Abstract
OBJECTIVES
Patients with left ventricular assist devices may experience external obstruction of the outflow graft through a gelatinous substance within the bend relief (BR; a stiff tube graft guiding the outflow graft). Preventative strategies have been missing. Having faced this problem, we decided to fenestrate the BR to avoid outflow graft obstruction (OGO).
METHODS
Since December 2010, 167 patients underwent left ventricular assist device implantation using HeartMate II or 3. BR fenestration was introduced on July 2018 (108 patients before, 59 after the introduction of BR fenestration). Follow-up computed tomography scans were obtained from all patients and were screened for OGO by 3 independent investigators. Results were correlated with log file history, echocardiographic and clinical outcomes.
RESULTS
Demographic data were comparable between groups, with mostly male patients. Patients with BR fenestration were older [63 (standard deviation (SD):10.6) vs 58 (SD: 10.7) years] and had shorter support duration [494 (SD: 383) vs 951 (SD: 875) days]. OGO was observed in 5 patients and occurred only in patients without fenestration. Importantly, it occurred late on postoperative Days 412, 462, 1043, 1184 and 1506. Three patients are still asymptomatic. Surgical revision was required in the other 2 patients for pump thrombosis or continuous low flow. One of them died 36 days after revision due to right heart failure.
CONCLUSIONS
Our results suggest that fenestration of the BR may be a preventative strategy to avoid external OGO. OGO occurred late, which suggests a careful long-term follow-up.
Keywords: Left ventricular assist device, External outflow graft obstruction
Not long ago, a rare pattern of outflow graft obstruction (OGO) inside the bend relief (BR) of left ventricular assist devices (LVAD) was described [1, 2].
INTRODUCTION
Not long ago, a rare pattern of outflow graft obstruction (OGO) inside the bend relief (BR) of left ventricular assist devices (LVAD) was described [1, 2]. The compression was found to be caused by a gelatinous substance between the outflow graft and the BR, which is thought to be due to ‘plasma sweating’ of the graft. OGO patients may be asymptomatic or present with variable symptoms including haemolysis, low flow alarms and heart failure. While the clinical impact of OGO is not fully understood, it can lead to life-threatening complications by progressive decrease of pump flow over time [3], leading to recurrent heart failure and potentially increasing the risk of pump thrombosis.
There are now a growing number of reports on OGO in the literature [4–6]. Various treatment strategies, such as surgical revision [1, 4] or interventional treatment with balloon intervention and/or stent implantation, have been reported [2, 5]. However, no preventative strategy has been suggested, yet.
Beginning in 2018, we fenestrated the BR of the HeartMate 3 during LVAD implantation, in order to reduce (or ideally prevent) the possibility of OGO formation as OGO has not been detected with the grid-like structured BR. We here compare our patients with fenestrated BR to earlier patients who were implanted without fenestration and report the results.
PATIENTS AND METHODS
Ethical statement
Our institutional review board (ethics committee, Friedrich-Schiller-Universität Jena, Jena Germany) approved the data for research and waived the need for individual informed consent (reference number: 2021-22368).
Patients
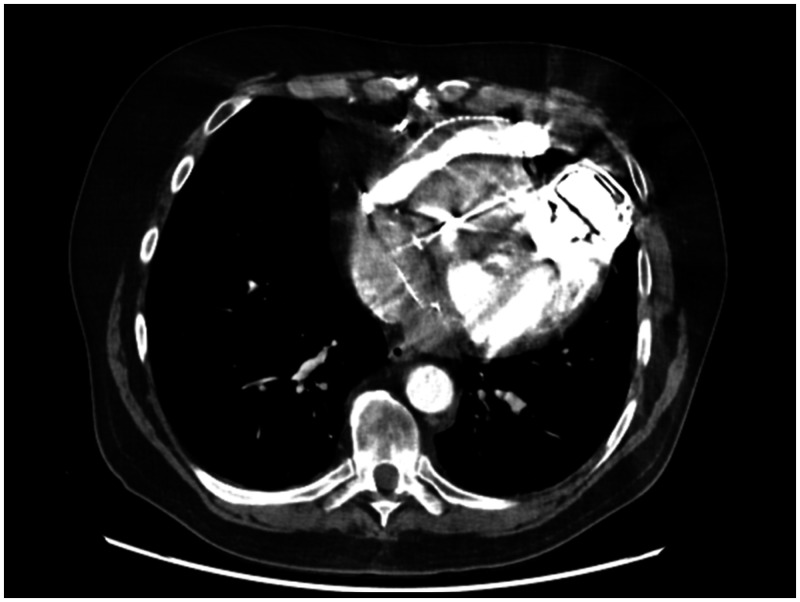
We retrospectively analysed all patients (n = 167) who underwent LVAD implantation with HeartMate II and 3 devices in our department between December 2010 and January 2022. In July 2018, we introduced a BR fenestration strategy, where we use a hole punch to create permeability as illustrated in Fig. 1. All HeartMate II and 3 patients, with or without fenestrated BR, were included in the analysis. Demographic characteristics, preoperative risk factors, intraoperative data, perioperative complications and outcomes were analysed.
Figure 1:
Photograph of our standardized fenestration of the bend relief. Fenestration was performed at 3 sides with 6 holes in each line using a 4.8 mm conventional punch (Medtronic).
All LVAD patients were seen for follow-up in the course of routine clinical visits (in stable patients 3–5 times per year) including analysis of LVAD log file history, echocardiographic and clinical data as well as regular angio computed tomography (CT) scans. The last one was 1 month for data lock. All patients underwent regular angio CT scans, there were no missing data. For all patients, the length of follow-up was 779 (771) days and ranged from 0 to 10 years. In the event of a patient’s death, the date of death was communicated to us by the emergency physician or the treating hospital. OGO was defined as an external compression of the outflow graft lumen inside the BR of ≥20% at its maximal point of reduction of the grafts’ predetermined cross-sectional area (assessed by CT scan). CT scans were independently screened by 2 ventricular assist device (VAD) surgeons and reviewed by an experienced radiologist. OGO was differentiated from thrombus when Hounsfield units were below 40 Hounsfield units (as the gelatinous substance has a lower absorption quality).
The surgical technique used for LVAD implantation was performed in all cases according to our standard operating procedure, except for the BR fenestration. Briefly, sternotomy was the standard approach (except in 6 cases) with pump implantation to the apex of the left ventricle and outflow graft anastomosis to the ascending aorta. The BR was fenestrated in a standardized manner (Fig. 1 and Video 1). Fenestration was performed at 3 sides with 6 holes in each line using a 4.8 mm conventional punch (Medtronic, Dublin, Ireland). Additional cardiac procedures (e.g. valve surgery, coronary bypass grafting, left atrial appendage closure, aortic surgery, extracorporeal life support (ECLS) explantation) were performed as indicated [6]. Pericardium was closed in all cases with native or bovine pericardium. Postoperative anticoagulation was performed as recommended [6], initially with heparin and later with oral anticoagulation (Phenprocoumon) and antiplatelet (in most cases Aspirin, alternatively Clopidogrel) therapy.
Statistics
Standard descriptive statistics were used to summarize the patients’ characteristics [e.g. categorical: absolute and relative frequencies/continuous: mean (standard deviation)]. The Kaplan–Meier method and competing risk analyses (applying the model by Fine&Gray) were used to analyse time-to-event data on survival and to estimate the cumulative incidence of OGO over time in the presence of competing risks (death, heart transplantation, LVAD exchange or decommissioning; supplementary material). Comparisons between the groups were performed using chi-squared or Fisher’s exact tests for nominal data types or Wilcoxon–Mann–Whitney tests for continuous, metric data types. All reported P-values of this exploratory study are 2-sided and not corrected for multiple comparisons. All statistical analyses were done with SPSS 27.0 (IBM, Armonk, New York, USA).
RESULTS
Table 1 shows the preoperative patient characteristics of the entire cohort separated into those with and those without BR fenestration. In the group without fenestrated BR (n = 108), 44% (n = 46) received HeartMate II and 37% (n = 60) HeartMate 3. In the group with fenestrated BR (n = 59), all patients received a HeartMate 3 device. Demographic data were comparable between the 2 groups. Most patients were male. Patients in the fenestrated BR group were older and suffered more often from ischaemic cardiomyopathy compared to those without fenestration. INTERMACS profiles were comparable among the 2 groups with the vast majority of patients in Class 1 and 2. One out of 6 patients received LVAD implantation from veno-arterial extracorporeal membrane oxygenation. Since we introduced the fenestration strategy in 2018, patients without fenestration had longer LVAD support times [951 (standard deviation: 875) vs 494 (standard deviation: 383) in the fenestrated group]. With the advent of the HeartMate 3, we no longer implanted HeartMate II. Therefore, only the group without BR fenestration contained HeartMate II patients. However, the BR is identical in both systems. Our surgical approach was through sternotomy in the majority of patients and the outflow graft anastomosis was always performed at the ascending aorta. About one-quarter of patients received LVAD implantation as redo procedure. Concomitant tricuspid valve repair was the most frequent additional procedure.
Table 1:
Perioperative patient characteristics
| Without fenestration (n = 108) | Fenestrated bend relief (n = 59) | P-value | |
|---|---|---|---|
| Age [years] | 58 (10.7) | 63 (10.6) | 0.002 |
| Male | 95 (88%) | 48 (81%) | 0.245 |
| ICM/DCM | 43 (40%)/57 (60%) | 35 (59%)/24 (41%) | 0.280 |
| INTERMACS | |||
| 1 | 24 (22%) | 16 (27%) | 0.776 |
| 2 | 17 (16%) | 11 (19%) | |
| 3 | 37 (34%) | 20 (34%) | |
| 4 | 30 (28%) | 12 (23%) | |
| Bridged with va-ECMO | 18 (17%) | 8 (14%) | 0.966 |
| DT/BTT | 46 (43%)/40 (37%) | 28 (47%)/11 (19%) | 0.037 |
| HMII/HM3 | 48 (44%)/60 (56%) | none/59 (100%) | <0.001 |
| Redo surgery | 29 (27%) | 14 (24%) | 0.647 |
| Full sternotomy | 102 (94%) | 59 (100%) | 0.088 |
| Outflow graft anastomosis | Ascending aorta, all cases | Ascending aorta, all cases | |
| Concomitant procedures | |||
| Tricuspid valve | 62 (57%) | 32 (54%) | 0.519 |
| Aortic valve | 6 (6%) | 11 (19%) | 0.008 |
| ASD/PFO closure | 16 (15%) | 5 (8%) | 0.201 |
| Temporary RVAD | 8 (7%) | 5 (8%) | 0.849 |
| LAA closure | 2 (1.9%) | 1 (21.7%) | 1.000 |
| CABG | 3 (2.7%) | 2 (3.4%) | 1.000 |
| Support duration | 951 (875) days (1–10 years) | 494 (383) days (0–4 years) | 0.022 |
| OGO | 5 (4.6%) | none | 0.163 |
Data are mean (standard deviation) or n (% of total).
ASD: atrial septal defect; BTT: bridge to transplant; CABG: coronary artery bypass grafting; DCM: dilatative cardiomyopathy; DT: destination therapy; HM 3: HeartMate 3™ (Abbott); HM II: HeartMate II™ (Abbott); ICM: ischaemic cardiomyopathy; INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support; LAA: left atrial appendage; PFO: patent foramen ovale; RVAD: right ventricular assist device; va-ECMO: veno-arterial extracorporeal membrane oxygenation.
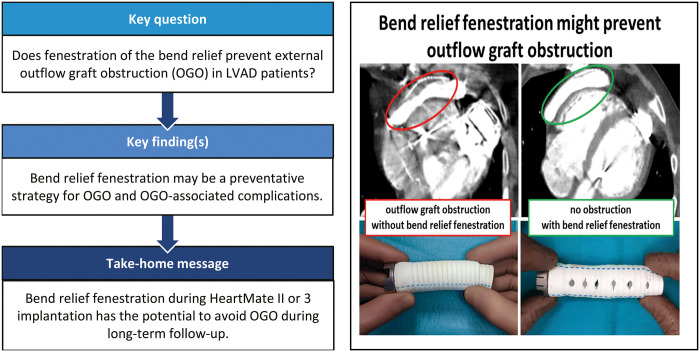
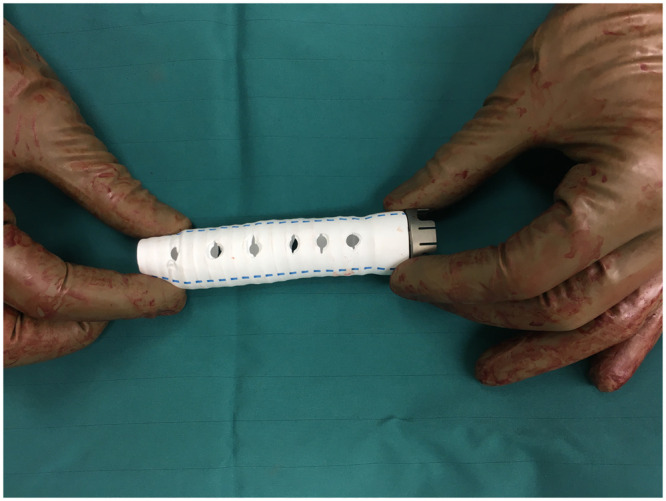
OGO occurred in 5 patients having received HeartMate II (n = 2) and HeartMate 3 (n = 3). Figure 2 shows a CT image of OGO illustrating the compression of the outflow graft inside the BR. Figure 3 shows an intraoperative picture of one of the 2 patients who were reoperated for OGO. The image shows the gelatinous substance pressing on the outflow graft inside the BR.
Figure 2:
Computed tomography angiogram of the outflow graft of a patient with outflow graft obstruction, showing compression of the outflow graft within the entire bend relief.
Figure 3:
Intraoperative picture of the incised bend relief during reoperation for a patient with a compressed outflow graft obstruction. Note the jelly-like substance under the bend relief on top of the Dacron graft.
Figure 4 shows Kaplan–Meier estimates of survival free from OGO for both groups. All OGOs occurred in the group without BR fenestration. The first OGO was diagnosed on postoperative Day 412. At this time, 45 patients were still at risk in the fenestrated group, but no OGO was detected. The other four OGOs were diagnosed on postoperative Days 462, 1043, 1184 and 1506. In the fenestrated group, there has been no observation of OGO thus far.
Figure 4:
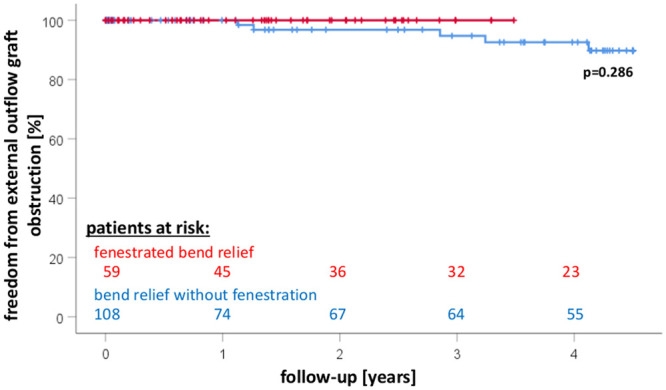
Kaplan–Meier estimates of survival free from outflow graft obstruction in left ventricular assist device patients with or without fenestration of the bend relief.
Table 2 shows the patient characteristics and outcomes of the five OGO patients. Patients were between 25 and 77 years old at time of LVAD implantation. These patients were implanted by 3 different surgeons. All patients were male and all suffered from dilated cardiomyopathy. OGO was detected by our routine CT scans. The degree of outflow graft obstruction ranged from 20% to 85%. In 3 patients, OGO was localized as short obstruction close to the pump. In the other 2 patients, it extended along the full length of the BR. Surgical revision was required in the 2 HeartMate II patients. Indication for reoperation was haemolysis and pump thrombosis in one and continuous low flow and worsening heart failure in the other. In both patients, the BR was incised and the outflow graft decompressed (Fig. 3). The first required a pump exchange due to concomitant pump thrombosis. This patient had a complicated course characterized by right heart failure and died on postoperative Day 36. The 3 OGO patients who did not receive surgery are still asymptomatic and are ongoing with regular follow-up. In one patient, the gelatinous substance that caused the compression was further examined. Histological assessment revealed fibrinoid like tissue.
Table 2:
Patient characteristics and outcomes of the 5 outflow graft obstruction patients
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age [years]a | 25 | 77 | 61 | 71 | 58 |
| Sex | Male | Male | Male | Male | Male |
| Aethiology | DCM | DCM | DCM | DCM | DCM |
| Indication | BTT | DT | BTT | DT | BTT |
| INTERMACS | 1 | 4 | 3 | 3 | 1 |
| HeartMate | II | II | 3 | 3 | 3 |
| OGO | |||||
| Onset [day] | 1184 | 1506 | 462 | 1043 | 412 |
| Degree | 60% | 85% | 50% | 20% | 65% |
| Location | Close to the pump | Full-length beneath BR | Full-length beneath BR | Close to the pump | Close to the pump |
| Therapy | Reoperation | Reoperation | None | None | None |
| Symptoms | Haemolysis | None | None | None | None |
| Outcome | Death (pod 36) | Ongoing (2864 daysb) | Ongoing (2206 daysb) | Ongoing (2052 daysb) | Ongoing (1767 daysb) |
Age at time of LVAD implantation.
Since implantation.
BR: bend relief; BTT: bridge to transplantation; DCM: dilatative cardiomyopathy; DT: destination therapy; ICM: ischaemic cardiomyopathy; INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support; OGO: outflow graft obstruction; pod: postoperative day.
DISCUSSION
We demonstrate in this analysis that fenestration of the BR may be a preventative strategy to avoid external outflow graft obstruction. OGO occurred late, which suggests a careful long-term follow-up.
OGO within the BR has initially been described in 2014 [1]. Since then, several centres have confirmed this observation [2, 3, 7, 8]. ‘Sweating’ of plasmatic components of the bloodstream has been suggested as mechanism for the gelatinous substance accumulating underneath the BR leading to a tamponade-type compression of the outflow graft [1]. While this suggestion appears plausible and is consistent with the macroscopic findings in the operating room (see Fig. 3), the time points of OGO detection may challenge this suggested mechanism. The earliest OGO detection in our patient cohort was on Day 412 after LVAD implantation and the latest about 4 years after LVAD implantation. However, in former days (before our first OGO cases), our follow-up did not consist of regular angio CT scan. CT scan was performed as the first patients became symptomatic, respectively, pump alarms occurred which could not be assessed by echo. This might explain the late detection of our first OGOs in the 2 HeartMate II patients. With these first cases of OGO, we adopted our follow-up protocol accordingly to regular CT scan which will probably lead to earlier detection in the future. Previous reports mostly described OGO in the second year of LVAD support [3]. However, time to OGO also varies in other reports with a wide range from 1 month to up to 5 years [3, 4]. This range may also be influenced by the fact that serial angio CT scans were also not routine in other centres. If sweating through the outflow graft is responsible for this observation, one may expect an earlier clinical appearance. Since detection occurred much later, one must assume that a chronic leakage problem exists and patients should be followed accordingly. We have therefore introduced yearly CT scans for our patients into our routine follow-up.
Interestingly, all observations were made in HeartMate devices. This is an important recognition because the HeartMate 3 is currently the leading available LVAD. The HVAD, as its biggest competitor, has recently been taken off the market. It is possible that OGO with other devices has not been published, yet. However, it is also possible that the stiff BR fully covering the outflow graft of the HeartMate devices may pose a risk specific to this device because the HeartMate BR is not permeable. We started the fenestration in 2018 as 3 of our ongoing LVAD patients presented with OGO at that time. Simultaneous to our experience increasing evidence for this type of VAD-related complication has been presented and published [3]. As OGO has not been described for LVADs with a grid-like designed BR (e.g. HVAD), we decided to fenestrate the HeartMate BR with the rationale to prevent accumulation of gelatinous mass within it and have not observed any OGO since. However, since OGO occurred late in our patients, we also still follow up all patients with fenestrated BR. Since 45 of the 59 patients with fenestration already exceeded the time on LVAD support at which the first OGO was detected in the other group, we are hopeful that our fenestration may be a permanent solution. However, longer follow-up is required.
In any case, to the best of our knowledge, this is the first report presenting results for a preventative strategy. All previous publications have focused on the diagnosis and management of patients with OGO [2, 4, 7, 9]. Surgical revision is an option for patients with clinical symptoms and different surgical or interventional approaches may be applied [2, 4, 7, 9]. Our patients received surgical revisions either for low pump flow and heart failure or for OGO associated with pump thrombosis. The latter case having received pump exchange and OGO relief prototypically illustrates the associated risks of this diagnosis, since the patient died 36 days after surgery due to right heart failure. So far, OGO incidence has been described with 3.6–4% of the implanted LVADs [10], but its true prevalence might be underestimated. As thousands of LVADs are implanted worldwide per year [11, 12], preventative strategies are urgently needed. We consider our data a first step into this important direction.
Limitations
Our study is limited by its retrospective nature and the fact that there is only a historical control group with heterogeneous characteristics (differences in age, device type and support duration). It is a single-centre study with a limited number of patients and events. There is only a mid-term follow-up in the fenestrated group so it is possible that OGO can also occur later in this group despite fenestration. The results can therefore only be taken as hypothesis generating and require confirmation in a multicentre setting as a randomized trial to further assess the mechanisms of OGO and its prevention. Thus, the here presented results should be considered preliminary.
CONCLUSION
Our results suggest that fenestration of the BR may be a preventative strategy to avoid external outflow graft obstruction. Outflow graft obstruction occurred late, which suggests a careful long-term follow-up.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
Data availability
The data underlying this article cannot be shared publicly as this has not been approved by the patients.
Author contributions
Gloria Faerber: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—original draft. Hristo Kirov: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing—review & editing. Imke Schwan: Data curation; Formal analysis; Investigation; Visualization; Writing—original draft. Stephanie Gräger: Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Validation; Visualization. Mahmoud Diab: Investigation; Validation; Writing—original draft. Sophie Tkebuchava: Formal analysis; Investigation; Methodology; Validation; Visualization. Torsten Doenst: Project administration; Resources; Software; Supervision; Validation; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Luca Di Marco, Francesco Onorati and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
Glossary
ABBREVIATIONS
- BR
Bend relief
- CT
Computed tomography
- LVAD
Left ventricular assist devices
- OGO
Outflow graft obstruction
- SD
Standard deviation
Contributor Information
Gloria Färber, Department of Cardiothoracic Surgery, Jena University Hospital, Friedrich Schiller University, Jena, Germany.
Hristo Kirov, Department of Cardiothoracic Surgery, Jena University Hospital, Friedrich Schiller University, Jena, Germany.
Imke Schwan, Department of Cardiothoracic Surgery, Jena University Hospital, Friedrich Schiller University, Jena, Germany.
Stephanie Gräger, Department of Radiology, Jena University Hospital, Friedrich Schiller University, Jena, Germany.
Mahmoud Diab, Department of Cardiothoracic Surgery, Jena University Hospital, Friedrich Schiller University, Jena, Germany.
Sophie Tkebuchava, Department of Cardiothoracic Surgery, Jena University Hospital, Friedrich Schiller University, Jena, Germany.
Torsten Doenst, Department of Cardiothoracic Surgery, Jena University Hospital, Friedrich Schiller University, Jena, Germany.
Presented at the 35th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 13–16 October 2021.
REFERENCES
- 1.Mehr AJ, Kwan MD, Kunavarapu C.. Thrombus detected in computed tomography angiography images of HeartMate II outflow graft: a cautionary tale. J Heart Lung Transplant 2014;33:1193–4. [DOI] [PubMed] [Google Scholar]
- 2.Abraham J, Remick JD, Caulfield T, Puhlman M, Evenson K, Ott G. et al. Left ventricular assist device outflow cannula obstruction treated with percutaneous endovascular stenting. Circ Heart Fail 2015;8:229–30. [DOI] [PubMed] [Google Scholar]
- 3.Barac YD, Nevo A, Schroder JN, Milano CA, Daneshmand MA.. LVAD outflow graft role in pump thrombosis. ASAIO J 2020;66:128–31. [DOI] [PubMed] [Google Scholar]
- 4.Alnabelsi T, Shafii AE, Gurley JC, Dulnuan K, Harris DD, Guglin M.. Left ventricular assist device outflow graft obstruction: a complication specific to polytetrafluoroethylene covering. a word of caution!. ASAIO J 2019;65:e58–62. [DOI] [PubMed] [Google Scholar]
- 5.Bhamidipati CM, Pal JD, Jones TK, McCabe JM, Reisman M, Smith JW. et al. Outflow graft obstruction treated with transcatheter management: a novel therapy for a new diagnosis. Ann Thorac Surg 2017;103:e101–4. [DOI] [PubMed] [Google Scholar]
- 6.Potapov EV, Antonides C, Crespo-Leiro MG, Combes A, Farber G, Hannan MM. et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur J Cardiothorac Surg 2019;56:230–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawabori M, Nordan T, Arkun K, Couper GS.. Left ventricular assist device outflow graft obstruction development in the original bend relief. Eur J Cardiothorac Surg 2020;58:1313. [DOI] [PubMed] [Google Scholar]
- 8.Posada D, Moayedi JG, Alhussein Y, Rodger M, Alvarez M, Wintersperger J. et al. Outflow graft occlusion of the HeartMate 3 left ventricular assist device. Circ Heart Fail 2017;10:e004275. [DOI] [PubMed] [Google Scholar]
- 9.Wert L, Kaufmann F, Solowjowa N, Dreysse S, Zimpfer D, Falk V. et al. Diagnosis and treatment strategies of outflow graft obstruction in the fully magnetically levitated continuous-flow centrifugal left ventricular assist device: a multicenter case series. ASAIO J 2021;67:e52–4. [DOI] [PubMed] [Google Scholar]
- 10.Nathan S, Ghotra AS, Rajagopal K, Patel C, Kumar S, Patel M. et al. Left ventricular assist device outflow graft obstruction: a case series. ASAIO J 2020;66:657–62. [DOI] [PubMed] [Google Scholar]
- 11.Beckmann A, Meyer R, Lewandowski J, Markewitz A, Gummert J.. German heart surgery report 2020: the annual updated registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 2021;69:294–307. [DOI] [PubMed] [Google Scholar]
- 12.Molina EJ, Shah P, Kiernan MS, Cornwell WK, Copeland H, Takeda K. et al. The Society of Thoracic Surgeons Intermacs 2020 annual report. Ann Thorac Surg 2021;111:778–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly as this has not been approved by the patients.