Abstract
Background
Studies of the relationship between vitamin D and physical functioning have had inconsistent results.
Methods
Physical functioning measures were collected for up to 2 years during a 2-stage, Bayesian, response-adaptive, randomized trial of 4 doses of vitamin D3 supplementation (200 [control], 1 000, 2 000, and 4 000 IU/day) to prevent falls. Two community-based research units enrolled adults aged ≥70 years, with elevated fall risk and serum 25-hydroxyvitamin D level of 10–29 ng/mL. The Pooled Higher Doses (PHD) group (≥1 000 IU/day, n = 349) was compared to the control group (n = 339) on changes in Short Physical Performance Battery (SPPB) score and its component tests, Timed Up-and-Go (TUG) test, 6-minute walk distance, and grip strength.
Results
The trial enrolled 688 participants. Mean age was 77.2 years, 56.4% were male, 79.7% White, and 18.2% Black. While the PHD and control groups both lost function over time on most outcomes, the 2 groups did not show differential change overall on any outcome. Incidence of transitioning to poor functioning on gait speed, SPPB score, or TUG test did not differ by dose group.
Conclusion
In older persons with low serum 25-hydroxyvitamin D level and elevated fall risk, high-dose vitamin D supplementation, ≥1 000 IU/day, did not improve measures of physical function compared to 200 IU/day.
Clinical Trial Registration
Keywords: Physical functioning, Randomized controlled trial, Vitamin D
Declines in mortality over the last half century have led to a greater proportion of the population living to old age, resulting in increased chronic disease and disability burden and corresponding increases in health care utilization and supportive and long-term care needs (1). The ability to function independently in the community is a goal of older individuals and a critically important public health issue. The most recent Medicare Current Beneficiary Survey (2) estimated that 15.1% of persons aged 65–74 years and 21.9% of those aged 85 years or older reported serious difficulty in carrying out 2 or more common activities such as walking, climbing stairs, dressing, or eating, or serious difficulty with cognition, seeing, or hearing.
Functional decline and disability in older persons have been attributed to demographic, health behavior, disease-specific, psychosocial, and biological risk factors (3). Several observational and interventional studies have also focused on vitamin D deficiency and its role in functional decline (4). There is biological plausibility for this association, with multiple biological pathways potentially explaining how vitamin D influences muscle and resultant mobility loss and falls (5). Vitamin D deficiency causes oxidative stress that leads to disruption of mitochondrial function and muscle atrophy, potentially working through the vitamin D receptor in muscle cells (6).
Observational studies have generally supported the hypothesis that vitamin D deficiency is associated with reduced physical functioning, but studies have yielded conflicting results. In a large cohort study representative of the older population in the Netherlands, low serum 25-hydroxyvitamin D [25(OH)D] was associated with poorer scores on the Short Physical Performance Battery (SPPB) and its individual components (balance, gait speed, chair rise time) and was predictive of decline in performance over a 3-year follow-up period (7). Data from the English Longitudinal Study on Aging showed that both low grip strength and SPPB score were associated with low serum levels of 25(OH)D (8). However, other studies have not been able to confirm the association of low levels of 25(OH)D with weak grip and leg strength and low physical performance on measures of balance, gait speed, and rising from a chair (9,10). In those studies reporting an association of low serum 25(OH)D with reduced physical function, there is the potential for reverse causality, with reduced physical function leading to less outdoor activity and therefore less sunlight exposure.
Randomized controlled trials (RCTs) have assessed the effect of treating older persons with vitamin D supplements on functioning, and these results have also been mixed. In a review of 15 RCTs in persons aged 60 years and older assessing multiple strength and functional measures, 9 trials did not show benefit of vitamin D supplementation, while 6 showed improvement in mobility or muscle strength (11). A meta-analysis of 7 of these RCTs that measured grip strength showed no association with vitamin D supplementation, whereas a separate meta-analysis of 5 trials that used the Timed Up-and-Go (TUG) test (12) showed a small increase (worsening) in the TUG time in those receiving vitamin D versus controls (11). Many past studies were small and included persons with normal serum levels of vitamin D. A recent, large RCT, which was not limited to persons with low vitamin D, showed no benefit of vitamin D3 supplementation for change in SPPB score (13).
In 2014, the National Institute on Aging funded STURDY (Study To Understand Fall Reduction and Vitamin D in You), a seamless 2-stage, Bayesian response-adaptive, randomized trial in community-dwelling older persons with low serum 25(OH)D and at elevated risk of falling (14,15). Pill-taking and follow-up of participants continued for 2 years or the end of trial, whichever occurred first. The primary outcome results of STURDY have been published (16). Vitamin D supplementation ≥1 000 IU/day did not prevent falls compared with 200 IU/day, with possible safety concerns at higher dosage levels, and did not improve gait speed, the prespecified secondary outcome of the trial. This report presents the results for additional measures of physical function and further explores the gait speed outcome.
Method
The trial protocol was approved by a Johns Hopkins University institutional review board. Written informed consent was provided by each participant. A data and safety monitoring board approved the protocol and monitored the trial. A description of trial methods has been published (14,15). Briefly, during the dose-finding stage, participants were randomly assigned to receive 200 (control), 1 000, 2 000, or 4 000 IU/day of vitamin D3 to identify which of the 3 noncontrol doses was most effective (the “best dose”) for preventing falls. After the best dose was identified, participants who had been randomly assigned to any of the noncontrol doses received the best dose, control participants continued receiving 200 IU/day, and new participants were randomly assigned 1:1 to the best or control dose.
Participants
Eligible participants were community-dwelling adults, aged ≥70 years, with elevated fall risk and serum 25(OH)D level 10–29 ng/mL (14). Elevated fall risk was defined by self-report of an injurious fall in the past year, ≥2 falls in the past year regardless of injury, fear of falling due to balance or walking problems, difficulty maintaining balance, or use of an assistive device when walking. Serum 25(OH)D level utilized the Endocrine Society definition for deficiency and insufficiency (17). Persons taking ≤1 000 IU/day of vitamin D supplements were allowed to screen for enrollment; if their 25(OH)D level while on their personal supplement qualified for enrollment, they were allowed to enroll if they agreed to maintain this dose during the trial. Participants enrolled at 2 community-based research units (Hagerstown, MD and Baltimore, MD), each at ~39° latitude.
Treatment
STURDY studied 4 doses of cholecalciferol (vitamin D3) supplements: 200 IU/day (control), 1 000 IU/day, 2 000 IU/day, and 4 000 IU/day. The rationale for and safety of these dose levels are explained elsewhere (14,16).
Pills for all doses had an identical appearance and were manufactured by Continental Vitamin Company (Vernon, CA). Duration of pill-taking and follow-up was 2 years or until the end of the trial, whichever came first.
Randomization
STURDY began randomizing participants to daily doses of 200 IU/day (control), 1 000 IU/day, 2 000 IU/day, or 4 000 IU/day of vitamin D3 on October 30, 2015 (16). Once 1 000 IU/day was identified on March 23, 2018 as the best noncontrol dose for preventing falls, participants randomized to 2 000 IU/day or 4 000 IU/day were switched to 1 000 IU/day, and new enrollees were randomized 1:1 to 1 000 IU/day or control. Randomization ended on February 11, 2019 and data collection ended on May 31, 2019. Participants and study personnel were masked to randomized dose, occurrence of adaptations, and the end of dose-finding.
Assessments
Functional performance tests included the SPPB (18), TUG test (12), 6-minute walk (19), and grip strength; each was completed at baseline and 3, 12, and 24 months after randomization.
Measures obtained from the SPPB include the overall score, the total balance stand time, gait speed, and the time to complete 5 chair rises. The overall SPPB score (range 0–12) is calculated as the sum of the balance, gait speed, and chair rise component subscores, each ranging from 0 to 4; higher scores indicate better physical performance. The total balance stand time is obtained by summing the times for the 3 stands comprising the balance subtest. Each balance stand requires a different foot position (side-by-side, semitandem, and tandem), and each stand is held as long as possible, up to 10 seconds. Gait speed is obtained from a timed usual-paced 4-m walk. The chair rise component consists of the participant, arms folded across the chest, rising from a seated position as quickly as possible, 5 times; the component terminates if 5 chair rises are not completed within 60 seconds.
The TUG test is a timed test of standing up from a chair, walking at a normal pace for 3 m, turning, returning to the chair, and sitting down; the test result is the time in seconds to complete all 5 parts. The test is terminated at 60 seconds if not completed by then.
In the 6-minute walk test, the participant is instructed to walk back and forth over a 10-m course for 6 minutes and to cover as much ground as possible; each end is marked with a cone which the participant has to walk around. Distance covered and time on the course are recorded.
Grip strength is measured with a hydraulic hand-held dynamometer (JAMAR Technologies, Hatfield, PA); the participant is asked to complete 3 squeezes with each hand, with 20 seconds of rest between squeezes. The maximum hold for the dominant hand is analyzed if available; if the participant is unable to squeeze with the dominant hand, the maximum hold for the nondominant hand is analyzed.
Statistical Analysis
Participants’ characteristics at study entry were examined using means and standard deviations for continuous variables and frequencies and percentages for categorical variables and were reported for each dose group analyzed and overall.
The primary analysis was a comparison of the Pooled Higher Doses (PHD) group (1 000, 2 000, and 4 000 IU/day groups) to the 200 IU/day group, an approach established in the analysis of the primary outcome (16). This comparison included all randomized participants regardless of dose assignment and used all available measurements of each outcome regardless of the dose used when the measure was obtained. In a sensitivity analysis, only those randomized to 1 000 IU/day (best dose) were compared to those randomized to 200 IU/day (control). An exploratory analysis examined each individual higher dose group relative to the control group using only participants randomized prior to the first adaptation of the randomization probabilities; this group is an unbiased population for comparison of each higher dose versus control because treatment assignment was not influenced by previously collected outcome data.
Change in each outcome from baseline to each time point was assessed in each dose group with a t-test. For each outcome, the difference between the PHD and control groups in the change from baseline was assessed with a longitudinal, mixed-effects linear regression model with the measure as the outcome and fixed effects including a single treatment term, 3 time point terms, and 3 treatment-by-time interaction terms, as well as a random intercept for participant. This model uses all available measures, including measures from participants with only a baseline measure. Difference between groups in change from baseline in an outcome was assessed overall with a 3-degree-of-freedom test of the combined 3 treatment-by-time interaction terms and at each time point with a 1-degree-of-freedom test of the time-specific treatment-by-time interaction term.
The sensitivity analysis comparing those randomized to 1 000 versus 200 IU/day and the analysis comparing each higher dose group versus control and limiting participants to those randomized prior to the first adaptation of the randomization probabilities used the same methods as above. In the latter analysis, the treatment group was represented with 3 terms and the number of treatment-by-time interaction terms increased to 9.
Transition to poor functioning was examined in the PHD and control groups for 3 physical functioning measures: gait speed, SPPB score, and TUG time. Two thresholds defining poor functioning were examined for gait speed (0.6 and 0.8 m/s) (20). Thresholds defining poor functioning on the SPPB score and TUG were 9 points and 12 seconds, respectively (21,22). For each measure analyzed, the analysis population was limited to participants whose performance at baseline on the measure was at least as good as the specified threshold, and the odds ratio (OR) for transition to poor functioning since baseline in the PHD group versus 200 IU/day groups at each testing time, 95% confidence interval (CI) for each OR, and the combined treatment-by-time interaction p value were calculated from corresponding simultaneous tests of general linear contrasts from a mixed-effects logistic regression model including 1 term for treatment, 3 terms for time point, 3 treatment-by-time interaction terms, and a random intercept for participant. Difference between groups in transition to poor functioning was assessed overall with a 3-degree-of-freedom test of the combined 3 treatment-by-time interaction terms.
Because 9 outcomes were evaluated using up to 4 analysis strategies, we used the Benjamini–Hochberg procedure (23) to control the false discovery rate to below 1 out of 9, the maximum number of comparisons evaluated with each strategy. Each table displays the unadjusted or nominal 2-sided p value for each 3-degree-of-freedom interaction test and the Benjamini–Hochberg adjusted p value and the false discovery rate (1/9 = 11.1%). An adjusted p is statistically significant if less than .11.
All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC), Stata 15 (StataCorp LLC, College Station, TX), or R-v3.6.0 (https://www.r-project.org/).
Results
Table 1 presents the baseline characteristics of the participants for the primary comparison groups, PHD and control, and overall; Supplementary Table 1 presents these characteristics for participants randomized to 1 000 IU/day and for each dose group in the cohort of participants randomized prior to the first adjustment of the randomization probabilities. The total study population (688 participants) was age 77.2 years on average, 56.4% male, and 18.2% Black. Participants showed evidence of functional limitations, with 36.8% having an SPPB score below 9, 11.8% having a gait speed below 0.6 m/s, and 29.6% having a TUG time of 12 or more seconds.
Table 1.
Characteristics of Randomized Participants at Enrollment
| Pooled Higher Doses* (N = 349) | 200 IU/day (N = 339) | All (N = 688) | |
|---|---|---|---|
| Age (years), mean ± SD | 77.2 ± 5.4 | 77.2 ± 5.4 | 77.2 ± 5.4 |
| Male, no. (%) | 190 (54.4%) | 198 (58.4%) | 388 (56.4%) |
| Race, no. (%)† | |||
| White | 267 (77.2%) | 276 (82.4%) | 543 (79.7%) |
| Black | 69 (19.9%) | 55 (16.4%) | 124 (18.2%) |
| Other | 16 (4.6%) | 7 (2.1%) | 23 (3.4%) |
| No. missing | 3 | 4 | 7 |
| Hispanic, Latino, or Spanish ethnicity, no. (%) | 5 (1.4%) | 3 (0.9%) | 8 (1.2%) |
| No. missing | 1 | 3 | 4 |
| Serum 25(OH)D (ng/mL) | |||
| 10–19, no. (%) | 100 (28.7%) | 100 (29.5%) | 200 (29.1%) |
| 20–29, no. (%) | 249 (71.3%) | 239 (70.5%) | 488 (70.9%) |
| Mean ± SD | 22.1 ± 4.9 | 22.1 ± 5.3 | 22.1 ± 5.1 |
| Median (Q25, Q75) | 23 (19, 26) | 23 (18, 27) | 23 (19, 26) |
| Taking a vitamin D supplement, no. (%) | 132 (37.8%) | 124 (36.6%) | 256 (37.2%) |
| Median (Q25, Q75) daily dose (IU) | 700 (400, 1 000) | 800 (414.5, 1 000) | 700 (400, 1 000) |
| Low physical activity‡, no. (%) | 43 (12.4%) | 47 (13.9%) | 90 (13.1%) |
| No. missing | 2 | 0 | 2 |
| SPPB total score§ | |||
| 0–8, no. (%) | 138 (39.5%) | 115 (33.9%) | 253 (36.8%) |
| 9–12, no. (%) | 211 (60.5%) | 224 (66.1%) | 435 (63.2%) |
| Mean ± SD | 8.6 ± 2.5 | 8.9 ± 2.3 | 8.7 ± 2.4 |
| SPPB total balance stand§ time (s) | |||
| Mean ± SD | 26.6 ± 6.2 | 26.9 ± 6.3 | 26.8 ± 6.2 |
| No. missing | 0 | 1 | 1 |
| SPPB gait speed (m/s)§ | |||
| <0.6 m/s | 46 (13.3%) | 35 (10.4%) | 81 (11.8%) |
| ≥0.6 m/s | 301 (86.7%) | 303 (89.6%) | 604 (88.2%) |
| Mean ± SD | 0.84 ± 0.23 | 0.89 ± 0.23 | 0.86 ± 0.24 |
| No. missing | 2 | 1 | 3 |
| SPPB chair stand time (s)§ | |||
| Mean ± SD | 15.7 ± 6.4 | 15.2 ± 5.0 | 15.4 ± 5.7 |
| No. missing | 39 | 31 | 70 |
| TUG time (s)‖ | |||
| <12 s | 228 (66.1%) | 251 (74.9%) | 479 (70.4%) |
| ≥12 s | 117 (33.9%) | 84 (25.1%) | 201 (29.6%) |
| Mean ± SD | 12.1 ± 5.9 | 11.1 ± 4.6 | 11.6 ± 5.3 |
| No. missing | 4 | 4 | 8 |
| 6-minute walk¶ | |||
| No. not starting walk | 43 | 28 | 71 |
| Those starting walk | |||
| No. | 306 | 311 | 617 |
| Distance (m), mean ± SD | 307 ± 89 | 322 ± 90 | 315 ± 90 |
| Those walking 6 minutes | |||
| No. (% of those starting) | 295 (96.4%) | 300 (96.5%) | 595 (96.4%) |
| Distance (m), mean ± SD | 313 ± 82 | 330 ± 81 | 321 ± 82 |
| Grip strength (kg)# | |||
| Females, no. in dose group | 159 | 141 | 300 |
| Mean ± SD | 18.9 ± 5.8 | 18.1 ± 6.5 | 18.5 ± 6.2 |
| No. missing | 7 | 3 | 10 |
| Males, no. in dose group | 190 | 198 | 388 |
| Mean ± SD | 29.2 ± 9.6 | 30.6 ± 9.0 | 29.9 ± 9.3 |
| No. missing | 2 | 4 | 6 |
Note: IU/day = international units per day; SD = standard deviation; SPPB = Short Physical Performance Battery; TUG = Timed Up-and-Go test.
*Pooled Higher Doses denotes the combined 1 000, 2 000, and 4 000 IU/day groups.
†More than one race could be reported by a participant; race was self-reported.
‡Physical activity level was considered low if <128 kcal/week (males) or <90 kcal/week (females).
§The SPPB is a 3-part assessment of physical functioning: balance testing, timed 4-m walk, and ability to stand up from a seated position in a chair; each part is scored 0–4 and the total SPPB score (range 0–12) is the sum of the 3 subscores. Higher scores indicate better physical function. The total balance stand time (range 0–30 s) combines the stand durations from the 3 balance tests. Gait speed was calculated as 4 m divided by the duration of the walk in seconds. The chair stand test outcome is the time required to complete 5 chair stands and the test is terminated at 60 seconds if not completed by that time.
‖The TUG test is a timed test of standing up from a chair, walking at a normal pace for 3 m, turning, returning to the chair, and sitting down; the test result is the time in seconds to complete all 5 parts. The test is terminated at 60 seconds if not completed by that time.
¶The 6-minute walk is a test of endurance; the participant is instructed to walk at maximum pace for 6 minutes and the score is distance covered. Each site used a straight-line course 10 m in length; each end was marked with a cone which the participant had to walk around. Total distance walked was recorded for each participant who started the walk.
#Grip strength is the maximum of 3 tries with the dominant hand of a hand-held dynamometer; if the dominant hand could not be tested, results for the nondominant hand were used.
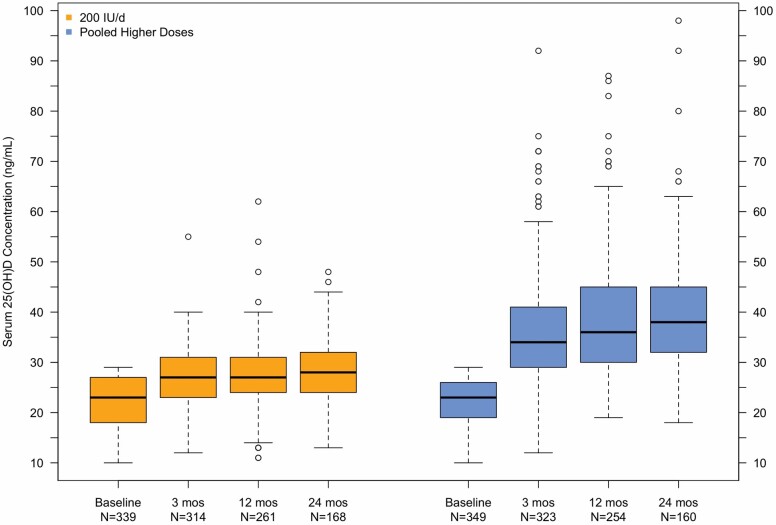
Figure 1 shows box plots of serum 25(OH)D levels at baseline and over follow-up in the control and PHD groups; mean level and change in serum 25(OH)D over time by treatment group are given in Supplementary Table 2. Median serum 25(OH)D level was 23 ng/mL for each group at enrollment; by 3 months after randomization to study pill, median 25(OH)D level was 34 ng/mL in the PHD group versus 27 ng/mL in the control group. This separation between the groups was maintained till the end of follow-up.
Figure 1.
Box plots of serum 25(OH)D at entry and 3, 12, and 24 months after randomization in the primary analysis population. Pooled Higher Doses denotes the combined 1 000, 2 000, and 4 000 IU/day groups.
Primary Comparison Physical Function Analyses
Changes in functional outcome measures from baseline to 3, 12, and 24 months are shown for the PHD group and the 200 IU/day group in Table 2 and Supplementary Table 3. On average, participants in both groups declined in functional performance over time, with significant within-group declines from baseline appearing in some measures at 12 months and in most measures at 24 months. An exception to this observation is the time required to complete 5 chair stands (Table 2); the average change within group was negative for both groups at all 3 time points, indicating faster completion of this test (better function) in follow-up versus at baseline, albeit by less than 2 seconds on average.
Table 2.
Change in Short Physical Performance Battery and Timed Up-and-Go Measures Since Randomization in the Primary Analysis Population
| 3 Months | 12 Months | 24 Months | p, Interaction* | |||||
|---|---|---|---|---|---|---|---|---|
| Pooled Higher Doses† | 200 IU/day | Pooled Higher Doses† | 200 IU/day | Pooled Higher Doses† | 200 IU/day | Unadj | Adj (significant if <.11) | |
| Total SPPB score (range 0–12)‡ | ||||||||
| No. of participants | 324 | 314 | 253 | 260 | 160 | 171 | ||
| BL, mean ± SD§ | 8.7 ± 2.5 | 8.9 ± 2.3 | 8.7 ± 2.4 | 9.0 ± 2.2 | 8.6 ± 2.4 | 9.0 ± 2.2 | ||
| Change (FU-BL), mean ± SD | −0.1 ± 1.7 | 0.0 ± 1.9 | −0.3 ± 2.0 | −0.2 ± 1.9 | −0.6 ± 2.3 | −0.8 ± 2.5 | ||
| p, change within group‖ | .33 | .88 | .04 | .06 | .001 | <.001 | ||
| p, difference between groups* | .67 | .76 | .76 | .91 | .91 | |||
| Total SPPB balance stand time (s)‡ | ||||||||
| No. of participants | 324 | 313 | 253 | 259 | 160 | 170 | ||
| BL, mean ± SD§ | 26.8 ± 6.0 | 26.9 ± 6.3 | 27.0 ± 5.6 | 27.3 ± 5.9 | 26.9 ± 5.9 | 26.7 ± 6.8 | ||
| Change (FU-BL), mean ± SD | 0.3 ± 5.4 | 0.1 ± 6.3 | −0.5 ± 6.6 | 0.1 ± 6.0 | −2.1 ± 7.4 | −1.3 ± 9.0 | ||
| p, change within group‖ | .25 | .77 | .21 | .84 | .001 | .07 | ||
| p, difference between groups* | .60 | .40 | .35 | .45 | .81 | |||
| SPPB gait speed (m/s)‡ | ||||||||
| No. of participants | 320 | 309 | 244 | 258 | 152 | 162 | ||
| BL, mean ± SD§ | 0.85 ± 0.23 | 0.89 ± 0.23 | 0.86 ± 0.24 | 0.90 ± 0.24 | 0.85 ± 0.24 | 0.93 ± 0.23 | ||
| Change (FU-BL), mean ± SD | −0.01 ± 0.17 | −0.01 ± 0.18 | −0.04 ± 0.17 | −0.04 ± 0.20 | −0.03 ± 0.16 | −0.09 ± 0.21 | ||
| p, change within group‖ | .32 | .58 | .001 | .001 | .007 | <.001 | ||
| p, difference between groups* | .97 | .75 | .04 | .15 | .45 | |||
| SPPB chair stand time (s)‡ | ||||||||
| No. of participants | 275 | 276 | 204 | 219 | 123 | 128 | ||
| BL, mean ± SD§ | 15.3 ± 6.1 | 15.1 ± 4.9 | 15.9 ± 6.8 | 15.1 ± 4.6 | 16.2 ± 7.6 | 14.8 ± 4.2 | ||
| Change (FU-BL), mean ± SD | −0.6 ± 6.3 | −0.2 ± 4.4 | −1.7 ± 7.3 | −0.3 ± 4.6 | −1.7 ± 8.1 | −0.6 ± 3.6 | ||
| p, change within group‖ | .14 | .38 | .001 | .30 | .02 | .05 | ||
| p, difference between groups* | .44 | .02 | .43 | .12 | .45 | |||
| TUG time (s)¶ | ||||||||
| No. of participants | 315 | 305 | 237 | 255 | 149 | 159 | ||
| BL, mean ± SD§ | 11.9 ± 5.9 | 11.0 ± 4.6 | 11.5 ± 4.9 | 10.8 ± 3.5 | 11.5 ± 4.1 | 10.4 ± 3.0 | ||
| Change (FU-BL), mean ± SD | −0.2 ± 2.6 | 0.3 ± 4.0 | 0.3 ± 2.6 | 0.5 ± 2.3 | 1.0 ± 3.0 | 1.1 ± 2.5 | ||
| p, change within group‖ | .11 | .15 | .11 | .001 | <.001 | <.001 | ||
| p, difference between groups* | .009 | .14 | .31 | .07 | .45 |
Note: BL = baseline; FU = follow-up; IU/day = international units per day; SD = standard deviation.
*A longitudinal mixed-effects linear regression model with fixed effects including a single term for treatment, 3 time point terms, and 3 treatment-by-time interaction terms and a random intercept for each participant was fit for each outcome; this model included all available measures of the outcome for all participants, including those with only a baseline measure. Each time-specific p value for difference between dose groups is derived from the corresponding treatment-by-time interaction term. The interaction test for overall difference between groups in differential change from baseline is from a 3-degree-of-freedom test of the combined 3 treatment-by-time interaction terms from the longitudinal model. The Benjamini–Hochberg procedure was used to control the false discovery rate to less than 1/9 (1 out of the maximum number of overall comparisons in each family of analyses comparing dose groups; an adjusted p value is statistically significant if <.11).
†Pooled Higher Doses denotes the combined 1 000, 2 000, and 4 000 IU/day groups.
‡The Short Physical Performance Battery (SPPB) is a 3-part assessment of physical functioning: balance testing, timed 4-m walk, and ability to stand up from a seated position in a chair; each part is scored 0–4, and the total SPPB score (range 0–12) is the sum of the 3 subscores. Higher scores indicate better physical function. The total balance stand time (range 0–30 seconds) combines the stand durations from the 3 balance tests. Gait speed was calculated as 4 m divided by the duration of the walk in seconds. The chair stand test outcome is the time required to complete 5 chair stands, and the test is terminated at 60 seconds if not completed by that time.
§Baseline values are shown for those with a follow-up measure at the given time point.
‖ p Values for time-specific change within group were derived from t-tests.
¶The Timed Up-and-Go (TUG) test is a timed test of standing up from a chair, walking at a normal pace for 3 m, turning, returning to the chair, and sitting down; the test result is the time in seconds to complete all 5 parts. The test is terminated at 60 seconds if not completed by that time.
The interaction test of difference between the PHD and the 200 IU/day groups in change from baseline overall was nonsignificant for the SPPB score (unadjusted p = .91) and SPPB total balance stand time (unadjusted p = .45; Table 2). The test of difference between groups overall was also not significant for SPPB gait speed (unadjusted p = .15) and SPPB chair stand time (unadjusted p = .12), despite apparent differences between groups in gait speed at 24 months and in chair stand time at 12 months (Table 2).
While the results for the TUG test yielded a difference between groups in change from baseline overall that was marginally statistically significant (unadjusted p = .07), the adjusted p = .45 was greater than the specified false discovery rate of 0.11. An apparent group difference in TUG time suggestive of improvement was limited to 3 months. At this time point, the PHD group mean change from baseline, −0.2 s ± 2.6, while not significantly different from 0, was in the direction of benefit, whereas the 200 IU/day group mean change from baseline (0.3 s ± 4.0), also not significantly different from 0, was in the direction of loss of function; this divergence of results gave a time-specific unadjusted p = .009 for difference between groups at 3 months (Table 2). Differences between the PHD and control groups in change from baseline overall also were nonsignificant for the 6-minute walk distance (all starting the walk, unadjusted p = .75, those walking 6 minutes unadjusted p = .82) and grip strength (females unadjusted p = .72, males unadjusted p = .42; Supplementary Table 3). The sensitivity analysis comparing those randomized to 1 000 IU/day to the 200 IU/day group also did not support a difference between dose groups in change over time in physical performance measures (Supplementary Table 4).
Secondary Analyses
Analyses of the cohort of those randomized prior to the first adjustment of randomization probabilities by each of the 4 vitamin D doses likewise documented no apparent benefit of the higher dose of vitamin D supplements (Supplementary Table 5). While the overall unadjusted p value for difference between dose groups in change in gait speed was .05, the adjusted p was .36, higher than the specified false discovery rate of .11, and the time-specific comparisons for each higher dose group versus the 200 IU/day group did not suggest consistent benefit in gait speed for any group over another.
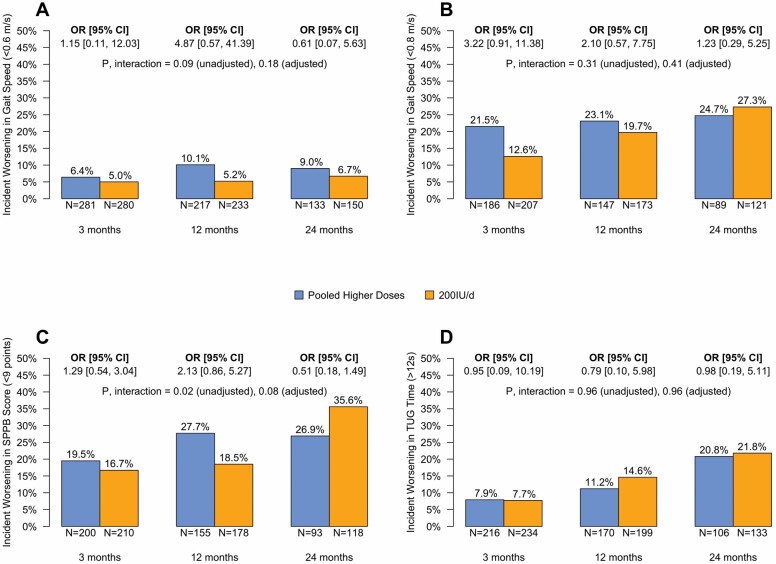
Incidence of transitioning to poor functioning in gait speed, SPPB, and TUG time in the PHD and 200 IU/day groups is shown in Figure 2. For the gait speed threshold of 0.6 m/s, the incidence of dropping below the threshold was greater in the PHD group versus control at 3, 12, and 24 months, but the overall difference between groups was not significant (unadjusted p = .09, adjusted p = .18, above the false discovery rate of 0.11). For the gait speed threshold of 0.8 m/s, the incidence of dropping below the threshold was higher in the PHD group than the 200 IU/day group at Months 3 and 12 but lower at 24 months. The statistically significant interaction for the SPPB score across the 3 time points (unadjusted p = .02, adjusted p = .08, below the false discovery rate of 0.11) was a result of higher incidence of decline in the PHD group at 3 and 12 months (OR [95% CI] for decline 1.29 [0.54–3.04] and 2.13 [0.86–5.27], respectively) and lower incidence of decline at 24 months (OR 0.51 [0.18–1.49]). The incidence of crossing the TUG threshold was nearly the same for both the PHD and control groups at each time point.
Figure 2.
Incident worsening in gait speed (A and B), SPPB score (C), and TUG time (D) in the primary analysis population. Each panel shows the percent of those with performance at least as good as the specified threshold level at baseline who worsen relative to the threshold during follow-up by dose (Pooled Higher Doses or 200 IU/day) and the Pooled Higher Doses versus 200 IU/day odds ratio (OR) of incident worsening by follow-up time. Participants whose baseline performance was worse than the specified threshold are excluded. The count below each bar is the number of participants in that dose group whose performance was at least as good as the threshold at baseline and who completed the measurement at the specified time point; the percent at the top of each bar is the percent of participants whose performance worsened at the specified time point. The Pooled Higher Doses versus 200 IU/day group OR of incident worsening for each time point is shown above each pair of bars with its 95% confidence interval (CI). The interaction p value shown on each plot tests whether the ORs differ over time. The Benjamini–Hochberg procedure was used to control the false discovery rate to less than 1/9 (1 out of the maximum number of overall comparisons in each family of analyses comparing dose groups; an adjusted p value is statistically significant if <.11). The ORs, 95% CIs, and p value are from a mixed-effects logistic regression model with the functional measure as the outcome and fixed effects including 1 term for treatment, 3 terms for time point, 3 treatment-by-time interaction terms, and a random intercept for each participant. Pooled Higher Doses denotes the combined 1 000, 2 000, and 4 000 IU/day groups. SPPB = Short Physical Performance Battery; TUG = Timed Up-and-Go test; IU = international units.
Discussion
Previous inconsistent results of both observational studies and clinical trials examining the impact of vitamin D on physical functioning and disability justified a large trial of an at-risk population to test whether vitamin D supplementation could reduce fall risk and preserve physical function. As previously reported (16), falls occurred at similar frequencies in those treated with 1 000 IU/day compared to 200 IU/day and change in gait speed did not differ overall in the PHD group versus 200 IU/day group. Our principal finding from additional analyses reported in this article is that there is no consistent evidence of any effect—benefit or harm—of vitamin D supplementation on physical function in a population of older adults at elevated risk of falls and with low serum levels of 25(OH)D.
Over the course of up to 24 months, there was no evidence of an overall difference in change in function between those receiving 1 000 IU/day or more (PHD group) and those receiving 200 IU/day in any of the outcomes examined. Sensitivity and exploratory analyses showed no consistent trends toward the benefit of any group over another, neither overall nor time-specific, in any of the physical function outcomes examined. A further test of the effect of vitamin D on transitioning across a critical threshold for gait speed, SPPB, and TUG (Figure 2) also did not show any evidence of a benefit in those taking 1 000 IU/day or higher versus 200 IU/day.
Many RCTs have assessed the possible benefit of vitamin D for strength and mobility, but comparing and aggregating results of these RCTs are difficult due to different inclusion criteria, treatment regimens, outcome measures, and follow-up times. In the review of 15 RCTs by Rosendahl-Riise et al. (11), almost all had a duration of 6 months or less, 7 added calcium to the vitamin D supplementation, and the meta-analysis was limited by the number of studies that used identical outcomes, only 7 included handgrip strength and only 5 included TUG. No beneficial effect was seen in either of those meta-analyses. A more recent meta-analysis using 19 studies also found no benefit for TUG and a small but insignificant improvement for grip strength (24). In subgroup analyses in that study, however, a significant effect was seen for grip strength with a higher dose of vitamin D (>1 000 IU/day), longer duration of the intervention, and lower baseline serum vitamin D level (<30 ng/mL). Another meta-analysis examined the effect of vitamin D on grip strength and found no effect in 17 RCTs with over 5 000 people (25). In 2 studies in that review of persons with severe vitamin D deficiency (<10 ng/mL), supplementation did show a significant effect on grip strength. A meta-analysis published in 2021 of 54 RCTs that addressed multiple physical function outcomes demonstrated that vitamin D supplementation was actually associated with poorer performance on knee extension strength, TUG test time, and the SPPB test (26). In a study of women with documented low 25(OH)D who were treated with supplemental vitamin D or placebo, there was no benefit of 2 800 IU/day of 25(OH)D versus placebo for grip strength, knee flexion strength, or TUG test, with a small but significant unfavorable effect seen for some outcome measures (27). Finally, an aggregation of 3 studies that used the TUG as an outcome showed a small but significant improvement in time to complete the test compared to controls (28). A recent, large multicountry European study did not measure TUG but found no benefit of vitamin D for SPPB score (13).
Adding the present study to previous RCTs, the predominant effect of vitamin D supplements on physical function remains null, although some studies and analyses show potential benefit. In STURDY, we found no difference between dose groups in change in gait speed overall, but saw a statistically significant benefit for gait speed at 24 months in the PHD group compared to 200 IU/day but not earlier, while other studies of shorter duration showed no benefit. It is possible that a long intervention, not attempted in most previous studies, is necessary to see benefit; however, with few other physical function outcomes showing improvement in our study at any time point, our findings do not support that a very long intervention will have a beneficial impact. Our examination of the effect of supplemental vitamin D on transitioning across a critical threshold for gait speed, SPPB, and TUG did not show any evidence of a benefit in those taking 1 000 IU/day or higher versus 200 IU/day.
The chief strengths of this study are enrollment of a vulnerable population, including only persons with low serum 25(OH)D who were at elevated risk of falling; the dose-finding stage that tested 3 doses; the assessment of multiple, well-validated functional endpoints with extensive usage in older populations; the multiple assessments with follow-up time extending to 24 months; and high rates of follow-up. If vitamin D supplementation had a strong effect on physical functioning in this population, this study was well designed to observe that effect.
The trial also has limitations. First, the control group received 200 IU/day of vitamin D because participants had low 25(OH)D levels at baseline; whether this small dose affected physical functioning is unknown. Second, it is possible that supplementing vitamin D would have had benefit for physical functioning if it were given in conjunction with an exercise program, which was not part of this study. For example, in a study of persons with very low 25(OH)D levels (≤16 ng/mL), TUG improved more in persons getting a vitamin D supplement plus resistance training compared to those receiving only vitamin D supplementation, although this outcome was not found for strength or SPPB score (29). A meta-analysis of 7 studies showed that combining vitamin D supplementation with exercise, compared to exercise alone, resulted in improved lower extremity strength but no difference in the SPPB or TUG test. (30). Finally, a more sophisticated method of assessing balance using a computerized force platform showed benefit in a trial of vitamin D supplementation (31), an effect that could have been missed using just the balance component of the SPPB.
In conclusion, this study did not show a benefit of vitamin D supplementation ≥1 000 IU/day compared to 200 IU/day for gait speed, the prespecified secondary outcome for the overall study, nor for multiple additional functional outcomes, consistent with much of the published literature.
Supplementary Material
Contributor Information
Jack M Guralnik, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Alice L Sternberg, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Christine M Mitchell, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Amanda L Blackford, Oncology Biostatistics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Jennifer Schrack, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Amal A Wanigatunga, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Erin Michos, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Stephen P Juraschek, Division of General Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Sarah Szanton, School of Nursing, Johns Hopkins University, Baltimore, Maryland, USA.
Rita Kalyani, Division of Endocrinology, Diabetes, & Metabolism, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Yurun Cai, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Lawrence J Appel, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University , Baltimore, Maryland, USA.
Funding
This trial was funded by the National Institute on Aging (NIA; grant U01AG047837) with support from the Office of Dietary Supplements, Mid-Atlantic Nutrition Obesity Research Center (grant P30DK072488), and Johns Hopkins Institute for Clinical and Translational Research (grant UL1TR003098). The funders had no role in the study report and no role in the decision to submit for publication. S.P.J. was supported by a National Institute of Diabetes and Digestive and Kidney Diseases training grant (T32DK007732) and a National Heart, Lung, and Blood Institute Career Development Award (K23HL135273). J.S. was supported by an NIA Career Development Award (K01AG048765). R.K. was supported by a National Institute of Diabetes and Digestive and Kidney Diseases Career Development Award (K23DK093583).
Conflict of Interest
None declared.
Author Contributions
All authors fulfilled authorship criteria, revised the final version of the manuscript, and gave their consent to publication.
Members of the STURDY Collaborative Research Group are listed in the Supplementary Material.
This article is subject to the NIH Public Access Policy.
References
- 1. Guralnik JM, Fried LP, Salive ME. Disability as a public health outcome in the aging population. Annu Rev Public Health. 1996;17:25–46. doi: 10.1146/annurev.pu.17.050196.000325 [DOI] [PubMed] [Google Scholar]
- 2. Centers for Medicare & Medicaid Services, Medicare Current Beneficiary Survey, Survey File. 2017. https://www.cms.gov/index.php/research-statistics-data-and-systemsresearchmcbsdata-tables/2017-medicare-current-beneficiary-survey-annual-chartbook-and-slides. Accessed November 20, 2020.
- 3. Michel JP, Ecarnot F. Integrating functional ageing into daily clinical practice. J Frailty Sarcopenia Falls. 2019;4(2):30–35. doi: 10.22540/JFSF-04-030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cesari M, Incalzi RA, Zamboni V, Pahor M. Vitamin D hormone: a multitude of actions potentially influencing the physical function decline in older persons. Geriatr Gerontol Int. 2011;11(2):133–142. doi: 10.1111/j.1447-0594.2010.00668.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maggio M, Lauretani F, De Vita F, et al. . Multiple hormonal dysregulation as determinant of low physical performance and mobility in older persons. Curr Pharm Des. 2014;20(19):3119–3148. doi: 10.2174/13816128113196660062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dzik KP, Kaczor JJ. Mechanisms of vitamin D on skeletal muscle function: oxidative stress, energy metabolism and anabolic state. Eur J Appl Physiol. 2019;119(4):825–839. doi: 10.1007/s00421-019-04104-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wicherts IS, van Schoor NM, Boeke AJ, et al. . Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92(6):2058–2065. doi: 10.1210/jc.2006-1525 [DOI] [PubMed] [Google Scholar]
- 8. Aspell N, Laird E, Healy M, Lawlor B, O’Sullivan M. Vitamin D deficiency is associated with impaired muscle strength and physical performance in community-dwelling older adults: findings from the English Longitudinal Study of Ageing. Clin Interv Aging. 2019;14:1751–1761. doi: 10.2147/CIA.S222143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verreault R, Semba RD, Volpato S, Ferrucci L, Fried LP, Guralnik JM. Low serum vitamin d does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc. 2002;50(5):912–917. doi: 10.1046/j.1532-5415.2002.50219.x [DOI] [PubMed] [Google Scholar]
- 10. Matheï C, Van Pottelbergh G, Vaes B, Adriaensen W, Gruson D, Degryse JM. No relation between vitamin D status and physical performance in the oldest old: results from the Belfrail study. Age Ageing. 2013;42(2):186–190. doi: 10.1093/ageing/afs186 [DOI] [PubMed] [Google Scholar]
- 11. Rosendahl‐Riise H, Spielau U, Ranhoff AH, Gudbrandsen OA, Dierkes J. Vitamin D supplementation and its influence on muscle strength and mobility in community‐dwelling older persons: a systematic review and meta‐analysis. J Hum Nutr Diet. 2017;30:3–15. doi: 10.1111/jhn.12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 13. Bischoff-Ferrari HA, Vellas B, Rizzoli R, et al. ; DO-HEALTH Research Group . Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324(18):1855–1868. doi: 10.1001/jama.2020.16909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Michos ED, Mitchell CM, Miller ER 3rd, et al. ; STURDY Collaborative Research Group . Rationale and design of the Study To Understand Fall Reduction and Vitamin D in You (STURDY): a randomized clinical trial of vitamin D supplement doses for the prevention of falls in older adults. Contemp Clin Trials. 2018;73:111–122. doi: 10.1016/j.cct.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michos ED, Mitchell CM, Miller ER 3rd, et al. ; STURDY Collaborative Research Group . Corrigendum to “Rationale and design of the Study To Understand Fall Reduction and Vitamin D in You (STURDY): a randomized clinical trial of vitamin D supplement doses for the prevention of falls in older adults” [Contemp Clin Trials. 73 (2018) 111–122]. Contemp Clin Trials. 2020;90:105936. doi: 10.1016/j.cct.2020.105936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Appel LJ, Michos ED, Mitchell C, et al. . The effects of four doses of vitamin D supplements on falls in older adults: a randomized clinical trial. Ann Int Med. 2021;174:145–156. doi: 10.7326/M20-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. ; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 18. Guralnik JM, Simonsick EM, Ferrucci L, et al. . A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 19. Guyatt GH, Sullivan MJ, Thompson PJ, et al. . The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 20. Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility—giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. 2014;311(20):2061–2062. doi: 10.1001/jama.2014.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bischoff HA, Stähelin HB, Monsch AU, et al. . Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32(3):315–320. doi: 10.1093/ageing/32.3.315 [DOI] [PubMed] [Google Scholar]
- 23. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57(1):289–290. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 24. Abshirini M, Mozaffari H, Kord-Varkaneh H, Omidian M, Kruger MC. The effects of vitamin D supplementation on muscle strength and mobility in postmenopausal women: a systematic review and meta-analysis of randomised controlled trials. J Hum Nutr Diet. 2020;33(2):207–221. doi: 10.1111/jhn.12717 [DOI] [PubMed] [Google Scholar]
- 25. Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22(3):859–871. doi: 10.1007/s00198-010-1407-y [DOI] [PubMed] [Google Scholar]
- 26. Bislev LS, Grove-Laugesen D, Rejnmark L. Vitamin D and muscle health: a systematic review and meta-analysis of randomized placebo-controlled trials. J Bone Miner Res. 2021;36(9):1651–1660. doi: 10.1002/jbmr.4412 [DOI] [PubMed] [Google Scholar]
- 27. Bislev LS, Langagergaard Rødbro L, Rolighed L, Sikjaer T, Rejnmark L. Effects of vitamin D3 supplementation on muscle strength, mass, and physical performance in women with vitamin D insufficiency: a randomized placebo-controlled trial. Calcif Tissue Int. 2018;103(5):483–493. doi: 10.1007/s00223-018-0443-z [DOI] [PubMed] [Google Scholar]
- 28. Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59(12):2291–2300. doi: 10.1111/j.1532-5415.2011.03733.x [DOI] [PubMed] [Google Scholar]
- 29. Bunout D, Barrera G, Leiva L, et al. . Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol. 2006;41(8):746–752. doi: 10.1016/j.exger.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 30. Antoniak AE, Greig CA. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: a systematic review and meta-analysis. BMJ Open. 2017;7(7):e014619. doi: 10.1136/bmjopen-2016-014619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cangussu LM, Nahas-Neto J, Orsatti CL, et al. . Effect of isolated vitamin D supplementation on the rate of falls and postural balance in postmenopausal women fallers: a randomized, double-blind, placebo-controlled trial. Menopause. 2016;23(3):267–274. doi: 10.1097/GME.0000000000000525 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.