Abstract
Intravenous access is difficult in some patients referred for 18F-FDG PET imaging. Extravasation at the injection site and accumulation in central catheters can lead to limited tumor 18F-FDG uptake, erroneous quantitation, and significant image artifacts. In this study, we compared the human biodistribution and dosimetry for 18F-FDG after oral and intravenous administrations sequentially in the same subjects to ascertain the dosimetry and potential suitability of orally administered 18F-FDG as an alternative to intravenous administration. We also compared our detailed intravenous 18F-FDG dosimetry with older dosimetry data. Methods: Nine healthy volunteers (6 male and 3 female; aged 19–32 y) underwent PET/CT imaging after oral and intravenous administration of 18F-FDG. Identical preparation and imaging protocols (except administration route) were used for oral and intravenous studies. During each imaging session, 9 whole-body PET scans were obtained at 5, 10, 20, 30, 40, 50, 60, 120, and 240 min after 18F-FDG administration (370 ± 16 MBq). Source organ contours drawn using CT were overlaid onto registered PET images to extract time–activity curves. Time-integrated activity coefficients derived from time–activity curves were given as input to OLINDA/EXM for dose calculations. Results: Blood uptake after orally administered 18F-FDG peaked at 45–50 min after ingestion. The oral-to-intravenous ratios of 18F-FDG uptake for major organs at 45 min were 1.07 ± 0.24 for blood, 0.94 ± 0.39 for heart wall, 0.47 ± 0.12 for brain, 1.25 ± 0.18 for liver, and 0.84 ± 0.24 for kidneys. The highest organ-absorbed doses (μGy/MBq) after oral 18F-FDG administration were observed for urinary bladder (75.9 ± 17.2), stomach (48.4 ± 14.3), and brain (29.4 ± 5.1), and the effective dose was significantly higher (20%) than after intravenous administration (P = 0.002). Conclusion: 18F-FDG has excellent bioavailability after oral administration, but peak organ activities occur later than after intravenous injection. These data suggest PET at 2 h after oral 18F-FDG administration should yield images that are comparable in biodistribution to conventional clinical images acquired 1 h after injection. Oral 18F-FDG is a palatable alternative to intravenous 18F-FDG when venous access is problematic.
Keywords: FDG, dosimetry, oral 18F-FDG
The radiotracer 18F-FDG is widely used to trace glucose metabolism. Although originally developed for brain imaging, 18F-FDG is most commonly used to image cancers, which generally have higher rates of glucose metabolism than most normal tissues (1). 18F-FDG is also used to image infections, inflammation, and myocardium viability (2–4). In virtually all these applications, 18F-FDG is given intravenously.
18F-FDG uptake typically rises over time in untreated tumors, whereas most normal tissues have gradually declining tracer uptake over time (5). For tumors, 18F-FDG PET imaging is commonly performed at 1 h after intravenous injection. The 1-h delayed static imaging with quantitation using SUV has played a significant role in the dissemination of PET technology. However, accurate quantitation assumes that the entire injected dose has reached the bloodstream and can be distributed throughout the body.
Although intravenous access is clearly simple and useful for routine 18F-FDG administration, many patients present with veins too poor or fragile for an intravenous line. This is a common occurrence in cancer patients undergoing extensive chemotherapy, because of venous inflammation or thrombosis, but poor venous access can occur in any patient (6–8). Although central venous catheters can be used, they are associated with thrombotic and infectious complications, and radiotracer will often adhere to their walls or tip, confounding interpretation and quantitation (9,10). In addition, many pediatric patients have a fear of injection. The pain and anxiety associated with injections can potentially result in brain activation, which may alter tracer distribution.
Extravasation of 18F-FDG at the injection site can also lead to poor uptake and major artifacts. Multiple attempts to gain intravenous access may not be successful, resulting in an inability to scan some patients. In such scenarios, there is a need for an alternative 18F-FDG administration route. Oral administration of 18F-FDG is an attractive alternative, provided it does not result in significant loss of information from scans or unfavorable dosimetry.
Martinez et al. first used the oral route for 18F-FDG administration in primates and humans (11). The investigators observed that the blood curve for oral administration had a longer uptake time, with a peak occurring at about 60 min and continuing for 120 min, compared with intravenous injection. They did not find much difference between the oral and intravenous routes in human brain images. They suggested performing radiation dosimetry studies, especially for gut and liver, before use in humans. Masud et al. compared brain images obtained after intravenous and oral 18F-FDG administration in healthy humans (12). The blood activity curve build-up phase after oral administration was slow and continued until around 110–120 min. They did not find a significant difference between the intravenous and oral methods in the brain images, except for later accumulation of 18F-FDG. Higashi et al. studied oral administration of 18F-FDG in normal rodents (13). They concluded that the fasting condition and 18F-FDG diluents and osmolality play a major role in 18F-FDG absorption from the gut. In a rodent model, 48 h of fasting and use of a hypotonic solution as a diluent for 18F-FDG yielded better absorption of 18F-FDG from the gut.
Franc et al. reported a case of a lung cancer patient who had to receive 18F-FDG orally because of nonpalpable veins (14). They observed high uptake in the mouth, esophagus, stomach, and bowel. Nair et al. compared oral and intravenous 18F-FDG administration methods in 2 healthy humans and 7 cancer patients (15). They claimed that all lesions were seen on both oral and intravenous images. The SUV on images from orally administered patients were 30%–60% lower than the SUV measured on images from patients undergoing intravenous administration. It was also observed that a larger amount of activity was retained in the gut. It was presumed that activity from the gut was eventually absorbed, but the uptake in normal organs was delayed after oral delivery compared with intravenous delivery.
The aim of the current study was to systematically compare the human biodistribution and dosimetry for 18F-FDG after oral and intravenous administrations in the same subjects to ascertain the potential applicability of orally administered 18F-FDG as an alternative to intravenous delivery.
MATERIALS AND METHODS
Subjects
This prospective study was approved by the Johns Hopkins Medicine Institutional Review Board (approval designation NA_00068464), and all subjects gave written informed consent. Healthy volunteers over 18 y of age were eligible to participate in this study. Volunteers were recruited using flyers placed at various locations on the Johns Hopkins medical campus, and modest financial compensation was provided for participation. Pregnant women and anyone taking a medication known to influence glucose metabolism (e.g., insulin or metformin) were excluded.
18F-FDG Preparation
18F-FDG was obtained from PETNET Solutions. Oral 18F-FDG was prepared by dissolving the targeted 370-MBq dose in approximately 500 mL of sugar-free fruit punch. This solution was given to participants in a sealed container with a straw so as to avoid spills. Participants were instructed to drink the entire volume within 5 min, followed by an additional 500 mL of water. Intravenous 18F-FDG was given in a 370-MBq dose per the standard clinical protocol.
Study Protocol
Eligible participants were asked to undergo 2 imaging sessions separated by at least 24 h and by no more than 14 d. Oral administration of 18F-FDG was always performed during the first visit, and intravenous administration during the second. For both visits, participants were instructed to fast for at least 6 h before the planned time of 18F-FDG administration. After reporting to the imaging center, participants underwent a brief, routine history and physical and were then asked to change into a hospital gown. During both imaging sessions, the same dose of 18F-FDG (targeted 370 MBq) was used and the same imaging procedure was performed, with the only difference being the route of 18F-FDG administration.
Imaging Parameters
PET/CT images were acquired using a Discovery RX VCT (GE Healthcare) PET/CT scanner. Whole-body PET/CT images were acquired 5, 10, 20, 30, 40, 50, 60, 120, and 240 min after both oral and intravenous administration of 18F-FDG. During each imaging session, the first 6 PET scans were acquired for 45 s per bed position and the last 3 scans were acquired for 255 s per bed position. Low-dose CT scans (120 kVp, 45 mA, 0.984 pitch, and 0.5-s tube rotation) were acquired before the start of the 5-, 60-, 120-, and 240-min PET scans, for a total of 4 CTs per imaging session. Images were obtained from the vertex through the mid thighs. Attenuation and scatter-corrected PET images were reconstructed using 3-dimensional ordered-subsets expectation maximization with 2 iterations, 21 subsets, and a 3-mm gaussian filter. The scanner was calibrated with respect to the same dose calibrator used for the 18F-FDG subject measurements, which was itself calibrated using a 68Ge reference source traceable to a national metrology institute. Routine phantom quality-control studies confirmed the quantitative accuracy of the PET images, at least for objects greater than around 22 mm in size.
Dosimetry
The PET/CT images from both imaging sessions were used to extract biodistribution data for oral and intravenous methods of 18F-FDG delivery, respectively. Low-dose CT was used to guide the manual delineation of each organ of interest using MIMvista (version 5.1; MIMvista Corp.). Volumes of interest were applied to the corresponding whole-body PET series to extract the mean source activity concentrations. Gastrointestinal organs were delineated into stomach contents, small intestine contents, upper large intestine contents, and lower large intestine contents. Whenever a source organ could not be drawn completely, the average activity concentration was multiplied by a standard phantom–based organ volume–density product (16). Activity concentration, normalized to administered activity, was plotted against time for each organ. Curve fitting was done using SAAM-II (version 1.2.1). Time-integrated activity coefficients were calculated per MIRD Committee formalism (17). The OLINDA/EXM 1.0 dosimetric software was used to obtain absorbed dose estimates and effective doses for each subject. The dynamic bladder model in the OLINDA/EXM was used to obtain a urinary bladder time-integrated activity coefficient with a voiding interval of 1.5 h and a biologic half-time obtained from the whole-body time–activity curve for each subject.
Statistical Methods
Differences in estimated absorbed dose between orally and intravenously administered 18F-FDG were assessed using paired t tests. All data analyses were performed using Excel (Microsoft Corp.), and in all cases, a P value of less than 0.05 was considered statistically significant.
RESULTS
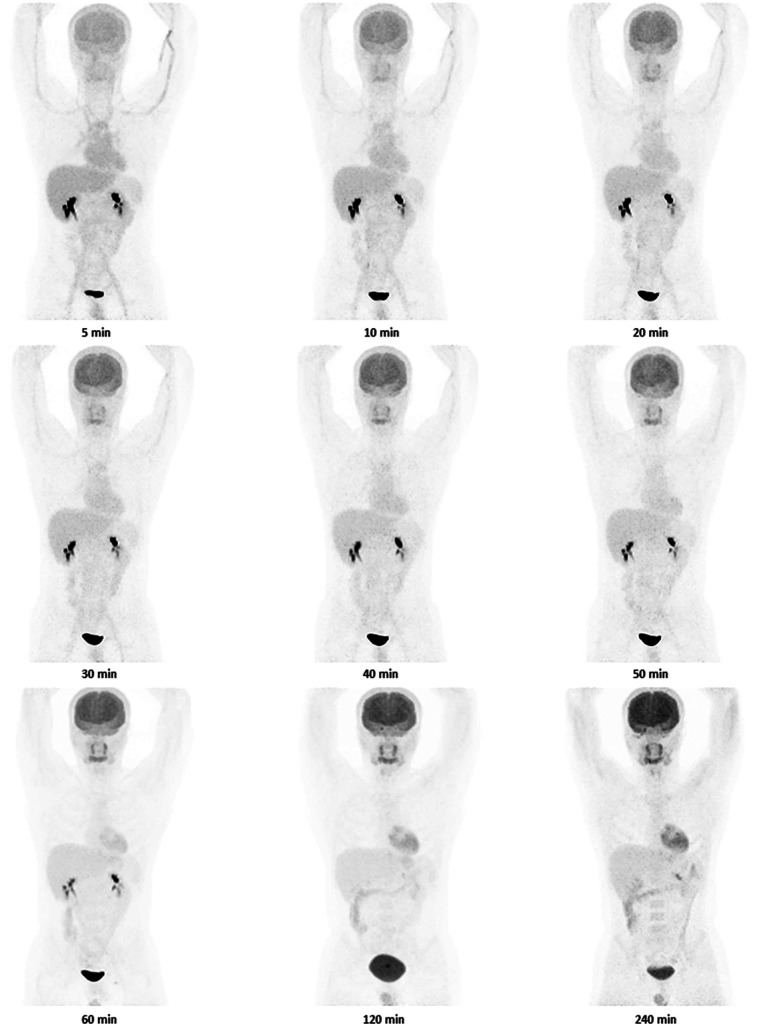
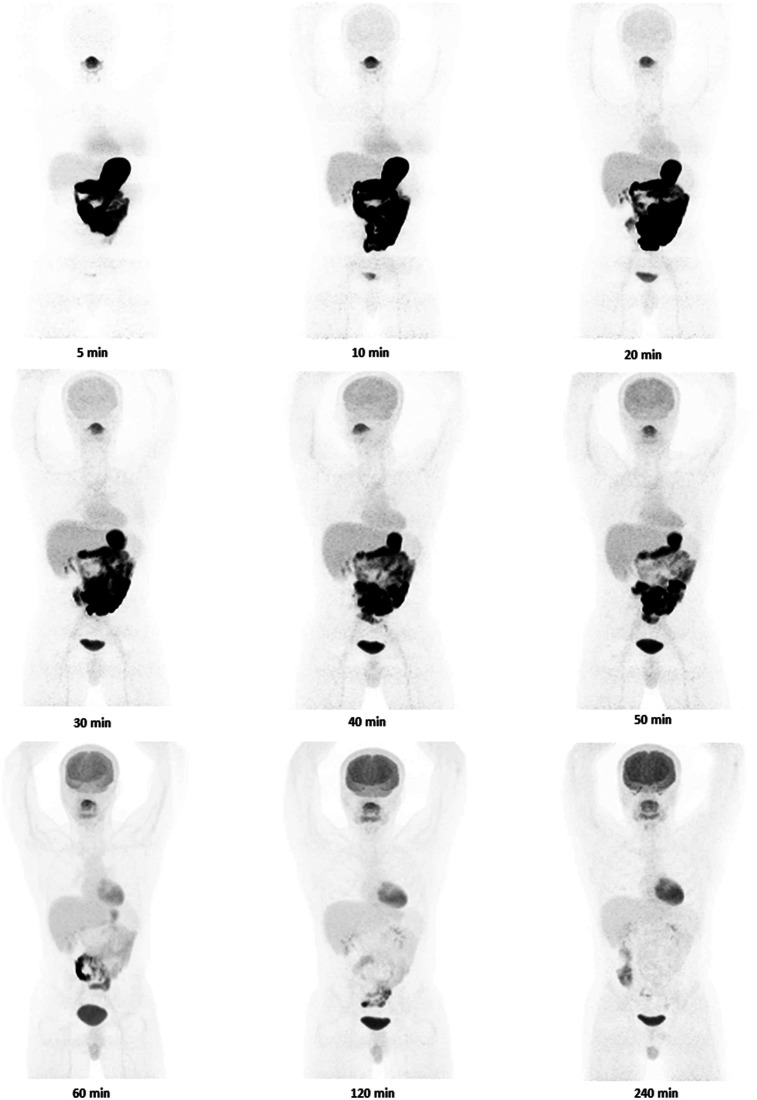
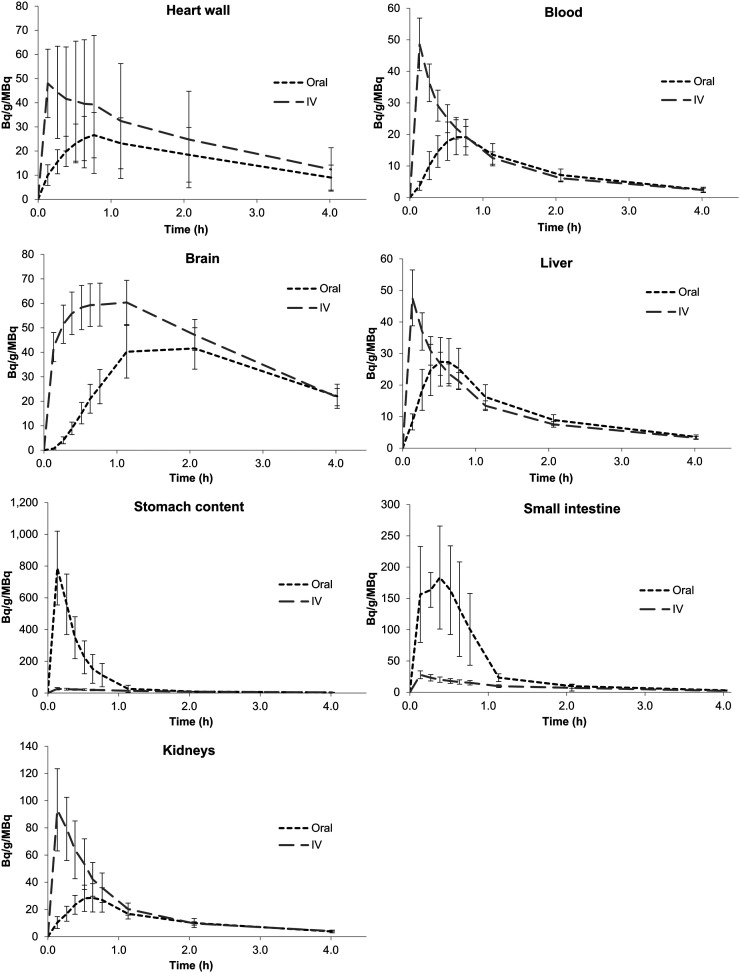
Nine healthy participants were included in this study (Table 1). Eight participants completed both oral and intravenous 18F-FDG imaging studies, and 1 participant completed oral 18F-FDG imaging but not intravenous 18F-FDG imaging. Figures 1 and 2 show images of a single volunteer after both routes of 18F-FDG administration at selected time points. A clear difference exists in abdominal imaging (with more tracer in the bowel after oral administration and less in the brain) at early time points. Figure 3 shows 18F-FDG biodistribution in 7 selected tissues, in becquerels per megabecquerel of administered activity per gram of tissue. In most organs (excluding the brain and bladder), the activity per gram of tissue at approximately 70 min was about the same for oral administration as for intravenous administration. Blood uptake after orally administered 18F-FDG peaked at 45–50 min.
TABLE 1.
Research Participant Characteristics
| Subject no. | Sex | Age (y) | Mass (kg) | Height (m) | BMI (kg/m2) | Orally administered activity (MBq) | Intravenously administered activity (MBq) |
| 1 | M | 32.0 | 91.0 | 1.78 | 28.7 | 395.9 | 373.7 |
| 2 | M | 29.0 | 66.0 | 1.80 | 20.4 | 370.0 | 373.7 |
| 3 | F | 19.0 | 54.0 | 1.65 | 19.8 | 388.5 | 373.7 |
| 4 | M | 23.0 | 77.0 | 1.78 | 24.3 | 355.2 | 370.0 |
| 5 | M | 22.0 | 84.0 | 1.83 | 25.1 | 377.4 | NA |
| 6 | M | 25.0 | 84.0 | 1.78 | 26.5 | 344.1 | 329.3 |
| 7 | M | 25.0 | 75.0 | 1.75 | 24.5 | 373.7 | 370.0 |
| 8 | F | 24.0 | 77.0 | 1.73 | 25.7 | 370.0 | 366.3 |
| 9 | F | 22.0 | 65.0 | 1.70 | 22.5 | 388.5 | 362.6 |
| Mean ± SD | 24.6 ± 3.9 | 74.8 ± 11.4 | 1.8 ± 0.1 | 24.2 ± 2.9 | 373.7 ± 16.5 | 364.9 ± 14.9 |
NA = not applicable.
FIGURE 1.
Images of 1 participant (subject 4 in Table 1) from specified time points after intravenous administration of 18F-FDG. Same color scale was used for all images.
FIGURE 2.
Images of 1 participant (subject 4 in Table 1) from specified time points after oral administration of 18F-FDG. Same color scale was used for all images.
FIGURE 3.
18F-FDG time–activity curves for selected source organs plotted as activity concentration normalized to administered activity.
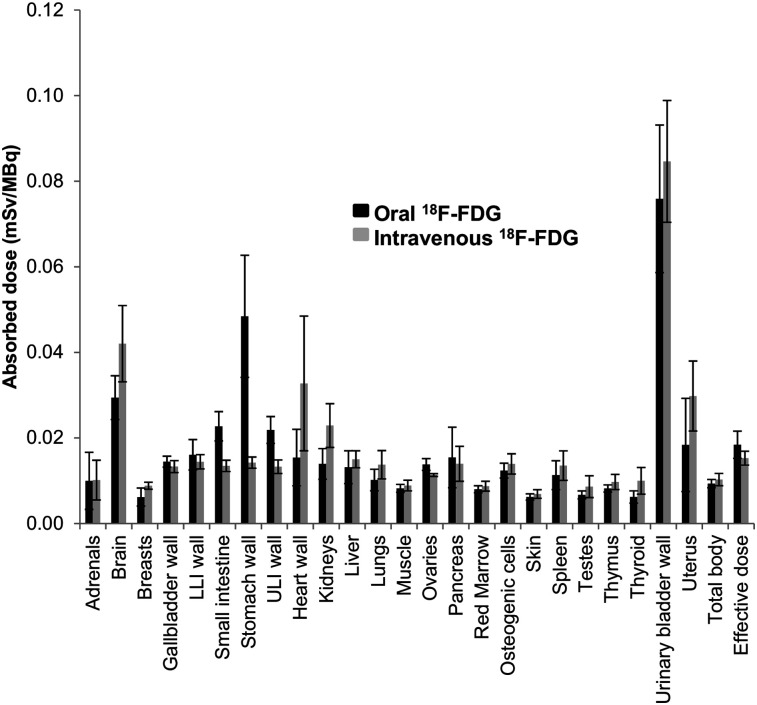
The mean oral-to-intravenous ratios of 18F-FDG uptake for major organs at 45 min were 1.07 ± 0.24 for blood, 0.94 ± 0.39 for heart wall, 0.47 ± 0.12 for brain, 1.25 ± 0.18 for liver, and 0.84 ± 0.24 for kidneys. Absorbed dose estimates for both routes of administration are shown in Table 2. Of the major organs, the highest absorbed dose after oral administration was in the urinary bladder wall, followed by stomach wall and then brain. The highest absorbed dose after intravenous administration was in the urinary bladder wall, followed by the brain and then the heart wall. The total effective dose was 20% higher for oral than for intravenous administration (0.018 ± 0.003 mSv/MBq vs. 0.015 ± 0.002 mSv/MBq, respectively; P = 0.002). High gastric and small-bowel uptake was visually identified through 1.5 h into the study.
TABLE 2.
Absorbed Dose Estimates for Orally and Intravenously Administered 18F-FDG
| Estimated absorbed dose (mSv/MBq) |
|||
| Target organ | Oral 18F-FDG | Intravenous 18F-FDG | P |
| Adrenals | 9.98E−03 ± 6.67E−03 | 1.02E−02 ± 4.65E−03 | 0.920 |
| Brain | 2.94E−02 ± 5.12E−03 | 4.20E−02 ± 8.91E−03 | 0.002 |
| Breasts | 6.19E−03 ± 2.11E−03 | 8.84E−03 ± 8.24E−04 | 0.089 |
| Gallbladder wall | 1.45E−02 ± 1.28E−03 | 1.33E−02 ± 1.40E−03 | 0.009 |
| Lower large intestine wall | 1.61E−02 ± 3.56E−03 | 1.44E−02 ± 1.66E−03 | 0.161 |
| Small intestine | 2.27E−02 ± 3.42E−03 | 1.35E−02 ± 1.32E−03 | <0.001 |
| Stomach wall | 4.84E−02 ± 1.43E−02 | 1.42E−02 ± 1.32E−03 | <0.001 |
| Upper large intestine wall | 2.19E−02 ± 3.14E−03 | 1.33E−02 ± 1.58E−03 | <0.001 |
| Heart wall | 1.54E−02 ± 6.60E−03 | 3.28E−02 ± 1.57E−02 | 0.056 |
| Kidneys | 1.39E−02 ± 3.58E−03 | 2.29E−02 ± 5.11E−03 | 0.002 |
| Liver | 1.32E−02 ± 3.83E−03 | 1.50E−02 ± 1.98E−03 | 0.404 |
| Lungs | 1.02E−02 ± 2.51E−03 | 1.38E−02 ± 3.31E−03 | <0.001 |
| Muscle | 8.23E−03 ± 9.23E−04 | 8.89E−03 ± 1.24E−03 | 0.033 |
| Ovaries | 1.38E−02 ± 1.37E−03 | 1.13E−02 ± 3.21E−04 | 0.128 |
| Pancreas | 1.54E−02 ± 7.08E−03 | 1.40E−02 ± 4.08E−03 | 0.310 |
| Red marrow | 8.01E−03 ± 8.24E−04 | 8.74E−03 ± 1.15E−03 | 0.014 |
| Osteogenic cells | 1.24E−02 ± 1.73E−03 | 1.39E−02 ± 2.37E−03 | 0.011 |
| Skin | 6.21E−03 ± 7.39E−04 | 6.90E−03 ± 1.01E−03 | 0.014 |
| Spleen | 1.13E−02 ± 3.37E−03 | 1.35E−02 ± 3.45E−03 | 0.028 |
| Testes | 6.70E−03 ± 9.25E−04 | 8.63E−03 ± 2.55E−03 | 0.192 |
| Thymus | 8.20E−03 ± 8.39E−04 | 9.72E−03 ± 1.76E−03 | 0.016 |
| Thyroid | 6.21E−03 ± 1.44E−03 | 9.99E−03 ± 3.12E−03 | 0.026 |
| Urinary bladder wall | 7.59E−02 ± 1.72E−02 | 8.46E−02 ± 1.42E−02 | 0.170 |
| Uterus | 1.84E−02 ± 1.09E−02 | 2.98E−02 ± 8.18E−03 | 0.077 |
| Total body | 9.33E−03 ± 9.67E−04 | 1.03E−02 ± 1.44E−03 | 0.010 |
| Effective dose | 1.84E−02 ± 3.18E−03 | 1.53E−02 ± 1.63E−03 | 0.002 |
Values are provided as mean ± SD.
Figure 4 compares the mean estimated absorbed dose to each organ for all participants after both methods of 18F-FDG administration. For most organs, the mean estimated absorbed dose was similar for intravenous versus oral administration, with the exception of the stomach wall, small intestine, heart wall, kidneys, and brain. After oral administration, mean activity in the stomach wall and small intestine was 3.4 and 1.7 times higher, respectively, than after intravenous administration. After intravenous administration, mean activity in the heart wall, kidneys, and brain was 2.1, 1.6, and 1.4 times higher, respectively, than after oral administration.
FIGURE 4.
Estimated radiation dose to each organ for both orally and intravenously administered 18F-FDG. LLI = lower large intestine; ULI = upper large intestine.
DISCUSSION
18F-FDG is a critically important tracer for PET imaging, with a wide and growing range of indications. Although intravenous delivery of 18F-FDG is normally very effective, difficult venous access, especially in cancer patients, is common. Indeed, standards have been developed that limit the number of attempted intravenous insertions by a single nurse to 2 in chemotherapy patients and to a total of 4 attempts using different individuals (7). Using an infusion catheter can be helpful, but catheters carry the risk of complications, including superior vena cava obstruction, infection, and occlusion, among others (9). Thus, intravenous access can sometimes be problematic and the availability of an additional tracer delivery route, for example, orally, can be logistically attractive when the time for the patient to complete the study is critical.
Given the importance of quantitative imaging, the ability to measure relative tracer uptake is highly dependent on knowing the amount of activity that was successfully administered. Patients with extravasated injections can have obvious alterations in SUV, due to either less tracer reaching the bloodstream or slowed absorption of tracer to the bloodstream. Both may affect quantitation. Oral 18F-FDG has the potential to allow for delivery and quantitation when it might otherwise be impossible to scan the patient with 18F-FDG. However, the repeatability of oral 18F-FDG uptake in humans has not been studied.
Although catheter infusion systems are attractive, 18F-FDG can stick to catheters or to the tip of a catheter or port, confounding imaging and quantitation. Indeed, 18F-FDG uptake in clots at the ends of catheters can cause confusion in some cases. Misdiagnosis of active lymphoma has occurred when tracer has actually been accumulated in the tip of a catheter or clot (18). In other situations, 18F-FDG uptake in a catheter tip has been considered a normal variant. Oral 18F-FDG potentially could provide advantages in such situations by limiting infusion-related 18F-FDG uptake. Oral administration potentially can avoid such confounding uptake and may be particularly relevant for attempts to assess infections in infusion catheters and ports, separating infused from accumulated activity, with the latter being much more relevant.
In tumor imaging, clinical studies suggest that many tumors, at least outside the immediate proximity of the bowel, can be imaged using 18F-FDG PET. Clearly, an orally administered 18F-FDG dose followed by PET/CT imaging has a higher probability of imaging tumor foci than does a scan that was cancelled because of lack of venous access. Indeed, oral 18F-FDG might be considered as “any port in a storm,” even if there is not a port.
A review of the literature (Table 3) shows that our intravenous dosimetry results are generally consistent with other 18F-FDG dosimetry reports but are perhaps more robust because they include a longer duration of imaging acquisition to determine biodistribution over time. Thus, they are probably somewhat more reliable than measurements using a more limited number of imaging data points. Interestingly, our data show somewhat lower dosimetry than, for example, the Food and Drug Administration–approved package insert.
TABLE 3.
Comparison of Dose Estimates Calculated in Current Study with Dose Estimates Reported for Other Published Intravenous 18F−FDG Dosimetry
| Site | Current study | FDA package insert (1) | Jones 1982 (21) | Mejia 1991 (22) | Deloar 1998 (23) | Hays 2002 (24) | Khamwan 2010 (25) | Velasques2010 (26) | Mattsson 2015 (27) |
| n | 9 | Unknown | 11 | 8 | 6 | Unknown | 35 | 97 | Unknown |
| Adrenals | 1.02E−02 ± 4.65E−03 | 1.3E−02* | 1.8E−02* | 1.6E−02* | 1.2E−02* | ||||
| Brain | 4.20E−02 ± 8.91E−03 | 1.9E−02 | 2.2E−02 | 2.9E−02 | 3.7E−02 | 3.5E−02 | 4.6E−02* | 3.8E−02 | |
| Breasts | 8.84E−03 ± 8.24E−04 | 9.2E−03* | 1.0E−02* | 1.0E−02* | 7.3E−03 | 7.7E−03 | 8.8E−03 | ||
| Gallbladder wall | 1.33E−02 ± 1.40E−03 | 1.3E−02 | 1.3E−02 | ||||||
| LLI wall | 1.44E−02 ± 1.66E−03 | 1.4E−02 | 1.8E−02* | 1.5E−02* | 1.4E−02 | ||||
| Small intestine | 1.35E−02 ± 1.32E−03 | 1.3E−02 | 1.7E−02* | 1.5E−02* | 1.2E−02 | ||||
| Stomach wall | 1.42E−02 ± 1.32E−03 | 1.3E−02 | 1.5E−02* | 1.2E−02 | 1.2E−02 | 1.1E−02 | |||
| ULI wall | 1.33E−02 ± 1.58E−03 | 1.2E−02 | 1.7E−02* | 1.5E−02* | 1.2E−02 | ||||
| Heart wall | 3.28E−02 ± 1.57E−02 | 5.9E−02* | 4.3E−02* | 4.5E−02* | 1.7E−02 | 3.5E−02* | 6.9E−02* | 6.7E−02* | |
| Kidneys | 2.29E−02 ± 5.11E−03 | 2.0E−02 | 2.3E−02* | 3.0E−02* | 2.8E−02* | 5.9E−03 | 2.7E−02* | 7.4E−02* | 1.7E−02 |
| Liver | 1.50E−02 ± 1.98E−03 | 1.6E−02* | 2.0E−02* | 2.3E−02* | 1.8E−02* | 8.4E−03 | 3.0E−02* | 2.4E−02* | 2.1E−02* |
| Lungs | 1.38E−02 ± 3.31E−03 | 1.7E−02* | 2.1E−02* | 1.1E−02 | 1.8E−02* | 8.4E−03 | 4.6E−03 | 1.5E−02* | 2.0E−02* |
| Muscle | 8.89E−03 ± 1.24E−03 | 1.1E−02* | 1.0E−02* | ||||||
| Ovaries | 1.13E−02 ± 3.21E−04 | 1.4E−02* | 1.4E−02* | 1.6E−02* | 1.5E−03* | 1.1E−02 | 1.4E−02* | ||
| Pancreas | 1.40E−02 ± 4.08E−03 | 2.6E−02* | 2.0E−02* | 2.6E−02* | 1.6E−03 | 1.3E−02 | |||
| Red marrow | 8.74E−03 ± 1.15E−03 | 1.3E−02* | 1.4E−02* | 1.2E−02* | 5.6E−03 | 1.7E−03 | 1.8E−02* | 1.1E−02* | 1.1E−02* |
| Osteogenic cells | 1.39E−02 ± 2.37E−03 | 4.1E−02* | 1.5E−02* | 8.0E−03 | 2.1E−02* | 1.1E−02 | 1.1E−02 | ||
| Skin | 6.90E−03 ± 1.01E−03 | 8.1E−03* | 1.1E−03 | 7.8E−03* | |||||
| Spleen | 1.35E−02 ± 3.45E−03 | 3.8E−02* | 4.3E−02* | 2.2E−02* | 1.4E−02* | 2.1E−03 | 1.5E−02* | 1.1E−02 | |
| Testes | 8.63E−03 ± 2.55E−03 | 1.1E−02* | 1.8E−02* | 1.5E−02* | 1.5E−02* | 1.5E−03 | 1.1E−02* | 1.1E−02* | 1.1E−02* |
| Thymus | 9.72E−03 ± 1.76E−03 | 1.2E−02* | 1.2E−02* | 1.2E−02* | |||||
| Thyroid | 9.99E−03 ± 3.12E−03 | 1.1E−02* | 1.3E−02* | 1.3E−02* | 1.0E−02* | 1.0E−02* | 1.0E−02* | ||
| Bladder wall | 8.46E−02 ± 1.42E−02 | 8.6E−02* | 1.2E−01* | 1.2E−01* | 3.1E−01* | 4.3E−02 | 6.4E−02 | 1.3E−01 | |
| Uterus | 2.98E−02 ± 8.18E−03 | 1.7E−02 | 1.9E−02 | 1.9E−02 | 1.8E−02 | ||||
| Total body | 1.03E−02 ± 1.44E−03 | 1.1E−02* | 1.2E−02* | 1.4E−02* | |||||
| Effective dose (mSv/MBq) | 1.53E−02 ± 1.63E−03 | 1.8E−02* | 2.4E−02* | 2.9E−02* | 1.5E−02 | 1.9E−02* |
Higher reported dose than in current study.
FDA = Food and Drug Administration; LLI = lower large intestine; ULI = upper large intestine.
All dose estimates are reported in mSv/MBq.
Our dosimetry data support a somewhat higher total-body residence time for 18F-FDG given orally than intravenously, likely because excretion is slower and later as there is activity remaining in the bowel for some time after injection that cannot be rapidly excreted. Other limited dosimetry exists for 18F-FDG given orally, but it is not strictly comparable. Shingaki et al. constructed 18F-FDG–laden capsules that were designed to dissolve in the gut (19). This variable clearance from the stomach and variable dissolution of the capsules make comparisons to our data difficult.
Oral 18F-FDG avoids the need to sedate or cause pain with an intravenous injection. Pain or stress may have effects on 18F-FDG biodistribution that could be confounding. Masud et al. showed quantitative differences in brain glucose metabolism between the oral and intravenous routes of 18F-FDG administration (12). They observed glucose metabolism to be significantly higher in the superior frontal gyrus, superior parietal lobule, lingual gyrus, and left cerebellar hemisphere in the intravenous group than in the oral group. Metabolically active areas were found in the superior, middle, and inferior temporal gyrus, parahippocampal gyrus, amygdaloid nucleus, pons, and cerebellum in the oral group, when compared with the intravenous group, perhaps because of brain stimulation caused by pain in the latter group.
Our study did not evaluate the diagnostic accuracy of 18F-FDG PET given orally. However, our dosimetry data support a higher total-body effective dose, by about 20%, for oral 18F-FDG. Our studies were conducted on healthy volunteers. Patients may indeed differ somewhat from the healthy volunteers. For example, profoundly delayed gastric emptying might be expected to delay the absorption of 18F-FDG given orally and require later imaging times. The delayed absorption of 18F-FDG from the bowel suggests that the optimal time for brain or tumor imaging after oral 18F-FDG is likely to be about 2 h after ingestion. Zhang et al. determined the optimal uptake time for imaging all organs but the brain to be 50–60 min, though this was based on a case report of 1 healthy volunteer (20). It is also probable that oral dosing would not be optimal for patients with tumors located in the upper abdomen or in the bowel wall. Such lesions might be more difficult to detect with oral than intravenous dosing. Additional systematic studies of oral 18F-FDG in patients with difficult venous access, or in need of evaluation of tissues or devices through which 18F-FDG is commonly injected, are warranted.
CONCLUSION
Oral 18F-FDG administration is feasible and results in excellent absorption and delivery of the radiotracer throughout the body. Peak uptake in normal tissues is somewhat delayed, and the overall absorbed radiation dose is about 20% higher after oral administration than for intravenous delivery. Oral 18F-FDG delivery should be considered if intravenous access is not feasible or desirable. Our study shows a 2-h uptake to be optimal for oral 18F-FDG and provide additional data on intravenous 18F-FDG dosimetry.
DISCLOSURE
This work was supported by grants from the National Cancer Institute (U01CA140204 and P30CA006973). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is orally administered 18F-FDG a suitable alternative to intravenously administered 18F-FDG?
PERTINENT FINDINGS: In a prospective study, 9 healthy participants underwent separate PET/CT imaging after oral and intravenous administrations of 18F-FDG. The total effective dose was significantly higher, by 20%, from orally administered 18F-FDG than from intravenously administered 18F-FDG.
IMPLICATIONS FOR PATIENT CARE: Oral administration of 18F-FDG is a reasonable option when venous access is difficult or impossible.
REFERENCES
- 1. Fludeoxyglucose F18 injection [package insert]. Washington, DC: Food and Drug Administration; 2000.
- 2.Czernin J, Phelps ME. Positron emission tomography scanning: current and future applications. Annu Rev Med. 2002;53:89–112. [DOI] [PubMed] [Google Scholar]
- 3.Basu S, Chryssikos T, Moghadam-Kia S, Zhuang H, Torigian DA, Alavi A. Positron emission tomography as a diagnostic tool in infection: present role and future possibilities. Semin Nucl Med. 2009;39:36–51. [DOI] [PubMed] [Google Scholar]
- 4.Ghesani M, Depuey EG, Rozanski A. Role of F-18 FDG positron emission tomography (PET) in the assessment of myocardial viability. Echocardiography. 2005;22:165–177. [DOI] [PubMed] [Google Scholar]
- 5.Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994;35:1308–1312. [PubMed] [Google Scholar]
- 6.Ingram P, Lavery I. Peripheral intravenous therapy: key risks and implications for practice. Nurs Stand. 2005;19:55–64. [DOI] [PubMed] [Google Scholar]
- 7.Pagnutti L, Bin A, Donato R, et al. Difficult intravenous access tool in patients receiving peripheral chemotherapy: a pilot-validation study. Eur J Oncol Nurs. 2016;20:58–63. [DOI] [PubMed] [Google Scholar]
- 8.Ehrhardt BS, Givens KEA, Lee RC. Making it stick: developing and testing the difficult intravenous access (DIVA) tool. Am J Nurs. 2018;118:56–62. [DOI] [PubMed] [Google Scholar]
- 9.Dychter SS, Gold DA, Carson D, Haller M. Intravenous therapy: a review of complications and economic considerations of peripheral access. J Infus Nurs. 2012;35:84–91. [DOI] [PubMed] [Google Scholar]
- 10.Bhargava P, Kumar R, Zhuang H, Charron M, Alavi A. Catheter-related focal FDG activity on whole body PET imaging. Clin Nucl Med. 2004;29:238–242. [DOI] [PubMed] [Google Scholar]
- 11.Martinez ZA, Colgan M, Baxter LR, Jr, et al. Oral 18F-fluoro-2-deoxyglucose for primate PET studies without behavioral restraint: demonstration of principle. Am J Primatol. 1997;42:215–224. [DOI] [PubMed] [Google Scholar]
- 12.Masud M, Yamaguchi K, Rikimaru H, et al. Evaluation of resting brain conditions measured by two different methods (i.v. and oral administration) with 18F-FDG-PET. Ann Nucl Med. 2001;15:69–73. [DOI] [PubMed] [Google Scholar]
- 13.Higashi T, Fisher SJ, Nakada K, Romain DJ, Wahl RL. Is enteral administration of fluorine-18-fluorodeoxyglucose (F-18 FDG) a palatable alternative to IV injection? Pre-clinical evaluation in normal rodents. Nucl Med Biol. 2002;29:363–373. [DOI] [PubMed] [Google Scholar]
- 14.Franc B, Carlisle MR, Segall G. Oral administration of F-18 FDG to evaluate a single pulmonary nodule by positron emission tomography in a patient with poor intravenous access. Clin Nucl Med. 2003;28:541–544. [DOI] [PubMed] [Google Scholar]
- 15.Nair N, Agrawal A, Jaiswar R. Substitution of oral 18F-FDG for intravenous 18F-FDG in PET scanning. J Nucl Med Technol. 2007;35:100–104. [DOI] [PubMed] [Google Scholar]
- 16.Cristy M, Eckerman KF. Specific Absorbed Fractions of Energy at Various Ages from Internal Photon Sources. Oak Ridge, TN: Oak Ridge National Laboratory; 1987. [Google Scholar]
- 17.Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry—standardization of nomenclature. J Nucl Med. 2009;50:477–484. [DOI] [PubMed] [Google Scholar]
- 18.Siösteen AK, Celsing F, Jacobsson H. FDG uptake in a catheter-related thrombus simulating relapse of lymphoma. Clin Nucl Med. 2005;30:338–339. [DOI] [PubMed] [Google Scholar]
- 19.Shingaki T, Takashima T, Wada Y, et al. Imaging of gastrointestinal absorption and biodistribution of an orally administered probe using positron emission tomography in humans. Clin Pharmacol Ther. 2012;91:653–659. [DOI] [PubMed] [Google Scholar]
- 20.Zhang K, Wang X, Hao L, Zhao Z, Han C. Dynamic observation of 18F-FDG uptake after oral administration in a healthy subject. J Nucl Med Technol. 2013;41:78–80. [DOI] [PubMed] [Google Scholar]
- 21.Jones SC, Alavi A, Christman D, Montanez I, Wolf AP, Reivich M. The radiation dosimetry of 2 [F-18]fluoro-2-deoxy-D-glucose in man. J Nucl Med. 1982;23:613–617. [PubMed] [Google Scholar]
- 22.Mejia AA, Nakamura T, Masatoshi I, Hatazawa J, Masaki M, Watanuki S. Estimation of absorbed doses in humans due to intravenous administration of fluorine-18-fluorodeoxyglucose in PET studies. J Nucl Med. 1991;32:699–706. [PubMed] [Google Scholar]
- 23.Deloar HM, Fujiwara T, Shidahara M, et al. Estimation of absorbed dose for 2-[F-18]fluoro-2-deoxy-D-glucose using whole-body positron emission tomography and magnetic resonance imaging. Eur J Nucl Med. 1998;25:565–574. [DOI] [PubMed] [Google Scholar]
- 24.Hays MT, Watson EE, Thomas SR, Stabin M. MIRD dose estimate report no. 19: radiation absorbed dose estimates from 18F-FDG. J Nucl Med. 2002;43:210–214. [PubMed] [Google Scholar]
- 25.Khamwan K, Krisanachinda A, Pasawang P. The determination of patient dose from 18F-FDG PET/CT examination. Radiat Prot Dosimetry. 2010;141:50–55. [DOI] [PubMed] [Google Scholar]
- 26.Velasques De Oliveira SM, Carlos MT, Carneiro MP, et al. Protocol for 18F-FDG quantification in PET-CT whole-body exams. Cell Mol Biol. 2010;56:44–46. [PubMed] [Google Scholar]
- 27.Mattsson S, Johansson L, Leide Svegborn S, et al. Radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. Ann ICRP. 2015;44:7–321. [DOI] [PubMed] [Google Scholar]