Abstract
Emerging evidence supports a hypothesized role for the α7-nicotinic acetylcholine receptor (α7-nAChR) in the pathophysiology of Alzheimer’s disease. 18F-ASEM (3-(1,4-diazabicyclo[3.2.2]nonan-4-yl)-6-18F-fluorodibenzo[b,d]thiophene 5,5-dioxide) is a radioligand for estimating the availability of α7-nAChR in the brain in vivo with PET. Methods: In this cross-sectional study, 14 patients with mild cognitive impairment (MCI), a prodromal stage to dementia, and 17 cognitively intact, elderly controls completed 18F-ASEM PET. For each participant, binding in each region of interest was estimated using Logan graphical analysis with a metabolite-corrected arterial input function. Results: Higher 18F-ASEM binding was observed in MCI patients than in controls across all regions, supporting higher availability of α7-nAChR in MCI. 18F-ASEM binding was not associated with verbal memory in this small MCI sample. Conclusion: These data support use of 18F-ASEM PET to examine further the relationship between α7-nAChR availability and MCI.
Keywords: nAChR, cholinergic, 18F-ASEM, dementia, PET
The α7-nicotinic acetylcholine receptor (α7-nAChR) may play a mechanistic role in the selective vulnerability of cholinergic cells to neurodegeneration in the earliest phases of Alzheimer’s disease (AD) (1). The α7-nAChR binds soluble 42-amino-acid β-amyloid peptide (Aβ42) with picomolar affinity (2), and in AD, the Aβ42–α7-nAChR interaction may result in dysregulated signal transduction, compromised synaptic plasticity, and a toxic, selective effect on cholinergic cells of the basal forebrain and its projection sites (3). AD mouse models support upregulation of α7-nAChR expression in the presence of Aβ42, which promotes further pathologic changes (4). In AD brain tissue, high levels of α7-nAChR colocalize with intracellular Aβ42 burden (5), and high α7-nAChR expression was found on basal forebrain (specifically nucleus basalis) cholinergic neurons (6). Relative to control tissue, a nonsignificant trend toward higher basal forebrain α7-nAChR expression was found in cases of mild cognitive impairment (MCI), a prodromal stage to dementia (6).
18F-ASEM (3-(1,4-diazabicyclo[3.2.2]nonan-4-yl)-6-18F-fluorodibenzo[b,d]thiophene 5,5-dioxide) is a PET radioligand for estimating the availability of α7-nAChR in the brain in vivo and has shown high specific binding in receptor blockade studies in mice (7) and baboons (8). 18F-ASEM PET has been used to study the α7-nAChR in healthy aging (9) and psychosis (10). In this pilot study, we used 18F-ASEM PET to test for higher in vivo availability of α7-nAChR in individuals with MCI than in cognitively intact, healthy controls. Associations between regional 18F-ASEM binding and memory were explored.
MATERIALS AND METHODS
Human Subjects
The Johns Hopkins Institutional Review Board approved this prospective study, which was conducted under an Investigational New Drug protocol approved by the U.S. Food and Drug Administration. Participants (nonsmokers over 65 y old) provided written informed consent. Participants completed a clinical assessment, MRI examination, and comprehensive neuropsychological evaluation as previously described (9). Included was the California Verbal Learning Test (11), which is sensitive to detecting memory impairment in MCI. All MCI participants had a global clinical dementia rating (CDR) (12) of 0.5 with a sum-of-boxes score not exceeding 2.5, and impaired memory on neuropsychological testing (at least 1 SD below normal performance on a memory test). Control participants were in stable health, with a global CDR of 0 (normal). Exclusion criteria included a global CDR of more than 0.5, a decline in activities of daily living, nicotine use in the past 6 mo, a neurological condition other than MCI, substance abuse, current use of medication with potential to affect radiotracer binding (i.e., acetylcholinesterase inhibitors and anticholinergics), or a contraindication to imaging.
The presence of an apolipoprotein E (APOE) ε4 allele is associated with cerebral amyloid burden (13). We classified participants as either an APOE ε4 carrier (presence of ≥1 ε4 allele) or an APOE ε4 noncarrier, using previously published genotyping methods (9).
Brain Imaging
18F-ASEM PET and MRI data were acquired, and PMOD (version 3.7) was used for image processing as previously described (9). MRI volumetric segmentation was conducted with the FreeSurfer software suite (http://surfer.nmr.mgh.harvard.edu/) or MRICloud (www.mricloud.org; for basal forebrain only). The regions of interest included cortical (frontal, parietal, temporal, occipital, cingulate, and cerebellar) and subcortical (hippocampus, thalamus, striatum, and basal forebrain) regions. Total intracranial volume was also estimated using FreeSurfer for comparison of regional atrophy between the 2 groups while accounting for individual variability in head size. Regional volume normalized to intracranial volume is referred to as regional volume ratio.
The kinetics of 18F-ASEM were modeled using Logan graphical analysis (14) with a metabolite-corrected arterial input function obtained from 90 min of dynamic data, generating a regional 18F-ASEM total distribution volume, VT (15). To address the effect of atrophy on 18F-ASEM binding in the brains of this elderly study population, we report regional VT values that were derived from PET data after partial-volume correction (PVC) using the algorithm of Müller-Gärtner et al. (16). VT estimates from images without PVC were secondary binding outcomes.
Statistical Analysis
Differences in 18F-ASEM VT between the control and MCI groups were tested using a linear mixed-effects regression model to accommodate within-subject assessment of brain regions that are associated to different degrees. Primary predictors included group, an index variable for brain region, and the 2-way interaction. Significance was set at a P value of less than 0.05 for this single mixed-effects regression model analysis. Pearson partial correlations were conducted to explore relationships within the MCI group between 18F-ASEM binding and performance on California Verbal Learning Test short- and long-delay free recall. Age was included in all models as a covariate because age may affect 18F-ASEM binding (9). When group differences emerged, subsequent models were run with the adjustment for not only age but also for race, sex, and APOE ε4 carrier status. Because there were 2 primary memory outcomes, the threshold for significance was set at a P value of less than 0.025 (=0.05/2) for examining these correlations.
RESULTS
Participants
Clinical characteristics, APOE ε4 carrier status, and relevant imaging parameters were similar between the control (n = 17) and MCI (n = 14) groups (Table 1). After age adjustment, patients with MCI had higher scores than controls on the sum-of-boxes CDR (P = 0.001) and lower scores on the 2 California Verbal Learning Test outcomes (each P < 0.025).
TABLE 1.
Clinical Characteristics and PET Parameters Among Healthy Controls and Patients with MCI
| Characteristic | Control (n = 17) | MCI (n = 14) | P |
| Age (y) | 76.4 ± 6.2 | 80.5 ± 5.5 | 0.06 |
| Sex (male) | 9 (53%) | 7 (50%) | 0.88 |
| Race (African American; Caucasian; Asian) | 4 (24%); 12 (71%); 1 (6%) | 7 (50%); 7 (50%); 0 (0%) | 0.24 |
| Education (y) | 17.1 ± 3.3 | 15.7 ± 4.3 | 0.35 |
| Body mass index | 26.5 ± 3.0 | 26.7 ± 3.2 | 0.92 |
| Genetic vulnerability, APOE ε4 carrier (yes) | 3 (18%) | 5 (36%) | 0.28 |
| Cognitive performance | |||
| Sum-of-boxes CDR | 0.0 ± 0.1 | 1.1 ± 0.7 | <0.001 |
| CVLT short-delay free recall | 11.2 ± 2.9 | 7.9 ± 4.0 | 0.02 |
| CVLT long-delay free recall | 11.8 ± 2.7 | 7.6 ± 5.0 | 0.02 |
| PET parameters | |||
| Molar activity (GBq/μmol) | 3,878.1 ± 2,614.6 | 3,327.1 ± 2,133.8 | 0.52 |
| Injected dose of radioactivity (MBq) | 516.2 ± 22.2 | 517.2 ± 17.7 | 0.89 |
| Injected mass (μg) | 0.08 ± 0.06 | 0.11 ± 0.12 | 0.43 |
CVLT = California Verbal Learning Test.
P values are from comparison using Student t test or χ2 test as appropriate except for comparison of cognitive performance, which was assessed using analysis of covariance with age as covariate. Qualitative data are expressed as numbers followed by percentages in parentheses; continuous data are expressed as mean ± SD.
Imaging
In age-adjusted analyses, there was a near-significant group × region interaction (F9,28 = 2.21, P = 0.05) indicating that there were group differences in some but not all MRI-based segmentation regional volumes. Specifically, the MCI group had smaller hippocampal (P = 0.001) and thalamic (P = 0.04) volumes than controls (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). The same pattern of differences was seen in regional volume ratios after age adjustment.
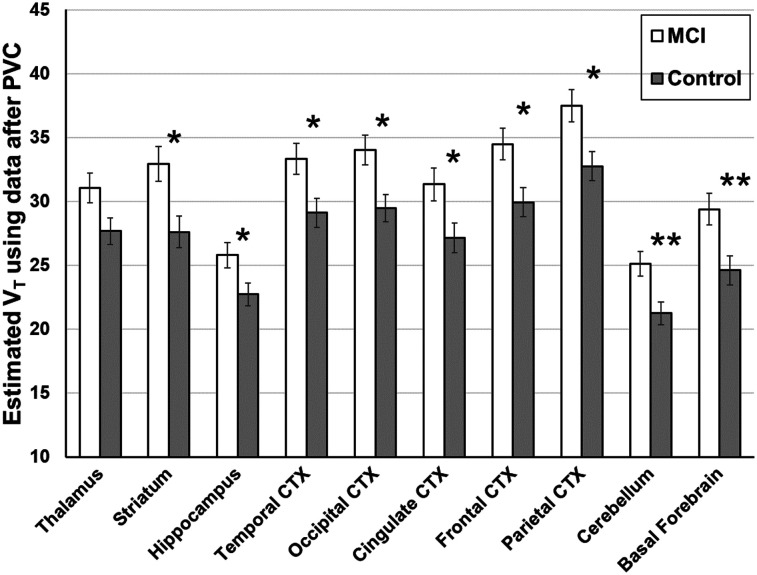
The metabolism of 18F-ASEM over the scan duration did not differ between groups at any time point. At 10, 45, and 90 min after injection, mean parent fractions were 69% ± 7%, 28% ± 9%, and 14% ± 5%, respectively, among the MCI group and 68% ± 6%, 29% ± 8%, and 15% ± 4%, respectively, among the controls. In age-adjusted analyses using images after PVC, the MCI group had a higher 18F-ASEM VT (estimated mean, 31.51; SE, 1.14) than did controls (estimated M, 27.23; SE, 1.04; F1,28 = 7.02, P = 0.01; Cohen’s d = 0.999; 95% confidence interval, 0.25–1.75), with the pattern of group differences similar across all regions (F9,28 = 1.37, P = 0.25; Fig. 1; Supplemental Table 2). Rerunning the model after further adjusting for race, sex, and APOE ε4 carrier status resulted in the same pattern of higher 18F-ASEM VT in MCI patients than in controls (F1,24 = 11.03, P = 0.003).
FIGURE 1.
Higher 18F-ASEM regional VT was found in 14 participants with MCI than in 17 cognitively intact healthy control participants, after accounting for age. Box plot diagram of 18F-ASEM VT was derived from images after PVC across 10 regions of interest. A linear mixed-effects regression model adjusting for age and brain region demonstrated higher VT in MCI patients than in controls. In secondary analyses, a regional difference for each region except thalamus was found between MCI patients and controls using individual linear regression models fit for region of interest and controlling for age (*P < 0.05; **P < 0.01). These differences in individual regions did not remain significant after Bonferroni adjustment for 10 regions tested, which required P < 0.005. VT is in units of mL cm−3. CTX = cortex.
Among secondary 18F-ASEM binding outcomes (Supplemental Table 3), a similar but nonsignificant binding pattern was observed using VT derived from data without PVC and age adjustment (F1,28 = 3.81, P = 0.06). After further adjusting for race, sex, and APOE ε4 carrier status, the group difference was significant (F1,24 = 9.06, P = 0.006).
Among patients with MCI, there were no associations between regional 18F-ASEM VT and California Verbal Learning Test performance (Supplemental Table 4).
DISCUSSION
Our 18F-ASEM PET data are consistent with higher availability of the α7-nAChR in MCI patients than in healthy controls across multiple brain regions. These pilot results align with postmortem studies reporting higher α7-nAChR levels in early stages of AD (6), as well as with animal models of AD (4,17). If validated as a mechanistically linked biomarker, high α7-nAChR availability may prove useful for earlier detection and therapeutic intervention in AD (18,19).
We acknowledge limitations in this study. First, VT reflects specifically bound and nondisplaceable (nonspecifically bound or free) radiotracer. Human 18F-ASEM PET data do not support a brain region without α7-nAChR that could be used as reference tissue for reporting the outcome of specifically bound (relative to nondisplaceable) 18F-ASEM. This widespread α7-nAChR distribution in human brain is similar to that reported in rhesus monkeys and differs from rodent brain (20). VT was derived from images after PVC that adjusts the PET signal for regional brain atrophy seen in neurodegeneration. Consistent with published MCI work and supporting use of PVC, we saw smaller hippocampal and thalamic volumes in MCI patients than in controls after age adjustment. However, a risk of using PVC is false elevation of regional VT estimates due to overcompensation by the method. In this study, model analysis using VT from images without PVC produced a similar, albeit nonsignificant, pattern of higher binding in MCI patients than in controls after age adjustment, which achieved significance after further adjustment for other factors (sex, race, and APOE ε4 carrier status). Second, whereas these findings suggest a role for the α7-nAChR in MCI, the cross-sectional study design and lack of amyloid markers in our pilot population limit the ability to assess causality or specificity to AD-mediated MCI (as opposed to MCI from non-AD pathology). The lack of association between 18F-ASEM VT and verbal memory impairment in this study may be due to possible inclusion of MCI participants with non-AD disease. Furthermore, here we focused on brain tissue, but the α7nAChR in cerebral vasculature may promote cerebral amyloid angiopathy (21), which is common in AD and linked to impaired memory. Complementary animal studies that manipulate α7-nAChR availability or activity should be pursued to evaluate causal, mechanistic relationships between the α7-nAChR and AD pathophysiology. Future studies designed to test the relationships between 18F-ASEM binding and neuropsychological performance in larger clinical samples are needed.
CONCLUSION
Our 18F-ASEM PET data support a higher availability of the α7-nAChR across several brain regions in individuals with MCI than in healthy individuals.
DISCLOSURE
This work was supported by the Henry N. Wagner, Jr., Endowment, a Johns Hopkins Doris Duke Foundation Early Clinician Investigator Award, and the Johns Hopkins Alzheimer Disease Research Center (P50 AG005146). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is the availability of the α7-nicotinic acetylcholine receptor (α7-nAChR) altered in brains of individuals with mild cognitive impairment (MCI)?
PERTINENT FINDINGS: In this cross-sectional study using PET, regional binding of 18F-ASEM that targets the α7-nAChR was compared between patients MCI and cognitively intact, elderly controls. Higher 18F-ASEM binding was observed in MCI compared with controls across all brain regions.
IMPLICATIONS FOR PATIENT CARE: 18F-ASEM is a promising radiotracer to test further the relationship between the availability of the α7-nAChR and MCI in vivo with PET.
Acknowledgments
We thank the Johns Hopkins PET Center for providing the 18F-ASEM.
REFERENCES
- 1.Hernandez CM, Dineley KT. Alpha7 nicotinic acetylcholine receptors in Alzheimer’s disease: neuroprotective, neurotrophic or both? Curr Drug Targets. 2012;13:613–622. [DOI] [PubMed] [Google Scholar]
- 2.Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. Beta-amyloid(1-42) binds to alpha7 nicotinic acetylcholine receptor with high affinity: implications for Alzheimer’s disease pathology. J Biol Chem. 2000;275:5626–5632. [DOI] [PubMed] [Google Scholar]
- 3.Parri HR, Hernandez CM, Dineley KT. Research update: alpha7 nicotinic acetylcholine receptor mechanisms in Alzheimer’s disease. Biochem Pharmacol. 2011;82:931–942. [DOI] [PubMed] [Google Scholar]
- 4.Dineley KT, Xia X, Bui D, Sweatt JD, Zheng H. Accelerated plaque accumulation, associative learning deficits, and up-regulation of alpha 7 nicotinic receptor protein in transgenic mice co-expressing mutant human presenilin 1 and amyloid precursor proteins. J Biol Chem. 2002;277:22768–22780. [DOI] [PubMed] [Google Scholar]
- 5.Nagele RG, D’Andrea MR, Anderson WJ, Wang HY. Intracellular accumulation of beta-amyloid(1-42) in neurons is facilitated by the alpha 7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience. 2002;110:199–211. [DOI] [PubMed] [Google Scholar]
- 6.Counts SE, He B, Che S, et al. Alpha7 nicotinic receptor up-regulation in cholinergic basal forebrain neurons in Alzheimer disease. Arch Neurol. 2007;64:1771–1776. [DOI] [PubMed] [Google Scholar]
- 7.Wong DF, Kuwabara H, Pomper M, et al. Human brain imaging of alpha7 nAChR with 18F-ASEM: a new PET radiotracer for neuropsychiatry and determination of drug occupancy. Mol Imaging Biol. 2014;16:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horti AG, Gao Y, Kuwabara H, et al. 18F-ASEM, a radiolabeled antagonist for imaging the alpha7-nicotinic acetylcholine receptor with PET. J Nucl Med. 2014;55:672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coughlin JM, Du Y, Rosenthal HB, et al. The distribution of the alpha7 nicotinic acetylcholine receptor in healthy aging: an in vivo positron emission tomography study with 18F-ASEM. Neuroimage. 2018;165:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlin J, Du Y, Crawford JL, et al. The availability of the alpha7 nicotinic acetylcholine receptor in recent-onset psychosis: a study using 18F-ASEM PET. J Nucl Med. December 20, 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Delis DC, Kramer JH, Kaplan E, Ober BA. CVLT: California Verbal Learning Test—Adult Version: Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 12.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 13.Risacher SL, Kim S, Shen L, et al. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI). Front Aging Neurosci. 2013;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logan J, Fowler JS, Volkow ND, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. [DOI] [PubMed] [Google Scholar]
- 15.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. [DOI] [PubMed] [Google Scholar]
- 16.Müller-Gärtner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. [DOI] [PubMed] [Google Scholar]
- 17.Jones IW, Westmacott A, Chan E, et al. Alpha7 nicotinic acetylcholine receptor expression in Alzheimer’s disease: receptor densities in brain regions of the APP(SWE) mouse model and in human peripheral blood lymphocytes. J Mol Neurosci. 2006;30:83–84. [DOI] [PubMed] [Google Scholar]
- 18.Takata K, Amamiya T, Mizoguchi H, et al. Alpha7 nicotinic acetylcholine receptor-specific agonist DMXBA (GTS-21) attenuates Abeta accumulation through suppression of neuronal gamma-secretase activity and promotion of microglial amyloid-beta phagocytosis and ameliorates cognitive impairment in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2018;62:197–209. [DOI] [PubMed] [Google Scholar]
- 19.Uteshev VV. The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur J Pharmacol. 2014;727:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han ZY, Zoli M, Cardona A, Bourgeois JP, Changeux JP, Le Novere N. Localization of 3H-nicotine, 3H-cytisine, 3H-epibatidine, and 125I-alpha-bungarotoxin binding sites in the brain of Macaca mulatta. J Comp Neurol. 2003;461:49–60. [DOI] [PubMed] [Google Scholar]
- 21.Clifford PM, Siu G, Kosciuk M, et al. Alpha7 nicotinic acetylcholine receptor expression by vascular smooth muscle cells facilitates the deposition of Abeta peptides and promotes cerebrovascular amyloid angiopathy. Brain Res. 2008;1234:158–171. [DOI] [PubMed] [Google Scholar]