Abstract
Objective
EnhanceFitness (EF) is an evidence‐based exercise program recommended for management of osteoarthritis (OA). However, access to EF is limited in rural areas. Accordingly, we evaluated the feasibility and acceptability of remotely delivered EF (tele‐EF) in rural, community‐dwelling older adults with symptomatic knee OA.
Methods
A single‐arm pilot trial of tele‐EF classes was conducted. Videoconferencing was used to livestream the instructor‐led, 1‐hour EF classes 3 days/week for 12 weeks. Outcomes were assessed at baseline and immediately post intervention.
Results
A total of 15 of 27 potential participants (55%) were screen eligible and enrolled into the trial. Participants had a median age of 70 years (interquartile range: 67‐75), and 14 (93%) were women. The median EF class attendance rate was 91% (interquartile range: 85%‐94%). Knee pain, as measured by the Knee Injury and Osteoarthritis Outcome Score (KOOS), improved significantly from baseline to the 12‐week end point (mean difference = −11.4 [95% confidence interval (CI): −20.9 to −2.0]; P = 0.02). In addition, participants’ self‐reported knee function improved significantly (mean difference in KOOS function score = −11.8 [95% CI: −18.4 to −5.2]; P < 0.01) as well as their physical capacity (mean difference in Timed Up and Go test time = 1.8 seconds [95% CI: 0.2‐3.4]; P = 0.03). All participants (100%) were very satisfied with tele‐EF classes, and 12 participants (86%) reported that their condition had much improved or very much improved since beginning the EF exercise program. Lastly, there were no serious adverse events.
Conclusion
Findings from this pilot trial indicate that tele‐EF is feasible and acceptable in rural older adults with knee OA.
INTRODUCTION
Knee osteoarthritis (OA) is highly prevalent and a leading cause of pain that limits physical functioning in older adults (1). Clinical practice guidelines recommend physical exercise for managing symptoms of knee OA (2). Despite these recommendations, studies have shown that participation in exercise is low in this population with a high risk for disability (3, 4, 5).
Recognizing the benefits of exercise for management of OA, the Centers for Disease Control and Prevention (CDC) has promoted evidence‐based exercise programs that are group based and led by instructors in the community (6, 7). However, access to evidence‐based exercise programs is severely limited in rural areas (8, 9). Many rural residents are unable to participate in community‐based exercise because of limited or lack of access to transportation and exercise facilities (10). Walking is a common choice for exercise among older adults (11), yet walking in rural areas is limited by lack of pedestrian infrastructure, including long distances between destinations and lack of sidewalks (12). Considering that rural communities have a higher burden of arthritis (13) and obesity (14), as well as a higher proportion of older adults, than nonrural areas (15), there is a critical need to adapt evidence‐based exercise programs for remote delivery.
We have partnered with Sound Generations, a nonprofit organization that licenses and manages EnhanceFitness (EF), to adapt their evidence‐based exercise program for remote delivery (16). EF is a multicomponent, instructor‐led, group exercise program that is recommended by the CDC for adults with OA (17). It is available in more than 800 sites nationally and is covered by certain Medicare Advantage plans (18, 19, 20), but it is generally not available in rural areas. Therefore, we sought to evaluate the feasibility and acceptability of remotely delivered EF (tele‐EF) among rural older adults with knee OA.
PATIENTS AND METHODS
In partnership with Sound Generations and Arbor Health (a rural‐serving health care system in Lewis County, Washington), we conducted a pilot trial of tele‐EF. This study was reviewed and approved by the Institutional Review Board of the University of Washington in Seattle (STUDY00011517). The trial was registered with ClinicalTrials.gov (NCT04881864).
Study design
This study was a single‐arm, 12‐week pilot trial of tele‐EF among rural older adults with knee OA. Outcomes were assessed at baseline and immediately post intervention.
Study participants and recruitment
Study participants were residents of Lewis County, Washington, which is a rural county based on the CDC's National Center for Health Statistics urban–rural classification (21). Inclusion criteria included (see Supplementary Material for screening questionnaire): age greater than or equal to 65 years, physician diagnosis of knee OA, symptomatic knee OA according to American College of Rheumatology criteria (22), knee pain–related difficulty with walking or going up and/or down stairs, community dwelling (not living in a nursing home), English speaking, and able to walk independently. The exclusion criteria were cognitive impairment determined by a Mini Montreal Cognitive Assessment score of <11 (23, 24) and any of the following in the past 6 months: cancer requiring treatment (except nonmelanoma skin cancer), heart attack, stroke, hip fracture, hip and/or knee replacement, spinal surgery, heart surgery, deep vein thrombosis, or pulmonary embolus.
To reach the target population, we developed a tool kit for Arbor Health to promote the study, including a study brochure, poster, draft letter to patients identified through electronic health records, and content for print advertisements and social media postings. Arbor Health used all components of the tool kit to promote the project, and the research team at the University of Washington fielded inquiries and screened potential participants by telephone. Recruitment occurred between December 18, 2020, and March 16, 2021. Verbal informed consent was obtained from all study participants. Compensation for participating in the study was $100.
Tele‐EF intervention
Zoom videoconferencing was used to livestream the EF classes that were led by an experienced, EF‐certified instructor. Classes lasted 1 hour and were held 3 days per week, Monday, Wednesday, and Friday, for 12 weeks. Each EF class used a standardized format that included a 5‐minute warm‐up phase, 20 minutes of moderate‐intensity aerobic training, a 5‐minute cooldown with balance exercises, 20 minutes of strength training, and 10 minutes of cooldown with stretching (18). Strength training involved progressive resistance exercises using adjustable 1‐ to 10‐pound ankle and wrist weights. All participants received the same set of adjustable weights from Arbor Health Hospital, where they completed functional assessments (see Secondary outcomes). In accordance with the EF protocol and training manual, the instructor modified exercises depending on the fitness level of individual participants, including doing exercises in the seated position, if necessary.
Consistent with the guidance for remotely delivering EF (25), an assistant helped the instructor and participants troubleshoot any technical challenges. In addition, the assistant monitored for safety and had participant emergency contact information available. Figure 1 illustrates that the EF instructor, participants, and assistant could all interact during livestreamed EF classes. To facilitate social interaction, the assistant opened the virtual classroom approximately 10 minutes prior to the start of class, and participants were able to join and see everyone in gallery view. Once it was time to start EF, the assistant spotlighted the EF instructor on the screen, who then began leading the class in exercise. Importantly, however, both the EF instructor and the assistant were able see all participants in gallery view to monitor for exercise form and any safety events. At the end of class, the assistant removed the spotlight to provide participants an opportunity to visit and ask the instructor questions for a few minutes.
Figure 1.
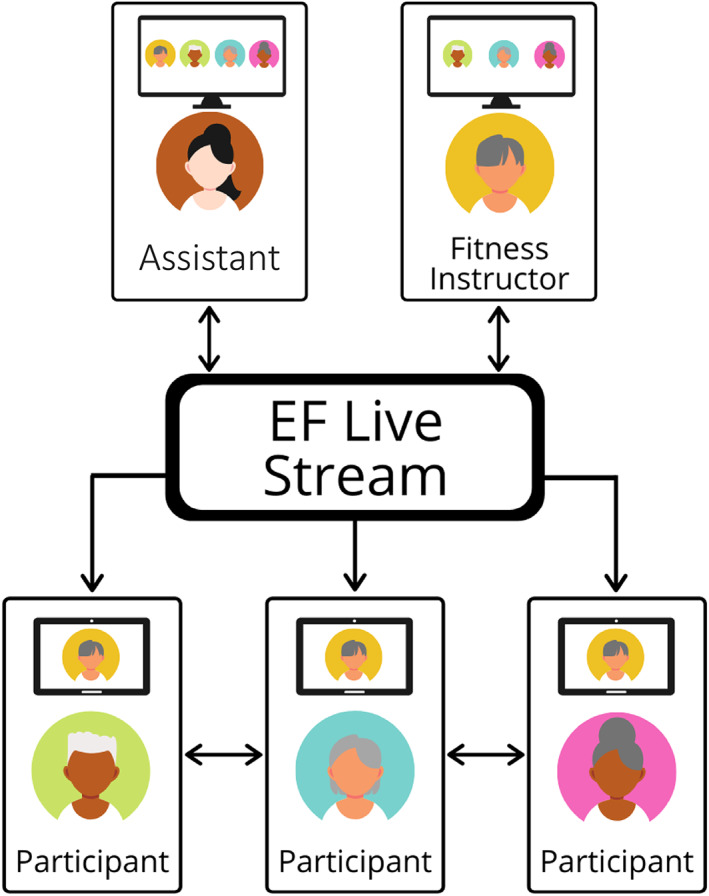
Illustration of remote delivery of Enhance®Fitness (EF) classes.
Prior to the start of classes, participants completed a technology needs assessment. Those who did not have appropriate equipment or sufficient access to broadband were given a cellular‐enabled tablet to participate in the study. One‐on‐one orientation meetings were held via Zoom with each study participant to familiarize them to the tele‐EF program and provide guided instruction on videoconferencing. When necessary, instructions on accessing and using Zoom were given by telephone prior to the orientation call. On average, the orientation meetings lasted 30 minutes (SD = 12). Tele‐EF classes began on March 17, 2021, and ended June 9, 2021.
Measures
Participant characteristics
Basic demographic and health characteristics were collected at baseline through an online questionnaire using the REDCap electronic data capture tools (26). Participants also answered standard questions about their OA and history of falling. The following definition of falling was provided: “By falling down we mean any fall, slip, or trip in which you lose your balance and land on the floor or ground or at a lower level.”
Feasibility and acceptability
Multiple metrics were used to assess the feasibility and acceptability of tele‐EF. The proportion of screened individuals who were eligible but refused to participate and the proportion of screened individuals who enrolled into the study are important feasibility measures. In addition, tele‐EF class attendance was systematically recorded. We also recorded any technology challenges encountered when participants engaged in tele‐EF classes, and if telephone support was necessary, the call length was recorded. To assess acceptability, we asked participants at the end of the trial, “How satisfied are you overall with the Enhance Fitness classes?” There were five response options ranging from “very dissatisfied” to “very satisfied.” Participants were also asked, “On a scale of 0‐10, with 0 being not helpful at all and 10 being very helpful, how helpful was this program in supporting you to increase your physical activity?”
Semistructured exit interviews were completed within 3 weeks of the tele‐EF program ending. An interview guide (see Supplementary Materials) was prepared to assess participants’ experiences with tele‐EF as well as advantages and disadvantages of tele‐EF. Interviews were completed by coauthor EVH (an experienced and trained interviewer), audio‐recorded, and transcribed and lasted on average 27 minutes (range: 17‐38 minutes).
Secondary outcomes
The collection of secondary outcomes was guided by expert recommendations for clinical trials of knee OA (27, 28). Multiple aspects of knee pain and physical function were measured with the Knee Injury and Osteoarthritis Outcome Score (KOOS), which includes five subscales with scores ranging from 0 to 100 (a higher score reflects better outcomes) (29). The Patient‐Reported Outcomes Measurement Information System 29‐Item profile measure (PROMIS‐29) was collected to assess health‐related quality of life across seven domains (30). Domain scores range from 0 to 100, with higher scores reflecting more of the concept being measured. To assess overall change in health, participants were asked to complete the single‐item Patient Global Impression of Change (PGIC) rating (“Since the start of the study, my overall status is…”). The PGIC has seven ordinal response options ranging from “very much worse” to “very much improved” (31, 32). Participants completed all questionnaires online via REDCap.
Performance‐based assessments of physical capacity were completed at Arbor Health Hospital by a trained physical therapist with more than 30 years of experience in functional assessment. Tests of physical capacity included the Timed Up and Go (TUG), Short Physical Performance Battery (SPPB), and single‐leg stand with eyes open (timed up to 30 seconds) tests. For the TUG test, participants stand up from a chair, walk 3 m at their usual pace, turn around, walk back to the chair, and sit back down (33). Timing starts when the participant begins to stand up from the chair and stops when they sit back down. Older adults who take greater than or equal to 12 seconds to complete the TUG test have greater risk of falling (vs. TUG time <12 seconds) (34). The SPPB test is composed of three tests of static balance (feet side by side, semitandem, and tandem for 10 seconds), usual gait speed over a 3‐ or 4‐m course (we used a 3‐m distance), and a five‐time sit‐to‐stand test (35). Each of the three components of the SPPB is scored on a 0 to 4 scale, and a total score is computed by summing the individual component scores (range 0‐12, higher scores indicate better function). An SPPB score less than 10 is associated with increased risk for mobility disability (vs. SPPB score ≥10) (36, 37). The physical therapist also measured height and weight.
Adverse events
Adverse events (AEs) were defined as any health event or injury that restricted a participant's activity for a day or required medical care, regardless of whether it was related to the tele‐EF classes. Serious AEs were defined as any event that causes hospitalization or death or is life threatening. Every week during the 12‐week intervention, participants completed an online questionnaire asking them about any activity‐limiting health events or falls each day during the past week. The study research coordinator contacted participants who reported an event or fall and obtained more information to determine its severity and relatedness to the study intervention.
Videoconference technology acceptance
A Technology acceptance model (TAM) scale was adapted to assess participant perceptions of the ease of use of, usefulness of, financial cost of, and intention to use videoconference technology for exercise. Similar to prior studies that adapted the TAM scale to assess use of mobile technology (38, 39), the scale used in the current study has 10 items with 7‐point Likert scale response options (see Supplementary Materials). There are four subscales, with higher scores reflecting more of the concept being measured.
Sample size and data analysis
A sample size of 14 participants was prespecified to gain real‐world experience implementing tele‐EF classes in the target population. Guidance on remote delivery of EF recommends class sizes of 10 to 12 (25). Resource constraints limited us to running a single livestream class for 12 weeks with an EF instructor and an assistant to monitor for safety and provide technology support. A group size of 12 is minimally recommended for pilot studies to assess for feasibility and provide minimal precision estimates of outcome measures (40). We estimated an attrition rate of 15%, retaining 12 of 14 for data analysis.
Descriptive statistics were computed for all variables. Paired t‐tests were used to examine within‐participant change (from baseline to post intervention) in the secondary outcome measures as well as changes in videoconference acceptance measures. All statistical analyses were conducted with Stata IC 16.1 (StataCorp).
The first three exit interview transcripts were read and coded by coauthors KVP and EVH. They discussed inconsistencies in coding until agreement was reached. EVH coded the remaining nine transcripts. The research team (KVP, EVH, and NMG) met to review codes and applied descriptive thematic analysis to identify features of participants’ experiences with tele‐EF (41, 42). Coding and analyses were completed using Microsoft Excel and Word.
RESULTS
Feasibility and acceptability metrics
A total of 27 individuals contacted the study team (Figure 2). Of these potential participants, 21 (77.8%) were eligible and 15 (55.6%) enrolled and completed the baseline assessments. There were no statistically significant differences in age or sex between those who did and those who did not enroll into the study. Among those who enrolled, 13 (86.7%) completed 12 weeks of tele‐EF classes and completed the postintervention assessments. One participant withdrew in week 5 of the study because of health issues unrelated to tele‐EF, and another withdrew in week 10 to travel after COVID‐19 restrictions were lifted. The study team was able to collect some self‐reported outcomes data from the participant who withdrew to travel but none from the participant who withdrew for health issues.
Figure 2.
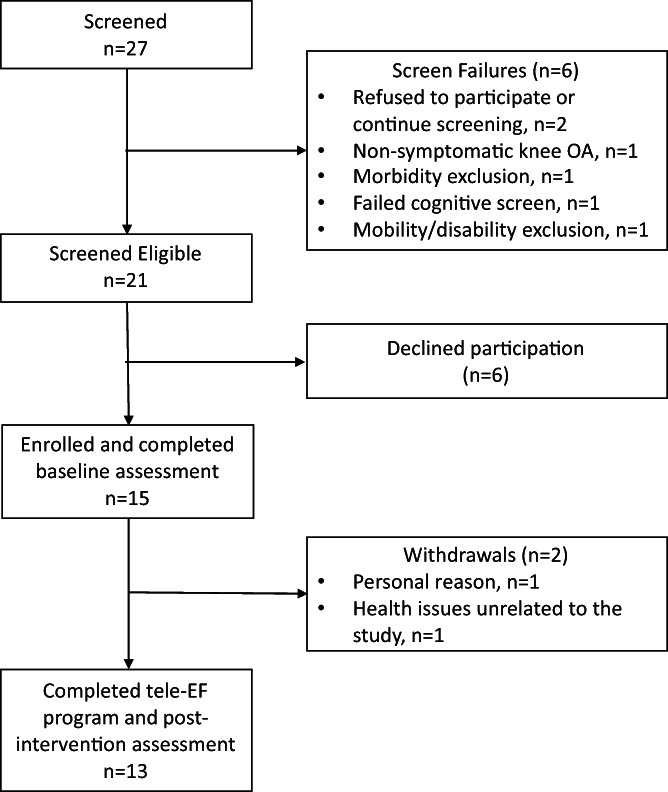
Flow of participants through the pilot trial of remotely delivered EnhanceFitness (tele‐EF). OA, osteoarthritis.
The technology assessment identified 11 (73.3%) participants who had home internet access and a device that could access Zoom, but 10 (66.6%) reported having sufficient broadband to stream video for 1 hour. Five (33.3%) participants were loaned a cellular‐enabled tablet to facilitate participation in tele‐EF.
Over the 12‐week intervention period, the median tele‐EF class attendance rate was 91.4% (interquartile range: 84.5%‐94.3%; n = 15). Fourteen telephone calls were made to address technology challenges that were encountered while participants attempted to engage in tele‐EF. The mean call length was 8.2 minutes (SD = 2.9, range = 5‐11). The majority (71.4%) of these technology support calls occurred during the first 2 weeks of tele‐EF classes. The most common technology challenge encountered was a lack of or insufficient internet connection.
In terms of acceptability, all participants (100%; n = 14) were very satisfied with tele‐EF classes. On the 0 to 10 scale for rating how helpful the tele‐EF program was for supporting participants to increase their physical activity, nine (64.3%) gave a rating of 10 (very helpful), whereas three (21.4%) and two (14.3%) of the remaining participants gave ratings of 8 and 9, respectively.
Participant characteristics
Table 1 shows the demographic and health characteristics of participants at baseline. Participants had a mean age of 71.8 years (SD = 5.8), 14 (93.3%) were women, 14 (93.3%) were White, 9 (60.0%) had less than a college education, 10 (71.4%) were obese, and all had multiple chronic conditions. Most participants had knee OA for more than 5 years. The co‐occurrence of hip and hand OA was common, and one third of participants had a history of knee replacement surgery. Notably, half of participants reported falling in the past year, and a third reported falling multiple times.
Table 1.
Participant characteristics at baseline (N = 15)
| Value | |
|---|---|
| Demographic characteristics | |
| Age in years, mean (SD) | 71.8 (5.8) |
| Women, n (%) | 14 (93.3) |
| Race, White, n (%) | 14 (93.3) |
| Education, n (%) | |
| High school graduate | 1 (6.7) |
| Some college or vocational school | 8 (53.3) |
| College graduate | 4 (26.7) |
| Master's degree | 2 (13.3) |
| Lives alone, n (%) | 3 (20.0) |
| Retired n (%) | 12 (80.0) |
| Health characteristics | |
| Duration of knee OA, mean (SD) | |
| 1‐5 years | 5 (38.5) |
| 6‐9 years | 2 (15.4) |
| ≥10 years | 6 (46.2) |
| Hip OA, n (%) | 5 (33.3) |
| Hand OA, n (%) | 10 (66.7) |
| Knee replacement, n (%) | 5 (33.3) |
| Hip replacement, n (%) | 2 (13.3) |
| Obese (BMI ≥30), n (%) | 10 (71.4) |
| Total number of medical conditions, n (%) | |
| 2 | 3 (20.0) |
| 3 | 6 (40.0) |
| ≥4 | 6 (40.0) |
| Fall history, n (%) | |
| Fell in the past year | 8 (53.3) |
| Fell multiple times in the past year | 5 (33.3) |
Abbreviations: BMI, body mass index; OA, osteoarthritis.
Secondary outcomes
Table 2 shows results for the secondary outcome measures. On the basis of the mean KOOS scores at baseline, participants had moderate to severe knee pain and knee‐related functional limitations. From baseline to the 12‐week end point, the mean KOOS scores for knee pain, knee function in daily function, and knee function in sport and recreation improved significantly (P < 0.05). The magnitude of improvements for knee pain and function in daily living are in the estimated range to be considered meaningfully important (43). However, there were no statistically significant changes in other knee symptoms (eg, stiffness) and knee OA–related quality of life. Anxiety, sleep disturbance, and pain interference, as measured by PROMIS‐29, improved significantly (P < 0.05). There were also improvements in self‐reported physical function, fatigue, and satisfaction with participation in social roles, but changes in these PROMIS‐29 domain scores were not statistically significant. The magnitude of improvements in PROMIS‐29 anxiety, physical function, and pain interference is in the estimated range to be considered meaningfully important in adults with knee OA (44). In terms of global rating of change, 12 participants (86%) reported that their condition had much improved or very much improved since beginning the tele‐EF program.
Table 2.
Change in outcome measures from baseline to the 12‐week end point
| Mean (SD) at baseline | Mean (SD) at 12‐week end point | Mean difference from baseline to 12‐week end point (95% CI) | P | |
|---|---|---|---|---|
| KOOS subscales (n = 14) | ||||
| Pain | 53.6 (21.2) | 65.0 (17.7) | −11.4 (−20.9 to −2.0) | 0.021 |
| Symptoms | 43.6 (13.8) | 43.7 (13.8) | −0.1 (−5.9 to 5.8) | 0.977 |
| Function in daily living | 57.6 (22.0) | 69.3 (16.3) | −11.8 (−18.4 to −5.2) | 0.002 |
| Function in sport and recreation | 17.5 (14.9) | 25.0 (20.0) | −7.5 (−14.3 to −0.7) | 0.032 |
| Quality of life | 42.4 (26.2) | 43.3 (25.1) | −0.9 (8.5 to −6.7) | 0.801 |
| PROMIS domains (n = 13) | ||||
| Anxiety | 51.4 (6.7) | 48.5 (7.4) | 2.9 (0.03 to 5.7) | 0.048 |
| Depression | 48.2 (7.3) | 48.1 (7.3) | 0.02 (−3.4 to 3.4) | 0.992 |
| Fatigue | 55.1 (10.6) | 52.8 (10.8) | 2.3 (−0.7 to 5.4) | 0.122 |
| Sleep disturbance | 54.4 (10.1) | 51.3 (4.6) | 3.0 (0.1 to 5.9) | 0.043 |
| Satisfaction with participation in social roles | 46.9 (12.0) | 49.4 (5.9) | −2.5 (−7.7 to 2.6) | 0.303 |
| Physical function | 38.8 (6.2) | 41.7 (6.9) | −2.9 (−6.0 to 0.1) | 0.060 |
| Pain interference | 58.3 (9.4) | 54.7 (8.4) | 3.6 (0.2 to 7.0) | 0.039 |
| Tests of physical capacity (n = 12) | ||||
| Timed Up and Go test in seconds | 12.6 (4.6) | 10.8 (3.7) | 1.8 (0.2 to 3.4) | 0.032 |
| Usual gait speed in m/second | 0.82 (0.20) | 0.82 (0.17) | −0.003 (−0.07 to 0.06) | 0.933 |
| Single‐leg stand test in seconds | 6.3 (5.9) | 7.4 (6.2) | −1.1 (−4.2 to 2.0) | 0.445 |
| 5‐time sit‐to‐stand test in seconds | 14.8 (4.1) | 12.3 (2.8) | 2.5 (1.2 to 3.9) | 0.002 |
| Short Physical Performance Battery | 9.2 (2.1) | 10.1 (2.1) | −0.9 (−1.7 to −0.1) | 0.027 |
Abbreviations: KOOS, Knee Injury and Osteoarthritis Outcome Score; PROMIS, Patient‐Reported Outcomes Measurement Information System.
The baseline mean scores for the TUG and SPPB tests indicated that the study sample, on average, had elevated risk of falling and mobility disability. Performance on these two tests of physical capacity as well as on the five‐time sit‐to‐stand test improved significantly from baseline to the 12‐week end point (P < 0.05). Improvements in TUG time and the SPPB score are in the range to be considered meaningfully important (45, 46). There were no statistically significant improvements in usual gait speed over 3 m or in balance performance.
AEs
Over the 12‐week intervention period, there were 15 AEs and no serious AEs. The most common AEs were pain flares, falls, and back pain. Only one of the AEs (a pain flare) was related to tele‐EF (participant reported exercising too intensively at the beginning of the study; pain flare resolved prior to the next EF class).
Acceptance of videoconference technology
Table 3 shows results from the adapted TAM scale used to assess perceptions of videoconference technology for exercising. From baseline to the 12‐week end point, there were statistically significant increases in participant perceptions of the ease of use and usefulness of videoconferencing for engaging in exercise (P < 0.05). At baseline, participants somewhat disagreed, on average, that the financial cost of videoconference technology would be a barrier. There was no change in this subscale at the end of 12 weeks of tele‐EF. Participants intention to use videoconferencing for exercise also did not change, but the average rating on this subscale was high already at baseline, likely reflecting participants willingness to participate in tele‐EF.
Table 3.
Changes from baseline to the 12‐week end point in acceptance of videoconference technology for exercising
| Technology acceptance model subscales a (n = 13) | Mean (SD) at baseline | Mean (SD) at 12‐week end point | Mean difference from baseline to 12‐week end point (95% CI) | P |
|---|---|---|---|---|
| Perceived ease of use | 5.3 (1.0) | 6.4 (0.8) | −1.1 (−1.6 to −0.6) | 0.001 |
| Perceived usefulness | 5.8 (0.9) | 6.6 (0.4) | −0.8 (−1.3 to −0.4) | 0.001 |
| Perceived financial cost as a barrier | 3.0 (1.5) | 2.8 (1.5) | 0.2 (−0.9 to 1.2) | 0.745 |
| Behavioral intention | 6.1 (5.8) | 6.3 (5.8) | −0.2 (−0.7 to 0.3) | 0.337 |
The adapted technology acceptance model measure is composed of 10 items that have a 1 to 7 response range, with higher scores reflecting greater agreement with the concept being measured.
Exit interviews
Semistructured interviews (n = 12) identified four key features of tele‐EF that participants valued. As illustrated by the quotes in Table 4, tele‐EF addressed environmental barriers to accessing community‐based exercise that rural older adults often face, including lack of consistently available programs, lack of transportation, and traveling long distances. For participants with caregiving responsibilities, the ability to exercise from home facilitated their participation because they had limited time to commit and needed to be available in case of caregiving‐related emergencies. In addition to accessibility, participants appreciated the group‐based livestream design of tele‐EF that facilitated accountability and helped sustain their motivation. Another important feature of tele‐EF was support that participants received not only from exercising with peers but also from the encouragement and guidance given by the instructor. Participants noted that the opportunity to ask the instructor questions allowed them to address concerns about their exercise form and facilitated continued participation among those with greater functional limitations (eg, modified exercises). Lastly, participants conveyed that they had joined the study to improve their knee OA–related pain and functional limitations and were gratified by the physical benefits of tele‐EF. The multicomponent exercise (ie, aerobic, strength, and balance training) was viewed positively and contributed toward improvement in overall health and functioning.
Table 4.
Key features and challenges of tele‐EF reported by rural older adults with knee osteoarthritis
| Quotes | |
|---|---|
| Accessibility: tele‐EF reduces environmental barriers to exercise. | “Well, it's a good incentive to exercise on a regular basis. Any other exercise classes that I would be able to get are very far away, so it's inconvenient to be able to travel 50 miles to go to an exercise class.” |
| “Oh, it's the lack of having to travel any distance to classes, one. Two, not having an instructor in the community, for quite a few years. There was really no alternative. If you're going to do the program, this is the way it needed to be done.” | |
| “I can stay at home and do it. I don't have to drive someplace and go do it. See, I don't drive anymore because of the seizure, so my husband would have to take me every place. For anything I have to do, he has to take me which is an inconvenience.” | |
| Accountability: tele‐EF classes facilitate accountability. | “In a way, a group kind of kept you motivated because if it was just a one‐on‐one, I think it would be easier to say, ‘Oh, I just won't do it today.’ But when it's a group, you kind of feel like, ‘Well, the whole group's doing it, I'll do it too.’” |
| “The ease of just doing it at home was great and the accountability that there's other people, showing up. And that you're going to do this, three times a week and that's how it is, you know, you're committed to it. If you don't have that social interaction of other people holding you accountable, you know, then you can kind of be a slacker.” | |
| Support: tele‐EF provides instructor and peer support. | “Well, they know what you're going through, what pain you're having. I don't know the history of everybody else that was in my group as far as whether they had surgery on their knees before or what. But having the camaraderie of doing it with other people really helps and gets you to want to go and to participate.” |
| “You feel the energy, even though I couldn't see the other participants [exercising], you felt the energy of people kind of chugging along with you so that's what I like about a class, rather than being individual.” | |
| “I like the positive very, very positive manner…kind of gentle encouragement to keep adding a few more weights, if possible.… I like that nobody ever was singled out for doing something wrong or not positioning right. Just a general positive attitude and feeling like we were part of something so was good.” | |
| “She [EF instructor] gave lots of different options, sometimes I stood and sometimes I sat depending on how I felt. And she always made you feel very comfortable with whatever way you chose to do and she would alternatively show us the ways to do it correctly, which I think is helpful because if you're not doing it correctly, it's not a great exercise. So I did feel that she was a great leader.” | |
| Physical benefits: tele‐EF improved functioning, pain, and other symptoms that had reinforcing effects. | “Advantages would be muscle strengthening, more movement in body function, as far as legs and arms and things. I think I was surprised that it was overall [body strengthening], I was thinking it was going to be just knees and come to find out it was overall.” |
| “I can move much better and walk better and just not feel stiff. Both knees are doing better than they were because they're just not as stiff and I haven't really had a lot of pain, which is really good.” | |
| “Probably the mobility, more than anything. And I have less pain with the arthritis if I'm moving about, you know than sitting in a chair or something.” | |
| “It just gives you more energy to be able to go throughout your day. And it continues. It doesn't just, ‘Well, the class is over so everything's ended.’ No. Your stamina keeps up. You just have a better outlook. It's just a good, uplifting thing to do.” | |
| Technology‐related challenges: tele‐EF requires equipment, internet connection, and technical support. | “It's hard to use when I didn't have a signal, or if it was going in and out. And in those cases, I would just keep doing what I thought we were doing until it would come back on.” |
| “There was some technical problems, but everyone just really worked on that.… In the beginning was a bit of a problem, but that all worked out and so yeah it was great.” | |
| “I would have enjoyed watching all of us because if you're in a class where you truly can see the other people.” |
Abbreviations: EF, EnhanceFitness; tele‐EF, remotely delivered EnhanceFitness.
In addition to positive features of tele‐EF, technology‐related challenges were also observed. Participants described experiencing occasional disruptions to tele‐EF because of interruptions in internet connection or limitations in bandwidth; however, this was also viewed as a routine experience living in a rural area. Also, several participants noted that they encountered difficulty accessing tele‐EF classes initially, but they were able to troubleshoot technology‐related problems and develop videoconferencing skills with the research team's support. Some participants expressed a desire for more opportunity for social interaction, whereas others expressed a preference to see other participants on their screen when exercising.
DISCUSSION
The current pilot study aimed to investigate the feasibility and acceptability of remote delivery of EF classes to rural, community‐dwelling older adults with knee OA. The results show that, in partnership with a rural‐serving health care system (Arbor Health), we were able to recruit a hard‐to‐reach population and enroll our target sample size over a 12‐week period that included the winter holidays. Among potential participants who contacted us, more than half were eligible and enrolled into the study. Also, the tele‐EF class attendance rate and study retention rate were high. Lastly, several measures of pain and physical function improved significantly from baseline to the 12‐week end point. Perceptions on the ease of use and usefulness of videoconference technology for exercising also improved. Taken together, these findings indicate that remote delivery of EF to rural older adults with knee OA is feasible and acceptable.
The CDC and other groups have recognized the need to improve equity in access to and delivery of evidence‐based OA management programs (6). A recent study found that experts rated land‐based home exercise the highest priority intervention for knowledge translation and dissemination to help decrease disparities in knee OA and improve outcomes in underserved populations, including rural communities (47). Given the results of the current study, remote delivery of EF classes is a promising intervention to address environmental barriers to exercise participation (10) and reduce the high burden of OA in the rural population (13). However, internet access is more limited in rural than nonrural areas. Indeed, five participants in the current study received a cellular‐enabled tablet to participate in tele‐EF. Although these participants lived in T‐Mobile's coverage area, one participant's signal strength was too weak in their home and they switched to a different cellular service provider. This experience highlights the real‐world challenge of delivering tele‐exercise to rural settings; however, the feasibility of disseminating tele‐EF in the United States is enhanced by recent appropriations to expand broadband in rural areas to support telehealth services (48, 49).
Importantly, the current study demonstrated that with appropriate support, rural older adults can effectively participate in tele‐exercise. Indeed, the technology support needs during tele‐EF classes in the current study were similar to the needs observed in an urban sample (16). In an initial study of tele‐EF among 44 older adults with knee OA in Seattle (16), 69% of support calls occurred in the first 2 weeks of tele‐EF, which is nearly identical to the 71% rate observed in the current study. Further, the median tele‐EF class attendance rates were also similar (≥90%) between the two studies (16). Interestingly, the initial orientation session held with each participant lasted 11 minutes longer, on average, in the current study than the previous one from Seattle (mean = 30 vs. 19 minutes, respectively). There were similar challenges addressed during the orientation (eg, navigating the camera's view, learning how to use Zoom functions [switching from speaker to gallery view], and learning common technology terms [swiping or scrolling]). This suggests that with a little extra training, rural older adults can participate in tele‐EF similarly as urban ones. These studies support the guidance issued by Sound Generations to hold an orientation session and have an assistant available to provide technical assistance during tele‐EF classes (25).
Engaging rural older adults in health promotion is challenging for a variety of reasons, including geographic barriers and transportation difficulties. However, considering that older adults are routinely seen in primary care clinics, partnering with rural‐serving health care systems can facilitate opportunities to promote physical activity. Indeed, our partnership with Arbor Health was critical to reaching the current study's target population. Anecdotally, some participants shared that receiving the recruitment letter from Arbor Health and checking with their physician helped assure them about the legitimacy of the study and tele‐EF program. Partnerships with health care systems can also help sustain programs. Arbor Health, for instance, obtained an EF license and is now offering tele‐EF classes on the basis of feedback received from study participants.
It is notable that half of the study participants at baseline reported falling in the past year and a third had fallen multiple times (Table 1). Further, the average time to complete the TUG test at baseline was greater than or equal to 12 seconds (Table 2), a threshold recommended by the CDC to identify those with a high risk of falling (https://www.cdc.gov/steadi/). This high prevalence of falls‐related risk factors is consistent with epidemiologic studies reporting that knee OA is associated with increased falls risk (50, 51, 52, 53). The magnitude of change in TUG time (1.8 seconds; Table 2) from baseline to the 12‐week end point was substantial and clinically meaningful (42), suggesting that participation in tele‐EF may reduce falls risk. Previous studies have demonstrated that participation in the in‐person EF program improves TUG time by 1.1 to 1.4 seconds (54, 55) and is associated with reduced risk of falls‐related injury (56). Indeed, EF is recognized as an evidence‐based falls prevention program (57). Future trials are needed to demonstrate the efficacy of tele‐EF for reducing falls risk in older adults with OA.
Several limitations should be considered when interpreting the current study results. First, this pilot study was not statistically powered to detect meaningful change in outcome measures. Also, there was no control group to compare changes in outcomes and evaluate the efficacy of tele‐EF. Second, the duration of the tele‐EF intervention was limited to 12 weeks (36 one‐hour exercise classes). Third, outcomes were only assessed immediately post intervention, without long‐term follow‐up. Lastly, the study's generalizability is limited because only one participant was male and we only partnered with one health care system in a single rural county in Washington State. Also, potential participants who did not use digital technology might not have contacted the study, although several study participants had limited technology experience.
To our knowledge, this is the first study to evaluate remote delivery of EF, an evidence‐based exercise program that is recommended by the CDC for OA management (17). The study findings indicate that with appropriate technology support, tele‐EF is feasible and acceptable to rural older adults with knee OA. However, additional research is needed with larger trials to evaluate the effectiveness of tele‐EF in this population with limited access to in‐person evidence‐based exercise programs.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Patel had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Patel, Gell.
Acquisition of data
Patel, Hoffman.
Analysis and interpretation of data
Patel, Hoffman, Phelan, Gell.
Supporting information
Disclosure Form
Appendix S1 Supporting Information
Appendix S2 Supporting Information
Appendix S3 Supporting Information
ACKNOWLEDGMENTS
We thank Diane Markman and Edwin Meelhuysen of Arbor Health as well as Paige Denison of Sound Generations for their strong support and partnership on this project.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr2.11452&file=acr211452‐sup‐0001‐Disclosureform.pdf.
ClinicalTrials.gov identifier: NCT04881864.
REFERENCES
- 1. Hunter DJ, Bierma‐Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. [DOI] [PubMed] [Google Scholar]
- 2. Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol 2020;72:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention (CDC) . Strength training among adults aged >/= 65 years–United States, 2001. MMWR Morb Mortal Wkly Rep 2004;53:25–8. [PubMed] [Google Scholar]
- 4. Dunlop DD, Song J, Semanik PA, Chang RW, Sharma L, Bathon JM, et al. Objective physical activity measurement in the osteoarthritis initiative: are guidelines being met? [full length article]. Arthritis Rheum 2011;63:3372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallis JA, Webster KE, Levinger P, Taylor NF. What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta‐analysis. Osteoarthritis Cartilage 2013, 21:1648–59. [DOI] [PubMed] [Google Scholar]
- 6. Osteoarthritis Action Alliance . A national public health agenda for osteoarthritis: 2020 update. URL: https://oaaction.unc.edu/wp-content/uploads/sites/623/2020/05/OA-Agenda-Final_04302020.pdf.
- 7. Kelley GA, Kelley KS, Hootman JM, Jones DL. Effects of community‐deliverable exercise on pain and physical function in adults with arthritis and other rheumatic diseases: a meta‐analysis. Arthritis Care Res (Hoboken) 2011;63:79–93. [DOI] [PubMed] [Google Scholar]
- 8. Towne SD Jr, Smith ML, Ahn S, Altpeter M, Belza B, Kulinski KP, et al. National dissemination of multiple evidence‐based disease prevention programs: reach to vulnerable older adults. Front Public Health 2015;2:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith ML, Towne SD, Herrera‐Venson A, Cameron K, Horel SA, Ory MG, et al. Delivery of fall prevention interventions for at‐risk older adults in rural areas: findings from a national dissemination. Int J Environ Res Public Health 2018;15:2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dobson F, Bennell KL, French SD, Nicolson PJ, Klaasman RN, Holden MA, et al. Barriers and facilitators to exercise participation in people with hip and/or knee osteoarthritis: synthesis of the literature using behavior change theory. Am J Phys Med Rehabil 2016;95:372–89. [DOI] [PubMed] [Google Scholar]
- 11. Amireault S, Baier JM, Spencer JR. Physical activity preferences among older adults: a systematic review. J Aging Phys Act 2018;27:128–39. [DOI] [PubMed] [Google Scholar]
- 12. Doescher MP, Lee C, Berke EM, Adachi‐Mejia AM, Lee CK, Stewart O. The built environment and utilitarian walking in small U.S. towns. Prev Med 2014;69:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boring MA, Hootman JM, Liu Y, Theis KA, Murphy LB, Barbour KE, et al. Prevalence of arthritis and arthritis‐attributable activity limitation by urban‐rural county classification ‐ United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013‐2016. JAMA 2018;319:2419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith AS, Trevelyan E. The older population in rural America: 2012–2016. Washington (DC): US Census Bureau; 2019. [Google Scholar]
- 16. Gell NM, Hoffman EV, Patel KV. Technology support challenges and recommendations for adapting an evidence‐based exercise program for virtual delivery to older adults: mixed‐methods study. JMIR Aging 2021;4:e27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . Physical activity programs. URL: https://www.cdc.gov/arthritis/interventions/physical-activity.html.
- 18. Belza B, Shumway‐Cook A, Phelan EA, Williams B, Snyder SJ, LoGerfo JP. The effects of a community‐based exercise program on function and health in older adults: the EnhanceFitness Program. J Appl Gerontol 2006;25:291–306. [Google Scholar]
- 19. Belza B, Snyder S, Thompson M, LoGerfo J. From research to practice: EnhanceFitness, an innovative community‐based senior exercise program. Top Geriatr Rehabil 2010;26:299–309. [Google Scholar]
- 20. Snyder SJ. Thompson M, Denison P. EnhanceFitness: a 20‐year dissemination history. Front Public Health 2015;2:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ingram DD, Franco SJ. 2013 NCHS urban‐rural classification scheme for counties. Vital Health Stat 2 2014:1–73. [PubMed] [Google Scholar]
- 22. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al, and. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 23. MoCA . URL: https://www.mocatest.org/.
- 24. Wong A, Nyenhuis D, Black SE, Law LS, Lo ES, Kwan PW, et al. Montreal cognitive assessment 5‐minute protocol is a brief, valid, reliable, and feasible cognitive screen for telephone administration. Stroke 2015;46:1059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sound Generations . EnhanceFitness remote class delivery guidance. Seattle: Sound Generations; 2020. [Google Scholar]
- 26. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith TO, Hawker GA, Hunter DJ, March LM, Boers M, Shea BJ, et al. The OMERACT‐OARSI core domain set for measurement in clinical trials of hip and/or knee osteoarthritis. J Rheumatol 2019;46:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dobson F, Hinman RS, Roos EM, Abbott JH, Stratford P, Davis AM, et al. OARSI recommended performance‐based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage 2013;21:1625–6. [DOI] [PubMed] [Google Scholar]
- 29. Roos EM, Toksvig‐Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS): validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes 2003;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS‐29 v2.0 profile physical and mental health summary scores. Qual Life Res 2018;27:1885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guy W. ECDEU assessment manual for psychopharmacology, revised, 1976. Rockville (MD): US Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 32. Patel KV, Amtmann D, Jensen MP, Smith SM, Veasley C, Turk DC. Clinical outcome assessment in clinical trials of chronic pain treatments. Pain Rep 2021;6:e784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- 34. Lusardi MM, Fritz S, Middleton A, Allison L, Wingood M, Phillips E, et al. Determining risk of falls in community dwelling older adults: a systematic review and meta‐analysis using posttest probability. J Geriatr Phys Ther 2017;40:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 36. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandinelli S, et al. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2009;64:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cajita MI, Hodgson NA, Budhathoki C, Han HR. Intention to use mHealth in older adults with heart failure. J Cardiovasc Nurs 2017;32:E1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hong SJ, Tam KY. Understanding the adoption of multipurpose information appliances: the case of mobile data services. Inf Syst Res 2006;17:162–179. [Google Scholar]
- 40. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287–91. [Google Scholar]
- 41. Sandelowski M. Whatever happened to qualitative description? [full length article]. Res Nurs Health 2000;23:334–40. [DOI] [PubMed] [Google Scholar]
- 42. Sandelowski M. What's in a name? Qualitative description revisited. Res Nurs Health 2010;33:77–84. [DOI] [PubMed] [Google Scholar]
- 43. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee AC, Driban JB, Price LL, Harvey WF, Rodday AM, Wang C. Responsiveness and minimally important differences for 4 patient‐reported outcomes measurement information system short forms: physical function, pain interference, depression, and anxiety in knee osteoarthritis. J Pain 2017;18:1096–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther 2011;41:319–27. [DOI] [PubMed] [Google Scholar]
- 46. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743–9. [DOI] [PubMed] [Google Scholar]
- 47. Houlding‐Braunberger E, Petkovic J, Lebel N, Tugwell P. Experts prioritize osteoarthritis non‐surgical interventions from Cochrane systematic reviews for translation into “Evidence4Equity” summaries. Int J Equity Health 2021;20:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coronavirus Aid , Relief, and Economic Security (CARES) Act of 2020, S 3548, 116th Cong (2020).
- 49. Consolidated Appropriations Act, 2021, HR 133, 116th Cong (2020).
- 50. Arden NK, Crozier S, Smith H, Anderson F, Edwards C, Raphael H, et al. Knee pain, knee osteoarthritis, and the risk of fracture. Arthritis Rheum 2006;55:610–5. [DOI] [PubMed] [Google Scholar]
- 51. Barbour KE, Sagawa N, Boudreau RM, Winger ME, Cauley JA, Nevitt MC, et al. Knee osteoarthritis and the risk of medically treated injurious falls among older adults: a community‐based US cohort study. Arthritis Care Res (Hoboken) 2019;71:865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Doré AL, Golightly YM, Mercer VS, Shi XA, Renner JB, Jordan JM, et al. Lower‐extremity osteoarthritis and the risk of falls in a community‐based longitudinal study of adults with and without osteoarthritis. Arthritis Care Res (Hoboken) 2015;67:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leveille SG, Jones RN, Kiely DK, Hausdorff JM, Shmerling RH, Guralnik JM, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA 2009;302:2214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shumway‐Cook A, Silver IF, LeMier M, York S, Cummings P, Koepsell TD. Effectiveness of a community‐based multifactorial intervention on falls and fall risk factors in community‐living older adults: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci 2007;62:1420–7. [DOI] [PubMed] [Google Scholar]
- 55. Fishleder S, Petrescu‐Prahova M, Harris JR, et al. Predictors of improvement in physical function in older adults in an evidence‐based physical activity program (EnhanceFitness). J Geriatr Phys Ther 2019;42:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Greenwood‐Hickman MA, Rosenberg DE, Phelan EA, Fitzpatrick AL. Participation in older adult physical activity programs and risk for falls requiring medical care, Washington State, 2005‐2011. Prev Chronic Dis 2015;12:E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. National Council on Aging . Evidence‐based falls prevention programs. URL: https://www.ncoa.org/article/evidence-based-falls-prevention-programs.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Appendix S1 Supporting Information
Appendix S2 Supporting Information
Appendix S3 Supporting Information


