Abstract
Our objective was to investigate the lesion detection rate of 18F-DCFPyL PET/CT, a prostate-specific membrane antigen (PSMA)–targeted PET agent, in patients with biochemically relapsed prostate cancer after primary local therapy. Methods: This was a prospective institutional review board–approved study of 90 patients with documented biochemical recurrence (median prostate-specific antigen [PSA], 2.5 ng/mL; range, 0.21–35.5 ng/mL) and negative results on conventional imaging after primary local therapies, including radical prostatectomy (n = 38), radiation (n = 27), or a combination of the two (n = 25). Patients on androgen deprivation therapy were excluded. Patients underwent whole-body 18F-DCFPyL PET/CT (299.9 ± 15.5 MBq) at 2 h after injection. The PSMA PET lesion detection rate was correlated with PSA, PSA kinetics, and original primary tumor grade. Results: Seventy patients (77.8%) showed positive PSMA PET results, with a total of 287 lesions identified: 37 prostate bed foci, 208 lesions in lymph nodes, and 42 in distant sites in bones or organs, Eleven patients had negative results, and 9 patients showed indeterminate lesions, which were considered negative in this study. The detection rates were 47.6% (n = 10/21), 50% (n = 5/10), 88.9% (n = 8/9), and 94% (n = 47/50) for PSA levels of >0.2 to <0.5, 0.5 to <1.0, 1 to <2.0, and ≥2.0 ng/mL, respectively. In postsurgical patients, PSA, PSA doubling time, and PSA velocity correlated with PET results, but the same was not true for postradiation patients. These parameters also correlated with the extent of disease on PET (intrapelvic vs. extrapelvic). There was no significant difference in the rate of positive scans between patients with higher-grade and lower-grade primary tumors (Gleason score of ≥4 + 3 vs. <3 + 4). Tumor recurrence was histology-confirmed in 40% (28/70) of patients. On a per-patient basis, positive predictive value was 93.3% (95% confidence interval, 77.6%–99.2%) by histopathologic validation and 96.2% (95% confidence interval, 86.3%–99.7%) by the combination of histology and imaging/clinical follow-up. Conclusion: 18F-DCFPyL PET/CT imaging offers high detection rates in biochemically recurrent prostate cancer patients and is positive in about 50% of patients with a PSA level of less than 0.5 ng/mL, which could substantially impact clinical management. In postsurgical patients, 18F-DCFPyL PET/CT correlates with PSA, PSA doubling time, and PSA velocity, suggesting it may have prognostic value. 18F-DCFPyL PET/CT is highly promising for localizing sites of recurrent prostate cancer.
Keywords: prostate cancer, biochemical recurrence, PSMA, DCFPyL, PET
After initial diagnosis, most patients with localized prostate cancer are treated with either radical prostatectomy, external-beam radiation, brachytherapy, or active surveillance (1,2). Despite definitive therapy with surgery or radiation, recurrent disease is relatively common, occurring in up to 50% of patients within 10 y (3). Prostate cancer recurrence is usually first suspected when a rise in serum prostate-specific antigen (PSA) is observed on posttreatment monitoring, often without findings on conventional imaging. This clinical scenario is defined as biochemical recurrence (BCR) and can precede clinically apparent disease by months or even years. Left untreated, BCR may progress to distant metastatic disease; early salvage therapy is therefore recommended while PSA is still low (4).
In the setting of BCR, the location of recurrence has clinical relevance; local recurrence alone usually carries a better prognosis, whereas nodal, bone, or visceral metastasis is associated with more aggressive disease behavior. Therefore, accurate identification of the site and extent of tumor recurrence is important for further treatment planning. Anatomic imaging methods have limited utility in this clinical scenario; CT and transrectal ultrasound have sensitivity between 25% and 54% for local recurrence detection, whereas MRI is somewhat better but still limited (5,6). Serum PSA, a biomarker for tumor mass, can reach high levels before the source becomes detectable on conventional imaging (7). These limitations have stimulated the development of new molecular imaging probes. Among these, positron-emitting probes targeting prostate-specific membrane antigen (PSMA) have been the most successful (8). PSMA is overexpressed in intermediate–to–high-grade tumors and demonstrates increased uptake with increasing tumor aggressiveness (8–10). To date, a variety of PSMA-targeted imaging probes have demonstrated improved sensitivity and specificity in BCR and metastatic disease compared with conventional imaging (11,12). The most experience has been reported for 68Ga-labeled probes, and experience with 18F-labeled PSMA tracers has been growing. The latter has the advantage of a longer half-life (110 min for 18F vs. 68 min for 68Ga), enabling centralized production in a cyclotron facility, and more favorable positron energies for imaging. 18F-DCFPyL (2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid) is a second-generation PSMA PET agent with high affinity for prostate cancer (13–15). Here, we report the results of a prospective study of 18F-DCFPyL PET/CT for detection and localization of recurrent disease in patients with BCR. Specifically, we aimed to investigate the tumor detection rate as a function of PSA and PSA kinetics. We hypothesized that 18F-DCFPyL PET/CT–positive scans are associated with more aggressive PSA kinetics than negative scans.
MATERIALS AND METHODS
Patient Selection and Study Design
We are reporting an interim analysis of the first 90 patients with biochemical relapsed prostate cancer in a prospective, Health Insurance Portability and Accountability Act–compliant, single-institution study, approved by the institutional review board, with written informed consent (NCT03181867). The study has an open protocol, actively enrolling 2 groups of participants. The first group consists of patients with newly diagnosed high-risk prostate cancer who are scheduled for prostatectomy or for a biopsy before radiation therapy (RT); recruitment of 55 patients is the aim for this group. The second group consists of patients with presumed prostate cancer relapse after prostate removal surgery or RT; recruitment of 275 patients is the aim for this group. The inclusion criterion was BCR prostatic adenocarcinoma, defined as a PSA level of more than 0.2 ng/mL in patients who underwent radical prostatectomy or at least 2 ng/mL greater than the nadir in post-RT patients, following the ASTRO-Phoenix criteria (16). PSA doubling time (PSAdt) and PSA velocity (PSAvel) were calculated. Patients had negative results on conventional imaging (CT and bone scanning) within the past 3–4 mo. The exclusion criteria included current androgen deprivation therapy (ADT) before enrollment, inability to tolerate a PET/CT scan, creatinine levels greater than 2 times the upper limit of normal, bilirubin levels greater than 2 times the upper limit of normal, or liver transaminase levels (alanine aminotransferase and aspartate aminotransferase) greater than 3 times the upper limit of normal, with the purpose of excluding patients with coexisting medical conditions that could potentially interfere with study procedures or results.
Biopsy of abnormal sites of uptake suggestive of disease was performed within approximately 2 mo of 18F-DCFPyL PET/CT, when feasible.
PET Imaging Protocol
18F-DCFPyL PET/CT imaging was performed on a 3-dimensional time-of-flight–mode GE Healthcare Discovery MI DR camera, with a 20-cm coronal and a 70-cm axial field of view. Data were reconstructed with 3-dimensional iterative maximum-likelihood expectation maximization using 29 subsets, 3 iterations, time-of-flight mode, point-spread-function regularization parameter 6.0, and a gaussian postprocessing filter with a 4.1-cm kernel. A second set of image reconstructions was done using the GE Healthcare Q-Clear reconstruction algorithm. The scanner uses CT-based attenuation correction, along with random, normalization, dead-time, and scatter correction (17) and anatomic coregistration.
18F-DCFPyL was synthesized using good manufacturing practices as previously described (13). Quality control testing was performed before injection to ensure the proper dose and specific activity. Radiochemical purity was 99.2% ± 0.4%, and specific activity was 1,842 ± 489 mCi/μmol.
Each patient received an intravenous bolus injection of 18F-DCFPyL with a mean injected activity of 299.3 MBq (8.09 mCi) (range, 229.4–325.6 MBq [6.2–8.8 mCi]), followed by a whole-body (head to toe) PET/CT scan at approximately 2 h after injection (3 min/bed position). Low-dose CT scans (120 kV, 60 mAs) were acquired before each PET scan for attenuation correlation and coregistration.
Safety
The patient’s vital signs, including heart rate and blood pressure, were obtained before 18F-DCFPyL injection at baseline, immediately after administration, 15 min after injection, and after imaging. Patients were monitored for adverse events during radiotracer administration, immediately after the scan, and on the next day via telephone query.
Imaging Interpretation
Two board-certified nuclear medicine physicians prospectively evaluated all imaging data independently, resolving any disagreements by consensus. 18F-DCFPyL PET/CT images were reviewed using a MIM workstation (version 6.9.2; MIM Software Inc.). Maximum-intensity-projection, axial, coronal, and sagittal PET, CT, and PET/CT images were reviewed. Any abnormal focal uptake greater than the surrounding background and not associated with physiologic uptake was considered suggestive of malignancy. PET-positive lesions were classified as local recurrence in the prostatectomy/prostate bed, pelvic/extrapelvic nodes, or organ/bone metastases. Indeterminate foci of uptake were considered negative for recurrence. Typical pitfalls on PSMA-ligand PET imaging, such as uptake in celiac and other dorsal ganglia roots, fractures, and degenerative changes, were considered negative (18). Semiquantitative PET parameters included SUVmax and PSMA-derived tumor volume generated by gradient-based segmentation. The gradient-based segmentation consisted of an edge-detection tool, generating an automated volume of interest, outlined on the basis of the boundaries of the DCFPyL-positive lesion, avoiding background of adjacent structures.
18F-DCFPyL PET–positive findings were validated by histologic confirmation from percutaneous biopsy of the suspected lesion or transrectal ultrasound–guided biopsy of intraprostatic sites within 4–6 mo from the PET imaging; lesion-targeted imaging comparing abnormal PET findings with pelvic MRI performed within 3 mo from PET, follow-up CT, 99mTc-bone scanning, or 18F-sodium fluoride PET/CT at 3–6 mo; or clinical follow-up (i.e., increase or decrease of PSA after treatment) when available.
Statistical Methods
18F-DCFPyL PET lesion detection rates were analyzed with regard to PSA, PSA kinetics, and primary tumor Gleason scores, using χ2 and Wilcoxon rank tests. Descriptive values are expressed as mean ± SD. Receiver-operating-characteristic analysis was performed to evaluate continuous PSA and PSA kinetics at the scan time as predictors of lesion identification, estimated by the area under the curve (AUC). At each PSA/PSA kinetics cutoff, true-positive rate and false-positive rate were defined as the proportion of 18F-DCFPyL PET/CT–positive and –negative results with PSA/PSA kinetics values above the cutoff. The Delong 95% confidence interval of AUC was calculated. The Youden index was calculated to determine the cutoffs that gave the optimal combination of sensitivity and specificity for lesion detection. 18F-DCFPyL PET variables derived from lesion-based analysis, including number of lesions, SUVmax, and tumor volume, were correlated with PSA and PSA kinetics by Spearman rank correlations. Per-patient positive predictive value was calculated, as confirmed by histologic validation or by the combination of histologic validation, multiparametric MRI within 1 mo, follow-up standard-of-care imaging at 3–6 mo after PET scanning, or follow-up PSA values after local or focal therapy. All tests were 2-sided, and P values of less than 0.05 were considered significant (19). Statistical and graphical analysis was performed using R software, version 3.2.5.
RESULTS
Patient Population
The institutional review board approved this study, and all subjects gave written informed consent. Ninety patients (mean age, 66 y; range, 50–81 y) underwent 18F-DCFPyL PET/CT. Patients had documented BCR prostate cancer, with a median PSA of 2.5 ± 5.9 ng/mL (range, 0.21–35.5 ng/mL). Prior primary local therapy consisted of radical prostatectomy (n = 38), RT (n = 27), or a combination of the two (n = 25). The time from therapy to scanning was 4.2 ± 3.9 y (range, 7 mo to 19 y). In total, 30 patients (33.3%) had first-line ADT, with a mean time of 4.3 y (range, 1.2 to 9.7 y) before imaging, but none were actively receiving ADT at the time of the scan. No patient experienced adverse events or clinically detectable pharmacologic effects after 18F-DCFPyL injection. Table 1 shows the patient characteristics.
TABLE 1.
Patient Characteristics
| Characteristic | Data |
| Total patients (n) | 90 |
| Primary local therapy (n) | |
| Radical prostatectomy ± RT, ± ADT | 63 |
| RT ± ADT | 27 |
| Age (y) | |
| Median | 66 |
| Range | 50–81 |
| Primary tumor Gleason (n) | |
| Gleason ≤ 6 | 13 |
| Gleason 7 | 32 |
| Gleason ≥ 8 | 45 |
| PSA (ng/mL) | |
| Median | 2.5 |
| Range | 0.21–35.5 |
| PSAdt (mo) | |
| Median | 7.0 |
| Range | 0.9−75.2 |
| PSAvel (ng/mL/y) | |
| Median | 2.0 |
| Range | 0.1–25 |
| Time from treatment to scan (mo) | |
| Median | 50.4 |
| Range | 7–228 |
Patient-Based Analysis
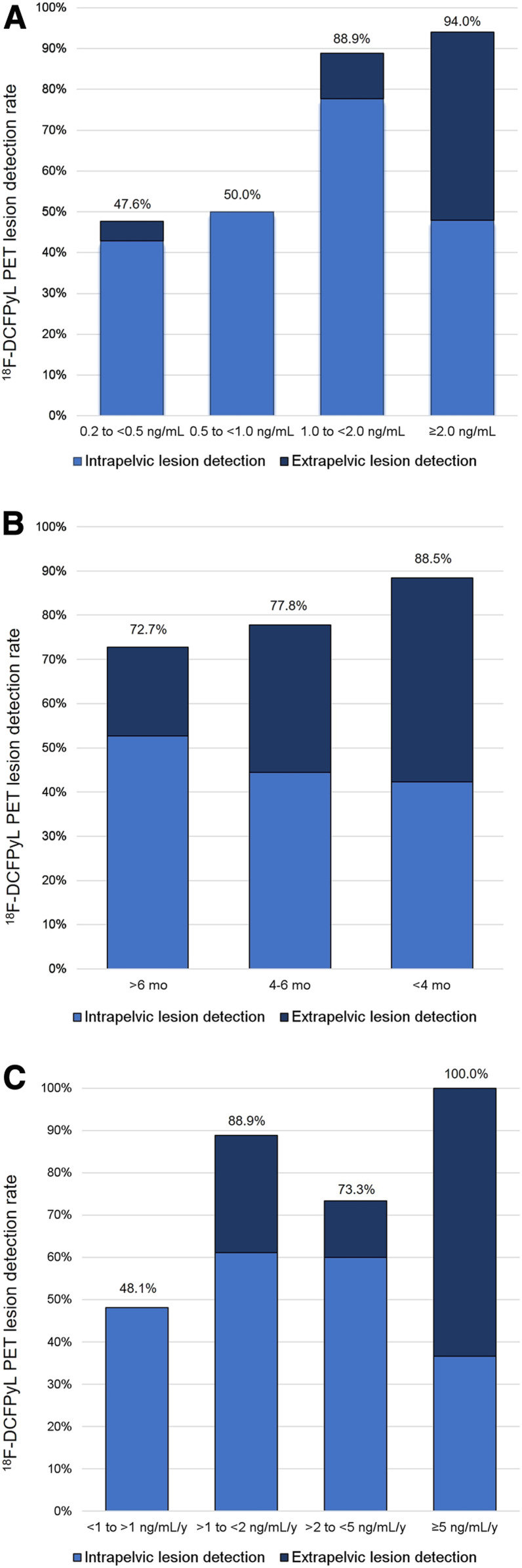
The overall 18F-DCFPyL PET lesion detection rate was 77.8% (70/90), identifying one or more sites suggestive of recurrent prostate cancer. Median PSA values were significantly lower in patients with negative findings than in those with positive findings (0.5 ± 2.1 vs. 3.1 ± 6.6 ng/mL, P < 0.001). The lesion detection rate correlated with an increase in PSA level. Stratified by PSA, 18F-DCFPyL PET lesion detection rates were 47.6% (n = 10/21), 50% (n = 5/10), 88.9% (n = 8/9), and 94% (n = 47/50) for a PSA level of >0.2 to <0.5, 0.5 to <1.0, 1 to <2.0, and ≥2.0 ng/mL, respectively (Fig. 1A). The subgroup analysis based on the patient’s primary initial therapy (prostatectomy vs. RT) demonstrated that for postprostatectomy patients, the detection rates were 47.6% (10/21), 62.5% (5/8), 87.5% (7/8), and 96.1% (25/26) at a PSA level of >0.2 to <0.5, 0.5 to <1.0, 1 to <2.0, and ≥2.0 ng/mL, respectively. For patients after RT, the detection rates were 0% (0/2), 100% (1/1), and 91.7% (22/24) at a PSA level of 0.5 to <1.0, 1 to <2.0, and ≥2.0 ng/mL, respectively. Most post-RT patients had a PSA level of more than 2 ng/mL, and there were no patients with a PSA level of between 0.2 and 0.5 ng/mL.
FIGURE 1.
18F-DCFPyL PET overall detection rates and intrapelvic and extrapelvic lesion detection rates, stratified by PSA (A), PSAdt (B), and PSAvel (C).
Overall, there was a slightly higher lesion detection rate (81.2% vs. 73.8%) in patients with higher primary tumor Gleason scores (≥4 + 3 vs. <3 + 4), but the difference was not statistically significant (P = 0.553). In patients imaged after prostatectomy, the detection rate for a Gleason score of ≤3 + 4 was not significant compared with those for a Gleason score of ≥4 + 3 (P = 0.359); thus, the 18F-DCFPyL PET/CT detection rate did not vary significantly with Gleason score.
Median PSAdt was 7 ± 12.5 mo (range, 0.9–75.2 mo). 18F-DCFPyL PET lesion detection rates were 72.7% (40/55), 77.8% (7/9), and 88.5% (23/26) for a PSAdt of >6 mo, 4–6 mo, and <4 mo, respectively (Fig. 1B). Having a lower PSAdt was not a strong predictor of having a positive 18F-DCFPyL PET result (P = 0.062).
PSAvel ranged from 0.1 to 25 ng/mL/y (median, 2.0 ± 4.9 ng/mL/y). 18F-DCFPyL PET lesion detection rates were 48.1% (13/27), 88.9% (16/18), 73.3% (11/15), and 100% (30/30) for a PSAvel of <1, >1 to <2, 2 to <5, and ≥5 ng/mL/y, respectively (Fig. 1C). PSAvel was able to distinguish between negative and positive scans (0.4 ± 0.9 vs. 3.6 ± 5.2, P < 0.001).
In the subgroup analysis based on primary local therapy, PSA, PSAdt, and PSAvel were all associated with 18F-DCFPyL PET lesion detection (P < 0.05) when patients had prostatectomy, whereas for those who received radiation, the distributions of PSA and PSAdt did not significantly differ between positive and negative scans. There was no significant difference between time from treatment and scan positivity (P = 0.148 and 0.198, respectively) for any of the patient subgroups.
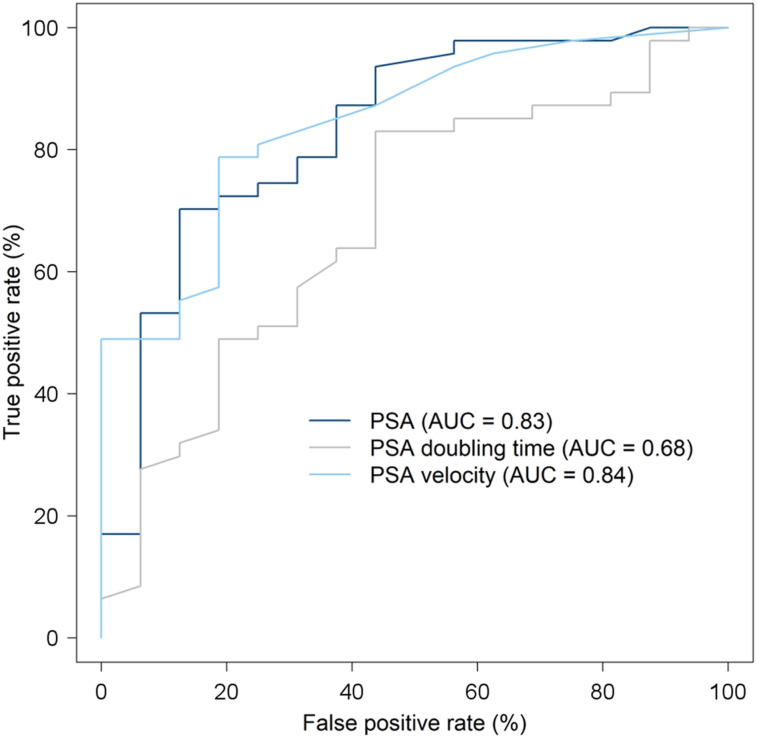
We assessed the benefit of performing 18F-DCFPyL PET/CT based on PSA value: receiver-operating-characteristic analysis was performed for the surgical patient cohort (n = 63) to assess the ability of PSA to distinguish between positive and negative scans (Fig. 2). The AUC was 0.83, and the optimal PSA cutoff to predict scan positivity was 0.81 ng/mL, which maximized the difference between true-positive rate and false-positive rate; that is, at this PSA value, the true-positive rate and false-positive rate were 0.70 and 0.13, respectively. For PSAdt, the AUC was 0.68, with an optimal cutoff of 9.7 mo corresponding to a true-positive rate of 0.83 and a false-positive rate of 0.44. For PSAvel, the AUC was 0.84, and the true-positive rate and false-positive rate were 0.79 and 0.19, for an optimal threshold of 0.85 ng/mL/y. A multivariable logistic model including both PSA and PSAdt as predictors, comparing AUCs, showed there was no significant difference from PSA alone (P = 0.06). Hence, there was no advantage of using the combination of PSA and PSAdt in predicting 18F-DCFPyL PET/CT results.
FIGURE 2.
Receiver-operating-characteristic analysis in prostatectomy patients (n = 63) for PSA, PSAdt, and PSAvel.
Regarding disease location, tumor sites detected by 18F-DCFPyL PET correlated with PSA (P < 0.001), PSAdt (P = 0.048), and PSAvel (P < 0.001), and the association was driven by the differences with respect to intrapelvic disease (i.e., prostatectomy bed with or without pelvic lymph nodes) versus extrapelvic disease (i.e., retroperitoneal nodes, supradiaphragmatic nodes, or bone or organ sites). Median PSA values were significantly lower for patients with intrapelvic than for those with extrapelvic disease (2.6 vs. 5.7 ng/mL; P < 0.001). There was a trend toward a higher PSAdt for patients with intrapelvic versus extrapelvic disease (7.2 vs. 5.5 mo; P = 0.051), and PSAvel was significantly lower in patients with intrapelvic than extrapelvic disease (1.5 vs. 7.3 ng/mL/y; P < 0.001). 18F-DCFPyL PET/CT detected disease confined to the pelvis in 47.7% (45/90) of patients, and stratifying by PSA, the intrapelvic detection rates were 42.9% (9/21), 50% (5/10), 77.8% (7/9), and 48% (24/50) at a PSA level of >0.2 to <0.5, 0.5 to <1.0, 1 to <2.0, and ≥2.0 ng/mL, respectively. Importantly, 55.5% of postradiotherapy patients had 18F-DCFPyL–positive lesions within the prostate bed, compared with 22.2% of postprostatectomy patients (P < 0.001). Extrapelvic disease detection was almost negligible (n = 1) for a PSA level of <1 ng/mL and increased to 11.1% (1/9) and 46% (23/50) when PSA increased to >1 to <2.0, and ≥2.0 ng/mL, respectively. Figure 1 shows the intrapelvic/extrapelvic detection rate compared with the overall detection rate, stratified by PSA. All patients with extrapelvic PET findings also had intrapelvic lesions, except for 3 patients with bone-only (n = 2) or lung (n = 1) lesions. Table 2 lists the locations of recurrent disease.
TABLE 2.
Tumor Sites Detected with 18F-DCFPyL PET/CT
| Tumor location* | No. of patients |
| Local recurrence (prostatectomy/prostate bed) | 29 (32.2%) |
| Lymph nodes | |
| Pelvic | 39 (43.3%) |
| Retroperitoneal | 14 (15.5%) |
| Distant supradiaphragmatic nodes | 3 (3.3%) |
| Bone | 9 (10%) |
| Organ (e.g., lungs, penile implant) | 5 (5.5%) |
There could be more than 1 lesion site per patient.
Lesion-Based Analysis
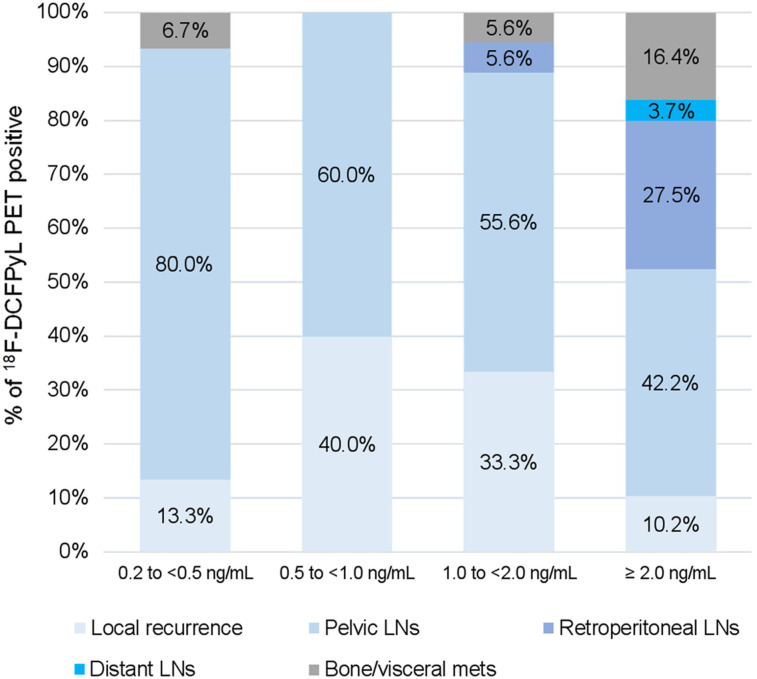
18F-DCFPyL PET/CT identified a total of 287 lesions: 37 in the prostatectomy/prostate bed, 208 in lymph nodes, and 42 in bone or organ sites. Figure 3 shows the percentage of 18F-DCFPyL–positive lesions by location, stratified by PSA. For a PSA level of <1 ng/mL, 18F-DCFPyL PET–positive lesions were mainly confined to the pelvis (Figs. 4 and 5), except for 1 patient with a PSA of 0.44 ng/mL and 1 bone lesion, which was biopsy-proven to be metastatic (Fig. 6). For a PSA level of 1 to <2 ng/mL, retroperitoneal nodes and bone/visceral sites were present in 5.6% (1/18) of the lesions, and for a PSA level of ≥2 ng/mL, retroperitoneal, distant nodes, and bone/visceral sites were present in 27.5% (67/244), 3.7% (9/244), and 16.4% (40/244) of the lesions, respectively.
FIGURE 3.
18F-DCFPyL–positive sites by location, stratified by PSA.
FIGURE 4.
A 60-y-old man with T2bN0, Gleason 4 + 3 prostate cancer, after prostatectomy. Time from treatment is 4 y, prescan PSA level is 0.24 ng/mL, and PSAdt is 16 mo. 18F-DCFPyL PET/CT demonstrates focal uptake (arrows) at right seminal vesicle resection site. Patient refused biopsy and proceeded with RT.
FIGURE 5.
A 63-y-old man with T3aN0, Gleason 4 + 3 prostate cancer after prostatectomy. Time from treatment is 0.7 y; prescan PSA level is 0.40 ng/mL, and PSAdt is 2.6 mo. 18F-DCFPyL PET/CT image shows single 3-mm left presacral node with focal intense uptake (arrows). This lesion was not biopsied, but we consider it less likely to be a dorsal nerve ganglion root because of its location and uptake intensity. Patient proceeded with salvage RT with presacral node boost and ADT. Recent PSA level was 0.02 ng/mL.
FIGURE 6.
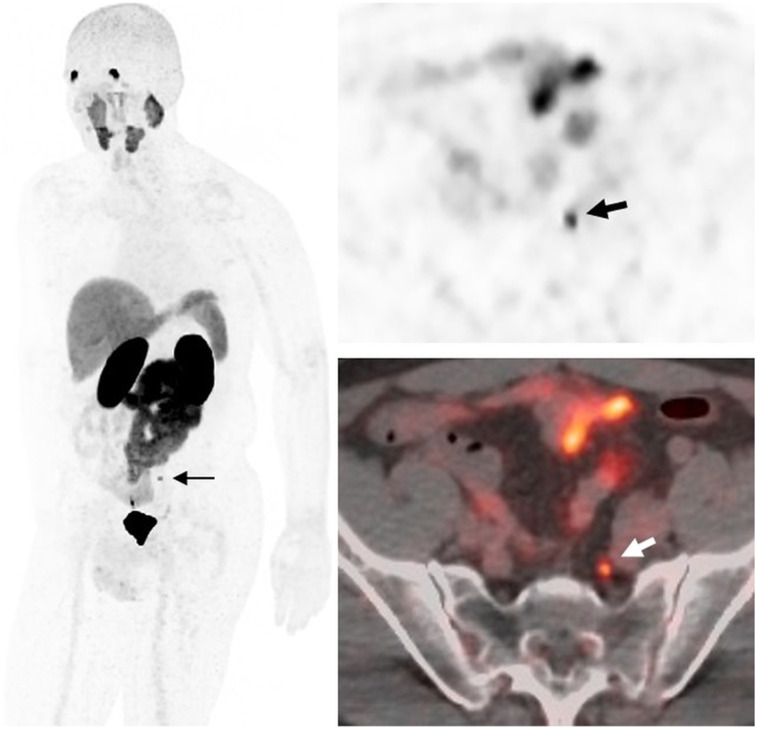
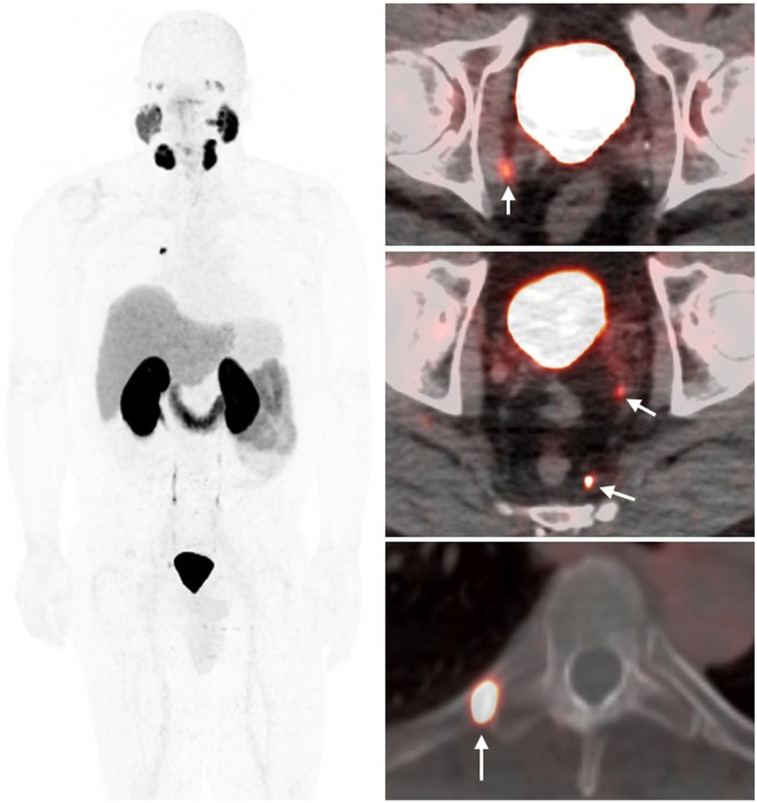
A 63-y-old man with T2cN1, Gleason 5 + 4 prostate cancer after prostatectomy. Time from treatment is 1.3 y; prescan PSA level is 0.44 ng/mL, and PSAdt is 2.2 mo. 18F-DCFPyL PET/CT demonstrates subcentimeter bilateral pelvic nodes and right fifth rib focus (arrows). Biopsy confirmed bone metastasis.
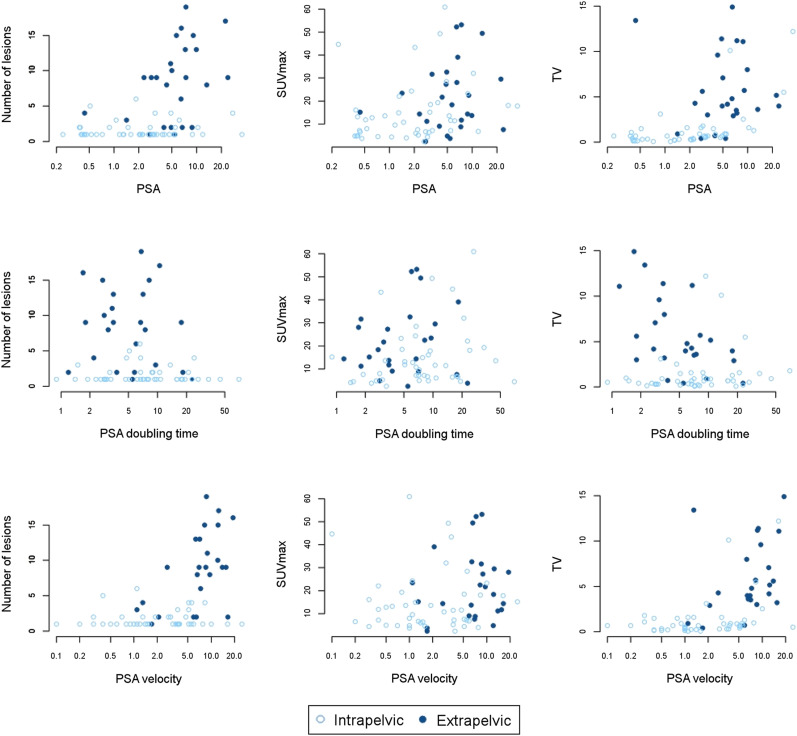
PSA values were associated with the number of 18F-DCFPyL–positive lesions and tumor volume (P < 0.001), whereas PSAdt was associated with the number of 18F-DCFPyL–avid lesions (P = 0.03). Some of these correlations were dependent on disease location; that is, there was a correlation between PSA and tumor volume for intrapelvic disease (r = 0.76) but not for extrapelvic disease (r = 0.03). There was no correlation between PSA and SUVmax for intra- or extrapelvic disease (Fig. 7).
FIGURE 7.
Correlation plots between PSA and PSA kinetics vs. lesion number, SUVmax, and tumor volume.
Histopathology and Patient Follow-up
Among 70 patients with positive scans, the presence of local recurrence or metastases was histologically confirmed in 40% (28/70) of cases for at least 1 site, either within the prostate bed (n = 17), lymph nodes (n = 8) or bones (n = 3). There were matched multiparametric MRI findings in 18.6% (13/70) of cases. Follow-up CT or bone scanning was available for 4.3% (3/70). PSA was undetectable after pelvic or node-boosted RT in 8.5% (7/70). Seventeen patients had lesions not amenable for biopsy or refused biopsy, and 2 had negative biopsy results (2 prostate foci). Therefore, the positive predictive value using histopathologic confirmation was 93.3% (95% confidence interval, 77.6%–99.2%; n = 30), whereas the positive predictive value using the histology–plus–follow-up standard reference was 96.2% (95% confidence interval, 86.3%–99.7%; n = 53).
Follow-up treatment information was available for 66 patients. Among these, 16 received local therapy: salvage radiation for 7, SBRT for 5, nodal/prostate resection for 3, and cryotherapy for 1. The other 43 patients were treated with systemic therapy: ADT alone for 17, ADT plus pelvic RT for 19, ADT plus palliative RT to the bone for 1, ADT plus chemotherapy for 2, and enrollment in a vaccine trial for 4; 7 patients continue active surveillance.
DISCUSSION
This was part of a prospective study assessing the performance of 18F-DCFPyL PET/CT in BCR prostate cancer patients after primary curative therapy. The patients had negative conventional-imaging results and a relatively low median PSA of 2.5 ng/mL (range, 0.21–35.5 ng/mL). This patient population is particularly difficult to assess because disease recurrence can vary from local recurrence to metastatic disease and the therapies for each disease category differ significantly. In this population, 18F-DCFPyL PET/CT had an overall lesion detection rate of about 78%. In a similar population, 18F-DCFPyL outperformed 18F-DCFBC, the first-generation 18F-PSMA compound, with a higher lesion detection rate (78% vs. 60%) mainly because of a higher tumor-to-background ratio and almost negligible blood-pool activity when compared with 18F-DCFBC (20).
Far more can be learned by stratifying the results according to PSA level. As with prior studies (21), the sensitivity of 18F-DCFPyL PET/CT increases with higher strata of PSA: lesion detection rates were 47.6%, 50%, 88.9%, and 94% for PSA levels of >0.2 to <0.5, 0.5 to <1.0, 1 to <2.0, and ≥2.0 ng/mL, respectively. Similar promising results have been demonstrated in patients with BCR using 68Ga-labeled PSMA ligands (21–24). In a recent metaanalysis of 4,790 patients with BCR, Perera et al. (23) showed an overall PSMA PET sensitivity of 77% and specificity of 97% on a per-patient analysis; for PSA categories 0–0.19, 0.2–0.49, 0.5–0.99, 1–1.99, and ≥2 ng/mL, the percentages of positive scans were 33%, 45%, 59%, 75%, and 95%, respectively. A similar overall detection rate of 75% was reported by Fendler et al. (24) in a prospective multicenter trial including 635 patients with BCR, and detection rates significantly increased with PSA: 38%, 57%, 84%, 86%, and 97% for PSA levels of <0.5, 0.5 to <1.0, 1.0 to <2.0, 2.0 to <5.0, and ≥5.0 ng/mL, respectively (24). In another prospective trial, lesion detection rates were reported to be significantly higher with PSMA PET/CT than with 18F-fluciclovine (56% vs. 26%) in patients with BCR at a PSA of less than 2 ng/mL (25).
18F-DCFPyL PET/CT exhibits similarly high lesion detection at very low PSA levels (0.2–0.5 ng/mL) compared with literature reports for 68Ga-PSMA-11 (∼48% vs. 46%–58%) (21,26). One of the few direct comparisons between 18F-DCFPyL and 68Ga-PSMA-11 PET was reported by Dietlein et al. (27), demonstrating comparable biodistributions for both tracers but slightly higher sensitivity for 18F-DCFPyL than for 68Ga-PSMA-11 (88% vs. 66%) when PSA ranged from 0.5 to 3.5 ng/mL. Another 18F version of a PSMA ligand, 18F-PSMA-1007, was evaluated by Giesel et al., who reported higher detection rates for patients with lower PSA values: 61.5%, 74.5%, 90.9%, and 94.0% for PSA levels of >0.2 to <0.5, 0.5 to <1.0, 1 to <2.0, and ≥2.0 ng/mL, respectively (22). These differences may be due to the fact that 18F-PSMA-1007 has minimal urinary excretion, whereas 18F-DCFPyL is renally excreted. With this agent, the interpretation of pelvic lesions near the urinary bladder may be somewhat easier with less confusion about activity in the ureters.
Because there was limited patient follow-up in this study, hard endpoints (appearance of metastases, death) are not available. However, using knowledge of other established prognostic biomarkers such as PSA, PSAdt, and PSAvel, one can reasonably infer the prognostic implications of PSMA. 18F-DCFPyL PET/CT results strongly correlated with PSA, PSAdt, and PSAvel in prostatectomy patients, but the same association was not seen in patients undergoing RT. This finding is likely due to the more complex kinetics of PSA in early recurrence after RT. In this context, despite few negative results (21), PSA kinetics was often found to be associated with a PSMA PET–positive detection rate (26,28). For instance, Eiber et al. reported increased detection rates with higher PSAvel, but no significant association was seen for PSAdt in a population of 248 patients with BCR after prostatectomy (26). In a recent smallest cohort of 51 patients, a higher PSAvel was also found to be associated with a higher detection rate in PSMA PET scans (28). In a large systematic metanalysis, Perera et al. reported that positive PSMA PET/CT scans increased with PSA and shorter PSAdt values (29).
An important result of our series is that PSA, PSAdt, and PSAvel correlate with the extent of disease (i.e., intrapelvic/extrapelvic) found on 18F-DCFPyL PET. Unifocal or multifocal 18F-DCFPyL–positive lesions within the pelvis were present in 48% of patients, whereas extrapelvic disease was found in 30% of subjects, almost exclusively when PSA was greater than 1 ng/mL. This is consonant with the clinical experience that control rates start to decline in patients with a PSA level of <1 ng/mL (30). This observation also agrees with previous published studies (31,32) evaluating the impact of PSMA PET in assessing patient management at both low and high PSA values: patients with low PSA values with PET-positive intrapelvic disease might benefit from influencing the radiation field by delineating dominant nodules to boost with salvage RT, whereas in patients with high PSA values, the presence of extrapelvic disease on PSMA PET could trigger systemic therapy. In our cohort, at PSA levels of more than 2 ng/mL PSMA PET was almost always positive for recurrence, and about 50% of the patients had distant metastasis, which could be an independent sign of poor outcome. Interestingly, primary tumor histopathologic grading (Gleason score) had little bearing on the rate of scan positivity or the intensity of uptake on 18F-DCFPyL PET, as is in accordance with previously published results (21,22).
Histologic confirmation was available for about 40% of patients whose scans were positive—a relatively high rate of confirmatory biopsies compared with other studies. However, there were a considerable number of positive sites without histologic validation. Unfortunately, this is a common problem in studies involving BCR prostate cancer patients because positive findings are often subcentimeter lesions deep within the pelvis, often in unsafe locations or otherwise not feasible or ethical to biopsy for a research purpose alone. Nonetheless, in our cohort, despite a few negative results mostly for technical reasons, the biopsies were uniformly positive for cancer.
One persistent finding across multiple studies of PSMA PET/CT is that even at very high PSA values, not all scans are positive. This finding suggests that a small but reliable fraction of patients with locally advanced disease do not express PSMA. It is possible that such tumors represent highly undifferentiated cancers or that they express other surface markers that may be amenable to imaging with another targeted agent.
Finally, we cannot evaluate the impact of 18F-DCFPyL PET/CT on treatment decisions because the scans were performed for research purposes and because patients received therapy based on PSA findings and not scan results. Nonetheless, it is foreseeable that PSMA PET could ultimately determine whether to use locoregional salvage or systemic palliative therapy, depending on whether the recurrence is in the pelvis or distant. This ability would be especially important at the early stages of BCR, when potential salvage RT options could still be curative, and likely would be most effective at PSA levels of <0.5–1.0 ng/mL (33).
CONCLUSION
18F-DCFPyL PET/CT successfully identifies PSMA-positive lesions compatible with prostate cancer in patients with BCR after primary local therapy when conventional-imaging results are negative. 18F-DCFPyL PET/CT has a high positivity rate even at low PSA values (<0.5 ng/mL). In postsurgical patients, 18F-DCFPyL PET/CT results correlate with established predictors of poor outcome, such as PSAdt. However, in postradiation patients the relationship is less clear. As with other studies, PSMA PET is not positive in all patients, and approximately 10% will have negative scans even at very high PSA values. 18F-DCFPyL PET/CT detection efficacy is comparable to previously published results with 68Ga-labeled-PSMA compounds, but 18F-DCFPyL has several logistic advantages because of its longer half-life.
DISCLOSURE
This project was funded in whole or in part by federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the lesion detection rate of 18F-DCFPyL, a PSMA-targeted PET agent, in biochemically relapsed prostate cancer patients after primary local therapy?
PERTINENT FINDINGS: Seventy of 90 patients (77.8%) who had been prospectively enrolled in the study showed a positive PSMA PET scan, with lesion detection rates of 47.6% (10/21), 50% (5/10), 88.9% (8/9), and 94% (47/50) for PSA levels of >0.2 to <0.5, 0.5 to <1.0, 1 to <2.0, and ≥2.0 ng/mL, respectively. Tumor recurrence was histologically confirmed in 40% (28/70) of the patients with positive PET findings.
IMPLICATIONS FOR PATIENT CARE: 18F-DCFPyL PET/CT successfully identifies tumor in patients with biochemically recurrent prostate cancer when conventional-imaging results are negative and, more importantly, at low PSA values (<0.5 ng/mL), which may substantially impact clinical management.
REFERENCES
- 1.Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–583. [DOI] [PubMed] [Google Scholar]
- 2.Pfister D, Bolla M, Briganti A, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol. 2014;65:1034–1043. [DOI] [PubMed] [Google Scholar]
- 3.Briganti A, Karnes RJ, Gandaglia G, et al. Natural history of surgically treated high-risk prostate cancer. Urol Oncol. 2015;33:163 e167-113. [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. [DOI] [PubMed] [Google Scholar]
- 5.Beer AJ, Eiber M, Souvatzoglou M, Schwaiger M, Krause BJ. Radionuclide and hybrid imaging of recurrent prostate cancer. Lancet Oncol. 2011;12:181–191. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008;179:906–910. [DOI] [PubMed] [Google Scholar]
- 7.Rouvière O, Vitry T, Lyonnet D. Imaging of prostate cancer local recurrences: why and how? Eur Radiol. 2010;20:1254–1266. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. [DOI] [PubMed] [Google Scholar]
- 9.Mannweiler S, Amersdorfer P, Trajanoski S, et al. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15:167–172. [DOI] [PubMed] [Google Scholar]
- 10.Ross JS, Sheehan CE, Fisher HA, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–6362. [PubMed] [Google Scholar]
- 11.Rowe SP, Gage KL, Faraj SF, et al. 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J Nucl Med. 2015;56:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe SP, Macura KJ, Ciarallo A, et al. Comparison of prostate-specific membrane antigen-based 18F-DCFBC PET/CT to conventional imaging modalities for detection of hormone-naive and castration-resistant metastatic prostate cancer. J Nucl Med. 2016;57:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of 18F-DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe SP, Macura KJ, Mena E, et al. PSMA-based 18F-DCFPyL-PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol. 2016;18:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorin MA, Rowe SP, Patel HD, et al. Prostate-specific membrane antigen targeted 18F-DCFPyL-positron emission tomography/computerized tomography for the preoperative staging of high risk prostate cancer: results of a prospective, phase II, single center study. J Urol. 2018;199:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roach M. III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. [DOI] [PubMed] [Google Scholar]
- 17.Surti S, Kuhn A, Werner ME, et al. Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med. 2007;48:471–480. [PubMed] [Google Scholar]
- 18.Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–217. [DOI] [PubMed] [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 20.Mena E, Lindenberg ML, Shih JH, et al. Clinical impact of PSMA-based 18F-DCFBC-PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur J Nucl Med Mol Imaging. 2018;45:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giesel FL, Knorr K, Spohn F, et al. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med. 2019;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions—a systematic review and meta-analysis. Eur Urol. February 14, 2019 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calais J, Ceci F, Eiber M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 27.Dietlein M, Kobe C, Kuhnert G, et al. Comparison of 18F-DCFPyL and 68Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aydin AM, Haberal B, Artykov M, Bilen CY, Yazici S. Clinicopathological predictors of positive 68Ga-PSMA-11-PET/CT in PSA-only recurrence of localized prostate cancer following definitive therapy. Ann Nucl Med. 2019;33:326–332. [DOI] [PubMed] [Google Scholar]
- 29.Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. [DOI] [PubMed] [Google Scholar]
- 30.Valicenti RK, Thompson I, Jr, Albertsen P, et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys. 2013;86:822–828. [DOI] [PubMed] [Google Scholar]
- 31.Calais J, Czernin J, Cao M, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rousseau E, Wilson D, Lacroix-Poisson F, et al. A prospective study on 18F-DCFPyL PSMA PET/CT imaging in biochemical recurrence of prostate cancer. J Nucl Med. 2019;60:1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King CR. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys. 2012;84:104–111. [DOI] [PubMed] [Google Scholar]