Abstract
For oncologic management or radiotherapy planning, reliable staging tools are essential. The recent development of quinoline-based ligands targeting cancer-associated fibroblasts demonstrated promising preclinical and clinical results. The current study aimed to evaluate the role of fibroblast activation protein inhibitor (FAPI) PET/CT as a first clinical analysis for primary malignancies within the lower gastrointestinal tract (LGT). Methods: 68Ga-FAPI PET/CT was performed on a cohort of 22 patients with LGT tumors, including 15 patients with metastatic disease, 1 patient with suspected local relapse, and 6 treatment-naïve patients. Uptake of 68Ga-FAPI-04 and 68Ga-FAPI-46 was quantified by SUVmax and SUVmean. After comparison with standard imaging, changes in tumor stage or localization and in oncologic or radiooncologic management were recorded. Results: The highest uptake of FAPI tracer was observed in liver metastases and anal cancer, with an SUVmax of 9.1 and 13.9, respectively. Because of low background activity in normal tissue, there was a high tumor-to-background ratio of more than 3 in most lesions. In treatment-naïve patients, TNM was changed in 50%, whereas in patients with metastases, new findings occurred in 47%. In total, FAPI imaging caused a high, medium, and low change in oncologic or radiooncologic management in 19%, 33%, and 29%, respectively. For almost every patient undergoing irradiation, target volume delineation was improved by 68Ga-FAPI PET/CT. Conclusion: The present study demonstrated that both primary and metastatic LGT tumors were reliably detected by 68Ga-FAPI PET/CT, leading to relevant changes in TNM status and oncologic or radiooncologic management. 68Ga-FAPI PET/CT seems to be a highly promising imaging agent for the diagnosis and management of LGT tumors, potentially opening new applications for tumor staging or restaging.
Keywords: FAPI, PET, gastrointestinal tract, fibroblast, oncologic management
Fibroblast activation protein (FAP), expressed by cancer-associated fibroblasts, is a type II transmembrane serine protease (1,2). The protein, which can be detected in a variety of malignant tumors, is able to activate cell signaling and contributes to tumor cell migration, tumor cell invasion, and tumor angiogenesis (3–5). With the development of FAP-targeting molecules, new imaging and therapeutic agents can be developed (6,7). Several preclinical studies identified the quinolone-based FAP inhibitor (FAPI), FAPI-04, as a promising radiotracer for malignant tumors that harbor a high number of activated fibroblasts (8,9). Meanwhile, novel derivates were synthesized with enhanced FAP binding and improved pharmacokinetics (10). Preliminary dosimetry estimates and biodistribution demonstrated rapid renal clearance and low uptake in background organs between 10 min and 3 h after injection. Furthermore, 68Ga-FAPI PET/CT had a tumor-to-background ratio equal to or better than that of 18F-FDG (11). However, reliable clinical data are still missing. In a first study, lower gastrointestinal tract (LGT) tumors such as colorectal or anal cancer demonstrated high uptake, with an SUVmax of 6–12 (12). Here, we report our first clinical experience with 68Ga-FAPI PET/CT in a cohort of patients with LGT tumors. After quantifying tracer uptake in primary tumors and metastases, we compared the results of 68Ga-FAPI PET/CT with those of conventional imaging, recording changes in tumor stage, metastasis localization, and oncologic or radiooncologic management.
MATERIALS AND METHODS
Patient Cohort
Our cohort consisted of 22 patients with LGT tumors. Written informed consent was obtained from all patients undergoing 68Ga-FAPI PET/CT on an individual-patient basis following the regulations of the German Pharmaceuticals Act, §13(2b). All patients were referred for the experimental diagnostics by their treating oncologists, who were facing a diagnostic challenge that could not be solved sufficiently with standard diagnostic imaging. Examples of such challenges are inconclusive results from standard imaging, such as MRI; insufficient tumor delineation for target-volume segmentation before irradiation; or the need to select target-positive patients for experimental last-line therapy with therapeutic FAPI conjugates. This retrospective study was approved by the local institutional review board, and all subjects gave written informed consent to participate.
Radiopharmaceuticals and 68Ga-FAPI PET/CT Imaging
Synthesis and labeling of both 68Ga-FAPI-04 and 68Ga-FAPI-46 followed the methods described by Lindner et al. (8) and Loktev et al. (9). A Biograph mCT Flow scanner (Siemens) was used for PET imaging. All scans were performed according to scan protocols as previously published (11,12). In summary, after low-dose CT without contrast, PET scans were acquired in 3-dimensional mode (matrix, 200 × 200), emission data were corrected, and reconstruction was performed. The injected activity for the 68Ga-FAPI examinations ranged from 111 to 298 MBq. PET scans were obtained 1 h after injection of the radiotracer. After injection, patients were asked about new symptoms, and vital signs were monitored starting at the time of tracer injection up to 30 min after the end of the examination.
Image Evaluation
The tracer biodistribution in patients was quantified by SUVmean and SUVmax at 1 h after injection.
For calculation of the SUV, circular regions of interest were drawn around the tumors on transaxial slices and automatically adapted to a 3-dimensional VOI with e.soft software (Siemens) at a 60% isocontour. The normal organs were evaluated with a 1-cm-diameter (for the small organs thyroid, parotid gland, myocardium, oral mucosa, and spinal cord) or 2-cm-diameter (brain, muscle, liver, spleen, kidney, fat, aortic lumen content, and lung) sphere placed inside the organ parenchyma. 68Ga-FAPI PET/CT scans were evaluated by 1 board-certified radiologist and 2 board-certified nuclear medicine physicians in consensus. Conventional imaging was interpreted by 2 board-certified radiologists in consensus without knowledge of the 68Ga-FAPI PET/CT results, thus establishing the pre-68Ga-FAPI PET/CT TNM classification. For all patients, changes in TNM stage, localization of metastases, and oncologic or radiooncologic management were recorded. This was done by documenting stage or tumor localization and oncologic or radiooncologic management before and after 68Ga-FAPI imaging by 2 nuclear medicine physicians and 1 radiation oncologist/medical oncologist. All findings and changes were interpreted in consensus. Changes in oncologic or radiooncologic management were graded by the level of impact: fundamental changes with regard to alteration of treatment type or treatment intent, significant changes within a treatment regime, and improvements in radiation oncology were classified as high, medium, and low, respectively.
Statistical Analysis
We performed descriptive analyses of patients and their tumor characteristics. For determination of SUVs, median and range were used. The correlation of FAPI uptake within or outside the tumor was determined using a 2-sided t test, with a P value of less than 0.05 being defined as statistically significant.
All statistical analyses were performed using SPSS Statistics (version 24; IBM) and Excel (version 15.41; Microsoft) for Mac (Apple).
RESULTS
The median age of the cohort was 62 y (range, 38–79 y). In total, 15 patients had known metastases, and 6 patients had new diagnoses. For 16 patients, 68Ga-FAPI PET/CT was performed for restaging because of suspected progressive disease, whereas the 6 patients with new diagnoses underwent PET imaging for primary staging. The patient characteristics are provided in Table 1.
TABLE 1.
Patient Characteristics
| Patient no. | Diagnosis | Cancer status | Sex | Age (y) | Tracer |
| 1 | Colon cancer | Metastasized | M | 68 | 68Ga-FAPI-04 |
| 2 | Sigmoid cancer | Metastasized | M | 68 | 68Ga-FAPI-04 |
| 3 | Sigmoid cancer | Metastasized | M | 55 | 68Ga-FAPI-04 |
| 4 | Rectal cancer | Metastasized | F | 44 | 68Ga-FAPI-04 |
| 5 | Rectal cancer | Metastasized | M | 66 | 68Ga-FAPI-04 |
| 6 | Anal cancer | Metastasized | F | 43 | 68Ga-FAPI-04 |
| 7 | Rectal cancer | Metastasized | M | 71 | 68Ga-FAPI-04 |
| 8 | Rectal cancer | Metastasized | M | 71 | 68Ga-FAPI-04 |
| 9 | Sigmoid cancer | Metastasized | F | 59 | 68Ga-FAPI-04 |
| 10 | Sigmoid cancer | Metastasized | F | 50 | 68Ga-FAPI-04 |
| 11 | Sigmoid cancer | Metastasized | F | 46 | 68Ga-FAPI-04 |
| 12 | Sigmoid cancer | Local relapse | F | 45 | 68Ga-FAPI-04 |
| 13 | Colon cancer | Metastasized | M | 69 | 68Ga-FAPI-04 |
| 14 | Colon cancer | Metastasized | M | 72 | 68Ga-FAPI-04 |
| 15 | Colon cancer | Metastasized | F | 46 | 68Ga-FAPI-04 |
| 16 | Colon cancer | Metastasized | M | 62 | 68Ga-FAPI-04 |
| 17 | Anal cancer | Treatment-naïve | M | 79 | 68Ga-FAPI-46 |
| 18 | Anal cancer | Treatment-naïve | F | 60 | 68Ga-FAPI-46 |
| 19 | Anal cancer | Treatment-naïve | F | 38 | 68Ga-FAPI-46 |
| 20 | Anal cancer | Treatment-naïve | F | 72 | 68Ga-FAPI-46 |
| 21 | Anal cancer | Treatment-naïve | F | 72 | 68Ga-FAPI-46 |
| 22 | Anal cancer | Treatment-naïve | F | 69 | 68Ga-FAPI-46 |
Safety
During and after 68Ga-FAPI PET/CT, no drug-related side effects occurred. PET imaging was tolerated well by all patients. Vital parameters remained stable, and no patient reported any new symptoms during the observation period.
68Ga-FAPI Uptake in Primary Tumors and Metastases
68Ga-FAPI PET/CT detected 6 primary tumors and 27 node, 14 liver, 12 soft-tissue, and 19 pulmonary metastases. In total, 72 68Ga-FAPI–positive metastases were observed in 22 patients (Table 2). One hour after injection, overall SUVmax and SUVmean for all primary tumors were 12.59 (±7.46) and 6.11 (±4.00), respectively. Uptake in colorectal cancers was intermediate (SUVmax, 8.6), whereas uptake in anal cancers was high (SUVmax, 13.9).
TABLE 2.
68Ga-FAPI–Positive Tumor Sites
| Site | n | SUVmax | SUVmean |
| Primary | 6 | 15.57 (±7.31) | 7.71 (±3.99) |
| Local relapse | 2 | 6.56 (±4.14) | 2.79 (±1.38) |
| Lymph node metastases | 27 | 8.33 (±4.33) | 4.18 (±2.33) |
| Pulmonary metastases | 19 | 7.01 (±2.98) | 3.31 (±1.45) |
| Tissue metastases (peritoneum, skin) | 12 | 6.91 (±2.91) | 3.50 (±1.74) |
| Liver metastases | 14 | 9.54 (±3.74) | 4.86 (±2.14) |
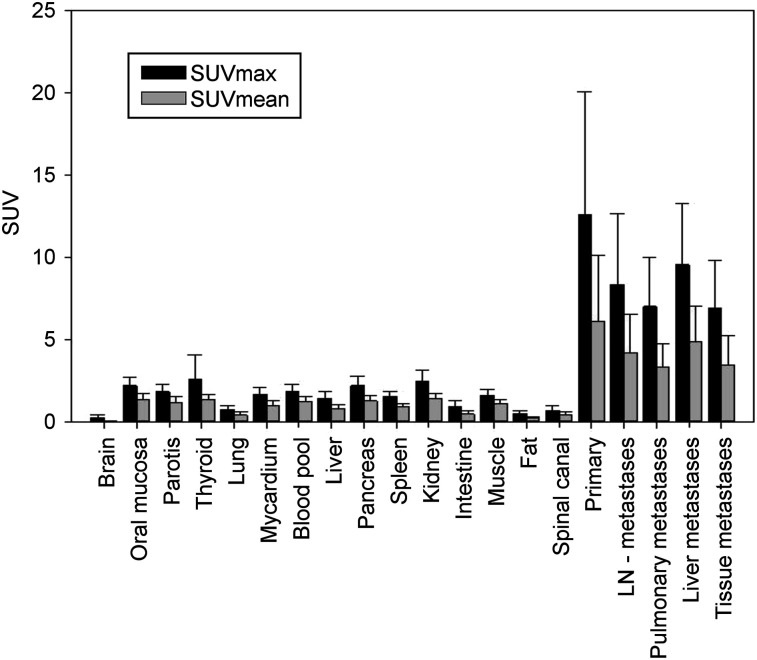
The median SUVmax and SUVmean of all metastases are 7.95 (±3.49) and 3.96 (±1.92), respectively. The highest 68Ga-FAPI uptake in metastases was observed for liver lesions, with an SUVmax of 9.1 (±3.6) and an SUVmean of 4.8 (±2.1). Background activity and activity in normal organs was low (SUVmax and SUVmean of 1.60 and 1.11, respectively, for muscle; 1.85 and 1.21, respectively, for blood pool; and 1.46 and 0.84, respectively, for normal liver parenchyma), leading to high tumor-to-background ratios of more than 3 with statistically significant differences from tumor activity (P ≤ 0.002 each) (Fig. 1). No clinically relevant differences were observed between the 2 68Ga-FAPI tracers.
FIGURE 1.
PET-based biodistribution analysis of 22 patients with 68Ga-FAPI PET, imaged at 1 h after injection. LN = lymph node.
Clinical Implications
The cohort of patients with colorectal cancer included 16 men and women with metastatic disease (15) or local relapse (1). Seven patients underwent CT, 1 patient MRI, and 6 patients both CT and MRI. One patient with colon cancer underwent 18F-FDG PET/CT for restaging, whereas 1 additional patient with colon cancer had no available conventional imaging data. 68Ga-FAPI PET/CT resulted in new findings in 7 of 15 patients (47%), which was caused mainly by the detection of metastases in a new organ system (7 patients). For 1 patient, a local relapse and nodal metastases were also detected. Furthermore, 68Ga-FAPI PET/CT was able to detect new lesions within the spleen (1 patient), lung (1 patient), and skull (1 patient). Further details are listed in Table 3. 68Ga-FAPI PET/CT resulted in a change in oncologic or radiooncologic management in 11 of 15 metastatic patients (73.3%), including 5 patients with a change in systemic therapy and 1 patient for whom the surgical approach was altered instead of systemic therapy. Local irradiation was performed instead of systemic therapy for 2 patients because of the 68Ga-FAPI imaging results (Table 4).
TABLE 3.
Changes in Tumor Stage or Further Findings According to 68Ga-FAPI PET/CT
| Characteristic | N |
| Metastatic disease | 15/21 |
| Overall change | 7/15 (47%) |
| Metastases in new organ system (lymphatic [2], splenic [1], hepatic [1], peritoneal [2], cutaneous [1]) | 7/7* |
| Absence of suggestive findings on conventional imaging | 1/7* |
| New detection of local relapse and nodal metastases | 1/7* |
| No change | 8/15 (53%) |
| Treatment-naïve patients | 6/21 |
| Overall change | 3/6 (50%) |
| N0/Nx to N1 | 2/3 |
| M1 to M0 | 1/3 |
| No change | 3/6 (50%) |
Some patients had multiple forms of changes.
TABLE 4.
Changes in Oncologic or Radiooncologic Management According to 68Ga-FAPI PET/CT
| Characteristic | N |
| Overall change | 17/21 |
| High impact | 4/21 (19%) |
| Palliative to curative intent | 1/4 |
| Systemic therapy to local irradiation | 2/4 |
| Systemic therapy to surgery | 1/4 |
| Medium impact | 7/21 (33%) |
| New systemic therapy | 5/7 |
| Radiotherapy: modified dose concept | 2/7 |
| Low impact (radiotherapy: improved target volume delineation) | 6/21 (29%) |
| No change | 4/21 |
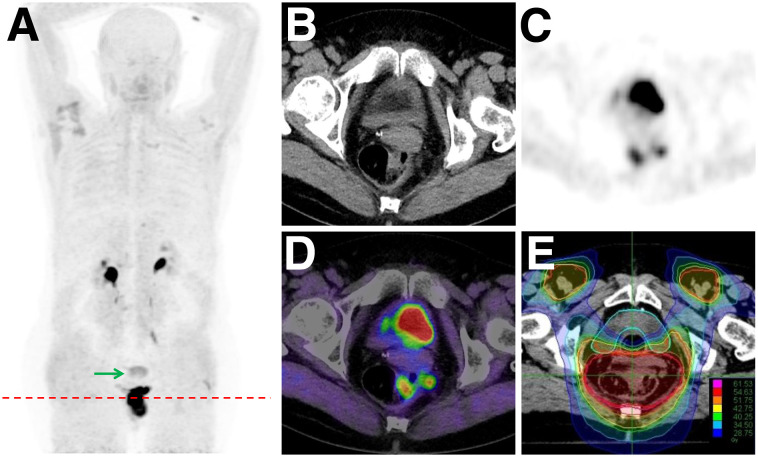
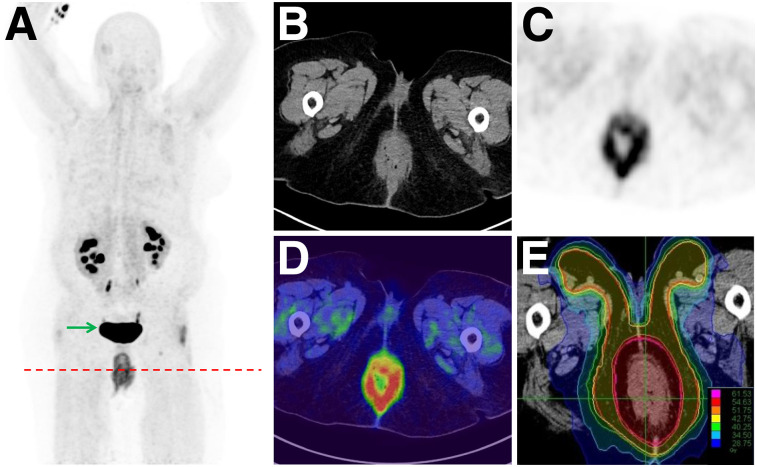
For all patients with treatment-naïve carcinoma of the anal canal, MRI was performed. 68Ga-FAPI PET/CT was performed before definitive chemoradiation. For 3 of 6 patients, a change in TNM classification occurred because of 68Ga-FAPI–positive nodes (2 patients with ill-defined results on MRI) or the absence of tracer uptake within suspected pulmonary lesions on CT (1 patient). These results led to changes in oncologic or radiooncologic management. For 2 patients, radiooncologic dose concepts were adapted. One of these patients underwent curative chemoradiation instead of palliative systemic therapy. Because of a high 68Ga-FAPI positivity within the primary tumor, boost volume delineation was improved for almost every patient (Figs. 2 and 3). One female patient was diagnosed with a multifocal anal cancer on 68Ga-FAPI PET/CT, leading to an adapted target boost volume during radiation therapy (Table 4).
FIGURE 2.
Inclusion of PET-positive pararectal node in boost area (E) after 68Ga-FAPI PET/CT (A: maximum-intensity projections; B: CT; C: PET; D: PET/CT) in patient with anal cancer. Arrow = nonspecific uptake in uterus.
FIGURE 3.
Adapted boost volume delineation (E) due to multifocal cancer detected by 68Ga-FAPI PET/CT in patient with anal cancer (A: maximum-intensity projections; B: CT; C: PET; D: PET/CT). Arrow = nonspecific uptake in bladder.
DISCUSSION
With more than 145,000 estimated new cases in the United States, colorectal cancer is the third most frequent cancer with regard to incidence and mortality (13). Modern oncologic and radiooncologic tumor management with individualized treatment approaches is necessary to improve clinical outcome while reducing treatment-related toxicity. However, effective tumor treatment depends on accurate and reliable diagnostic imaging. In 1990, Garin-Chesa et al. observed a high expression of fibroblast activation protein in colorectal tumor tissue (14). Meanwhile, several studies suggested that LGT tumors commonly harbor cancer-associated fibroblasts expressing fibroblast activation protein (15–18). A first trial evaluating the uptake of 68Ga-FAPI in a cohort of patients with 28 different malignant tumors observed promising results with regard to colorectal and anal cancer (12).
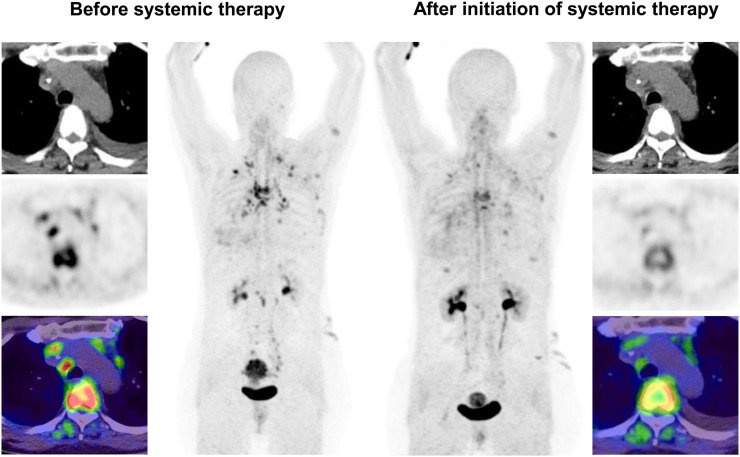
Our study demonstrated that 68Ga-FAPI PET/CT was able to detect both primary tumors and metastases arising from the LGT. Although the current SUVs for colorectal tumors were comparable to the results of Kratochwil et al., a slightly higher tracer uptake was observed for anal cancer: Evaluation of patients with carcinoma of the anal canal undergoing 68Ga-FAPI PET/CT yielded a high SUVmax of 13.9, compared with intermediate uptake in previous findings (12). A heterogeneity of expression and a low case number are the likely explanations for these differences. For metastases, the highest uptake was found in liver metastases, which are common in colorectal cancer. The use of a FAPI tracer may be advantageous for patients with suspected liver metastases, because of the low background of the normal liver (SUVmax, 1.5), leading to a potential high detection rate. This is in accordance with initial results obtained by Giesel et al., who found hepatic background to be significantly lower when using 68Ga-FAPI than 18F-FDG (11). Thus, 68Ga-FAPI imaging might be helpful for local therapies such as stereotactic body radiotherapy by improving accuracy. The integration of precise diagnostic means for target volume delineation in radiation oncology maximizes therapeutic benefit while minimizing side effects. The results of PET-based radiotherapy with 68Ga-FAP1 PET planning of liver metastases showed promising results, with a change in target volume definition in 84% (19). Furthermore, the use of 4-dimensional PET/CT allowed a significant decrease of planning target volume in a small cohort of 8 patients (20). Thus, 68Ga-FAPI PET/CT may contribute to improved radiotherapy planning and more precise staging or restaging for patients with colorectal cancer (Fig. 4).
FIGURE 4.
In patient with metastatic carcinoma of sigmoid colon, decrease in 68Ga-FAPI uptake in bone and nodal metastases after initiation of systemic therapy (ipilimumab, nivolumab, and bevacizumab) indicates treatment response.
In our cohort, an alteration of target volume delineation was caused in every case also for treatment-naïve patients with anal cancer. To avoid tumor relapses and improve overall survival, the use of 68Ga-FAPI PET/CT for treatment planning may guide boost volume definition or decrease elective node irradiation, which could reduce side effects. Preliminary data suggest that treatment outcome may be improved significantly by targeting cancer-associated fibroblasts (FAPI-guided radiotherapy) as a supplementary treatment to conventional irradiation (21). Furthermore, PET imaging may also contribute to improved clinical outcome by aggressive, local treatment approaches for patients with oligometastatic or oligoprogressive disease. For almost 20% of our cohort, a significant change in oncologic or radiooncologic management was caused by 68Ga-FAPI PET/CT, leading to local treatment approaches. In a previously published trial by Palma et al., patients with 1–5 oligometastatic lesions undergoing stereotactic body radiotherapy had a significantly better overall survival than patients undergoing standard-of-care palliative treatment after a median follow-up of 25 mo (22). However, even though 68Ga-FAPI PET/CT may open new applications in tumor staging and oncologic management for LGT tumors, larger and prospective trials are needed to provide more data evaluating the role of this new hybrid imaging technology in daily clinical practice.
To the best of our knowledge, this was the first study evaluating the clinical role of 68Ga-FAPI PET/CT in LGT tumors. As such, it had several limitations. The patient cohort was small and retrospective. Moreover, no histologic verification was possible for most lesions. However, the study nonetheless showed the potential of this agent to identify LGT disease and should encourage further studies on larger cohorts.
CONCLUSION
For patients with LGT malignancies, 68Ga-FAPI PET/CT was able to demonstrate promising results with regard to uptake and restaging. These properties make the agent useful for guiding radiation therapy of LGT tumors. Given its short uptake time and the fact that patient do not need to fast before the study, 68Ga-FAPI PET/CT could be ideal for oncologic or radiooncologic management and may open new applications for individualizing treatments for patients with LGT tumors.
DISCLOSURE
A potential financial conflict of interest is present for Clemens Kratochwil, Uwe Haberkorn, Thomas Lindner, and Frederik Giesel because of a patent application for quinoline-based FAP-targeting agents for imaging and therapy in nuclear medicine. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does 68Ga-FAPI PET/CT have an impact on the oncologic or radiooncologic management of patients with malignant gastrointestinal tract tumors?
PERTINENT FINDINGS: A cohort of 22 patients with malignancies of the LGT was evaluated. Changes in tumor stage or localization and in oncologic or radiooncologic management were recorded after a comparison with standard imaging. Although the TNM stage was changed in 50% of patients (treatment-naïve), and for 47% of all patients with metastatic disease new findings were obtained, a change in oncologic or radiooncologic management occurred in 81% because of 68Ga-FAPI imaging.
IMPLICATIONS FOR PATIENT CARE: 68Ga-FAPI PET/CT was able to demonstrate promising results with regard to uptake and restaging and may open new applications for individualizing treatments for patients with malignant tumors of the LGT.
Acknowledgments
We gratefully acknowledge all participating patients.
REFERENCES
- 1.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA. 1990;87:7235–7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rettig WJ, Garin-Chesa P, Healey JH, et al. Regulation and heteromeric structure of the fibroblast activation protein in normal and transformed cells of mesenchymal and neuroectodermal origin. Cancer Res. 1993;53:3327–3335. [PubMed] [Google Scholar]
- 3.Chen WT, Kelly T. Seprase complexes in cellular invasiveness. Cancer Metastasis Rev. 2003;22:259–269. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, Wang S, Kelly T. Seprase promotes rapid tumor growth and increased microvessel density in a mouse model of human breast cancer. Cancer Res. 2004;64:2712–2716. [DOI] [PubMed] [Google Scholar]
- 5.Kelly T. Fibroblast activation protein-alpha and dipeptidyl peptidase IV (CD26): cell-surface proteases that activate cell signaling and are potential targets for cancer therapy. Drug Resist Updat. 2005;8:51–58. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann C, Babich JW, Joyal J, Marquis J, Wang J-C, inventors; Molecular Insight Pharmaceuticals, Inc., assignee. Selective seprase inhibitors. U.S. patent application 2010/0098633 A1. April 22, 2010.
- 7.Lindner T, Loktev A, Giesel F, et al. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm Chem. 2019;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindner T, Loktev A, Altmann A, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–1422. [DOI] [PubMed] [Google Scholar]
- 9.Loktev A, Lindner T, Mier W, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. 2018;59:1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loktev A, Lindner T, Burger EM, et al. Development of novel FAP-targeted radiotracers with improved tumor retention. J Nucl Med. March 8, 2019 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giesel FL, Kratochwil C, Lindner T, et al. 68Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med. 2019;60:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kratochwil C, Flechsig P, Lindner T, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 14.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA. 1990;87:7235–7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry LR, Lee HO, Lee JS, et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:1736–1741. [DOI] [PubMed] [Google Scholar]
- 16.Henriksson ML, Edin S, Dahlin AM, et al. Colorectal cancer cells activate adjacent fibroblasts resulting in FGF1/FGFR3 signaling and increased invasion. Am J Pathol. 2011;178:1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotti F, Jarrar AM, Pai RK, et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J Exp Med. 2013;210:2851–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tommelein J, Verset L, Boterberg T, et al. Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer. Front Oncol. 2015;5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffen IG, Wust P, Rühl R, et al. Value of combined PET/CT for radiation planning in CT-guided percutaneous interstitial high-dose-rate single-fraction brachytherapy for colorectal liver metastases. Int J Radiat Oncol Biol Phys. 2010;77:1178–1185. [DOI] [PubMed] [Google Scholar]
- 20.Riou O, Serrano B, Azria D, et al. Integrating respiratory-gated PET-based target volume delineation in liver SBRT planning, a pilot study. Radiat Oncol. 2014;9:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Tang Y, Tan Y, et al. Cancer-associated fibroblasts in radiotherapy: challenges and new opportunities. Cell Commun Signal. 2019;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. [DOI] [PubMed] [Google Scholar]