Abstract
Forkhead box O3 (FOXO3) is a candidate longevity gene. Urban residents are also positively associated with longer life expectancy. We conducted a gene–environment interaction to assess the synergistic effect of FOXO3 and urban/rural environments on mortality. We included 3 085 older adults from the Chinese Longitudinal Healthy Longevity Survey. We used single-nucleotide polymorphisms (SNPs) rs2253310, rs2802292, and rs4946936 to identify the FOXO3 gene and classified residential locations as “urban” and “rural.” Given the open cohort design, we used the Cox-proportional hazard regression models to assess the mortality risk. We found the minor allele homozygotes of FOXO3 to have a protective effect on mortality (HR [95% CI] for rs4946936 TT vs CC: 0.807 [0.653–0.996]; rs2802292 GG vs TT: 0.812 [0.67–0.985]; rs2253310 CC vs GG: 0.808 [0.667–0.978]). Participants living in urban areas had a lower risk of mortality (HR of the urban vs the rural: 0.854 [0.759–0.962]). The interaction between FOXO3 and urban and rural regions was statistically significant (pinteraction < .01). Higher air pollution (fine particulate matter: PM2.5) and lower residential greenness (Normalized Difference Vegetation Index [NDVI]) both contributed to higher mortality. After adjusting for NDVI and PM2.5, the protective effect size of FOXO3 SNPs was slightly attenuated while the protective effect size of living in an urban environment increased. The effect size of the beneficial effect of FOXO3 on mortality is roughly equivalent to that of living in urban areas. Our research findings indicate that the effect of places of residence and genetic predisposition of longevity are intertwined.
Keywords: Air pollution, Greenness, Healthy aging, Longevity gene, Urban environment
Mammals have 4 Forkhead box O (FOXO) genes, including FOXO1, FOXO3, FOXO4, and FOXO6. FOXO proteins are important transcription factors. They turn stimuli arising from insulin, growth factors, nutrients, and oxidative stress into gene expressions (1). FOXOs are associated with longevity through the upregulation of target genes involved in stress resistance, metabolism, cell cycle arrest, and apoptosis (2). FOXO is thought to act against aging and age-related diseases, including cardiovascular disease, type 2 diabetes, cancer, and neurodegenerative diseases (3). Genetic variation of the FOXO3 gene and human longevity association were initially described in a group of American Men of Japanese ancestry in Hawaii. The investigators found an odds ratio (OR) of 2.75 for homozygous minor versus homozygous major alleles between the longevity cases and controls of younger population (4). Subsequently, a plethora of studies in human populations showed FOXO3 to be associated with longevity and better health span across diverse human populations (5). The mechanism of the FOXO3 gene is complex and multifaceted. A previous review article suggests that genetic factors account for approximately one third of the variability in the human life span (6). Thus, FOXO3 is not necessarily deterministic for better healthspan, and its interaction with the environment may play a role. We previously reported the interaction between FOXO and residential greenness (7). So far, there are no other studies on the interaction of FOXO3 with urban environmental factors.
Empirical evidence points to urban residents having better health than their rural counterparts around the world, especially in developed countries (8,9). This could be due to better access to medical treatment, better sanitation, nutrition, education, and income. At the same time, recent urbanization in developing countries often coincides with higher air pollution due to industrial activities and decreasing residential greenness because of city expansion. The effect of these environmental factors on premature mortality has been studied extensively. Air pollution is one of the highest causes of disability-adjusted life years globally, and residential greenness has been shown to be related to older adults’ health (10,11). Still, the global burden of disease for low greenness access lacks gene–environmental interaction evidence.
Because life span has complex environmental and genetic underpinnings, we aim to study the effects of FOXO3 and the urban–rural disparity on mortality. Our analysis considers demographic, lifestyle, and environmental (air pollution and residential greenness) factors. First, we aim to evaluate the main effect of FOXO3 and residence on mortality in a Chinese older population. Second, we aim to see if there is evidence of effect modification by air pollution or greenness levels. Third, we aim to look specifically at effect modification age and gender through interaction terms and stratified analyses.
Method
Study Population
We used the Chinese Longitudinal Healthy Longevity Survey (CLHLS) data. The study is an open cohort, with participants entering in 2008 and following up to 2014, surveyed roughly biennially. The geographical distribution encompassed 23 out of 34 provinces in China, covering a wide range of urban and rural regions, with socioeconomic and climatic diversity. There were 3 554 participants with genotype sequencing data first interviewed in 2008. We excluded 133 participants aged younger than 65 years or belonging to ethnic minorities and 336 participants with missing data in residence, environmental factors, and other covariates. The final study sample consisted of 3 085 participants.
Genotyping
Beijing Genomics Institute carried out a replication study for 13 228 individuals using a well-designed and customized chip, targeting about 27 000 longevity-phenotype-related single-nucleotide polymorphisms (SNPs) based on the prior CLHLS genome-wide association study. We extracted the FOXO3 genotypic data of the 2008 cohort from this replication study. The single SNP association analysis, genotype association analysis, linkage disequilibrium, and haplotype association analysis of CLHLS FOXO data were presented in a previous study (12). We used the same tagging SNPs rs4946936, rs2802292, and rs2253310 to identify the FOXO3 gene as theirs (12). We abbreviated them as FOXO3_rs4946936, FOXO3_rs2802292, and FOXO3_rs2253310, respectively, in this study. The minor/major alleles were T/C for FOXO3_rs4946936, G/T for FOXO3_rs2802292, and C/G for FOXO3_rs2253310, respectively.
Environmental Exposure Assessment
There were 3 categories of residence in CLHLS: village, town, and city. We further classified “City” and “Town” as urban areas and “Village” as rural areas. Using each participant’s geographical residential location, we calculated greenness and air pollution exposures.
We used the Normalized Difference Vegetation Index (NDVI) with a 500-m radius around the residence to quantify the residential greenness. NDVI quantifies vegetation by measuring the difference between near-infrared (which vegetation strongly reflects) and red light (which vegetation absorbs). NDVI ranges from −1.0 to +1.0, with higher values indicating higher levels of vegetative density or more abundant greenness. Based on the satellite image from the Moderate-Resolution Imaging Spectroradiometer in the National Aeronautics and Space Administration’s Terra Satellite, we calculated NDVI from near-infrared radiation minus visible radiation divided by near-infrared radiation plus visible radiation. We linked the individual’s residential address at the time of the interview with the imagery according to the longitude and latitude to get NDVI. We calculated the contemporaneous NDVI to assess acute exposure to greenness, defined as the nearest month NDVI to the month of death for individuals who had died or the last interview month for those alive or lost to follow-up (13).
Ground-level PM2.5 concentrations were estimated by combining aerosol optical depth retrievals from the National Aeronautics and Space Administration’s Moderate-Resolution Imaging Spectroradiometer, Multiangle Imaging Spectroradiometer, and Sea-viewing Wide field-of-view Sensor satellite instruments; vertical profiles derived from the GEOS-Chem chemical transport model; and calibration to ground-based observations of PM2.5 using geographically weighted regression (14). The resultant PM2.5 concentration estimates were highly consistent (R² = 0·81) with out-of-sample 10-fold cross-validated PM2.5 concentrations from monitors (14). We matched the annual average PM2.5 concentrations in a 1 km × 1 km grid to each participant’s residence (15). We calculated the 3-year average PM2.5, which was found to have the strongest association with mortality among older adults in China (15).
Covariates
We included baseline age, gender, marital status, education, smoking status, drinking status, and physical activity (16,17). We classified marital status into 2 categories: Currently married and living with spouse as “married” and widowed/separated/divorced/never married/married but not living with spouse as “not married.” We used the schooling year to evaluate education level. We divided the regular exercise, smoking, and alcohol drinking status into 3 categories: “Current,” “Former,” and “Never.” For example, participants were asked, “Do you do exercise regularly at present (planned exercise like walking, playing balls, running, and so on)?” and/or “Did you do exercise regularly in the past?” We defined the regular exercise status as “Current” for participants who answered “Yes” to the first question, “Former” for who answered “No” to the first question and “Yes” to the second question, and “Never” for who answered “No” to both questions.
Statistical Model
We conducted Cox-proportional hazard regression models for every FOXO3 SNPs (FOXO3_rs2253310, FOXO3_rs2802292, and FOXO3_rs4946936) and residence to evaluate their single effect on mortality. We assessed the interaction of FOXO3 SNPs and residence by adding their product term. We adjusted for age, gender, marital status, education, smoking status, drinking status, and physical activity in all models. We draw the adjusted survival curve based on the expected survival curves calculated based on the Cox model separately for subpopulations (18). As a sensitivity analysis, we additionally adjusted for NDVI and PM2.5, FOXO3 × NDVI and residence × NDVI, or FOXO3 × PM2.5 and residence × PM2.5. We also examined their associations stratified by gender and age. We set the nominal significance level at 0.05. We presented the exact p value, adjusted hazard ratios (HRs), and 95% confidence intervals (CIs) to indicate the effect size of exposures on mortality. We used R 4.0.3 and SAS university edition to perform all the analyses.
Results
The baseline mean age of the 3 085 participants was 84.9 (SD: 11.3) years, 1 634 (53%) were female, and 32.3% lived in urban areas (classified as city or town). In 12 696 person-years of follow-up, we saw 1 439 mortality events. The distributions of the 3 SNPs of FOXO3 were even across populations of different demographic characteristics (except for age groups and gender), indicating mendelian randomization. Participants in rural areas received fewer years of childhood education, reported a lower frequency of exercise behavior, and lived around places with higher residential greenness than urban areas. The distribution of age, sex, marriage status, smoking, alcohol drinking, and ambient PM2.5 exposure level was similar between rural and urban residents (Table 1).
Table 1.
Study Population Characteristics at Baseline
| Variables | Minor Allele of FOXO3_rs2802292 | Residence | Overall | |||
|---|---|---|---|---|---|---|
| 0 (N = 1 552) | 1 (N = 1 248) | 2 (N = 285) | Rural (N = 2 090) | Urban (N = 995) | (N = 3 085) | |
| Sex: n (%) | ||||||
| Male | 718 (46.3) | 587 (47.0) | 146 (51.2) | 975 (46.7) | 476 (47.8) | 1 451 (47.0) |
| Female | 834 (53.7) | 661 (53.0) | 139 (48.8) | 1 115 (53.3) | 519 (52.2) | 1 634 (53.0) |
| Age | ||||||
| Mean (SD) | 84.5 (11.4) | 85.5 (11.1) | 84.7 (11.0) | 84.8 (11.2) | 85.2 (11.3) | 84.9 (11.3) |
| Median [Min, Max] | 86.0 [65.0, 107] | 87.0 [65.0, 110] | 86.0 [65.0, 107] | 86.0 [65.0, 107] | 87.0 [65.0, 110] | 86.0 [65.0, 110] |
| Age group: n (%) | ||||||
| 65–79 | 444 (28.6) | 324 (26.0) | 76 (26.7) | 584 (27.9) | 260 (26.1) | 844 (27.4) |
| 80–89 | 502 (32.3) | 392 (31.4) | 95 (33.3) | 679 (32.5) | 310 (31.2) | 989 (32.1) |
| 90–99 | 428 (27.6) | 374 (30.0) | 83 (29.1) | 574 (27.5) | 311 (31.3) | 885 (28.7) |
| 100+ | 178 (11.5) | 158 (12.7) | 31 (10.9) | 253 (12.1) | 114 (11.5) | 367 (11.9) |
| Education year: n (%) | ||||||
| 0 year | 966 (62.2) | 775 (62.1) | 166 (58.2) | 1 377 (65.9) | 530 (53.3) | 1 907 (61.8) |
| ≥1 year | 586 (37.8) | 473 (37.9) | 119 (41.8) | 713 (34.1) | 465 (46.7) | 1 178 (38.2) |
| Marriage: n (%) | ||||||
| Married | 608 (39.2) | 449 (36.0) | 108 (37.9) | 775 (37.1) | 390 (39.2) | 1 165 (37.8) |
| Not married | 944 (60.8) | 799 (64.0) | 177 (62.1) | 1 315 (62.9) | 605 (60.8) | 1 920 (62.2) |
| Exercise: n (%) | ||||||
| Current | 439 (28.3) | 341 (27.3) | 81 (28.4) | 411 (19.7) | 450 (45.2) | 861 (27.9) |
| Former | 96 (6.2) | 66 (5.3) | 17 (6.0) | 108 (5.2) | 71 (7.1) | 179 (5.8) |
| Never | 1 017 (65.5) | 841 (67.4) | 187 (65.6) | 1 571 (75.2) | 474 (47.6) | 2 045 (66.3) |
| Smoking: n (%) | ||||||
| Current | 340 (21.9) | 278 (22.3) | 65 (22.8) | 469 (22.4) | 214 (21.5) | 683 (22.1) |
| Former | 204 (13.1) | 173 (13.9) | 34 (11.9) | 263 (12.6) | 148 (14.9) | 411 (13.3) |
| Never | 1 008 (64.9) | 797 (63.9) | 186 (65.3) | 1 358 (65.0) | 633 (63.6) | 1 991 (64.5) |
| Alcohol drinking: n (%) | ||||||
| Current | 355 (22.9) | 280 (22.4) | 58 (20.4) | 480 (23.0) | 213 (21.4) | 693 (22.5) |
| Former | 174 (11.2) | 113 (9.1) | 23 (8.1) | 204 (9.8) | 106 (10.7) | 310 (10.0) |
| Never | 1 023 (65.9) | 855 (68.5) | 204 (71.6) | 1 406 (67.3) | 676 (67.9) | 2 082 (67.5) |
| Contemporaneous NDVI in 0.1 unit | ||||||
| Mean (SD) | 4.19 (2.00) | 4.39 (1.97) | 4.20 (1.98) | 4.64 (1.93) | 3.51 (1.89) | 4.27 (1.99) |
| Median [Min, Max] | 4.00 [−1.52, 9.04] | 4.22 [0.111, 8.82] | 3.93 [−0.212, 8.36] | 4.60 [−0.212, 8.69] | 3.14 [−1.52, 9.04] | 4.08 [−1.52, 9.04] |
| Three-year average PM2.5 in 10 μg/m³ | ||||||
| Mean (SD) | 5.07 (1.41) | 5.04 (1.37) | 4.84 (1.46) | 5.08 (1.39) | 4.94 (1.41) | 5.04 (1.40) |
| Median [Min, Max] | 5.20 [0, 9.81] | 5.07 [0, 9.81] | 4.85 [0, 8.13] | 5.21 [0, 9.80] | 4.94 [0, 9.81] | 5.14 [0, 9.81] |
| Residence: n (%) | ||||||
| Rural | 1 037 (66.8) | 863 (69.2) | 190 (66.7) | — | — | 2 090 (67.7) |
| Urban | 515 (33.2) | 385 (30.8) | 95 (33.3) | — | — | 995 (32.3) |
| Minor allele of FOXO3_rs2802292: n (%) | ||||||
| 0 | — | — | — | 1 037 (49.6) | 515 (51.8) | 1 552 (50.3) |
| 1 | — | — | — | 863 (41.3) | 385 (38.7) | 1 248 (40.5) |
| 2 | — | — | — | 190 (9.1) | 95 (9.5) | 285 (9.2) |
| Minor allele of FOXO3_rs2253310: n (%) | ||||||
| 0 | 1 546 (99.6) | 23 (1.8) | 0 (0) | 1 048 (50.1) | 521 (52.4) | 1 569 (50.9) |
| 1 | 6 (0.4) | 1 222 (97.9) | 1 (0.4) | 850 (40.7) | 379 (38.1) | 1 229 (39.8) |
| 2 | 0 (0) | 3 (0.2) | 284 (99.6) | 192 (9.2) | 95 (9.5) | 287 (9.3) |
| Minor allele of FOXO3_rs4946936: n (%) | ||||||
| 0 | 1 519 (97.9) | 151 (12.1) | 4 (1.4) | 1 114 (53.3) | 560 (56.3) | 1 674 (54.3) |
| 1 | 33 (2.1) | 1 082 (86.7) | 73 (25.6) | 831 (39.8) | 357 (35.9) | 1 188 (38.5) |
| 2 | 0 (0) | 15 (1.2) | 208 (73.0) | 145 (6.9) | 78 (7.8) | 223 (7.2) |
Note: NDVI = Normalized Difference Vegetation Index; PM = particulate matter.
The major allele homozygote, heterozygote, and minor allele homozygote proportion for FOXO3_rs4946936 were 54.3%, 38.5%, and 7.2%, respectively, for FOXO3_rs2802292 were 50.3%, 40.5%, and 9.2%, respectively, for FOXO3_rs2253310 were 50.9%, 39.8, and 9.3%, respectively (Table 1). In Cox-proportion hazard regression model, we adjust for age, sex, and lifestyle factors. We found the minor allele homozygotes of FOXO3 to be associated with protective effect against mortality compared to major allele homozygotes (HR [95% CI] for FOXO3_rs4946936 TT vs CC: 0.807 [0.653–0.996], for FOXO3_rs2802292 GG vs TT: 0.812 [0.67–0.985], and for FOXO3_rs2253310 CC vs. GG: 0.808 [0.667–0.978]; Table 2).
Table 2.
The Association Between FOXO3 SNPs, Residence, and Mortality
| Term | Model—Residence | Model—FOXO | Model—FOXO + Residence | Model—FOXO × Residence | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | β (SE) | p | |
| FOXO3_rs4946936 (CC as reference) | |||||||||
| TC | 0.947 (0.849–1.056) | .328 | 0.941 (0.844–1.049) | .272 | 0.932 (0.819–1.060) | −0.071 (0.066) | .282 | ||
| TT | 0.807 (0.653–0.996) | .046 | 0.801 (0.649–0.989) | .04 | 0.657 (0.503–0.859) | −0.419 (0.136) | .002 | ||
| Residence (Rural as reference) | 0.854 (0.759–0.962) | .009 | 0.851 (0.756–0.957) | .007 | 0.805 (0.688–0.941) | −0.217 (0.08) | .007 | ||
| FOXO3_rs4946936 TC × residence—urban | 1.029 (0.808–1.311) | 0.028 (0.124) | .818 | ||||||
| FOXO3_rs4946936 TT × residence—urban | 1.846 (1.194–2.854) | 0.613 (0.222) | .006 | ||||||
| FOXO3_rs2802292 (TT as reference) | |||||||||
| TG | 0.956 (0.857–1.066) | .420 | 0.951 (0.853–1.061) | .371 | 0.958 (0.843–1.090) | −0.043 (0.066) | .517 | ||
| GG | 0.812 (0.670–0.985) | .034 | 0.806 (0.665–0.977) | .028 | 0.646 (0.506–0.825) | −0.437 (0.125) | <.001 | ||
| Residence (rural as reference) | 0.854 (0.759–0.962) | .009 | 0.851 (0.756–0.957) | .007 | 0.807 (0.686–0.95) | −0.214 (0.083) | .01 | ||
| FOXO3_rs2802292 TG × residence—urban | 0.970 (0.761–1.236) | −0.031 (0.124) | .805 | ||||||
| FOXO3_rs2802292 GG × residence—urban | 1.998 (1.342–2.975) | 0.692 (0.203) | .001 | ||||||
| FOXO3_rs2253310 (GG as reference) | |||||||||
| GC | 0.942 (0.845–1.051) | .285 | 0.938 (0.840–1.046) | .247 | 0.943 (0.829–1.073) | −0.058 (0.066) | .375 | ||
| CC | 0.808 (0.667–0.978) | .029 | 0.801 (0.661–0.97) | .023 | 0.643 (0.505–0.820) | −0.441 (0.124) | <.001 | ||
| Residence (rural as reference) | 0.854 (0.759–0.962) | .009 | 0.85 (0.755–0.957) | .007 | 0.805 (0.685–0.946) | −0.216 (0.082) | .009 | ||
| FOXO3_rs2253310 GC × residence—urban | 0.973 (0.763–1.241) | −0.027 (0.124) | .828 | ||||||
| FOXO3_rs2253310 CC × residence—urban | 1.996 (1.342–2.968) | 0.691 (0.202) | .001 |
Notes: HR = hazard ratio; CI = confidence interval; SNP = single-nucleotide polymorphism. All models adjusted for baseline age, sex, education, marriage, smoking, alcohol drinking, and exercise.
As expected, those who lived in urban areas had a lower risk of mortality (HR [95% CI]: 0.854 [0.759–0.962]) compared to rural areas (Table 2). Correspondingly, higher contemporaneous NDVI was associated with a lower risk of mortality (HR [95% CI] for 0.1 unit of NDVI: 0.885 [0.861–0.909]). Higher 3-year average PM2.5 was associated with a higher risk of mortality (HR [95% CI] for each 10 μg/m³ increase of PM2.5: 1.13 [1.086–1.175]; Supplementary Table 1).
In our effect modification analysis, we found statistically significant interactions between FOXO3 SNP minor allele homozygote and urban versus rural residential locations (Table 2). Interestingly, the protective effect of FOXO3 SNP minor allele homozygote was only evident in rural areas but not in urban areas (Table 3). A significant interaction also existed between FOXO3 SNP and NDVI, but not between FOXO3 SNP and PM2.5. The protective effect of urban residence did not shown or reversed among those carrying two minor alleles (Table 4).
Table 3.
Hazard Ratios (95% CI) of FOXO3 SNPs on Mortality in the Interaction Model on the Condition of Rural and Urban Residence
| Rural Areas | Urban Areas | |||
|---|---|---|---|---|
| n | HR (95% CI) | n | HR (95% CI) | |
| FOXO3_rs4946936 | ||||
| CC | 1 114 | Reference | 560 | Reference |
| TC | 831 | 0.932 (0.819–1.060) | 357 | 0.959 (0.781–1.177) |
| TT | 145 | 0.657 (0.503–0.859)* | 78 | 1.214 (0.86–1.713) |
| FOXO3_rs2802292 | ||||
| TT | 1 037 | Reference | 515 | Reference |
| TG | 863 | 0.958 (0.843–1.09) | 385 | 0.929 (0.757–1.142) |
| GG | 190 | 0.646 (0.506–0.825)* | 95 | 1.291 (0.943–1.767) |
| FOXO3_rs2802292 | ||||
| GG | 1 048 | Reference | 521 | Reference |
| GC | 850 | 0.943 (0.829–1.073) | 379 | 0.918 (0.747–1.129) |
| CC | 192 | 0.644 (0.505–0.820)* | 95 | 1.284 (0.938–1.758) |
Notes: HR = hazard ratio; CI = confidence interval; SNP = single-nucleotide polymorphism. All models adjusted for baseline age, sex, education, marriage, smoking, alcohol drinking, and exercise.
*Denotes statistical significance.
Table 4.
The HRs (95% CI) for Mortality by Urban and Rural Residence in the Interaction Model on the Condition of Different Genotypes of FOXO3 SNPs
| Rural vs Urban (reference) | ||
|---|---|---|
| n rual/nurban | HR (95% CI) | |
| FOXO3_rs4946936 CC | 1 114/560 | 1.243 (1.063–1.454) |
| FOXO3_rs4946936 TC | 831/357 | 1.208 (0.999–1.461) |
| FOXO3_rs4946936 TT | 145/78 | 0.673 (0.448–1.013) |
| FOXO3_rs2802292 TT | 1 037/515 | 1.239 (1.053–1.457) |
| FOXO3_rs2802292 TG | 863/385 | 1.277 (1.060–1.539) |
| FOXO3_rs2802292 GG | 190/95 | 0.620 (0.431–0.892) |
| FOXO3_rs2253310 GG | 1 048/521 | 1.242 (1.057–1.459) |
| FOXO3_rs2253310 GC | 850/379 | 1.276 (1.057–1.540) |
| FOXO3_rs2253310 CC | 192/95 | 0.622 (0.433–0.895) |
Notes: HR = hazard ratio; CI = confidence interval; SNP = single-nucleotide polymorphism. All models adjusted for baseline age, sex, education, marriage, smoking, alcohol drinking, and exercise.
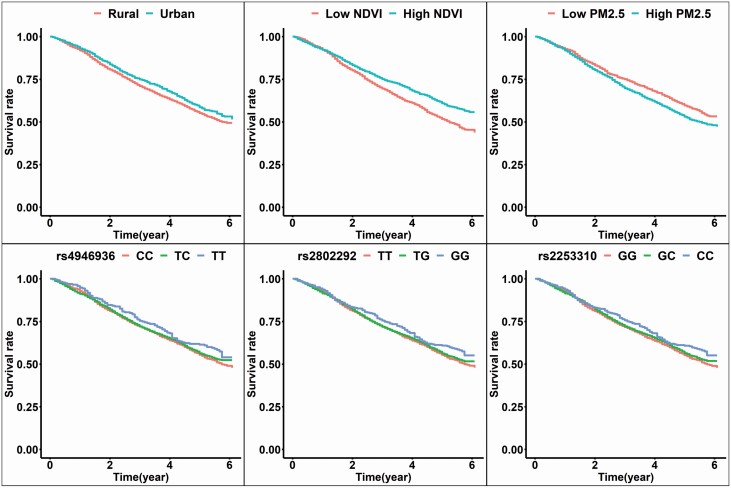
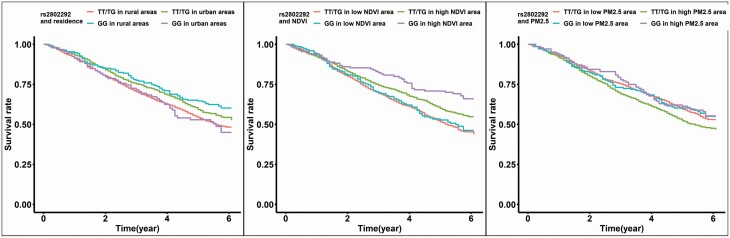
The adjusted Kaplan–Meier’s survival curve plots out the mortalities over the years of follow-up for FOXO3 and residential locations (Figures 1 and 2). Residing in urban regions, higher contemporaneous NDVI, lower PM2.5, and minor allele homozygote of FOXO3 had higher survival rates than their counterparts (Figure 1). Minor allele homozygote carriers of FOXO3_rs2802292 had a higher survival rate in rural areas than urban areas, and those nonminor allele homozygote carriers had a higher survival rate in urban areas than rural areas. For the combination of NDVI and FOXO3, minor allele homozygote of FOXO3_rs2802292 had a higher survival rate than those without minor allele homozygote in high NDVI areas. At the same time, there was no significant difference among the genotypes in the low NDVI area. The protective effect of the minor allele homozygote of FOXO3_rs2802292 was evident in the high PM2.5 area, not in low PM2.5. The harmful effect of high PM2.5 showed only in participants without minor allele homozygote of FOXO3_rs2802292 (Figure 2). Adjustment of NDVI and PM2.5 attenuated some effects (Supplementary Table 1).
Figure 1.
The adjusted survival curve of the residence, NDVI, PM2.5, and FOXO3 SNPs. Note: The model was adjusted for baseline age, sex, schooling year, residence, marriage, exercise, smoking, and drinking alcohol. The adjusted survival curve was based on the average predictions for each stratum by using the R package survminer. NDVI = Normalized Difference Vegetation Index; PM = particulate matter.
Figure 2.
The adjusted survival curve of the combination of FOXO3_292 and the residential factors. Note: The model was adjusted for baseline age, sex, schooling year, residence, marriage, exercise, smoking, and drinking alcohol. The adjusted survival curve was based on the average predictions for each stratum by using the R package survminer.
After adding the term of FOXO3 × NDVI and residence × NDVI in the model, the interaction of FOXO3 and residence persisted. The interaction of FOXO3 and NDVI was significant, while there was no significant interaction between residence and NDVI. In the model with interaction terms of FOXO3 × residence, FOXO3 × PM2.5, and residence × PM2.5, the interaction of FOXO3 and residence persisted. The interaction of FOXO3 and PM2.5, residence and PM2.5 were both not significant. The 3-way interaction of gender × FOXO3 × residence was not significant. In the stratified analyses by gender, the main protective effect of FOXO only showed in the female, not in the male. The effect of residence was a little stronger in the male than the female. The interaction between FOXO3_rs2802292/rs2253310 and residence was significant in the female and male, and the interaction between FOXO3_rs4946936 and residence was only significant in the female (Supplementary Tables 2 and 3). In the stratified analyses by age, FOXO3 only showed a borderline protective effect on mortality risk among participants aged 85 or older, and urban living only had a protective effect among participants aged younger than 85. The interaction between FOXO3_rs2802292/rs2253310 and residence was significant in both age groups (Supplementary Tables 4 and 5).
Discussion
By utilizing a longitudinal cohort design where our participants reside in diverse settings, we can ascertain and compare genetic and environmental effects. We found a significant effect of FOXO3 on mortality in a cohort of older individuals in China. We also found people living in urban areas had a protective impact on mortality compared to rural regions. While some people with FOXO3 minor allele have a genetic advantage in longevity, that advantage is similar to the benefit of living in an urban area, comparing the hazard ratios. In our stratified analyses, the effect of the gene was more pronounced in rural areas, females, and participants aged 85 or older.
Our study is the first to concurrently compare the effect of the environment and genetic determinants of longevity. On the environmental side, much empirical evidence points to urban residents having better health outcomes than rural residents in countries spanning both high, middle, and low-income levels. The number of people and the proportion of the world population now living in cities account for 55% of the world’s population (19). In China, urban residents account for 63.9% of the population (20). Cities can be a positive force for health, such as sanitation and water safety, access to medical care, and greater food diversity. Negative urban environmental factors that affect health include air and noise pollution, lack of green space, and toxic waste.
Environment, genetics, and stochastic processes all contribute to longevity. Our findings on FOXO3 replicated prior research findings of a strong protective effect, as FOXO3 has been associated with human longevity in many populations. The OR and 95% CI for the longevity of homozygous minor allele versus homozygous major allele were 2.75 (1.51–5.02) for FOXO3_rs2802292 in a Japanese American men cohort (4), 2.44 (1.38–4.34) for rs2802288 (a proxy of FOXO3_rs2802292) in the Southern Italian Centenarian males (21), and 1.53 (1.06–2.21) for rs2802288 in a German population (22). The OR (95%CI) for the longevity of one or two minor alleles versus homozygous major allele carriers were 1.65 (1.19–2.30) in the male and 1.67 (1.27–2.18) in the female for FOXO3_rs2802292, and FOXO3_rs2253310 and FOXO3_rs4946936 had similar results in the Han Chinese population (12). These prior studies were of case–control (longevity vs nonlongevity) study design and all indicated those carrying minor alleles of FOXO SNPs were more likely to be long-lived than those carrying major alleles. A CLHLS study compared the centenarians and people who died before 100 years old in the follow-up and found those who carried the minor allele of any SNP of FOXO3_rs494693, FOXO3_rs2802292, FOXO3_rs2253310 may have 62%–67% and 61%–73% higher probability of survival from middle age to age 100 years and older (23). In our prospective cohort study design, we confirmed the protective effect of FOXO3 SNPs in longitudinal cohort analyses (HRs were around 0.8 for each of the 3 FOXO3 SNPs). The cohort study design of our analysis allows us to assess the relative effect and effect modification of environmental factors.
It is possible that the built environment can interact with the gene effect. We found the protective effect of FOXO3 SNP minor allele homozygote was only evident in rural areas but not in urban areas. Future studies are needed on the etiology of the effects of FOXO3 in residential locations. For the most part, rural residents are more sensitive to air pollution than urban residents in China. Previous studies demonstrated that socioregional factors such as urban/rural residency might influence the expression of heritable tendencies related to alcohol use (24), schizophrenia (25), and subclinical depressive symptoms (26). These factors may be influential on FOXO3 gene expression, or that FOXO3 may modify the etiology of these diseases. In a previous study, we found that the association between FOXO3 SNP and mortality was more substantial in areas with higher greenness (7). In developed countries, indigenous adult all-cause mortality, cervical cancer mortality, trauma mortality, and incidence of myocardial infarction were all significantly lower in urban areas than rural areas in a review on populations of Australia, Canada, New Zealand, and the United States (9). The rural mortality penalty is large and growing based on a study at the county level of the United States, and the findings indicated that the effects of rurality on mortality were not the result of spuriousness produced by place-based differences in race, education, income, and poverty (8). A review studied health in relation to aspects of urbanization across developing countries and found urbanization was positively but not significantly associated with life expectancy and urban–rural differences in mortality from communicable diseases depending on the disease studied (27). A previous study based on CLHLS 2002–2014 wave found that older adults in urban areas had 11% lower risks of mortality (HR = 0.89, p < .01) than their rural counterparts adjusting for demographic factors, and this association was explained away by socioeconomic factors (28). We confirmed the rural mortality penalty with a similar HR (HR [95% CI]: 0.854 [0.759–0.962]), and we found this association persisted after considering environmental factors including PM2.5 and NDVI.
Our study has many strengths. First, our study is nested within a large cohort study, which collected information on many potential confounders and lifestyle attributes of the study participants. Compared to many other genetic and health studies using a case–control study design, our findings should be more robust. Second, our study population is diverse and is spread throughout many different regions of China, covering a wide range of socioeconomic and climatic regions. This diversity not only allows us to look for effect modification but also allows us to see dose–response relationships due to the heterogeneity of exposures. Third, our study has many years of follow-up, allowing us to calculate long-term follow-up effects. Fourth, our study is an early adopter of remote sensing technology in calculating environment exposures, which is less prone to bias.
Our study has several limitations due to the study design and resolution of data. First, while our research study is based on a large cohort, we do not have molecular insight into how air pollution and residential greenness are interacting with FOXO3 on a genetic level or on a protein level. Second, our cohort is composed of a group of older adults, which means we cannot generalize our findings to younger populations. Third, we did not consider the change of residence. Although the older adults were unlikely to move, the residence rurality can change after years’ follow-up. Future studies can evaluate the association between the time-varying residence and mortality. Furthermore, our cohort resides in one country, so whether a similar relationship exists in other populations needs validation.
Conclusions
In our study cohort of older Chinese, we replicated the finding of a protective effect of FOXO3 against mortality. Our results indicated that FOXO3 and residence in urban environments have synergistic effects on health. Our epidemiology findings provide insight into possible activation hypotheses and gene–environment interactions.
Supplementary Material
Acknowledgments
The authors thank all the participants and workers of the Chinese Longitudinal Healthy Longevity Study (CLHLS).
Contributor Information
John S Ji, Vanke School of Public Health, Tsinghua University, Beijing, China.
Linxin Liu, Vanke School of Public Health, Tsinghua University, Beijing, China.
Lijing L Yan, Global Health Research Center, Duke Kunshan University, Kunshan, China.
Yi Zeng, Center for Healthy Aging and Development Studies, National School of Development, Peking University, Beijing, China; Center for the Study of Aging and Human Development, Duke Medical School, Durham, North Carolina, USA.
Funding
The Chinese Longitudinal Healthy Longevity Study (CLHLS) data sets analyzed in this article are jointly supported by the National Key R&D Program of China (2018YFC2000400), National Natural Sciences Foundation of China (72061137004, 71490732), the US National Institute of Aging of National Institute of Health (P01AG031719) and the Duke University School of Medicine and Duke-NUS Medical School (Duke/Duke-NUS/RECA(Pilot)/2019/0051).
Conflict of Interest
None declared.
References
- 1. Daitoku H, Sakamaki J, Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein–protein interactions. Biochim Biophys Acta. 2011;1813(11):1954–1960. doi: 10.1016/j.bbamcr.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 2. Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27(16):2276–2288. doi: 10.1038/onc.2008.21 [DOI] [PubMed] [Google Scholar]
- 3. Morris BJ, Willcox DC, Donlon A, Willcox BJ. FOXO3: a major gene for human longevity—a mini-review. 2015;61(6):515–525. doi: 10.1159/000375235 [DOI] [PMC free article] [PubMed]
- 4. Willcox BJ, Donlon TA, He Q, et al. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105(37):13987–13992. doi: 10.1073/pnas.0801030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanese P, Forte G, Disciglio V, Grossi V, Simone C. FOXO3 on the road to longevity: lessons from SNPs and chromatin hubs. Comput Struct Biotechnol J. 2019;17:737–745. doi: 10.1016/j.csbj.2019.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brooks-Wilson AR. Genetics of healthy aging and longevity. Hum Genet. 2013;132(12):1323–1338. doi: 10.1007/s00439-013-1342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu L, Zhu A, Shu C, Zeng Y, Ji JS. Gene–environment interaction of FOXO and residential greenness on mortality among older adults. Rejuvenation Res. 2021;24(1):49–61. doi: 10.1089/rej.2019.2301 [DOI] [PubMed] [Google Scholar]
- 8. Cosby AG, McDoom-Echebiri MM, James W, Khandekar H, Brown W, Hanna HL. Growth and persistence of place-based mortality in the United States: the rural mortality penalty. Am J Public Health. 2019;109(1):155–162. doi: 10.2105/AJPH.2018.304787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carson E, Sharmin S, Maier AB, Meij JJ. Comparing indigenous mortality across urban, rural and very remote areas: a systematic review and meta-analysis. Int Health. 2018;10(4):219–227. doi: 10.1093/inthealth/ihy021 [DOI] [PubMed] [Google Scholar]
- 10. Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuan Y, Huang F, Lin F, Zhu P, Zhu P. Green space exposure on mortality and cardiovascular outcomes in older adults: a systematic review and meta-analysis of observational studies. Aging Clin Exp Res. 2021;33(7):1783–1797. doi: 10.1007/s40520-020-01710-0 [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Wang WJ, Cao H, et al. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet. 2009;18(24):4897–4904. doi: 10.1093/hmg/ddp459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ji JS, Zhu A, Bai C, et al. Residential greenness and mortality in oldest-old women and men in China: a longitudinal cohort study. Lancet Planet Health. 2019;3(1):e17–e25. doi: 10.1016/S2542-5196(18)30264-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Donkelaar A, Martin RV, Brauer M, et al. Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environ Sci Technol. 2016;50(7):3762–3772. doi: 10.1021/acs.est.5b05833 [DOI] [PubMed] [Google Scholar]
- 15. Li T, Zhang Y, Wang J, et al. All-cause mortality risk associated with long-term exposure to ambient PM2·5 in China: a cohort study. Lancet Public Health. 2018;3(10):e470–e477. doi: 10.1016/S2468-2667(18)30144-0 [DOI] [PubMed] [Google Scholar]
- 16. Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet. 2017;389(10079):1619–1629. doi: 10.1016/S0140-6736(17)30548-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeng Y, Chen H, Ni T, et al. Interaction between the FOXO1A-209 genotype and tea drinking is significantly associated with reduced mortality at advanced ages. Rejuvenation Res. 2016;19(3):195–203. doi: 10.1089/rej.2015.1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Therneau TM, Crowson CS, Atkinson Jan EJ. Adjusted survival curves.2015. https://cran.r-project.org/web/packages/survival/vignettes/adjcurve.pdf. Accessed October 1, 2021.
- 19. Ezzati M, Webster CJ, Doyle YG, Rashid S, Owusu G, Leung GM. Cities for global health. BMJ. 2018;363:k3794. doi: 10.1136/bmj.k3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Bureau of Statistics. Main data of the seventh national population census. http://www.stats.gov.cn/english/PressRelease/202105/t20210510_1817185.html. Published 2021. Accessed June 3, 2021.
- 21. Anselmi CV, Malovini A, Roncarati R, et al. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12(2):95–104. doi: 10.1089/rej.2008.0827 [DOI] [PubMed] [Google Scholar]
- 22. Flachsbart F, Caliebe A, Kleindorp R, et al. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106(8):2700–2705. doi: 10.1073/pnas.0809594106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng Y, Cheng L, Chen H, et al. Effects of FOXO genotypes on longevity: a biodemographic analysis. J Gerontol A Biol Sci Med Sci. 2010;65(12):1285–1299. doi: 10.1093/gerona/glq156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcohol Clin Exp Res. 2001;25(5):637–643. doi: 10.1111/j.1530-0277.2001.tb02261.x. [DOI] [PubMed] [Google Scholar]
- 25. van Os J, Hanssen M, Bak M, Bijl RV, Vollebergh W. Do urbanicity and familial liability coparticipate in causing psychosis? Am J Psychiatry. 2003;160(3):477–482. doi: 10.1176/appi.ajp.160.3.477 [DOI] [PubMed] [Google Scholar]
- 26. Jokela M, Lehtimäki T, Keltikangas-Järvinen L. The influence of urban/rural residency on depressive symptoms is moderated by the serotonin receptor 2A gene. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(7):918–922. doi: 10.1002/ajmg.b.30555 [DOI] [PubMed] [Google Scholar]
- 27. Eckert S, Kohler S. Urbanization and health in developing countries: a systematic review. World Health Popul. 2014;15(1):7–20. doi: 10.12927/whp.2014.23722 [DOI] [PubMed] [Google Scholar]
- 28. Zhao Y, Xu X, Dupre ME, Xie Q, Qiu L, Gu D. Individual-level factors attributable to urban–rural disparity in mortality among older adults in China. BMC Public Health. 2020;20(1):1–11. doi: 10.1186/s12889-020-09574-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.