Abstract
Background
Motor function affects ability to perform daily activities and maintain independence. Yet, the interrelatedness of upper and lower extremity motor impairments and the magnitude of their contribution to slow gait and mobility difficulty are not well investigated.
Methods
Participants in the Baltimore Longitudinal Study of Aging (N = 728, aged 50–99) completed motor and physical function tests including grip and knee extension strength, pegboard, finger tapping, standing balance, chair stands, fast-paced 400-m walk, and usual gait speed. Slow gait was defined as usual gait speed <1.0 m/s. Mobility difficulty was defined as self-reported difficulty walking ¼ mile or climbing stairs. Structural equation modeling examined the interrelationships of motor measures and their contributions to slow gait and mobility difficulty, adjusting for demographics and comorbidities.
Results
Poorer manual dexterity (−0.571 standard deviation [SD] units, p < .001) and lower muscle strength (upper and lower extremity; −0.447 SD units, p = .014) were most strongly associated with slow gait speed, followed by slower chair stand pace (−0.195 SD units, p = .002) and greater lap time variation (0.102 SD units, p = .028). Lower muscle strength (−0.582 SD units, p = .001) was most strongly associated with mobility difficulty, followed by slower chair stand pace (−0.322 SD units, p < .001), slower gait speed (−0.247 SD units, p < .001), and poorer standing balance (−0.190 SD units, p = .043).
Conclusions
Components of manual dexterity and strength were the strongest correlates of slow gait and mobility difficulty in mid-to-late life. Longitudinal studies examining relationships between changes in these motor parameters and mobility are needed to elucidate possible causal effects.
Keywords: Gait speed, Mobility difficulty, Motor function, Physical function, Structural equation modeling
Older adults experience motor function decline early in the disablement process, affecting performance of daily activities and the ability to maintain independence (1–3). These age-related motor impairments include muscle weakness, slower gait speed, greater movement variability, and poor balance control (4–8), which may reflect deteriorating sensorimotor control. Over the past several decades, motor impairments have been linked to increased risk of falls, disability, and mortality (5–7,9–12). In addition, substantial research has found motor impairments are associated with a greater likelihood of cognitive decline that may precede development of dementia by several years (10–12). Thus, motor impairments may serve as early markers of an emerging low-functioning phenotype that precedes physical and cognitive impairments in older adults.
Several studies have examined a variety of motor and physical performance measures in relation to mobility disability and falls risk. A composite measure of physical performance, the Short Physical Performance Battery (SPPB), has been widely used to examine physical function incorporating lower extremity strength via capacity to repeatedly rise from a chair without using one’s arms, standing balance, and gait speed (13). Lord and colleagues also developed the Physiological Profile Assessment to assess physiological capacities needed for motor performance using multiple factors including vision (contrast sensitivity), muscle strength, proprioception, reaction time, and balance (14). These and other summary measures of motor and physical performance enable prediction of adverse health outcomes including falls, disability, and mortality (13–15), but do not elucidate potential pathways and interconnections between motor impairments and mobility function. Additionally, these summary measures target older and generally more debilitated persons. Focusing on higher-functioning middle-aged and older adults with more challenging functional assessments may improve our understanding of the motor impairments that precede the onset and progression of early declines in functional abilities, when intervention and rehabilitative efforts are more likely to be successful.
Structural equation modeling (SEM), a multivariate analysis technique, enables researchers to examine relationships among latent variables as unobserved theoretical constructs and between observed and latent variables. It treats 1 or more variables simultaneously as predictors and outcomes, depending on the theoretical framework (16). Although SEM has been used in medical and health sciences research, few studies have used SEM to model the connection between motor impairments and physical function in older adults (17–19). Moreover, a comprehensive understanding of upper and lower extremity motor impairments is lacking in higher-functioning middle-aged and older adults. As slow gait speed has been associated with various adverse health outcomes including cognitive decline and mortality (5), quantifying associations between and among a broad sampling of motor function measures and mobility difficulty may facilitate identification of the predominant contributors to reduced and declining mobility in mid-to-late life. This study aims to: (a) delineate the prevalence of motor and physical function impairments in middle-aged and older adults, and (b) examine the interrelationships among multiple motor and physical performance measures and their contributions to slow gait speed and mobility difficulty using SEM.
Method
Study Participants
The Baltimore Longitudinal Study of Aging (BLSA) was established in 1958 and conducted by the National Institute on Aging Intramural Research Program. This longitudinal study continuously enrolls community-dwelling volunteers, aiming to explore the interdependence of aging and disease processes and their mutual impact on physical and cognitive performance. Participants are free of major chronic conditions and cognitive and functional impairment at the time of enrollment. A detailed description of the study design has been published elsewhere (20). Extensive data on health characteristics, cognitive assessments, and physical function tests are collected during clinical visits every 1–4 years depending on age (every 4 years if <60, every 2 years if 60–79, and annually if ≥80). The study protocol was approved by the National Institutes of Health Intramural Institutional Review Board. Informed consent was obtained from all participants. This analysis included BLSA participants aged ≥ 50 who completed motor and physical function tests between 2012 and 2018. Participants with a history of stroke or Parkinson’s disease were excluded due to potential interference with motor performance.
Measurements
Strength
Isokinetic knee extension strength was measured as quadriceps peak torque (Nm) using a BioDex dynamometer at an angular velocity of 3.14 rad/s (180°/s) over 60° of movement. The maximum of 3 trials was used in the analysis. Hand grip strength was measured in kilograms (kg) force using a Jamar Hydraulic hand dynamometer, with 3 trials on each hand. The maximum of 3 trials on each hand and the greatest grip strength in either hand was used in this analysis.
Manual dexterity
The Purdue Pegboard, a test of visuomotor integration and manual dexterity, reflects the coordination of movement in the upper extremities and has been associated with disability (21). Participants pick up pegs with a single hand and place them sequentially into small holes on the board as quickly as possible for 30 seconds. The number of pegs placed was recorded for 2 trials per hand and averaged for the dominant and nondominant hand separately.
Fine motor function
The finger tapping test consists of simple and complex tapping tests (22). For the simple finger tapping test, participants are instructed to tap as quickly as possible for 10 seconds using the index finger of the dominant or nondominant hand separately. The average time per tap over 3 trials for each hand was used in the analysis. For the complex finger tapping test, both hands are used in an alternating fashion to tap as quickly as possible for 10 seconds. The average time per tap over 3 trials for the complex tapping test was used for analysis.
Physical functioning
Physical functioning was measured using an expanded version of the SPPB initially developed in the Health, Aging and Body Composition Study (23) to minimize ceiling effects and to assess physical performance for high-functioning older adults. In the BLSA version, participants are instructed to stand up from a straight-backed chair with folded arms across the chest and sit down 10 times as quickly as possible. The rate for repeated chair stands (chair stands/s) was calculated. The standing balance test consists of 3 progressively more difficult standing tests, semi-tandem, full-tandem, and single-leg stance, held for 30 seconds for each. Time to hold each pose was used in the analysis. Usual gait speed was measured with 2 trials of a 6-m walk test and the faster trial was used for analysis.
The long-distance corridor walk (LDCW) test was used to measure endurance walking performance (23). Participants were instructed to complete a 2.5-minute warm-up walk at their usual, comfortable pace, immediately followed by a 400-m walk performed as quickly as possible. The course was 20 m long and marked by orange traffic cones at both ends. Participants walked back and forth along the course for ten 40-m laps. The time to complete each lap was recorded and lap time variation was defined as the detrended standard deviation (SD) of residuals of lap time over 10 laps, as previously described (24). Briefly, linear random-effects models with random intercepts and slopes were conducted to regress lap time on lap number from 1 to 10 within individuals. The SD of the residuals was obtained as lap time variation. Participants who did not complete the LDCW due to inability to complete or medical concern were missing lap time variation.
Mobility difficulty was defined as self-reported difficulty walking ¼ mile or climbing 10 steps (25). To capture motor deficits at an earlier stage, slow gait speed was defined as usual gait speed <1.0 m/s, a threshold that is higher in the functional spectrum, but also associated with mobility limitation, hospitalization, and mortality (5,26).
Covariates
Sociodemographic characteristics including age, sex, race, and education level were reported by participants in the health interview. Height and weight were assessed using a stadiometer and a calibrated scale, respectively. Body mass index (BMI) was calculated as kilograms per meter squared (kg/m2). Total lean mass and total fat mass were assessed using total body dual-energy X-ray absorptiometry (Prodigy Scanner, GE, Madison, WI) with Encore Software (27). Number of comorbidities were calculated based on self-reported chronic conditions including cardiovascular disease, pulmonary disease, liver disease, kidney disease, neuropathy, hypertension, diabetes, cancer, and lower extremity arthritis. Global mental status was assessed using the Mini-Mental State Examination (MMSE) (28).
Statistical Analysis
The data distributions were checked for outliers for all variables. Two extreme outlying values in grip strengths and 1 extreme outlying value (above 99 percentiles) in the simple finger tapping test were coded as missing to avoid potential misclassification. The means and SDs or frequencies and percentages of demographics, health characteristics, and motor and physical function tests were examined. Independent t tests or chi-square tests were used to examine age and sex differences in sociodemographic and health characteristics, and motor and physical function performance. All continuous motor and physical function variables were dichotomized, such that poorer performance in these tests represents motor and physical function impairments. Cut points were determined based on previous studies when possible. If no published cut points were available, cut points were defined using data distributions or receiver operating characteristic (ROC) curve analysis. For instance, the cutoff point for grip strength was 20 kg for women and 32 kg for men, as previously used (29). Usual gait speed slower than 1.0 m/s was defined as slow gait speed (5,26,30). The optimal cut points for body weight-normalized knee extension strength/weight ratio were determined using ROC analysis to predict slow gait speed (<1.0 m/s) when it reached the minimal value of Euclidean distance: (1 − sensitivity)2 + (1 − specificity)2. The cut-point was 0.78 Nm/kg for women and 0.92 Nm/kg for men. Low endurance performance was defined as 400-m walk time slower than 5 minutes (30). The cut-points for other tests were based on data distributions (the lowest or highest quintile): (a) pegboard test ≤ 10 pegs, (b) finger tapping simple and complex >0.207 seconds and >0.201 seconds, respectively, (c) chair stand pace <0.42 stands/s, and (d) lap time variation >1.15 seconds.
The SEM was comprised of measurement models for several relevant constructs, and the structural relationships among the constructs. Before estimating the full SEM, we tested a correlated-factors measurement model with 4 latent factors (muscle strength, fine motor function, manual dexterity, and standing balance) using confirmatory factor analysis (CFA) to ensure proper fit. Muscle strength was measured by grip strength and knee extension strength. Fine motor function was measured by the simple finger tapping test in the dominant hand and nondominant hands, and complex finger tapping test. Manual dexterity was modeled using indicators for the Purdue pegboard test in the dominant hand and nondominant hand. Standing balance was measured by semi-tandem, full-tandem, and single-leg stand time which could range from 0 to 90. To facilitate estimation of all factor loadings and in keeping with common practice in SEM, the variances of these 4 latent variables were constrained to be 1. The p value for χ 2 statistic, root mean square error of approximation (RMSEA), comparative fit index (CFI), and Tucker–Lewis index (TLI) were used as model indices to assess model fits. A p value > .05 for the χ 2 statistic, RMSEA ≤ 0.05, CFI and TLI ≥ 0.95 were considered good model fits (31).
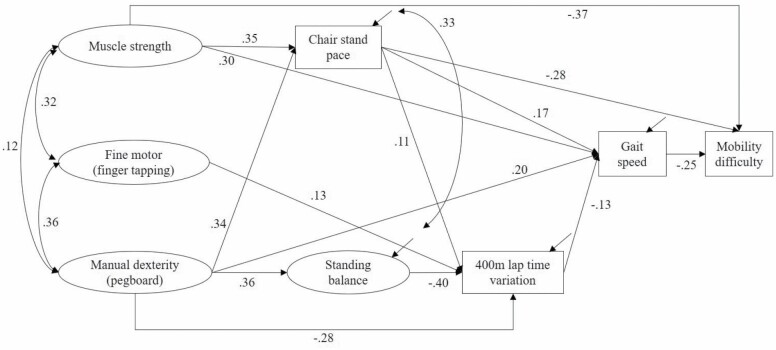
The full SEM consisted of the measurement models for the 4 aforementioned factors, and structural relationships between them and other variables. In a first model (Figure 2), latent factors for muscle strength, fine motor function, and manual dexterity were treated as exogenous variables; chair stand pace, latent variable standing balance time, lap time variation, and slow gait speed as a dichotomous outcome were endogenous variables. In a second SEM (Figure 3), mobility difficulty as the outcome variable was additionally added to the model. Weighted least square estimation with theta parameterization was used. In these 2 models, we used continuous motor and physical function measures. In separate models, we additionally tested SEM using dichotomous motor and physical function impairments.
Figure 2.
Structural equation model (SEM) of associations between motor and physical function measures and slow gait speed (<1.0 m/s) adjusted for age, sex, race, height, and weight. All loadings are standardized coefficients and significant at p < .05. Nonsignificant paths are omitted in this diagram. N = 728. χ 2(65) = 233.28, root mean square error of approximation (RMSEA) = 0.060, comparative fit index (CFI) = 0.965.
Figure 3.
Structural equation model (SEM) of associations between motor and physical function measures and mobility difficulty adjusted for age, sex, race, height, and weight. Mobility difficulty was defined as self-reported difficulty in walking ¼ mile or climbing stairs. All loadings are standardized coefficients and significant at p < .05. Nonsignificant paths are omitted in this diagram. N = 728. χ 2(70) = 229.26, root mean square error of approximation (RMSEA) = 0.056, comparative fit index (CFI) = 0.967.
For continuous motor measures, finger tapping time was multiplied by −1 to have the same direction as other motor variables (higher values indicate better function) and to help interpretation of path coefficients. All SEM models were adjusted for age, sex, race, BMI, and number of comorbidities. We additionally conducted a sensitivity analysis adjusting for MMSE score, total fat mass, and total lean mass. The total, direct, and indirect associations from each motor and physical function measure to the outcome variables were examined using Bootstrapping methods (32). For model modification, suggestions for improving model fit according to modification indices were considered if the changes had theoretical logic. Multigroup SEM analysis was further performed to examine sex differences in the relationship between motor measures and outcome variables (slow gait speed or mobility difficulty).
The significance level α was set as 0.05. Descriptive statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). The SEM analysis was performed using Mplus 8.4 (Muthén & Muthén, Los Angeles, CA).
Results
The final analytic sample consisted of 728 participants after excluding those with stroke or Parkinson’s disease (Supplementary Figure 1). Over half (56.2%) of participants were women and around two-thirds (66.6%) were White. The sociodemographic characteristics, health conditions, and physical functioning tests in all participants and in women and men only are presented in Table 1. After dichotomizing continuous motor and physical functioning variables, we found that the percentage of poorer performance in these tests, defined as having motor and physical function impairments, ranged from 1.8% for the semi-tandem stand to 47.8% for the single-leg stand (Table 2). Impairment in motor and physical function was evident across all age groups for all measures except semi- and full-tandem standing balance, with greater prevalence at older age (Figure 1).
Table 1.
Sample Characteristics, Health Conditions, and Physical Functioning Performance in Middle-Aged and Older Adults in the Baltimore Longitudinal Study of Aging (N = 728)
| Characteristics | N | Total | Women (n = 409) | Men (n = 319) | p Value* |
|---|---|---|---|---|---|
| Mean ± SD or n (%) | |||||
| Age (years) | 728 | 74.1 ± 10.7 | 73.2 ± 10.5 | 75.3 ± 10.8 | .008 |
| Race | 728 | .131 | |||
| White | 485 (66.6) | 260 (63.6) | 225 (70.5) | ||
| Black | 192 (26.4) | 119 (29.1) | 73 (22.9) | ||
| Other | 51 (7.0) | 30 (7.3) | 21 (6.6) | ||
| Education (years) | 726 | 17.6 ± 2.7 | 17.3 ± 2.5 | 18.0 ± 2.8 | <.001 |
| BMI (kg/m2) | 727 | 27.3 ± 4.9 | 26.8 ± 5.2 | 28.0 ± 4.5 | .001 |
| Total fat mass (kg) | 705 | 27.4 ± 10.6 | 28.2 ± 10.9 | 26.5 ± 10.2 | .031 |
| Total lean mass (kg) | 705 | 45.8 ± 9.9 | 38.8 ± 4.9 | 54.9 ± 6.9 | <.001 |
| MMSE score | 693 | 28.5 ± 1.6 | 28.8 ± 1.3 | 28.1 ± 1.9 | <.001 |
| Number of comorbidities† | 728 | 1.3 ± 1.2 | 1.2 ± 1.1 | 1.5 ± 1.3 | .006 |
| Pegboard test (# of pegs) | |||||
| Dominant hand | 707 | 12.3 ± 2.2 | 12.9 ± 2.0 | 11.6 ± 2.3 | <.001 |
| Nondominant hand | 708 | 11.9 ± 2.1 | 12.2 ± 2.0 | 11.5 ± 2.2 | <.001 |
| Mean | 704 | 12.1 ± 2.0 | 12.6 ± 1.9 | 11.6 ± 2.1 | <.001 |
| Finger tapping test (s) | |||||
| Simple dominant | 720 | 0.183 ± 0.03 | 0.185 ± 0.03 | 0.180 ± 0.03 | .016 |
| Simple nondominant | 719 | 0.194 ± 0.03 | 0.196 ± 0.03 | 0.190 ± 0.03 | .007 |
| Simple mean | 720 | 0.188 ± 0.03 | 0.191 ± 0.03 | 0.185 ± 0.03 | .008 |
| Complex | 717 | 0.168 ± 0.06 | 0.168 ± 0.06 | 0.167 ± 0.06 | .849 |
| Grip strength (kg) | |||||
| Left | 722 | 28.1 ± 10.8 | 22.2 ± 6.8 | 35.6 ± 10.3 | <.001 |
| Right | 724 | 29.2 ± 10.7 | 23.2 ± 6.6 | 36.9 ± 10.1 | <.001 |
| Maximum | 725 | 30.2 ± 10.8 | 24.0 ± 6.6 | 38.1 ± 10.0 | <.001 |
| Knee extension strength (Nm) | |||||
| Left | 662 | 70.3 ± 28.3 | 57.7 ± 19.6 | 86.6 ± 29.5 | <.001 |
| Right | 655 | 70.7 ± 27.3 | 58.8 ± 18.5 | 85.6 ± 29.1 | <.001 |
| Maximum | 727 | 74.1 ± 28.2 | 61.5 ± 19.1 | 90.1 ± 29.8 | <.001 |
| Standing balance time (s) | |||||
| Semi-tandem stand time | 727 | 29.6 ± 3.0 | 29.6 ± 2.9 | 29.6 ± 3.0 | .997 |
| Did not hold for 30 s | 13 (1.8) | 7 (1.7) | 6 (1.9) | .860 | |
| Full-tandem stand time | 728 | 27.1 ± 7.9 | 27.1 ± 7.9 | 27.1 ± 7.9 | .969 |
| Did not hold for 30 s | 101 (13.9) | 55 (13.5) | 46 (14.4) | .706 | |
| Single-leg stand time | 728 | 19.5 ± 12.4 | 20.3 ± 12.3 | 18.4 ± 12.6 | .051 |
| Did not hold for 30 s | 348 (47.8) | 180 (44.0) | 168 (52.7) | .020 | |
| Chair stand pace (stands/s) | 725 | 0.54 ± 0.19 | 0.54 ± 0.2 | 0.54 ± 0.2 | .818 |
| 400-m endurance walk | 712 | ||||
| Did not complete | 43 (5.9) | 27 (6.6) | 16 (5.0) | .368 | |
| Total time (s) | 685 | 289.5 ± 69.0 | 293.7 ± 67.3 | 284.1 ± 70.8 | .068 |
| Lap time variation (s) | 685 | 0.91 ± 0.45 | 0.91 ± 0.5 | 0.92 ± 0.4 | .741 |
| Usual gait speed (m/s) | 728 | 1.13 ± 0.2 | 1.12 ± 0.2 | 1.15 ± 0.2 | .077 |
| Mobility difficulty | 728 | 59 (8.1) | 30 (7.3) | 29 (9.1) | .389 |
Notes: BMI = body mass index; MMSE = Mini-Mental State Examination; SD = standard deviation.
*Independent t tests were used to compare motor and physical function in women and men. The bolded p values indicate statistically significant at p < .05.
†Number of chronic conditions including cardiovascular disease, pulmonary disease, liver disease, kidney disease, neuropathy, hypertension, diabetes, cancer, and lower extremity arthritis.
Table 2.
Motor and Physical Function Impairment Across Age Groups in Middle-Aged and Older Adults in the Baltimore Longitudinal Study of Aging (N = 728)
| Motor and Physical Function Impairment | Total | Age Groups (years) | Trend p Value* | |||
|---|---|---|---|---|---|---|
| n (%) | 50–59 n = 89 (12.2%) |
60–69 n = 160 (22.0%) |
70–79 n = 243 (33.4%) |
≥80 n = 236 (32.4%) |
||
| Poor manual dexterity (≤10 pegs) | 117 (16.62) | 1 (1.12) | 3 (1.90) | 32 (13.62) | 81 (36.49) | <.001 |
| Slow finger tapping simple (>0.207 s) | 147 (20.42) | 1 (1.14) | 22 (13.75) | 48 (20.00) | 76 (32.76) | <.001 |
| Slow finger tapping complex (>0.201 s) | 143 (19.94) | 3 (3.37) | 16 (10.00) | 53 (22.27) | 71 (30.87) | <.001 |
| Poor grip strength | 170 (23.45) | 1 (1.12) | 4 (2.50) | 59 (24.58) | 106 (44.92) | <.001 |
| Women (<20 kg) | ||||||
| Men (<32 kg) | ||||||
| Poor knee extension strength | 249 (34.30) | 7 (7.87) | 34 (21.25) | 86 (35.68) | 122 (51.69) | <.001 |
| Women (<0.78 Nm/kg) | ||||||
| Men (<0.92 Nm/kg) | ||||||
| Poor standing balance (unable to hold for 30 s) | ||||||
| Semi-tandem | 13 (1.79) | 0 | 0 | 2 (0.82) | 11 (4.66) | .001 |
| Full-tandem | 101 (13.87) | 0 | 4 (2.50) | 18 (7.41) | 79 (33.47) | <.001 |
| Single-leg | 348 (47.80) | 4 (4.49) | 35 (21.88) | 117 (48.15) | 192 (81.36) | <.001 |
| Unable to complete 10 chair stand | 22 (3.03) | 0 | 1 (0.63) | 6 (2.47) | 15 (6.44) | .002 |
| Chair stand (<0.42 stands/s) | 150 (20.69) | 4 (4.49) | 16 (10.00) | 43 (17.70) | 87 (37.34) | <.001 |
| Unable to complete 400-m walk | 43 (5.91) | 1 (1.12) | 2 (1.25) | 12 (4.94) | 28 (11.86) | <.001 |
| Slow 400-m walking (>5 min) | 220 (32.12) | 2 (2.27) | 20 (12.66) | 71 (30.74) | 127 (61.06) | <.001 |
| High lap time variation (>1.15 s) | 136 (19.85) | 2 (2.27) | 15 (9.49) | 46 (19.91) | 73 (35.10) | <.001 |
| Slow gait speed (<1.0 m/s) | 175 (24.04) | 7 (7.87) | 19 (11.88) | 45 (18.52) | 104 (44.07) | <.001 |
| Mobility difficulty† | 59 (8.10) | 1 (1.12) | 7 (4.38) | 19 (7.82) | 32 (13.56) | <.001 |
Notes:
*Mantel–Haenzel chi-square tests for trend (1 df) were used for the analysis. The bolded p values indicate statistically significant at p < .05.
†Mobility difficulty was defined as self-reported difficulty in walking ¼ mile or climbing stairs.
Figure 1.
Prevalence of motor and physical function impairment across age groups.
The measurement model consisted of latent factors muscle strength (grip strength and knee extension strength), fine motor function (finger tapping), manual dexterity (pegboard test), and standing balance (semi- and full-tandem and single-leg stands). According to CFA results, the semi-tandem time was removed due to a low factor loading (0.33) as an indicator of standing balance. The factor loadings for indicators ranged from 0.65 (full-tandem stance) to 0.95 (knee extension strength; Supplementary Figure 2).
In the SEM Model 1 using continuous indicators for motor and physical performance and slow gait speed (<1.0 m/s) as the outcome, the fully adjusted model showed a good model fit (Figure 2). Latent factor muscle strength had a direct and an indirect association via chair stand pace with slow gait speed. Manual dexterity had direct associations with chair stand pace, standing balance, lap time variation, and slow gait speed. Fine motor function was only directly associated with lap time variation (Figure 2). Comparing all the standardized coefficients for direct, indirect, and total associations (Supplementary Table 1), we found the strongest predictor of slow gait speed was manual dexterity (total associations = −0.571 SD units, 95% confidence interval [CI]: −0.743, −0.414), followed by latent factor muscle strength (−0.447 SD units, 95% CI: −0.823, −0.109), chair stand pace (−0.195 SD units, 95% CI: −0.320, −0.071), and lap time variation (0.102 SD units, 95% CI: 0.011, 0.193). The coefficients indicate that, for example, 1 SD unit increase in manual dexterity was associated with 0.571 unit decrease in z-score of the probability of having slow gait speed. Further adjustment in the subset of participants (N = 670) with MMSE score, total fat mass, and total lean mass did not substantially change the results (data not shown). The multigroup analysis suggested that paths from motor function to slow gait speed may differ by sex. The direct associations from chair stand pace and lap time variation to slow gait speed were both stronger in men than in women (data not shown).
In the SEM Model 2 with mobility difficulty as the outcome, the fully adjusted model had a good model fit (Figure 3). Latent factor muscle strength was directly associated with chair stand pace, usual gait speed, and mobility difficulty. Manual dexterity was directly associated with chair stand pace, standing balance, and lap time variation, and indirectly associated with mobility difficulty. Chair stand pace was directly associated with usual gait speed and mobility difficulty (Figure 3). Comparing all the standardized coefficients for total associations (Supplementary Table 2), we found the strongest predictor of mobility difficulty was muscle strength (total associations = −0.582 SD units, 95% CI: −0.927, −0.252), followed by chair stand pace (−0.322 SD units, 95% CI: −0.500, −0.174), usual gait speed (−0.247 SD units, 95% CI: −0.379, −0.116), and standing balance (−0.190 SD units, 95% CI: −0.381, −0.019). Further adjusting for MMSE score, total fat mass, and total lean mass did not substantially change the results. Multigroup analysis showed that there were no statistically significant sex differences for the effects of motor and physical function on mobility difficulty.
Additional models using dichotomous motor and physical function variables indicated similar results. Poor muscle strength (standardized coefficient = 0.536, 95% CI: 0.231, 0.967) had the strongest association with slow gait speed, followed by poor manual dexterity (0.401, 95% CI: 0.124, 0.631), slow chair stands pace (0.174, 95% CI: −0.123, 0.360), and high lap time variation (0.176, 95% CI: −0.025, 0.326; Supplementary Table 3). Poor muscle strength (0.685, 95% CI: 0.330, 1.312) and poor standing balance (0.488, 95% CI: 0.122, 0.861) were significantly associated with mobility difficulty (Supplementary Table 4).
Discussion
This study characterized the prevalence and interrelationships of upper and lower extremity motor and physical function impairments that begin in midlife and increase with advancing age. Findings suggest that manual dexterity and both measures of upper and lower extremity strength are among the strongest predictors of slow gait speed. Muscle strength, chair stands, and standing balance were the strongest predictors of mobility difficulty. Collectively, these results indicate that poor manual dexterity which reflects neurocoordination of hand movement may play an important role in reduced functional performance in mid-to-late life. These findings enhance previous recognition and understanding of motor function, especially strength and manual dexterity, as markers of mobility difficulty beginning in midlife, and provide a platform for future longitudinal research into the functional consequences of both gross and fine motor impairments and possibly the health conditions leading to these impairments.
Our study findings are consistent with previous research that mobility difficulty is attributable to impairments across multiple systems and contributors to mobility limitation (eg, slowed gait) are multifactorial (33). Mobility issues involve several factors including loss of muscle mass or reduced muscle strength, poor balance control, neuromuscular impairment, and cognitive decline (34,35). To this end, lower levels of motor and physical function may not only reflect reduced functional integrity of cortical and subcortical brain motor regions, but also impaired sensory, cognitive, and/or muscle function. Our study facilitates understanding of the relationships among and between multiple motor and physical performance measures and provides insights for identifying an “at-risk” low-functioning phenotype. This in turn can help identify more upstream intervention targets to potentially prevent functional decline at an early stage, maintaining functional independence, and improving quality of life in older adults.
Our study found manual dexterity was associated with multiple physical performance tests including chair stand, standing balance, 400-m walk variability, and usual gait speed. Manual dexterity reflects the ability to accurately and rapidly grasp and manipulate small objects, which requires not only musculoskeletal function but also neurological function (36). Several studies have found that manual dexterity is associated with cognitive function (37,38), but the relationship with mobility performance has rarely been investigated. Although the Pegboard test, a measure of visuomotor integration and manual dexterity, has been used for motor function assessment in patients with early-stage Parkinson’s disease, it is generally overlooked in healthy older adults (39). Our study found that 36.5% of participants aged ≥80 had poor manual dexterity compared to 1.1% and 1.9% of those aged 50–59 and 60–69, respectively. Notably, the latent factor manual dexterity was one of the strongest predictors of slow gait speed. Previous research also showed that manual dexterity deteriorates rapidly with aging and can affect mobility in later life (40). Further longitudinal studies may help elucidate the impacts of manual dexterity on limitations in physical function and gait speed and its potential use as an early screening tool.
Muscle weakness, especially poor lower extremity strength, has been associated with mobility disability and functional decline in older adults (9,41). Although lower extremity strength, often measured using knee extension strength, is crucial in various physical tasks such as rising from a chair, walking, or climbing steps, upper extremity strength also plays a role in functional performance. Several studies have found grip strength to predict cardiometabolic diseases, disability, and mortality (42–44). Also, knee extension strength may not always be a stronger predictor of functional performance than grip strength and both measures are important predictors of functional limitations (45). In line with previous research, our study found that the latent factor muscle strength, which represented both upper and lower extremity strength, was a strong predictor of slow gait speed and mobility difficulty. The dramatic increase in the prevalence of poor grip strength in participants aged ≥80 compared to older adults aged 50–69 also reflects the substantial decline in muscle strength with aging which in turn affects mobility in older adults. Of note, although standing balance time was not significantly associated with slow gait speed in this study, the performance of the chair stand test as a measure of lower extremity function is strongly affected by dynamic balance. Thus, the significant relationship between chair stand pace and mobility may be partially attributed to balance. Future studies should incorporate both static and dynamic balance separately in SEM to further elucidate the contributions of balance to mobility difficulty in older adults.
Our study used markers with high (eg, 400-m fast walk) and low measurement ceilings function (eg, gait speed) to limit ceiling effects and capture early markers of lower motor and physical function in middle-aged and older adults. The 400-m endurance walk can differentiate walking capacity at a higher level for well-functioning older adults and has been associated with cardiovascular disease, mobility limitation, and mortality (25,46). Our study found that 61.1% of participants aged ≥80 had low walking endurance compared to 2.3% of those in the 50–59 age group. To this end, walking endurance may act as a sensitive measure to discriminate early motor impairments among older adults with higher functioning capacity (25,46). We also included a novel measure of gait variability (ie, lap time variation) to improve discrimination/detection of early motor impairments. The computation of lap time variation controls for the effect of fatigue-related slowing on variability, which facilitates our understanding of walking variability during an endurance walking test (24,47). Lap time variation has also been associated with poor executive function especially psychomotor slowing (24). Thus, a better understanding of the variability of movement not only helps identify older adults at risk of mobility limitation, but also informs future research on motor impairments as an early marker of adverse health outcomes including cognitive decline and dementia.
The study findings should be considered with limitations. First, the temporality and casual relationships cannot be determined due to the cross-sectional design; longitudinal studies using SEM to examine motor and physical function tests associations with meaningful decline in gait speed or development of mobility difficulty are warranted. Second, the BLSA is a well-functioning and well-educated cohort which reduces confounding by disease burden, but limits generalizability. Further, although 26% of the study sample was Black, we did not have sufficient power to examine racial differences that may account for previously reported differences in gait speed and function (48). Replication of these results in other cohorts is warranted. Third, motor function tests involving lateral movement were not available in the BLSA. Other domains of movement should be incorporated in future studies.
In conclusion, our study sheds light on the associations connecting motor and physical function to mobility difficulty in middle-aged and older adults. Motor and physical function in both upper and lower extremities contribute to poor functional performance. Specifically, muscle strength and manual dexterity appear to be the most prominent factors associated with mobility in this well-functioning cohort. The remarkable finding of manual dexterity indicates that other than musculoskeletal function, neurological function also plays an important role in mobility performance, particularly gait speed, and should be incorporated in composite measures of physical function in healthy older adults. Further, the prevalence of impairments in motor and physical function begins in midlife and increases dramatically with older age. Early identification of motor and physical function impairments may thus help inform interventions to prevent onset or delay progression of disability earlier in the disablement process when treatments are more likely to be effective. Future longitudinal studies are needed to understand trajectories of early impairments in these functional tests and their contribution to mobility and functional decline.
Supplementary Material
Contributor Information
Yurun Cai, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Qu Tian, Intramural Research Program, National Institute on Aging, Baltimore, Maryland, USA.
Alden L Gross, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Center on Aging and Health, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Hang Wang, Center on Aging and Health, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Jian-Yu E, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Wilmer Eye Institute, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Yuri Agrawal, Department of Otolaryngology - Head and Neck Surgery, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Eleanor M Simonsick, Intramural Research Program, National Institute on Aging, Baltimore, Maryland, USA.
Luigi Ferrucci, Intramural Research Program, National Institute on Aging, Baltimore, Maryland, USA.
Jennifer A Schrack, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Center on Aging and Health, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Funding
This work was supported by the National Institutes of Health (NIH) grant R01AG061786. This study was also supported in part by the Intramural Research Program, National Institute on Aging, NIH.
Conflict of Interest
E.M.S., L.F., and J.A.S. currently serve on the editorial board of Journals of Gerontology Medical Sciences (JGMS). All other authors have no conflict of interest to declare.
References
- 1. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rantanen T, Guralnik JM, Ferrucci L, Leveille S, Fried LP. Coimpairments: strength and balance as predictors of severe walking disability. J Gerontol A Biol Sci Med Sci. 1999;54(4):M172–M176. doi: 10.1093/gerona/54.4.m172 [DOI] [PubMed] [Google Scholar]
- 3. Buchman AS, Wilson RS, Yu L, Boyle PA, Bennett DA, Barnes LL. Motor function is associated with incident disability in older African Americans. J Gerontol A Biol Sci Med Sci. 2016;71(5):696–702. doi: 10.1093/gerona/glv186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(suppl. 2):37–41. doi: 10.1093/ageing/afl084 [DOI] [PubMed] [Google Scholar]
- 5. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893 [DOI] [PubMed] [Google Scholar]
- 7. Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62(9):983–988. doi: 10.1093/gerona/62.9.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004;52(7):1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x [DOI] [PubMed] [Google Scholar]
- 9. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980–986. doi: 10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Camicioli R, Howieson D, Oken B, Sexton G, Kaye J. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998;50(5):1496–1498. doi: 10.1212/wnl.50.5.1496 [DOI] [PubMed] [Google Scholar]
- 12. Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006;63(12):1763–1769. doi: 10.1001/archneur.63.12.1763 [DOI] [PubMed] [Google Scholar]
- 13. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 14. Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83(3):237–252. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 15. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 16. Ullman JB, Bentler PM. Structural equation modeling. In Weiner IB, eds. Handbook of Psychology. doi: 10.1002/0471264385.wei0224 [DOI] [Google Scholar]
- 17. Beran TN, Violato C. Structural equation modeling in medical research: a primer. BMC Res Notes. 2010;3:267. doi: 10.1186/1756-0500-3-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menz HB, Lord SR, Fitzpatrick RC. A structural equation model relating impaired sensorimotor function, fear of falling and gait patterns in older people. Gait Posture. 2007;25(2):243–249. doi: 10.1016/j.gaitpost.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 19. Dai B, Ware WB, Giuliani CA. A structural equation model relating physical function, pain, impaired mobility (IM), and falls in older adults. Arch Gerontol Geriatr. 2012;55(3):645–652. doi: 10.1016/j.archger.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 20. Kuo PL, Schrack JA, Shardell MD, et al. A roadmap to build a phenotypic metric of ageing: insights from the Baltimore Longitudinal Study of Aging. J Intern Med. 2020;287(4):373–394. doi: 10.1111/joim.13024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32(3):234–247. doi: 10.1037/h0061266 [DOI] [PubMed] [Google Scholar]
- 22. Tian Q, Chastan N, Thambisetty M, Resnick SM, Ferrucci L, Studenski SA. Bimanual gesture imitation links to cognition and olfaction. J Am Geriatr Soc. 2019;67(12):2581–2586. doi: 10.1111/jgs.16151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group . Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644–M649. doi: 10.1093/gerona/56.10.m644 [DOI] [PubMed] [Google Scholar]
- 24. Tian Q, Simonsick EM, Resnick SM, Shardell MD, Ferrucci L, Studenski SA. Lap time variation and executive function in older adults: the Baltimore Longitudinal Study of Aging. Age Ageing. 2015;44(5):796–800. doi: 10.1093/ageing/afv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group . Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644–M649. doi: 10.1093/gerona/56.10.m644 [DOI] [PubMed] [Google Scholar]
- 26. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x [DOI] [PubMed] [Google Scholar]
- 27. Moore AZ, Caturegli G, Metter EJ, et al. Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62(2):230–236. doi: 10.1111/jgs.12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 29. Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):559–566. doi: 10.1093/gerona/glu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simonsick EM, Newman AB, Visser M, et al. ; Health, Aging and Body Composition Study . Mobility limitation in self-described well-functioning older adults: importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63(8):841–847. doi: 10.1093/gerona/63.8.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schreiber JB, Nora A, Stage FK, Barlow EA, King J. Reporting structural equation modeling and confirmatory factor analysis results: a review. J Educ Res. 2006;99(6):323–338. doi: 10.0.12.128/JOER.99.6.323-338 [DOI] [Google Scholar]
- 32. Nevitt J, Hancock GR. Performance of bootstrapping approaches to model test statistics and parameter standard error estimation in structural equation modeling. Struct Equ Model A Multidiscip J. 2001;8(3):353–377. doi: 10.1207/S15328007SEM0803_2 [DOI] [Google Scholar]
- 33. Brown CJ, Flood KL. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310(11):1168–1177. doi: 10.1001/jama.2013.276566 [DOI] [PubMed] [Google Scholar]
- 34. Freiberger E, Sieber CC, Kob R. Mobility in older community-dwelling persons: a narrative review. Front Physiol. 2020;11:881. doi: 10.3389/fphys.2020.00881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721–733. doi: 10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krampe RT. Aging, expertise and fine motor movement. Neurosci Biobehav Rev. 2002;26(7):769–776. doi: 10.1016/s0149-7634(02)00064-7 [DOI] [PubMed] [Google Scholar]
- 37. Carment L, Abdellatif A, Lafuente-Lafuente C, et al. Manual dexterity and aging: a pilot study disentangling sensorimotor from cognitive decline. Front Neurol. 2018;9:910. doi: 10.3389/fneur.2018.00910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kobayashi-Cuya KE, Sakurai R, Sakuma N, et al. Hand dexterity, not handgrip strength, is associated with executive function in Japanese community-dwelling older adults: a cross-sectional study. BMC Geriatr. 2018;18(1):192. doi: 10.1186/s12877-018-0880-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dan X, Liu J, Doyon J, Zhou Y, Ma J, Chan P. Impaired fine motor function of the asymptomatic hand in unilateral Parkinson’s disease. Front Aging Neurosci. 2019;11:266. doi: 10.3389/fnagi.2019.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vieluf S, Godde B, Reuter EM, Voelcker-Rehage C. Age-related differences in finger force control are characterized by reduced force production. Exp Brain Res. 2013;224(1):107–117. doi: 10.1007/s00221-012-3292-4 [DOI] [PubMed] [Google Scholar]
- 41. Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58(11):2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x [DOI] [PubMed] [Google Scholar]
- 42. Peterson MD, Duchowny K, Meng Q, Wang Y, Chen X, Zhao Y. Low normalized grip strength is a biomarker for cardiometabolic disease and physical disabilities among U.S. and Chinese Adults. J Gerontol A Biol Sci Med Sci. 2017;72(11):1525–1531. doi: 10.1093/gerona/glx031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sallinen J, Stenholm S, Rantanen T, Heliövaara M, Sainio P, Koskinen S. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc. 2010;58(9):1721–1726. doi: 10.1111/j.1532-5415.2010.03035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sasaki H, Kasagi F, Yamada M, Fujita S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120(4):337–342. doi: 10.1016/j.amjmed.2006.04.018 [DOI] [PubMed] [Google Scholar]
- 45. Martien S, Delecluse C, Boen F, et al. Is knee extension strength a better predictor of functional performance than handgrip strength among older adults in three different settings? Arch Gerontol Geriatr. 2015;60(2):252–258. doi: 10.1016/j.archger.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 46. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 47. Tian Q, Ferrucci L, Resnick SM, et al. The effect of age and microstructural white matter integrity on lap time variation and fast-paced walking speed. Brain Imaging Behav. 2016;10(3):697–706. doi: 10.1007/s11682-015-9449-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blanco I, Verghese J, Lipton RB, Putterman C, Derby CA. Racial differences in gait velocity in an urban elderly cohort. J Am Geriatr Soc. 2012;60(5):922–926. doi: 10.1111/j.1532-5415.2012.03927.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.