Abstract
Objective
Despite proven benefits, less than half of patients with rheumatoid arthritis (RA) are treated using a treat‐to‐target (TTT) strategy. Our objective was to identify critical discrepancies between rheumatologist and patient mental models related to the treatment of RA to inform interventions designed to increase implementation of TTT.
Methods
We developed rheumatologist and patient mental models using the Mental Models Approach to Risk Communication. We conducted semistructured interviews to elicit views related to RA treatment decisions with 14 rheumatologists and 30 patients with RA. We also included responses (n = 284) to an open‐ended question on a survey fielded to augment qualitative descriptions from the interviews. Interviews were transcribed and coded independently by two members of the research team.
Results
Rheumatologist and patient mental models for RA treatment are significantly more complex than the TTT model. Both consider domains (system factors and patient readiness) outside of disease activity measurement, target setting, and risk versus benefit assessment in their decision‐making. Furthermore, specific factors were found to be unique to each model. For example, the physician model stresses the importance of evaluating disease activity over time and patient adherence. In contrast, patients discussed the impact of chronic disease weariness, medication‐related fatigue, the importance of feeling adequately informed, and stress associated with changing medications.
Conclusion
We found several discrepancies primarily related to information gaps and differences in how patients and physicians value trade‐offs that can serve as specific targets to improve patient–physician communication and ultimately inform interventions to improve uptake of TTT.
Significance & Innovations.
Mental models refer to knowledge, beliefs, and/or attitudes that develop over time in response to an individual's experiences, values, and worldviews.
Rheumatologist and patient mental models regarding the treatment of RA include distinct factors.
The discrepancies between rheumatologist and patient mental models identified can be used to inform interventions to improve implementation of TTT.
INTRODUCTION
Despite proven benefits and widespread endorsement, implementation of treat‐to‐target (TTT) strategies in rheumatoid arthritis (RA) across the United States and Europe is low, with most studies demonstrating appropriate escalation of treatment in fewer than 50% of patients in the setting of elevated disease activity (1, 2, 3, 4, 5, 6). Discordance between rheumatologists’ global impressions and composite disease activity scores, as well as patient comorbidities, influences adherence to TTT (2, 7). Nonetheless, TTT rates are low even among patients with moderate to high levels of disease activity attributable to ongoing inflammation who do not have contraindications to escalating care (2).
System‐related factors, including poor access to rheumatologists, time constraints, and inadequate insurance, are known barriers to implementing TTT (2, 8). Additional important barriers include discordances between patients’ and clinicians’ assessments of disease activity, thresholds to change treatment, and evaluation of risk (2, 8, 9, 10, 11). For these reasons, patient reluctance to add or switch disease‐modifying antirheumatic drugs (DMARDs) is the most commonly cited reason for not adhering to TTT in clinical practice (2, 11).
The objective of this study was to better understand rheumatologists’ and patients’ decision‐making regarding TTT using the Mental Models Approach to Risk Communication (MMARC) derived from decision science (12). Mental models refer to the networks of knowledge, beliefs, and/or attitudes that develop over time in response to an individual's experiences, values, and worldviews. The MMARC provides a rigorous framework to develop interventions that address critical discrepancies between the mental models held by different populations, in this context, rheumatologists and patients. The MMARC has been applied to improve decision‐making across several health domains, including breast cancer (13), sexually transmitted infections (14), and vaccines (15). In this study, we sought to develop rheumatologist and patient models related to treatment decisions in RA to identify key discrepancies that can be targeted in future interventions to improve implementation of TTT. Because treatment options that are consistent with one's mental models are likely to be accepted, whereas those that are conflicting are more likely to be rejected, defining and addressing gaps between these models is necessary to minimize patient reluctance as a barrier to TTT.
PATIENTS AND METHODS
Participants
We interviewed rheumatologists in the United States with expertise in TTT. Rheumatologists were invited by email to participate and did not receive any compensation.
Patients were recruited via email invitations for either an in‐depth interview or a subsequent online survey (to quantify the prevalence of the beliefs found in the patient interviews) through ArthritisPower, a research registry created by Global Healthy Living Foundation (GHLF)/CreakyJoints and The University of Alabama at Birmingham (16). Informed consent was provided online through an internal GHLF survey. Eligible patients were at least 21 years of age, fluent in English, lived in the United States, had a physician diagnosis of RA, were taking DMARD(s), and were currently under the care of a rheumatologist. Patients participating in the interviews and survey were compensated with $50 and $25 gift cards, respectively. The research protocol was approved by the Institutional Review Boards at Advarra, Carnegie Mellon University, and Yale University School of Medicine.
The online survey (see Supplementary Material) was developed to further reflect the concepts that emerged in the patient interviews, helping provide quantitative data regarding the relative importance of the beliefs identified in the qualitative interviews. Although the quantitative data from the surveys will be reported elsewhere, we include selected responses from an open‐ended question on the follow‐up survey, “Is there anything else you'd like to mention that wasn't covered in this survey?” to augment qualitative descriptions that emerged from the interviews. The survey items were asked on a 5‐point Likert‐type scale, with labels corresponding to equal‐spaced intervals to allow for parametric analyses, and were completed via the Qualtrics survey platform.
Interviews
The Carnegie Mellon University team (JD and ML) created the initial drafts of the interview guides, and then the rest of the research team (BH, SJB, JRC, LRH, WBN, CW, SV, and LF) provided edits and comments prior to pilot testing. The rheumatologist interview guide was pilot tested with two rheumatologists at Yale, and the patient interview guide was pilot tested with six patients with RA recruited by the GHLF. The patient participants provided comments that were summarized, discussed, and addressed by the research team. The interview guide and survey were then edited to reflect the patient participants’ input. Interview guides are included in Supplementary Figure 1. Interviews were conducted (and recorded) over the phone by trained interviewers with the rheumatologists (BH knew three of the interviewees professionally) and patients (ML did not know the interviewees) and transcribed verbatim by a professional service.
One coder (LF) divided each transcript into discrete blocks so that ideas were separated into individual codable statements. Two members of the research team (LF and BH) independently analyzed the transcripts and the open‐text responses from the survey using the identical model and codes. We coded interview statements and open‐text responses according to a representation of factors (grouped by domain) influencing implementation of TTT and added new elements to represent concepts that emerged in the interviews. The full research team discussed how best to integrate each new concept into the model before using it as a code to apply to future transcripts. No new codes emerged in the last two interviews, suggesting that saturation was reached. The coders met at regular intervals to review, compare, and resolve discrepancies. Final agreement between coders was high (κ ≥ 0.95). Based on the codes that were assigned to each set of interviews, we created one model representing how rheumatologists conceptualize TTT and another representing how patients conceptualize decisions related to their treatment.
RESULTS
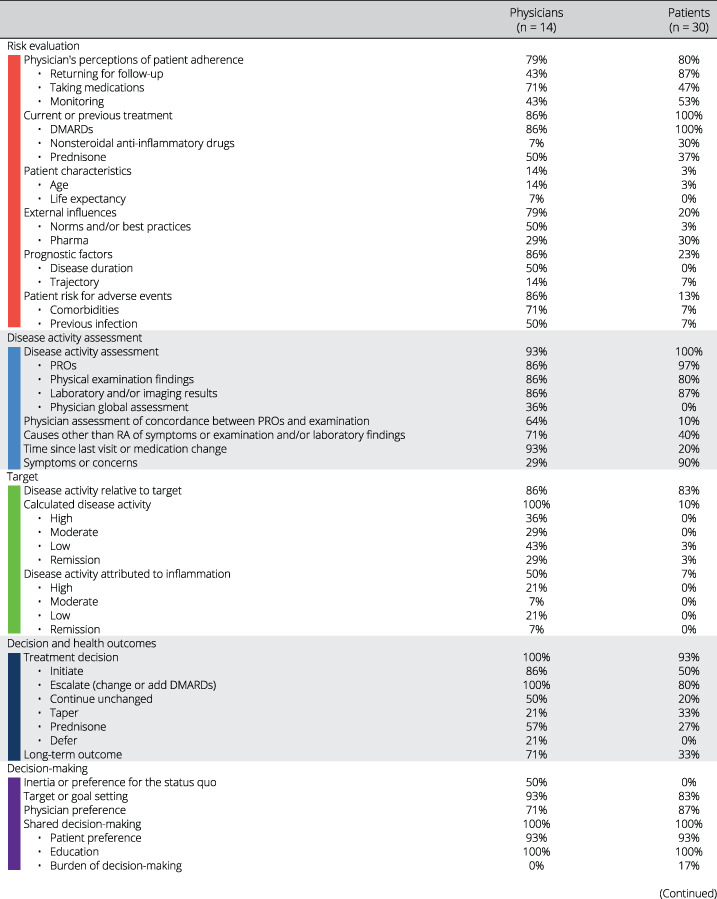
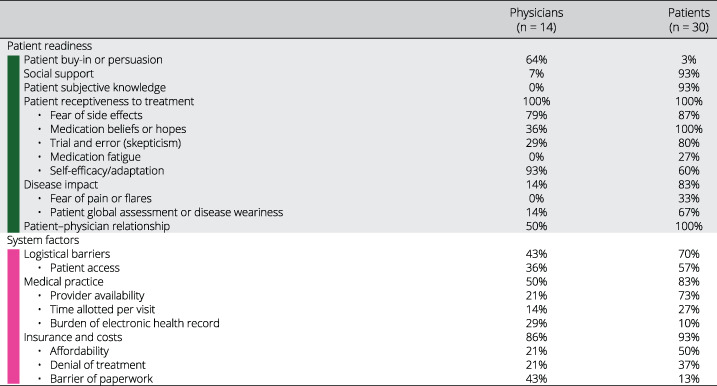
We present findings descriptively, highlighting common threads and codes that emerged, with representative quotations. We only present concepts that emerged for more than one respondent to prevent overinterpreting idiosyncratic ideas. We included the concepts reported by only one respondent in the Supplementary Material. We report the prevalence of each concept mentioned using the corresponding code (Table 1).
Table 1.
Prevalence of concepts discussed
|
|
Abbreviations: DMARD, disease‐modifying antirheumatic drug; PRO, patient‐reported outcome; RA, rheumatoid arthritis.
Comparison of models
The TTT model is specific and prescriptive: measure disease activity and adjust treatment to achieve or maintain a target. Figure 1 represents this basic model and the factors influencing TTT reported by the rheumatologists in this study that have already been discussed in previous studies, including patient comorbidities, medication lag, and discrepancies between disease activity score and medical doctor (MD) global assessment, which all account for warranted variability in implementation of TTT. However, mental models of treatment decision‐making in RA are much more complex, as illustrated in Figures 2 and 3.
Figure 1.
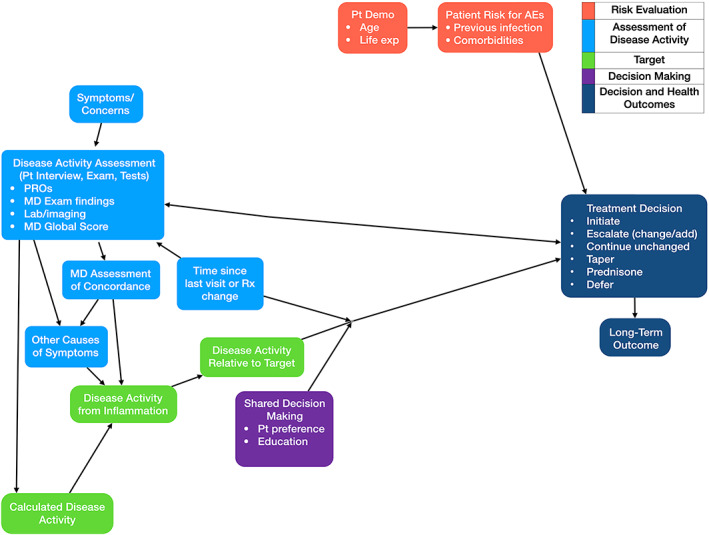
Factors accounting for warranted variability in implementation of TTT. The thickness of each outline reflects how many respondents mentioned the corresponding concept. Blank nodes represent concepts that were not mentioned by more than one respondent in that population. Bullet points (representing elements of the parent concept) are omitted from nodes where they were not mentioned by more than one respondent in that population. Arrows depict proposed predictive relationships between concepts and do not imply empirically supported causal relationships. AEs, adverse events; Demo, demographics; MD, medical doctor; PROs, patient‐reported outcomes; Pt, patient; Rx, medication; TTT, treat‐to‐target.
Figure 2.
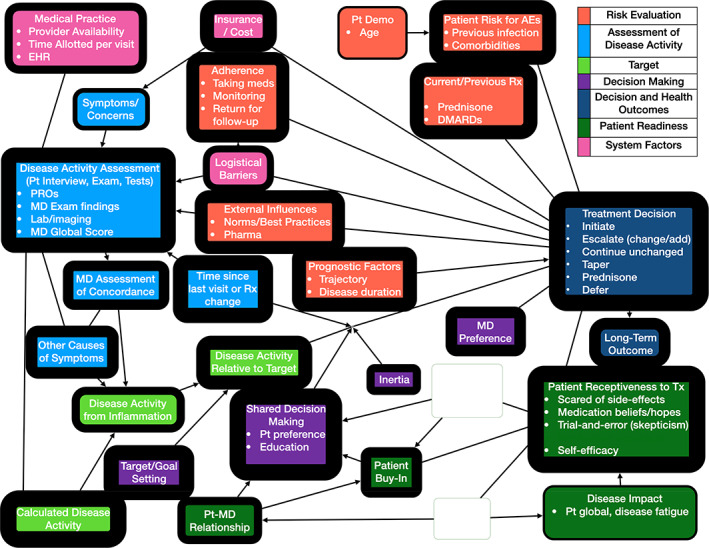
Rheumatologist mental model of TTT. The thickness of each outline reflects how many respondents mentioned the corresponding concept. Blank nodes represent concepts that were not mentioned by more than one respondent in that population. Bullet points (representing elements of the parent concept) are omitted from nodes where they were not mentioned by more than one respondent in that population. Arrows depict proposed predictive relationships between concepts and do not imply empirically supported causal relationships. AEs, adverse events; Demo, demographics; EHR, electronic health record; MD, medical doctor; PROs, patient‐reported outcomes; Pt, patient; Rx, medication; TTT, treat‐to‐target; Tx, treatment.
Figure 3.
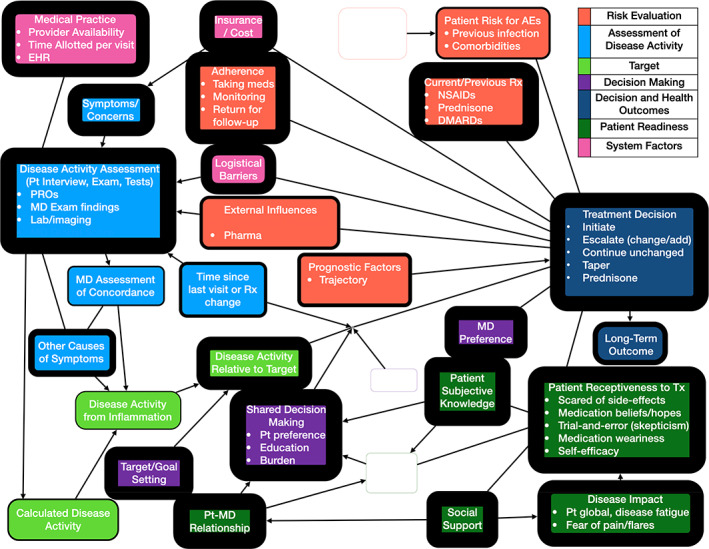
Patient mental model of RA treatment. The thickness of each outline reflects how many respondents mentioned the corresponding concept. Blank nodes represent concepts that were not mentioned by more than one respondent in that population. Bullet points (representing elements of the parent concept) are omitted from nodes where they were not mentioned by more than one respondent in that population. Arrows depict proposed predictive relationships between concepts and do not imply empirically supported causal relationships. AEs, adverse events; Demo, demographics; EHR, electronic health record; MD, medical doctor; PROs, patient‐reported outcomes; Pt, patient; RA, rheumatoid arthritis; Rx, medication; Tx, treatment.
Rheumatologist model
For the rheumatologist model, we conducted in‐depth interviews with 14 rheumatologists (nine women, five men): 13 with recognized expertise in RA across 11 academic medical centers in 10 states and one in private practice. The rheumatologist mental model includes factors included in Figure 1 and two other domains: system factors and patient readiness. In addition, four factors were added to the risk evaluation domain: prognostic factors, adherence, external influences, and current or previous treatment. With respect to prognostic factors, several physicians stated that treatment decisions should be made based on the overall trajectory of disease activity rather than the disease activity score obtained at the point of care. Physicians’ impression of expected patient adherence also influenced decision‐making. External influences reflected both the positive pressure to adhere to clinical practice guidelines and the negative impact of perceived overselling of TTT by pharmaceutical companies. Patient experience (amount of benefit as well as intolerance) on current and previous medications (non‐steroidal anti‐inflammatory drugs (NSAIDs), prednisone, and DMARDs) was also frequently discussed as an important factor in decision‐making. The added patient readiness domain addresses patient reluctance to start, add, or change DMARDs and the additional time required to increase patient buy‐in to escalating care (eg, to effectively educate patients regarding the rationale to TTT and to address erroneous medication beliefs). System‐related barriers to implementing TTT at the patient level (eg, logistical barriers such as difficulty obtaining laboratory tests or attending follow‐up visits due to lack of transportation or work requirements), practice level (lack of provider availability, restricted visit times), and insurance level (completing paperwork for authorization, affordability, and denial of treatment) were all discussed by the rheumatologists.
Patient model
For the patient interviews, a total of seven emails were sent between January 21, 2020, and March 4, 2020, to a total of 410 recipients within the ArthritisPower registry based on eligibility. The emails had an open rate of 52.4% and a click rate of 34.4% of people who opened the email; ultimately, 30 patients completed the interviews: 17 were women; age ranged from 29 to 81 years (mean [SD] 56.9 [13.7]); and 21 self‐identified as White, seven as Black or African American, two as Native American or Alaska Native, and one as “other.” Black patients were purposefully oversampled to ensure adequate input from a population that may be more risk averse (17, 18).
For the patient surveys, we sent 16,321 email invitations, each with a unique survey link; 4681 emails were opened (28.7%) and 1354 survey links were accessed (8.3% of invitations, 28.9% of opened emails), resulting in 804 completed online eligibility screenings. From the completed screenings, 640 patients were eligible for participation and were provided with a survey link tying their responses to their eligibility screening. Among 640 patients who completed the online survey, 44% (n = 284) submitted open‐ended responses that were included in the analysis; 88% were women, and age ranged from 23 to 93 years (mean [SD] 58.6 [11.3]). Most (n = 252) self‐identified as White, nine as Black or African American, three as Native American or Alaska Native, two as Asian, 13 as multiracial, and five as “other.”
The patient model includes the same domains as the physician model. However, there were discrepancies in the factors that were discussed. Notably absent from the patient mental model is the cornerstone of TTT: generating a disease activity score based on a validated instrument. Patients rarely mentioned the procedures by which physicians assess disease activity relative to a target, reflecting the relative lack of transparency of this process to them. In contrast, the patient readiness domain is very prominent in the patient mental model. This domain includes two factors not addressed in the physician model: disease impact (emphasizing factors over and above pain and functional status) and patient subjective knowledge (ie, perception of being informed). The current and anticipated impact of RA on emotional, mental, and physical well‐being featured prominently in patients’ decision‐making. Chronic disease weariness and medication fatigue were uniquely discussed by patients. They described the importance of having adequate knowledge, including from sources outside their rheumatologist, to be able to make treatment changes. Several discussed the burden of decision‐making associated with considering the trade‐offs related to continuing with the status quo versus changing or adding DMARDs. The uncertainty regarding whether and how much one will benefit from a new DMARD and the risk of experiencing new side effects were frequently mentioned as adverse aspects of changing medications. The importance of the patient–physician relationship and support (in terms of encouraging use of medications and providing tangible assistance, such as transportation) were included in both models, but both were discussed much more frequently by patients.
Interviews
Table 1 presents all factors raised in the interviews. The following sections highlight the concepts that have been less well described in the literature.
Rheumatologist interviews
Risk evaluation: disease trajectory
Some described the known variability in disease activity inherent to RA and questioned basing treatment decisions on a single assessment at the point of care: “[M]aybe at the next visit they'll go down without altering therapy, so I try to look at the trajectory of where people are at rather than just a single moment in time” (MD5).
External influences
Although the benefits of TTT were recognized by all, some cited the potential unwelcome influence of pharmaceutical companies: “[I]t was promoted and pushed a lot by pharmaceutical companies to escalate utilization of their therapies” (MD2); “There is a part of me that thinks the TTT approach is really to sell more drugs and is very pharmaceutically driven” (MD4); “[The] pharmaceutical companies want us to treat aggressively because they sell more drugs” (MD6).
Current and previous treatment
Rheumatologists described how the patients’ previous DMARD experience factored into their treatment decisions: “I think we're doing the patients and the rheumatologists a disservice by suggesting you can achieve low disease activity or remission after you fail the first biologic or small molecule plus methotrexate” (MD8).
Adherence
Poor adherence to starting or continuing medication as well as to returning for follow‐up visits was also discussed as a barrier to TTT: “[Adherence is] a huge issue and one of the things that we always talk about” (MD3). Another physician paraphrased: “You gave me that medication and I did not take it because I got concerned and I did not want to call you” (MD10). Some described specific strategies to address poor adherence: “I'm going to probably have them come back in two weeks because I don't want to have them try something and then give up on it and not come back” (MD1); “I think we need to do a better job of eliciting why, is it the bus fare to get to the hospital, to get to the lab, working full time and not being able to get to the lab during their hours” (MD4).
Assessment of disease activity
Rheumatologists widely acknowledged the importance of using disease activity scores as part of the TTT paradigm: “[H]old one's feet to the fire to maximize care” (MD2). Some also criticized the strategy as being too prescriptive. “There's a risk there because if you only play things by the numbers…then you must change the therapy” (MD9). Others insinuated that TTT was too prescriptive and noted the limitations of practicing medicine according to an algorithm: “One cannot give a robot a Clinical Disease Activity Index and then have the robot follow an algorithm” (MD3).
Decision‐making: inertia and preference for the status quo
Physicians discussed how physicians and patients may be complacent in “continuing the same therapy” (MD4) because it is “easier” (MD6). “The fellow will…say, ‘Mrs. X says she is fine.’ I'll say, ‘Does she have any synovitis?’ ‘Well, yes,…but it's chronic and she says she's fine.’ If patients don't complain,…sometimes we don't take action” (MD6). Physicians also described patients as being reluctant to change the status quo. One rheumatologist paraphrased a patient with active RA: “I don't want to change anything, I'm going on a trip or I'm happy with the meds right now and I'm getting by” (MD4).
Patient readiness: use of laboratory tests and imaging
Physicians discussed the role of inflammatory markers and imaging in their own decisions about whether to escalate care and also described using these assessments as a “bartering tool” (MD4) to persuade patients: “Now I probably would use imaging to convince that patient that we need to go a little further” (MD6); “I show them their x‐rays compared to what a normal x‐ray looks like and emphasize that if we see this much change over a year or two, imagine over 10 years how much that could be magnified and that could translate into deformities in your hands, not being able to use them” (MD9).
Patient interviews
This section provides a more detailed description of selected concepts emphasized by patients as represented in their mental model (Figure 3 and Table 1).
Patient readiness: subjective knowledge
Active participation in TTT requires that patients feel adequately informed. Almost all patients emphasized the need to obtain information from sources beyond their rheumatologists prior to considering treatment changes: “I get on the internet and I'll ask lots of questions, look at websites on this. And then, I ask the doctor lots of questions” (patient interview 10, age 66, male); “I feel like there's an opportunity to learn from others (other patients). I know more what to expect out of treatment and…how much better should I be feeling” (patient interview 5, age 35, male).
Medication fatigue
Some patients described medication fatigue as a significant barrier to starting or continuing RA medication: “I am tired of medicine. That's why it's hard for me to start taking medicine” (patient interview 1, age 40, female); “I was really tired of pills” (patient interview 20, age 58, female); “So tired of the pills. I figure I've taken over 30,000 pills since I was diagnosed” (patient survey 147, age 30, female).
Medication beliefs
Illness perceptions related to RA and its treatment also influenced the patient's model. Some perceived being seronegative as a significant obstacle because of perceived uncertainty related to their diagnosis or differences in response to treatment (compared with seropositive patients): “[A]s a seronegative RA patient, I find it very difficult to find the right combination of medications to manage RA” (patient survey 4, age 53, female); “It's hard to accept taking medication for RA when blood work does not support a diagnosis of RA” (patient survey 193, age 64, female); “Having seronegative RA can make treatment decisions more difficult” (patient survey 35, age 64, female). Some held beliefs that they could achieve an acceptable outcome while limiting medical treatment: “I sort of have this goal that I would like to get completely medication‐free” (patient interview 16, age 64, female). Many patients viewed diet as an essential component of their treatment. Some hoped to find a diet that would allow them to discontinue medication: “I've really changed my diet a lot and doing an anti‐inflammatory diet, and hoping this stuff goes away” (patient interview 9, age 65, male). Others expressed interest in diet but acknowledged its limitations: “I wonder about dietary type things, whether there is something that could be done with my diet that would make some of the inflammation go away. Part of me is skeptical about the whole diet thing” (patient interview 16, age 64, female).
Decision‐making burden of changing treatment
Patients described significant trade‐offs between staying with the status quo and starting a new medication: “The best time to change medication is when you don't have any other changes going on in your life, because you can't predict how you're going to react to the medication and what kind of side effects you're going to have, so it could disrupt anything else you have planned” (patient interview 30, age 67, female); “My hope is that this medicine will prevent further joint damage and I'll maintain some of what I still have. If that doesn't happen, I'm going to be very bitter that the last few mobile years of my life have been spent in 2 days a week brain fog without sun, wine, and a head full of thick hair” (patient survey 278, age 44, female). Some emphasized the burden of decision‐making: “If it might stop working, what will happen? I have to think about every little thing. To me they're not small decisions” (patient interview 1, age 40, female); “I certainly had to weigh out each option and decide what was best…do you go for the high‐risk medication so that you have a better quality of life or do you have less potential future impacts from the drugs with your health and more…poor quality life?” (patient interview 14, age 40, female). Moreover, many of these descriptions included emotions with negative valence, such as fear: “It's sometimes a scary thought of changing meds, because of the fear of it not working and starting all over” (patient survey 80, age 42, female); “I have cycled through several medications and it is always scary when you come to the end of one medication's effectiveness because you don't know for sure whether the next you try will work or cause side effects more problematic than the pain and stiffness (of RA)” (patient survey 91, age 50, female).
Skepticism about a trial and error approach
Patients expressed varied opinions about their rheumatologists’ approaches to treatment. Some voiced an accurate understanding of the limitations of current practice: “We had to find out what worked and what didn't” (patient interview 1, age 40, female); “My understanding was that she was following what is sort of the accepted approach” (patient interview 3, age 64, male); “It is difficult to find the ‘right’ medication as we are all different and have different responses to meds” (patient survey 48, age 75, female).
However, many were skeptical about their rheumatologist's trial and error approach: “I guess if it was up to him and I didn't turn it down, we would be playing musical chairs [with medication]” (patients interview 5, age 35, male); “It's more like throwing spitballs at the wall to see which one will work” (patient interview 12, age 57, male); “It was an experiment, and he would add medication, he'd change the medication…I felt like a lab rat” (patient interview 10, age 66, male).
Risk evaluation: external influences
Like physicians, some patients also described concerns regarding the influence of pharmaceutical companies on clinicians’ decision‐making: “The medications I was given based on maybe outside influence on the doctors from pharmaceutical companies rather than good science. I have always felt doctors tended to change to whatever the ‘newest’ drug advertised was” (patient survey 96, age 69, female); “I would just like to say, I feel most rheumatologists prescribe medications solely on what the drug companies say” (patient survey 153, age 56, female); “Why don't more doctors try to find natural remedies? Is it because the drug companies would lose too much money?” (patient survey 286, age 59, male).
DISCUSSION
In this study, we developed rheumatologist and patient mental models related to RA treatment. We sought to compare and highlight discrepancies that may be targeted to improve risk communication and uptake of TTT. As in previous studies, lack of access to rheumatology care for initial and follow‐up visits, limitations of using disease activity scores (eg, elevated scores not reflective of RA disease activity), time constraints, patient preference, and insurance and cost were identified as potential barriers to TTT. However, both rheumatologists and patients described additional factors that are important to consider.
Although rheumatologists all acknowledged how a TTT strategy improves delivery of care in RA, several expressed concerns that the model may be too prescriptive. Some rheumatologists also questioned the value of applying TTT to patients who have not responded to one or more biologic or targeted synthetic DMARDs. This view is in keeping with the American College of Rheumatology 2021 guidelines for the treatment of RA, in which TTT is strongly recommended for patients who have not been previously treated with a biologic or targeted synthetic DMARD but is conditionally recommended for those who have had an inadequate response to one of these medications (19). Several queried whether decisions to escalate treatment should be based on a disease activity score measured at a single time point, suggesting that monitoring disease activity over time (eg, by using mobile technology) may provide valuable supplemental information to support TTT decision‐making (20). Moreover, repeated measures may also act as a nudge to overcome both physician inertia and patient reluctance to abandon the status quo.
Patients brought up several issues that can be addressed to improve patient–physician communication and implementation of TTT. First, many emphasized a strong preference to obtain information from distinct sources when making treatment decisions. They described the importance of obtaining accurate and up to date medical information from their rheumatologists, as well as the need to learn about patient experiences from their peers. The latter was seen by many as a critical component in their decision‐making process that could not be satisfied by health professionals. Although the use of patient testimonials can have both positive and negative impacts on decision‐making, it is important to recognize that many patients require this information to engage in the TTT process with their rheumatologist. Therefore, rheumatologist referral to trustworthy resources may expedite TTT in some patients.
Regardless of the lack of data supporting specific diets or dietary restrictions in patients with RA, the desire to treat illness through dietary manipulation is ubiquitous. The interviews suggest that it is critical for rheumatologists to meet patients where they are. For example, by explaining how optimizing diet may complement, but not substitute for DMARDs, is likely to be more constructive than dismissing dietary interventions because of the lack of supporting evidence.
Some patients described a significant downside to switching DMARDs. The potential harm in switching represents a key discrepancy between how patients and physicians value the trade‐offs inherent to TTT. Recognizing patients’ difficulty of starting a new medication with uncertain benefits and potential new side effects is a prospective target to facilitate uptake of TTT and presents a scenario in which learning from other patients’ experiences may be particularly valuable.
Although the interviews led to an improved understanding of how rheumatologists and patients conceptualize decision‐making in RA, there are several important limitations to our study. First, the patients interviewed were volunteers who responded to an email invitation to participate. Thus, their views are not necessarily generalizable to the greater RA population. Most notably, they are more likely to have strong information preferences and prefer taking an active role in their care because they were recruited from an online patient community. Another limitation is that our study population was predominantly female. This may be partially attributed to sex differences in the prevalence of RA as well as to the increased likelihood of women opting into studies than men (21). Additionally, the vast majority of patients who completed the survey were White, which is often seen with patient registries or organizations. Taking into account the racial and ethnic disparities in RA for minority populations further underscores the importance of increasing racial and ethnic minority group representation (22, 23). Lastly, although we report how frequently each concept was coded, these data do not reflect the prevalence of specific factors in the rheumatologist or patient population.
The purpose of the MMARC is to identify key discrepancies between rheumatologist and patient mental models related to treatment decisions in RA to improve communication and change behavior. In this study, we found several discrepancies primarily related to information gaps and differences in how patients and physicians value trade‐offs in decision‐making regarding TTT. Specifically, patients who struggle to initiate, add, or switch DMARDs emphasized the importance of learning from their peers prior to being ready to engage in shared decision‐making with their rheumatologists. To address this need, we filmed videos of patients with RA recounting their experiences facing similar decisions. Our next steps are to determine how best to incorporate these patient narratives into clinical practice.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Liana Fraenkel had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Hsiao, Downs, Lanyon, Blalock, Curtis, Harrold, Nowell, Wiedmeyer, Venkatachalam, Fraenkel
Acquisition of data
Hsiao, Downs, Lanyon, Nowell, Venkatachalam, Fraenkel
Interpretation and analysis of data
Hsiao, Downs, Lanyon, Blalock, Curtis, Harrold, Nowell, Wiedmeyer, Venkatachalam, Fraenkel
Supporting information
Disclosure Form:
Supplementary Figure 1. Interview guide for patient interviews.
Supplementary Figure 2. Rheumatologist mental model of TTT.
Supplementary Figure 3. Patient mental model of TTT.
ACKNOWLEDGMENTS
We would like to acknowledge the tremendous contribution of the participants who participated in the in‐depth interviews and survey as well as the Global Healthy Living staff who helped set up the patient recruitment process for the qualitative interviews: Danielle Ali, Jessica Boles, Kelly Gavigan, and Laura Stradford.
No potential conflicts of interest relevant to this article were reported.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr2.11443&file=acr211443‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Harrold LR, Harrington JT, Curtis JR, Furst DE, Bentley MJ, Shan Y, et al. Prescribing practices in a US cohort of rheumatoid arthritis patients before and after publication of the American College of Rheumatology treatment recommendations. Arthritis Rheum 2012;64:630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zak A, Corrigan C, Yu Z, Bitton A, Fraenkel L, Harrold L, et al. Barriers to treatment adjustment within a treat to target strategy in rheumatoid arthritis: a secondary analysis of the TRACTION trial. Rheumatology (Oxford) 2018;57:1933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Solomon DH, Losina E, Lu B, Zak A, Corrigan C, Lee SB, et al. Implementation of treat‐to‐target in rheumatoid arthritis through a learning collaborative: results of a randomized controlled trial. Arthritis Rheumatol 2017;69:1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Markusse IM, Dirven L, Han KH, Ronday HK, de Sonnaville PBJ, Kerstens PJ, et al. Evaluating adherence to a treat‐to‐target protocol in recent‐onset rheumatoid arthritis: reasons for compliance and hesitation. Arthritis Care Res (Hoboken) 2016;68:446–53. [DOI] [PubMed] [Google Scholar]
- 5. Hetland ML, Jensen DV, Krogh NS. Monitoring patients with rheumatoid arthritis in routine care: experiences from a treat‐to‐target strategy using the DANBIO registry. Clin Exp Rheumatol 2014;32 Suppl 85:S141–6. [PubMed] [Google Scholar]
- 6. Gvozdenovic E, Allaart CF, van der Heijde D, Ferraccioli G, Smolen JS, Huizinga TW, et al. When rheumatologists report that they agree with a guideline, does this mean that they practise the guideline in clinical practice? Results of the International Recommendation Implementation Study (IRIS). RMD Open 2016;2:e000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ford JA, Solomon DH. Challenges in implementing treat‐to‐target strategies in rheumatology. Rheum Dis Clin North Am 2019;45:101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harrold LR, Reed GW, John A, Barr CJ, Soe K, Magner R, et al. Cluster‐randomized trial of a behavioral intervention to incorporate a treat‐to‐target approach to care of US patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2018;70:379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fraenkel L, Cunningham M. High disease activity may not be sufficient to escalate care. Arthritis Care Res (Hoboken) 2014;66:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Studenic P, Radner H, Smolen JS, Aletaha D. Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis Rheum 2012;64:2814–23. [DOI] [PubMed] [Google Scholar]
- 11. Tymms K, Zochling J, Scott J, Bird P, Burnet S, de Jager J, et al. Barriers to optimal disease control for rheumatoid arthritis patients with moderate and high disease activity. Arthritis Care Res (Hoboken) 2014;66:190–6. [DOI] [PubMed] [Google Scholar]
- 12. Morgan MG, Fischhoff B, Bostrom A, Atman C. Risk communication: a mental models approach. 1st ed. Cambridge (UK): Cambridge University Press; 2002. [Google Scholar]
- 13. Downs JS, Bruine de Bruin W, Fischhoff B, Hesse B, Maibach E: How people think about cancer: a mental models approach. In: Heath RL, O'Hair D, editors. Handbook of risk and crisis communication. New York: Routledge; 2009. p. 507–24. [Google Scholar]
- 14. Downs JS, Bruine de Bruin W, Fischhoff B, Murray PJ. Behavioral decision research intervention reduces risky sexual behavior. Curr HIV Res 2015;13:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Downs JS, de Bruin WB, Fischhoff B. Parents' vaccination comprehension and decisions. Vaccine 2008;26:1595–607. [DOI] [PubMed] [Google Scholar]
- 16. Nowell WB, Merkel PA, McBurney RN, Young K, Venkatachalam S, Shaw DG, et al. Patient‐powered research networks of the Autoimmune Research Collaborative: rationale, capacity, and future directions. Patient 2021;14:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Constantinescu F, Goucher S, Weinstein A, Fraenkel L. Racial disparities in treatment preferences for rheumatoid arthritis. Med Care 2009;47:350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Constantinescu F, Goucher S, Weinstein A, Smith W, Fraenkel L. Understanding why rheumatoid arthritis patient treatment preferences differ by race. Arthritis Rheum 2009;6:413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fraenkel L, Bathon JM, England BR, St Clair EW, Aryssi T, Carandang K, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2021;73:924–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seppen BF, L'Ami M J, Duarte dos Santos Rico S, Ter Wee MM, Turkstra F, Roorda LD, et al. A smartphone app for self‐monitoring of rheumatoid arthritis disease activity to assist patient‐initiated care: protocol for a randomized controlled trial. JMIR Res Protoc 2020;9:e15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kvien TK, Uhlig T, Odegard S, Heiberg MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci 2006;1069:212–22. [DOI] [PubMed] [Google Scholar]
- 22. Greenberg JD, Spruill TM, Shan Y, Reed G, Kremer JM, Potter J, et al. Racial and ethnic disparities in disease activity in patients with rheumatoid arthritis. Am J Med 2013;126:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strait A, Castillo F, Choden S, Li J, Whitaker E, Falasinnu T, et al. Demographic characteristics of participants in rheumatoid arthritis randomized clinical trials: a systematic review. JAMA Netw Open 2019;2:e1914745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form:
Supplementary Figure 1. Interview guide for patient interviews.
Supplementary Figure 2. Rheumatologist mental model of TTT.
Supplementary Figure 3. Patient mental model of TTT.


