1271–1276. doi: 10.3109/09638288.2011.641657 [DOI] [PubMed] [Google Scholar]
- 8. Gerrard P. The hierarchy of the activities of daily living in the Katz index in residents of skilled nursing facilities. J Geriatr Phys Ther. 2013;36(2):87–91. doi: 10.1519/JPT.0b013e318268da23 [DOI] [PubMed] [Google Scholar]
- 9. Jagger C, Arthur AJ, Spiers NA, Clarke M. Patterns of onset of disability in activities of daily living with age. J Am Geriatr Soc. 2001;49(4):404–409. doi: 10.1046/j.1532-5415.2001.49083.x [DOI] [PubMed] [Google Scholar]
- 10. Gill TM, Guo Z, Allore HG. The epidemiology of bathing disability in older persons. J Am Geriatr Soc. 2006;54(10):1524–1530. doi: 10.1111/j.1532-5415.2006.00890.x [DOI] [PubMed] [Google Scholar]
- 11. Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x [DOI] [PubMed] [Google Scholar]
- 12. Penninx BW, Messier SP, Rejeski WJ, et al. Physical exercise and the prevention of disability in activities of daily living in older persons with osteoarthritis. Arch Intern Med. 2001;161(19):2309–2316. doi: 10.1001/archinte.161.19.2309 [DOI] [PubMed] [Google Scholar]
- 13. Pahor M, Guralnik JM, Ambrosius WT, et al. ; LIFE Study Investigators . Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin LG, Freedman VA, Schoeni RF, Andreski PM. Trends in disability and related chronic conditions among people ages fifty to sixty-four. Health Aff (Millwood). 2010;29(4):725–731. doi: 10.1377/hlthaff.2008.0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin LG, Schoeni RF. Trends in disability and related chronic conditions among the forty-and-over population: 1997-2010. Disabil Health J. 2014;7(1 suppl):S4–S14. doi: 10.1016/j.dhjo.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown RT, Diaz-Ramirez LG, Boscardin WJ, Lee SJ, Williams BA, Steinman MA. Association of functional impairment in middle age with hospitalization, nursing home admission, and death. JAMA Intern Med. 2019;179(5):668–675. doi: 10.1001/jamainternmed.2019.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown RT, Diaz-Ramirez LG, Boscardin WJ, Lee SJ, Steinman MA. Functional impairment and decline in middle age: a cohort study. Ann Intern Med. 2017;167(11):761–768. doi: 10.7326/M17-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cimino T, Steinman MA, Mitchell SL, et al. The course of functional impairment in older homeless adults: disabled on the street. JAMA Intern Med. 2015;175(7):1237–1239. doi: 10.1001/jamainternmed.2015.1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller DK, Wolinsky FD, Malmstrom TK, Andresen EM, Miller JP. Inner city, middle-aged African Americans have excess frank and subclinical disability. J Gerontol A Biol Sci Med Sci. 2005;60(2):207–212. doi: 10.1093/gerona/60.2.207 [DOI] [PubMed] [Google Scholar]
- 20. Wloch EG, Kuh D, Cooper R. Is the hierarchy of loss in functional ability evident in midlife? Findings from a British birth cohort. PLoS One. 2016;11(5):e0155815. doi: 10.1371/journal.pone.0155815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liang J, Bennett JM, Shaw BA, et al. Gender differences in functional status in middle and older age: are there any age variations? J Gerontol B Psychol Sci Soc Sci. 2008;63(5):S282–S292. doi: 10.1093/geronb/63.5.s282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardy SE, Dubin JA, Holford TR, Gill TM. Transitions between states of disability and independence among older persons. Am J Epidemiol. 2005;161(6):575–584. doi: 10.1093/aje/kwi083 [DOI] [PubMed] [Google Scholar]
- 24. Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291(13):1596–1602. doi: 10.1001/jama.291.13.1596 [DOI] [PubMed] [Google Scholar]
- 25. Gill TM, Kurland BF. Prognostic effect of prior disability episodes among nondisabled community-living older persons. Am J Epidemiol. 2003;158(11):1090–1096. doi: 10.1093/aje/kwg237 [DOI] [PubMed] [Google Scholar]
- 26. Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48(4):445–469. doi: 10.1016/s0277-9536(98)00370-0 [DOI] [PubMed] [Google Scholar]
- 27. Breitner JC, Welsh KA, Gau BA, et al. Alzheimer’s disease in the National Academy of Sciences-National Research Council Registry of Aging Twin Veterans. III. Detection of cases, longitudinal results, and observations on twin concordance. Arch Neurol. 1995;52(8):763–771. doi: 10.1001/archneur.1995.00540320035011 [DOI] [PubMed] [Google Scholar]
- 28. Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):i162–i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 30. He XZ, Baker DW. Body mass index, physical activity, and the risk of decline in overall health and physical functioning in late middle age. Am J Public Health. 2004;94(9):1567–1573. doi: 10.2105/ajph.94.9.1567m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai L, Schenker N, Lubitz J. Analysis of functional status transitions by using a semi-Markov process model in the presence of left-censored spells. J R Stat Soc Ser C Appl Stat. 2006;55(4):477–491. doi: 10.1111/j.1467-9876.2006.00548.x [DOI] [Google Scholar]
- 32. Jackson C. Multi-state models for panel data: the msm package for R. J Stat Softw. 2011;38. doi: 10.18637/jss.v038.i08 [DOI] [Google Scholar]
- 33. Southern DA, Faris PD, Brant R, et al. ; APPROACH Investigators . Kaplan–Meier methods yielded misleading results in competing risk scenarios. J Clin Epidemiol. 2006;59(10):1110–1114. doi: 10.1016/j.jclinepi.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 34. Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58(4):783–787. doi: 10.1111/j.1532-5415.2010.02767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4(2):103–112. doi: 10.1177/1536867X0400400201 [DOI] [Google Scholar]
- 36. Devlin T, Weeks B. Spline functions for logistic regression modeling. In: Proceedings of the 11th Annual SAS Users Group International Conference. Cary, NC: SAS Institute, Inc.; 1986:646–651. [Google Scholar]
- 37. Harrell FE. Hmisc: Harrell Miscellaneous. R Package Version 4.5-0. https://cran.r-project.org/web/packages/Hmisc/index.html. Accessed June 15, 2021.
- 38. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 39. Brown RT, Komaiko KD, Shi Y, et al. Bringing functional status into a big data world: validation of national Veterans Affairs functional status data. PLoS One. 2017;12(6):e0178726. doi: 10.1371/journal.pone.0178726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naik AD, Concato J, Gill TM. Bathing disability in community-living older persons: common, consequential, and complex. J Am Geriatr Soc. 2004;52(11):1805–1810. doi: 10.1111/j.1532-5415.2004.52513.x [DOI] [PubMed] [Google Scholar]
- 41. Brown RT, Kiely DK, Bharel M, Mitchell SL. Geriatric syndromes in older homeless adults. J Gen Intern Med. 2012;27(1):16–22. doi: 10.1007/s11606-011-1848-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferrucci L, Guralnik JM, Simonsick E, Salive ME, Corti C, Langlois J. Progressive versus catastrophic disability: a longitudinal view of the disablement process. J Gerontol A Biol Sci Med Sci. 1996;51(3):M123–M130. doi: 10.1093/gerona/51a.3.m123 [DOI] [PubMed] [Google Scholar]
- 43. Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56(10):1839–1844. doi: 10.1111/j.1532-5415.2008.01923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–1066. doi: 10.1056/NEJMsa012528 [DOI] [PubMed] [Google Scholar]
- 45. Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19(10):1027–1033. doi: 10.1111/j.1525-1497.2004.40016.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.