Abstract
Objective
Using a large, de‐identified electronic health record database with over 3.2 million patients, we aimed to identify trends of systemic lupus erythematosus (SLE) medication use during pregnancy and birth outcomes from 1989 to 2020.
Methods
Using a previously validated algorithm for SLE deliveries, we identified 255 pregnancies in patients with SLE and 604 pregnancies in controls with no known autoimmune diseases. We examined demographics, medications, SLE comorbidities, and maternal and fetal outcomes in SLE and control deliveries.
Results
Compared with control deliveries, SLE deliveries were more likely to be complicated by preterm delivery (odds ratio [OR]: 6.71; 95% confidence interval [CI]: 4.31‐10.55; P < 0.001) and preeclampsia (OR: 3.22; 95% CI: 1.83‐5.66; P < 0.001) after adjusting for age at delivery, race, and parity. In a longitudinal analysis, medication use during SLE pregnancies remained relatively stable, with some increased use of hydroxychloroquine over time but no increase in aspirin use. For SLE deliveries, preterm delivery and preeclampsia rates remained stable.
Conclusion
We observed rates of preeclampsia and preterm delivery in SLE that were five times higher than the general population and higher compared with other prospective SLE cohorts. Furthermore, we did not observe improved outcomes over time with preeclampsia and preterm delivery. Despite increasing evidence for universal use of hydroxychloroquine and aspirin, we did not observe substantially higher use of these medications over time, particularly for aspirin. Our results demonstrate the continued need to prioritize educational and implementation efforts to improve adverse pregnancy outcomes in SLE.
Significance & Innovations.
Although there are multiple prospective cohort and administrative database studies in systemic lupus erythematosus (SLE) pregnancies, we are among the first to use an electronic health record (EHR) database to assess real‐world outcomes in SLE deliveries.
Using a unique, longitudinal EHR database, we identified a large cohort of SLE and control deliveries across three decades.
We are among the first to assess trends of delivery outcomes in SLE and SLE medication use in pregnancy.
INTRODUCTION
Systemic lupus erythematosus (SLE) pregnancies have been studied using prospective cohorts that are often limited by small sample sizes (1, 2). These studies capture patients with SLE followed in the rheumatology clinic that agree to participate in research studies. Population studies are mainly from European populations that may not reflect the racial diversity seen in US SLE pregnancies (3, 4, 5). Administrative database studies typically increase the sample sizes but often lack granular data to ensure accurate case status (6, 7). These studies often focus on inpatient data and use one count of an SLE billing code from discharge diagnoses despite multiple studies showing that one count of an SLE billing code does not accurately identify SLE cases (8, 9, 10) or SLE pregnancies (11). Electronic health record (EHR) studies bridge these gaps by having relatively large sample sizes (12) and dense, longitudinal data not just on a patient's SLE but also on other comorbidities, medications, and pregnancy outcomes. Furthermore, EHR studies allow researchers to follow patients across multiple care settings and provide easy to accrue, low‐cost, real‐world data on patients who may not enroll in research studies.
Both prospective (1, 2, 13) and administrative database studies (6, 7) along with meta‐analyses (14, 15, 16) in SLE pregnancies demonstrate an increased risk of adverse fetal and maternal outcomes. An administrative study using the National Inpatient Sample database from 1998 to 2015 reported that SLE pregnancy outcomes improved over time, notably both maternal and fetal death (7). This study was limited, however, by using only one count of an SLE billing code and inpatient data to identify SLE pregnancies. Furthermore, this study lacked granular data on race, SLE medication use, SLE comorbidities, and preterm delivery. Using a previously validated algorithm for SLE deliveries (11), we identified SLE deliveries in a large EHR along with controls with no known autoimmune diseases. We examined demographics, medications, SLE comorbidities, and maternal and fetal outcomes in SLE deliveries from 1989 to 2020. We assessed trends over time of SLE medications prescribed during pregnancy.
PATIENTS AND METHODS
Synthetic Derivative
Research was conducted in compliance with the Helsinki Declaration. Because a de‐identified database was used, the study was deemed to be non‐human subjects research, and informed consent was waived. After obtaining approval from the Vanderbilt University Medical Center (VUMC) Institutional Review Board, we identified possible SLE and control pregnancies from the Synthetic Derivative. The Synthetic Derivative is a de‐identified copy of our EHR with longitudinal data since 1989 and has been previously described (12). Briefly, the Synthetic Derivative contains over 3.2 million subjects with clinical data from VUMC inpatient, outpatient, primary care, and subspecialty care encounters. Telephone encounters, nursing communications, medications, laboratory, radiology, and pathology data are also available. Records external to VUMC are not available.
Identifying SLE and control patients
Within the Synthetic Derivative, we identified potential SLE pregnancies using a previously validated and published algorithm that requires at least four codes from the SLE International Classification of Diseases Ninth Revision (ICD‐9) (710.0) or International Classification of Diseases Tenth Revision–Clinical Modification (ICD‐10‐CM) (M32.1, M32.8, or M32.9) and one or more ICD‐9 or ICD‐10‐CM pregnancy or delivery‐related codes (11). This previously validated algorithm uses primarily delivery‐related codes with the goal to identify SLE deliveries or births in the EHR. These delivery codes have been previously validated in other chronic diseases (17, 18, 19) and are in Supplemental Table 1. A flowchart of SLE pregnancy selection is shown in Figure 1A. Our algorithm has a positive predictive value of 81% and a sensitivity of 95% and has been internally and externally validated (11). We performed chart review on all possible SLE deliveries identified by the algorithm to ensure that they had SLE diagnosed by an internal or external rheumatologist. Deliveries to mothers who had possible SLE were excluded. We counted SLE nephritis and antiphospholipid antibody syndrome (APS) if they were documented in clinical notes during that specific SLE pregnancy. We performed chart review and used keywords of “nephritis” and “antiphospholipid antibody syndrome.” We required diagnosis by an internal or external rheumatologist or nephrologist for SLE nephritis and diagnosis by an internal or external rheumatologist or hematologist for APS. For APS, we performed chart review to ensure that patients had both positive antiphospholipid antibodies and a qualifying thrombotic or obstetric event.
Figure 1.
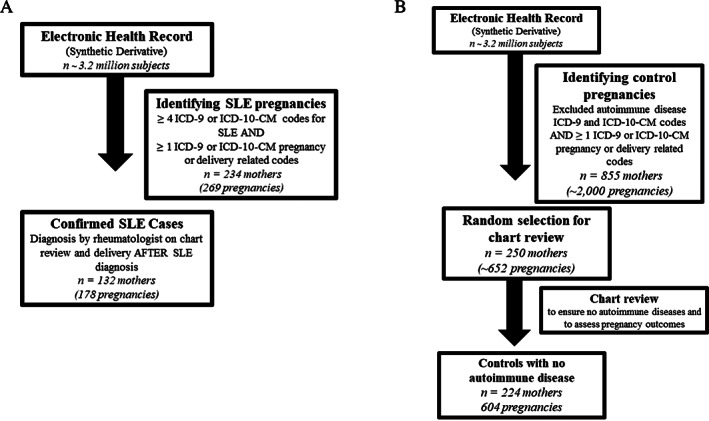
Flowchart of patient selection. (A) SLE pregnancies were selected from the EHR using four or more SLE ICD‐9 (710.0) or ICD‐10‐CM (M32.1 or M32.8 or M32.9) codes while also requiring one or more ICD‐9 or ICD‐10‐CM pregnancy or delivery‐related codes. A full list of these codes is in Supplemental Table 1. We then required chart review to confirm SLE diagnosis by a rheumatologist resulting in 174 SLE cases with 255 pregnancies and 132 SLE cases with 178 pregnancies with restricting to deliveries after SLE diagnosis. (B) Control pregnancies were selected from the EHR using the same pregnancy or delivery‐related codes used for the SLE pregnancies aforementioned along with requiring that controls not have codes for autoimmune diseases. We then chart‐reviewed a random 250 controls to ensure no autoimmune disease and to assess pregnancy outcomes, resulting in 224 mothers with 604 pregnancies. EHR, electronic health record; ICD, International Classification of Diseases; SLE, systemic lupus erythematosus.
Lastly, we examined for medication use during pregnancy with a focus on SLE medications such as antimalarials, corticosteroids, and immunosuppressants as well as aspirin and anticoagulants. In addition to reviewing the patient's medication lists from notes, we performed chart review on the full text or entirety of Obstetrics and gynecology and rheumatology notes to determine medication use during pregnancy and then performed chart review on admission and discharge notes to ascertain medication use at delivery.
Controls were identified in the Synthetic Derivative and did not have ICD‐9 codes under the 710.* heading “Diffuse diseases of connective tissue,” the 714.* heading “Rheumatoid arthritis and other inflammatory polyarthropathies” or ICD‐10 codes M05.* (“Rheumatoid arthritis with rheumatoid factor”), M06.* (“Other rheumatoid arthritis”), M32 (“SLE”), M33.* (Dermatopolymyositis), M34.* (“Systemic sclerosis”), M35.* (“Other systemic involvement of connective tissue”), or M36.* (“Systemic disorders of connective tissue in diseases classified elsewhere”). Controls were “medical home patients” who received longitudinal care at VUMC with at least three outpatient visits within 5 years to ensure that the density of records was similar to that of cases (20, 21, 22). We then ensured that the controls also had 1 or more pregnancy or delivery codes, the same codes used for the SLE deliveries. We then randomly selected 250 of these controls for chart review to ensure the absence of any autoimmune disease and assess delivery outcomes. We performed chart review and excluded controls where autoimmune disease was documented as present or diagnosed during that pregnancy. A flowchart of control pregnancy selection is shown in Figure 1B.
Outcomes assessed
Race and ethnicity were based on both self‐report and administrative data. Prior studies have validated that these EHR race assignments reflect genetic ancestry (23). For SLE and control deliveries, we used the obstetrician diagnosis of preeclampsia, severe preeclampsia, eclampsia, and HELLP syndrome, as documented in clinical notes. Pregnancies were defined as all gestations, including spontaneous abortions. Deliveries were defined as gestations of 20 weeks or more, which included stillbirths, preterm births, and full‐term births. We considered preeclampsia only in pregnancies with a gestation of 20 weeks or more. Preterm delivery was defined as live births with a gestation of less than 37 weeks. Miscarriage was defined as fetal demise at less than 20 weeks, and stillbirth was defined as fetal demise at 20 weeks or more. The duration of SLE was calculated as the time from the first SLE billing code in the EHR or the date documented by a rheumatology note (whichever came first) to the delivery date for that particular pregnancy. Hypertension, diabetes mellitus, and gestational diabetes were all assessed from delivery discharge notes.
Statistical analyses
We performed cross‐sectional and longitudinal analyses of delivery outcomes in both SLE cases and controls. For SLE outcomes, we assessed outcomes in all SLE pregnancies but also compared outcomes that occurred before versus after SLE diagnosis. Our unit of analysis was not a patient with SLE but the SLE delivery. We assessed timing of SLE diagnosis in relation to each delivery. If a delivery occurred prior to the SLE diagnosis date, then it was analyzed as a delivery before SLE. Similarly, if a delivery occurred after the SLE diagnosis date, it was analyzed as a delivery after SLE. We compared categorial variables using χ2 or Fisher's exact test and compared continuous variables using the Mann‐Whitney U test, as there were non‐normal distributions in the data. We performed logistic regression in all deliveries to estimate the association of SLE case status with preterm delivery and preeclampsia after adjusting for age at delivery, race, and parity, defined as number of pregnancies. We also performed logistic regression in SLE deliveries that occurred after SLE diagnosis to measure the association of SLE disease covariates with preterm delivery and preeclampsia adjusting for age at delivery and race. For logistic regression models, odds ratios (ORs) and 95% confidence intervals (95% CIs) are reported. For sample size for our models with preterm delivery, we estimated having 70 SLE deliveries with preterm delivery. Applying the rule of 10‐15 outcomes per one covariate, we estimated having between four and seven covariates in the model to prevent overfitting. For the outcome of preeclampsia with 36 SLE deliveries with preeclampsia, we estimated having two to three covariates. Because both SLE and control mothers could contribute multiple pregnancies, and because parity can impact pregnancy outcomes, we conducted sensitivity analyses in which we only analyzed the first pregnancy for SLE and control mothers if they had multiple pregnancies. In addition, we used the gee package in R to construct a generalized estimating equation (GEE) with an exchangeable correlation structure to account for correlation between multiple pregnancies per patient. Two‐sided P values less than 0.05 were considered significant. Analyses were conducted using R version 4.0.2.
RESULTS
Comparison of SLE and control pregnancies
We identified 255 pregnancies in 174 SLE cases: 178 pregnancies occurring after SLE diagnosis, 60 occurring before SLE diagnosis, and 17 with missing data regarding SLE diagnosis date. We started with 250 control mothers and excluded 2 mothers with missing data and 24 mothers with autoimmune disease discovered on chart review. A list of the autoimmune diseases is included in Supplemental Table 2. We then analyzed 224 control mothers with 604 pregnancies. SLE and control pregnancies are compared in Table 1. The racial background was similar in pregnancies to SLE cases and controls and was predominantly White. More control versus SLE pregnancies occurred in Hispanic mothers (11% vs. 5%, P < 0.01). Mean age at delivery was similar in controls compared with SLE cases (27.0 ± 6.6 vs. 27.3 ± 6.0, P = 0.67). Compared with SLE cases, controls had more pregnancies (3.1 ± 2.0 vs. 2.3 ± 1.6, P < 0.001) and deliveries (2.3 ± 1.3 vs. 1.7 ± 0.9, P = 0.001), defined as gestations of 20 weeks or more. SLE cases and controls had similar rates of live births (82% vs. 83%, P = 0.72). Compared with controls, SLE cases were more likely to have adverse delivery outcomes, including higher rates of cesarean section (47% vs. 31%, P < 0.001), preeclampsia (23% vs. 9%, P < 0.001), and preterm delivery (47% vs. 14%, P < 0.001). Preeclampsia was more likely to occur preterm in SLE cases versus controls (84% vs. 28%, P < 0.001). Both mean gestational age (32.9 ± 8.2 weeks vs. 35.0 ± 9.8 weeks, P < 0.001) and mean birthweights (2.5 ± 1.0 kg vs. 3.2 ± 0.7 kg, P < 0.001) were significantly lower in SLE cases compared with controls. Compared with control pregnancies, SLE pregnancies were more likely to be complicated by hypertension (33% vs. 10%, P < 0.001) and diabetes mellitus (5% vs. 2%, P = 0.05) but not gestational diabetes (7% vs. 6%, P = 0.67).
Table 1.
Comparison of pregnancies to SLE cases and controls
| Characteristics | Pregnancies to SLE cases | Pregnancies to controls | P value |
|---|---|---|---|
| (n = 255) a | (n = 604) a | ||
| Mean age at delivery ± SD (y) | 27.3 ± 6.0 | 27.0 ± 6.6 | P = 0.67 |
| Race (%) | |||
| White | 66% | 59% | P = 0.11 |
| African American | 30% | 38% | |
| Asian | 2% | 2% | |
| Other/Multi‐race | 2% | 1% | |
| Ethnicity (%) | |||
| Hispanic | 5% | 11% | P < 0.01 |
| Mean duration in the EHR (y) ± SD | 13.3 ± 8.3 | 12.0 ± 7.6 | P = 0.18 |
| Parity | |||
| Mean number of pregnancies per mother ± SD | 2.3 ± 1.6 | 3.1 ± 2.0 | P < 0.001 |
| Mean number of deliveries per mother ± SD | 1.7 ± 0.9 | 2.3 ± 1.3 | P = 0.001 |
| Pregnancy outcomes | |||
| Live birth (%) | 82% (194/238) | 83% (502/602) | P = 0.72 |
| Cesarean section (%) | 47% (97/207) | 31% (168/546) | <0.001 |
| Mean gestational age ± SD (wk) | 32.9 ± 8.2 | 35.0 ± 9.8 | <0.001 |
| Mean birthweight ± SD (kg) | 2.5 ± 1.0 | 3.2 ± 0.7 | <0.001 |
| Mean Apgar score at 1 min ± SD | 7.1 ± 2.3 | 7.9 ± 1.3 | 0.07 |
| Mean Apgar score at 5 min ± SD | 8.3 ± 1.5 | 8.8 ± 0.7 | 0.01 |
| Preterm (%) | 47% (90/191) | 14% (71/497) | <0.001 |
| Preeclampsia (%) | 23% (45/199) | 9% (32/368) | <0.001 |
| Preeclampsia occurring preterm (%) | 84% (38/45) | 28% (9/32) | <0.001 |
Abbreviations: EHR, electronic health record; SLE, systemic lupus erythematosus.
N refers to number of pregnancies. For SLE cases, there were 255 pregnancies to 174 mothers with SLE. For controls, there were 602 pregnancies to 224 control mothers.
Because both SLE and control patients could contribute multiple pregnancies, we performed a sensitivity analysis in which we only analyzed the first pregnancy for SLE and control mothers if they had multiple pregnancies. Using this methodology, rates for adverse outcomes did not significantly change in either SLE or control patients compared with our original analyses (Supplemental Tables 3 and 4).
Pregnancy outcomes for mothers with SLE in our study are compared with prior SLE prospective cohorts and the general population in Supplemental Table 5. Compared with prospective SLE pregnancy cohorts, adverse SLE pregnancy outcomes in our EHR study, particularly preterm delivery, were higher (51% vs. 30%). Compared with the general population, our SLE cases had almost 5 times higher rates of preterm delivery (51% vs. 10%) and preeclampsia (25% vs. 5%) (24, 25). Comparing controls in our study to the general population, controls had slightly higher rates of adverse pregnancy outcomes.
Comparison of SLE pregnancies before and after SLE diagnosis
We then compared the 60 SLE pregnancies that occurred before SLE diagnosis to the 178 pregnancies that occurred after SLE diagnosis (Table 2). Overall, there were similar rates of adverse pregnancy outcomes in SLE pregnancies that occurred before and after SLE diagnosis. Rates of preterm deliveries (40% vs. 51%, P = 0.24) and preeclampsia (19% vs. 25%, P = 0.42) were similar in pregnancies that occurred before versus after SLE diagnosis. Mean duration of SLE disease for pregnancies that occurred after SLE diagnosis was 5.4 ± 5.1 years with a median of 4.1 years. For pregnancies that occurred before SLE diagnosis, SLE was diagnosed on average 6.5 ± 5.9 years later with a median of 5.8 years after delivery. There were 10 pregnancies for which the SLE diagnosis occurred within 1 year after the delivery. In deliveries after SLE diagnosis, adverse outcomes, including preeclampsia and preterm delivery, were lower at 5 years or longer after SLE diagnosis compared with 0‐2 and 2‐5 years after SLE diagnosis (Supplemental Table 6).
Table 2.
Comparison of pregnancy outcomes before vs. after SLE diagnosis
| Pregnancy outcomes | SLE pregnancies before SLE diagnosis | SLE pregnancies after SLE diagnosis | P value |
|---|---|---|---|
| (n = 60) | (n = 178) | ||
| Mean age at delivery ± SD (y) | 25.9 ± 6.7 | 28.6 ± 5.6 | 0.02 |
| Live birth (%) | 87% (48/55) | 80% (139/173) | 0.24 |
| Cesarean section (%) | 43% (19/44) | 47% (72/152) | 0.62 |
| Mean gestational age ± SD (wk) | 31.4 ± 10.3 | 31.6 ± 9.2 | 0.58 |
| Mean birthweight ± SD (kg) | 2.6 ± 1.2 | 2.4 ± 1.0 | 0.55 |
| Apgar score at 1 min ± SD | 6.5 ± 2.6 | 7.2 ± 2.3 | 0.60 |
| Apgar score at 5 min ± SD | 7.9 ± 1.4 | 8.3 ± 1.5 | 0.23 |
| Preterm (%) | 40% (17/42) | 51% (70/138) | 0.24 |
| Preeclampsia (%) | 19% (8/42) | 25% (36/144) | 0.42 |
| Preeclampsia occurring preterm (%) | 88% (7/8) | 94% (34/36) | 0.48 |
Abbreviation: SLE, systemic lupus erythematosus.
Characteristics of SLE pregnancies after SLE diagnosis
Of the SLE pregnancies that occurred after SLE diagnosis, 32% were complicated by SLE nephritis during the pregnancy and 20% by APS. Of the patients with SLE with APS, 52% had an obstetrical complication, and 48% had a thrombotic complication. Of the preterm SLE births that occurred after SLE diagnosis, 73% were medically induced owing to hypertensive disorders or SLE flare whereas 27% were spontaneous.
Medication use in SLE pregnancies
We then assessed medication use during pregnancy in pregnancies that occurred after SLE diagnosis (Supplemental Table 7). Medication use was 63% for corticosteroids, 40% for hydroxychloroquine, 14% for azathioprine, 32% for aspirin, and 23% for anticoagulants.
Longitudinal analyses of SLE medication use
We then examined medication use longitudinally from 1989 to 2020 in pregnancies that occurred after SLE diagnosis (Figure 2). Overall, SLE medication use during pregnancy was low. For hydroxychloroquine, use increased over time, with peak use at around 70% of SLE pregnancies in 2015. Use of corticosteroids stayed relatively consistent at 60%‐70%. For azathioprine, use was low at 13% to 20% and stayed relatively consistent. Aspirin use decreased over time ranging from as high as almost 70% in 1995 down to 20% from 2015 to 2020.
Figure 2.
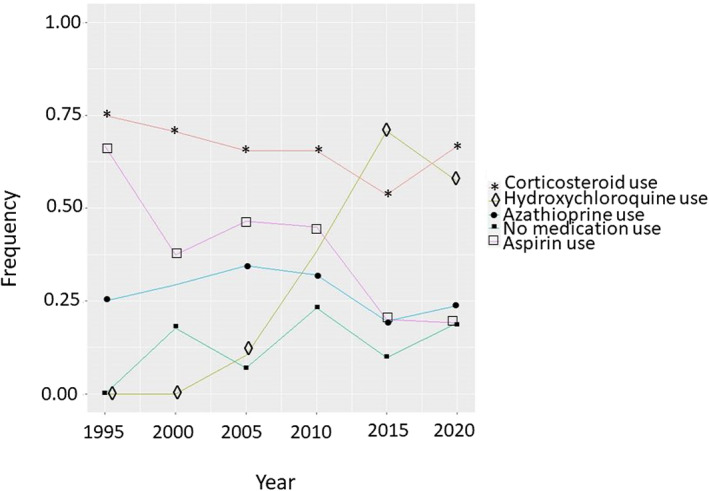
Trends of medication use during SLE pregnancies. The x‐axis shows year SLE pregnancy occurred. The y‐axis shows the proportion of pregnancies with medication use in that particular year. Only SLE pregnancies that occurred after SLE diagnosis were included. For corticosteroids (red line with the stars), we see a relatively steady rate over time. For antimalarials (green line with diamonds), we see an increase in time, with a peak rate of about 70%. For aspirin (purple line with squares), we see a decline in use over time. For azathioprine (blue line with circles), we see low use and a relatively steady rate over time. SLE, systemic lupus erythematosus.
Longitudinal analyses of pregnancy outcomes in SLE cases and controls
We examined pregnancy outcomes longitudinally from 1989 to 2020 in SLE pregnancies that occurred after SLE diagnosis (Figure 3A). In general, pregnancy outcomes were stable over the study period. Live birth and preeclampsia rates were both stable. Cesarean section and preterm delivery rates both increased and then somewhat decreased over time. We also examined these same outcomes in all SLE pregnancies with similar results. We examined control pregnancy outcomes longitudinally from 1989 to 2020 (Figure 3B). Similar to the SLE pregnancies, rates of adverse pregnancy outcomes in the controls remained relatively stable over the study period. Compared with SLE pregnancies, control pregnancies had lower rates of cesarean section, preterm delivery, and preeclampsia.
Figure 3.
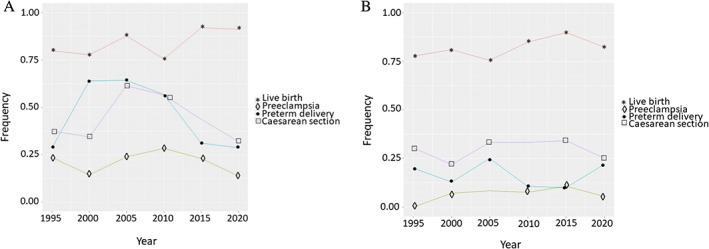
Trends of SLE and control pregnancy outcomes. (A) The x‐axis shows year pregnancy occurred. The y‐axis shows the proportion of pregnancies with the outcome in that particular year. Only SLE pregnancies that occurred after SLE diagnosis were included. Trends of SLE pregnancy outcomes were relatively stable over time, including live birth (red line with stars) and preeclampsia (green line with diamonds). Preterm delivery (blue line with circles) and cesarean section (purple line with squares) increased and then decreased. (B) Trends of control pregnancy outcomes were relatively stable over time. Compared with SLE pregnancies, control pregnancies had lower rates of cesarean section, preterm delivery, and preeclampsia. SLE, systemic lupus erythematosus.
Models for SLE and control pregnancies
We performed a logistic regression model for preeclampsia in SLE and control pregnancies (Table 3). After adjusting for age at delivery and parity, SLE case status (OR: 3.22; 95% CI: 1.83‐5.66; P < 0.001) and African American race (OR: 1.58; 95% CI: 1.08‐2.29; P = 0.02) were both significantly associated with preeclampsia. We also performed a logistic regression to evaluate the risk of preterm deliveries in SLE and control pregnancies. After adjusting for age at delivery, race, and parity, SLE case status (OR: 6.71; 95% CI: 4.31‐10.55; P < 0.001) was significantly associated with preterm deliveries. Estimates from the GEE were nearly identical to those from the main logistic regression models, while the models examining the first pregnancy only also showed significant associations of similar magnitude (Supplemental Table 8).
Table 3.
Models of preeclampsia and preterm delivery in SLE and control pregnancies
| Population | Preeclampsia | Preterm delivery | ||
|---|---|---|---|---|
| Raw OR (95% CI) | Adjusted OR a (95% CI) | Raw OR (95% CI) | Adjusted OR a (95% CI) | |
| SLE vs. control pregnancies | 3.42 (2.03‐5.76) | 3.22 (1.83‐5.66) | 8.27 (5.43‐12.59) | 6.71 (4.31‐10.55) |
| SLE pregnancies only | ||||
| Nephritis vs. non‐nephritis | 1.45 (0.66‐3.17) | 1.01 (0.41‐2.46) | 1.48 (0.74‐2.98) | 1.29 (0.60‐2.77) |
| Corticosteroid user vs. non‐user | 1.13 (0.46‐2.81) | 1.00 (0.38‐2.77) | 1.35 (0.62‐2.95) | 1.43 (0.63‐3.29) |
Abbreviations: OR, odds ratio; SLE, systemic lupus erythematosus.
Adjusted for age at delivery, race, and parity, defined as number of pregnancies.
Models for SLE pregnancies
In unadjusted analyses, there was no association of SLE nephritis with either preeclampsia (OR: 1.45; 95% CI: 0.66‐3.17; P = 0.35) or preterm delivery (OR: 1.48; 95% CI: 0.74‐2.98; P = 0.27) (Table 3). There was also no association of corticosteroid use during pregnancy with either preeclampsia (OR: 1.13; 95% CI: 0.46‐2.81; P = 0.79) or preterm delivery (OR: 1.35; 95% CI: 0.62‐2.95; P = 0.45). After adjusting for age at delivery, race, and parity, corticosteroid use was not associated with preeclampsia (OR: 1.00; 95% CI: 0.38‐2.77; P = 0.99). As there was significant collinearity between corticosteroid use and SLE nephritis, we could not add SLE nephritis to the aforementioned model. In a separate model adjusting for age at delivery, parity, and SLE nephritis, African American race (OR: 2.18; 95% CI: 1.16‐4.21; P = 0.02) was significantly associated with preeclampsia. After adjusting for age at delivery, race, and parity, corticosteroid use was not associated with preterm delivery (OR: 1.43; 95% CI: 0.63‐3.29; P = 0.39). In a separate model, after adjusting for age at delivery, race, and parity, SLE nephritis was also not associated with preterm delivery (OR: 1.29; 95% CI: 0.60‐2.77; P = 0.52).
DISCUSSION
We examined adverse pregnancy outcomes and SLE medication use in a large EHR SLE cohort. As expected, patients with SLE were more likely to have adverse pregnancy outcomes compared with our EHR controls and the US general population. Unfortunately, adverse pregnancy outcomes in patients with SLE, including preterm delivery and preeclampsia, have not improved over time. Furthermore, recommended medications in SLE pregnancy such as aspirin and hydroxychloroquine have not increased over time despite increased evidence supporting their use in pregnancy.
Preterm delivery and preeclampsia did not significantly change from 1989 to 2020. Live birth and cesarean section rates were similar across the study duration. Similar to the SLE pregnancies, outcomes in the control pregnancies were also stable, including live birth, cesarean section, preeclampsia, and preterm delivery. These results contrast with findings in a large administrative study that used the National Inpatient Sample to assess SLE pregnancy outcomes from 1998 to 2015 (7). This study identified SLE pregnancies using a one‐time code of either SLE ICD‐9 or ICD‐10‐CM codes. They report that inpatient infant death mortality decreased over the study duration in both SLE and non‐SLE pregnancies. Rates of preeclampsia or eclampsia decreased in SLE pregnancies but increased in non‐SLE pregnancies. For cesarean section, rates increased in both SLE and non‐SLE pregnancies but increased more significantly in non‐SLE pregnancies. The National Inpatient Sample study could not assess SLE comorbidities, SLE medications, or preterm delivery. We hypothesize that the different findings in the National Inpatient Sample study (7) are most likely related to different phenotyping efforts for defining SLE cases. While we initially used billing codes to identify possible SLE pregnancies, we required four or more SLE billing codes, not just one, as was done in the National Inpatient Sample study. We then performed chart review on all our potential SLE pregnancies to ensure they had an SLE diagnosis given by a rheumatologist. This type of chart review was not possible in the National Inpatient Sample study. It is possible that the National Inpatient Sample study may include hospitals with less severe SLE cases than our tertiary care hospital. In addition, another methodologic difference in the NIS study was that the unit of analysis was the hospitalization rather than the patient, as unique patient identifiers are not available in the NIS. Our unit of analysis was an SLE delivery.
As expected, there were worse outcomes in our SLE pregnancies compared with our controls, as has been previously reported (14, 15). Compared with controls, SLE pregnancies were significantly more likely to have higher rates of cesarean sections, preeclampsia, and preterm deliveries. In adjusted models for age at delivery, race, and parity, SLE case status was strongly associated with both preeclampsia and preterm deliveries. Comparing SLE pregnancies in our study to other SLE prospective cohorts, SLE pregnancies in our study had higher rates of preeclampsia and preterm deliveries (26). We hypothesize that these higher rates of adverse pregnancy outcomes could be because our patients are from a tertiary care center. There may be referral bias such that the sickest patients are referred to our center with access to specialty services, including a higher acuity level neonatal intensive care unit. Our controls did have slightly higher rates of preeclampsia and preterm delivery compared with the general population. The higher rates of adverse pregnancy outcomes in our SLE pregnancies compared with other prospective cohorts are also likely related to patient selection. In one large, prospective cohort study, patients with SLE with high disease activity were excluded (26). In our study, we did not restrict which SLE pregnancies were analyzed and only required that patients with SLE be diagnosed with SLE by a rheumatologist.
Rates of adverse pregnancy outcomes were relatively the same before SLE diagnosis compared with after SLE diagnosis. This finding was unexpected as we anticipated increased rates of adverse pregnancy outcomes in pregnancies after SLE diagnosis but not before SLE diagnosis. We hypothesized that these rates could be similar if patients had SLE diagnosed shortly after a pregnancy. Our results, however, showed that this was not the case. In pregnancies that occurred before SLE diagnosis, on average, SLE was diagnosed 6.5 years after pregnancy. There were only 10 pregnancies in which SLE was diagnosed within 1 year after pregnancy. Other studies (27, 28, 29), including a study in a predominantly African American SLE population (30), demonstrated similar findings of increased rates of adverse pregnancy outcomes both before and after SLE diagnosis. A large Swedish registry showed increased adverse pregnancy outcomes in pregnancies that occurred both 0‐2 years and 2‐5 years prior to SLE diagnosis (29). We hypothesize along with these studies that a preclinical disease state likely exists in SLE that can negatively impact pregnancy outcomes. As SLE autoantibodies can precede clinical presentation, the presence of autoantibodies, particularly antiphospholipid antibodies, could explain this preclinical disease state with increased adverse pregnancy outcomes prior to SLE diagnosis. In our data, for example, we observed that in all 11 spontaneous abortions, the presence of antiphospholipid antibodies predated the spontaneous abortions and even predated an APS diagnosis in 4 spontaneous abortions.
We also found low SLE medication use in our SLE pregnancies throughout the study duration except for frequent use of corticosteroids. For hydroxychloroquine, use increased, with highest use at around 70% in 2015. Increasing evidence demonstrates beneficial effects of hydroxychloroquine use in SLE pregnancies for both the mother and the fetus (31, 32, 33, 34, 35, 36, 37, 38, 39). The American College of Rheumatology (ACR) released reproductive health guidelines in 2020 that recommend all pregnant patients with SLE use hydroxychloroquine during pregnancy unless there are contraindications (40). Our study demonstrated low rates of aspirin use, with most recent use at only 20%. Because aspirin is an over‐the‐counter medication, it may not be reported the same way as prescription medications, resulting in an underreporting of aspirin use in our data. We did, however, perform chart review on the full text or entirety of notes and not just the medication list to try and capture all medications recommended. These low rates are despite increasing evidence and recommendations from the American College of Obstetrics and Gynecology and the US Preventive Health Task Force in 2014 that aspirin use in high‐risk patients reduces risk of preeclampsia (2, 41, 42, 43, 44) and a randomized controlled clinical trial in 2017 showing that low‐dose aspirin reduced risk of preterm preeclampsia compared with placebo (45). The ACR reproductive health guidelines in 2020 conditionally recommended that all pregnant patients with SLE be on low‐dose aspirin to reduce risk of hypertensive disorders during pregnancy (40). While most of the SLE pregnancies in our cohort occurred before the 2020 guidelines, our results demonstrate the continued gaps of care, with a need to increase both hydroxychloroquine and aspirin use in SLE pregnancies.
Corticosteroid use during pregnancy and SLE nephritis have both been associated with risk of preterm delivery and preeclampsia (46, 47). Although we had adequate power to detect a result of similar magnitude, we observed no association of corticosteroid use and SLE nephritis with either preterm delivery or preeclampsia. We hypothesize that the high background rates of both preeclampsia and preterm delivery in our non‐nephritis and non‐corticosteroid deliveries may have attenuated any relative measures of association.
Although we have a relatively large, unique EHR cohort of SLE deliveries, there are limitations to our study. We conducted our study at a single center in the Southeastern United States, so our results may not be generalizable to SLE in other regions. Our study was conducted at a tertiary referral care center that could bias our results to more severe outcomes. The validated algorithm we used to assemble our SLE and control cohorts uses primarily delivery‐related billing codes to focus on deliveries and births, so we may not have captured all pregnancies, particularly early spontaneous abortions, in our dataset. Because our study used EHR data, we do not have access to SLE disease activity or damage measures. These measures are not collected routinely in clinical practice and thus are not available currently in the EHR. Date of SLE diagnosis is also not systematically collected in the EHR, so for 56 SLE deliveries, we estimated SLE disease duration from first SLE code, which may underestimate SLE disease duration but did not significantly impact our overall mean SLE disease duration. Because our EHR is de‐identified, we do not have available data on education level, income, or insurance status. These social determinants can impact pregnancy outcomes (1, 2, 16, 48). We acknowledge missingness in our EHR data as records from outside our institution are not available. This missingness could cause us to underestimate adverse pregnancy outcomes. Furthermore, assessing adverse pregnancy outcomes at time of the delivery hospitalization may also cause us to underestimate adverse pregnancy outcomes.
In conclusion, we identified a large EHR cohort of SLE and control pregnancies across three decades. Unfortunately, rates of adverse outcomes in SLE pregnancies have not improved over time. Similarly, rates of aspirin and hydroxychloroquine use have not improved over time despite increasing evidence that supports their use. These results highlight persistent gaps in management strategies for SLE pregnancies and the continued need to direct resources to improve care in these high‐risk patients.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Barnado had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Barnado, Osmundson.
Acquisition of data
Barnado, Hubbard, Green, Camai.
Analysis and interpretation of data
Barnado, Hubbard, Wheless, Osmundson.
Supporting information
Disclosureform:
Supplementary Table 1 Delivery‐Related Billing Codes.
Supplementary Table 2 List of autoimmune diseases in controls.
Supplementary Table 3 Adverse pregnancy outcomes in patients with systemic lupus erythematosus including first pregnancy only.
Supplemental Table 4. Adverse pregnancy outcomes in controls including first pregnancy only.
Supplemental Table 5. Comparison of adverse pregnancy outcomes.
Supplemental Table 6. Outcomes in deliveries after SLE diagnosis.
Supplemental Table 7. Ever medication use during SLE pregnancies.
Supplemental Table 8. Different modeling methods for outcomes in SLE and control deliveries.
ACKNOWLEDGMENTS
None.
Supported by National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant K08‐AR‐072757‐01 (Dr. Barnado), the Rheumatology Research Foundation K Supplement Award (Dr. Barnado), NIH/National Institute on Drug Abuse (NIDA) grant K23‐DA‐047476 (Dr. Osmundson), NIH/National Center for Research Resources (NCRR) grant UL1‐RR‐024975 (Vanderbilt University Medical Center [VUMC]), and NIH/National Center for Advancing Translational Sciences (NCATS) grant ULTR‐000445 (VUMC).
April Barnado, MD, MSCI, Janie Hubbard, BA, Sarah Green, BA, Alex Camai, BA, Lee Wheless, MD, PhD, Sarah Osmundson, MD, MS: Vanderbilt University Medical Center, Nashville, Tennessee.
No potential conflicts of interest relevant to this article were reported.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr2.11447&file=acr211447‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Andrade RM, McGwin G, Alarcón GS, Sanchez ML, Bertoli AM, Fernández M, et al. Predictors of post‐partum damage accrual in systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (XXXVIII). Rheumatology (Oxford) 2006;45:1380–4. [DOI] [PubMed] [Google Scholar]
- 2. Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD, et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med 2015;163:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palmsten K, Simard JF, Chambers CD, Arkema EV. Medication use among pregnant women with systemic lupus erythematosus and general population comparators. Rheumatology (Oxford) 2017;56:561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skorpen CG, Lydersen S, Gilboe IM, Skomsvoll JF, Salvesen KA, Palm O, et al. Influence of disease activity and medications on offspring birth weight, pre‐eclampsia and preterm birth in systemic lupus erythematosus: a population‐based study. Ann Rheum Dis 2018;77:264–9. [DOI] [PubMed] [Google Scholar]
- 5. Zusman EZ, Sayre EC, Avina‐Zubieta JA, De Vera MA. Patterns of medication use before, during and after pregnancy in women with systemic lupus erythematosus: a population‐based cohort study. Lupus 2019;28:1205–13. [DOI] [PubMed] [Google Scholar]
- 6. Clowse MEB, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol 2008;199:127.e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta B, Luo Y, Xu J, Sammaritano L, Salmon J, Lockshin M, et al. Trends in maternal and fetal outcomes among pregnant women with systemic lupus erythematosus in the united states: a cross‐sectional analysis. Ann Intern Med 2019;171:164–71. [DOI] [PubMed] [Google Scholar]
- 8. Moores KG, Sathe NA. A systematic review of validated methods for identifying systemic lupus erythematosus (SLE) using administrative or claims data. Vaccine 2013;31 Suppl 10:K62–73. [DOI] [PubMed] [Google Scholar]
- 9. Barnado A, Casey C, Carroll RJ, Wheless L, Denny JC, Crofford LJ. Developing electronic health record algorithms that accurately identify patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2017;69:687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jorge A, Castro VM, Barnado A, Gainer V, Hong C, Cai T, et al. Identifying lupus patients in electronic health records: Development and validation of machine learning algorithms and application of rule‐based algorithms. Semin Arthritis Rheum 2019;49: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barnado A, Eudy AM, Blaske A, Wheless L, Kirchoff K, Oates JC, et al. Developing and validating methods to assemble systemic lupus erythematosus births in the electronic health record. Arthritis Care Res (Hoboken) 2020;74:849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large‐scale de‐identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 2008;84:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clowse MEB, Magder LS, Witter F, Petri M. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum 2005;52:514–21. [DOI] [PubMed] [Google Scholar]
- 14. Bundhun PK, Soogund MZ, Huang F. Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: a meta‐analysis of studies published between years 2001‐2016. J Autoimmun 2017;79:17–27. [DOI] [PubMed] [Google Scholar]
- 15. He WR, Wei H. Maternal and fetal complications associated with systemic lupus erythematosus: an updated meta‐analysis of the most recent studies (2017‐2019). Medicine (Baltimore) 2020;99:e19797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marder W. Update on pregnancy complications in systemic lupus erythematosus. Curr Opin Rheumatol 2019;31:650–8. [DOI] [PubMed] [Google Scholar]
- 17. Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am J Obstet Gynecol 2006;194:992–1001. [DOI] [PubMed] [Google Scholar]
- 18. Boulet SL, Okoroh EM, Azonobi I, Grant A, Hooper WC. Sickle cell disease in pregnancy: maternal complications in a Medicaid‐enrolled population. Matern Child Health J 2013;17:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang S, Cardarelli K, Shim R, Ye J, Booker KL, Rust G. Racial disparities in economic and clinical outcomes of pregnancy among Medicaid recipients. Matern Child Health J 2013;17:1518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schildcrout JS, Denny JC, Bowton E, Gregg W, Pulley JM, Basford MA. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther 2012;92:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barnado A, Carroll RJ, Casey C, Wheless L, Denny JC, Crofford LJ. Phenome‐wide association studies uncover a novel association of increased atrial fibrillation in male patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2018;70:1630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gandelman JS, Khan OA, Shuey MM, Neal JE, McNeer E, Dickson A, et al. Increased incidence of resistant hypertension in patients with systemic lupus erythematosus: a retrospective cohort study. Arthritis Care Res (Hoboken) 2020;72:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dumitrescu L, Ritchie MD, Brown‐Gentry K, Pulley JM, Basford M, Denny JC, et al. Assessing the accuracy of observer‐reported ancestry in a biorepository linked to electronic medical records. Genet Med 2010;12:648–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 2019;124:1094–112. [DOI] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention . Preterm birth. 2021. URL: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm.
- 26. Buyon JP, Kim MY, Salmon JE. Predictors of pregnancy outcomes in patients with lupus. Ann Intern Med 2016;164:131. [DOI] [PubMed] [Google Scholar]
- 27. Petri M, Allbritton J. Fetal outcome of lupus pregnancy: a retrospective case‐control study of the Hopkins Lupus Cohort. J Rheumatol 1993;20:650–6. [PubMed] [Google Scholar]
- 28. Dhar JP, Essenmacher LM, Ager JW, Sokol RJ. Pregnancy outcomes before and after a diagnosis of systemic lupus erythematosus. Am J Obstet Gynecol 2005;193:1444–55. [DOI] [PubMed] [Google Scholar]
- 29. Arkema EV, Palmsten K, Sjowall C, Svenugsson E, Salmon JE, Simard JF. What to expect when expecting with systemic lupus erythematosus (SLE): a population‐based study of maternal and fetal outcomes in SLE and pre‐SLE. Arthritis Care Res (Hoboken) 2016;68:988–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barnado A, Wheless L, Meyer AK, Gilkeson GS, Kamen DL. Pregnancy outcomes among African‐American patients with systemic lupus erythematosus compared with controls. Lupus Sci Med 2014;1:e000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leroux M, Desveaux C, Parcevaux M, Julliac B, Gouyon JB, Dallay D, et al. Impact of hydroxychloroquine on preterm delivery and intrauterine growth restriction in pregnant women with systemic lupus erythematosus: a descriptive cohort study. Lupus 2015;24:1384–91. [DOI] [PubMed] [Google Scholar]
- 32. Eudy AM, Siega‐Riz AM, Engel SM, Franceschini N, Howard AG, Clowse MEB, et al. Effect of pregnancy on disease flares in patients with systemic lupus erythematosus. Ann Rheum Dis 2018;77:855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clowse MEB, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 2006;54:3640–7. [DOI] [PubMed] [Google Scholar]
- 34. Diav‐Citrin O, Blyakhman S, Shechtman S, Ornoy A. Pregnancy outcome following in utero exposure to hydroxychloroquine: a prospective comparative observational study. Reprod Toxicol 2013;39:58–62. [DOI] [PubMed] [Google Scholar]
- 35. Hwang JK, Park HK, Sung YK, Hoh JK, Lee HJ. Maternal outcomes and follow‐up of preterm and term neonates born to mothers with systemic lupus erythematosus. J Matern Fetal Neonatal Med 2018;31:7–13. [DOI] [PubMed] [Google Scholar]
- 36. Kroese SJ, de Hair MJH, Limper M, Lely AT, van Laar JM, Derksen RH et al. Hydroxychloroquine use in lupus patients during pregnancy is associated with longer pregnancy duration in preterm births. J Immunol Res 2017;2017:2810202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Georgiou PE, Politi EN, Katsimbri P, Sakka V, Droso AA. Outcome of lupus pregnancy: a controlled study. Rheumatology (Oxford) 2000;39:1014–9. [DOI] [PubMed] [Google Scholar]
- 38. Teh CL, Wong JS, Ngeh NK, Loh WL. Systemic lupus erythematosus pregnancies: a case series from a tertiary, East Malaysian hospital. Lupus 2009;18:278–82. [DOI] [PubMed] [Google Scholar]
- 39. Ruffatti A, Tonello M, Hoxha A, Sciasica S, Cuadrado MJ, Lation JO, et al. Effect of additional treatments combined with conventional therapies in pregnant patients with high‐risk antiphospholipid syndrome: a multicentre study. Thromb Haemost 2018;118:639–46. [DOI] [PubMed] [Google Scholar]
- 40. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol 2020;72:529–56. [DOI] [PubMed] [Google Scholar]
- 41. LeFevre ML; US Preventive Services Task Force. Low‐dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161:819–26. [DOI] [PubMed] [Google Scholar]
- 42. Abheiden CN, Blomjous BS, Kroese SJ, Bultink IE, Fritsch‐Stork RD, Titia Lely A, et al. Low‐molecular‐weight heparin and aspirin use in relation to pregnancy outcome in women with systemic lupus erythematosus and antiphospholipid syndrome: a cohort study. Hypertens Pregnancy 2017;36:8–15. [DOI] [PubMed] [Google Scholar]
- 43. Moroni G, Doria A, Giglio E, Imbasciati E, Tani C, Zen M, et al. Maternal outcome in pregnant women with lupus nephritis. A prospective multicenter study. J Autoimmun 2016;74:194–200. [DOI] [PubMed] [Google Scholar]
- 44. Imbasciati E, Tincani A, Gregorini G, Doria A, Moroni G, Cabiddu G, et al. Pregnancy in women with pre‐existing lupus nephritis: predictors of fetal and maternal outcome. Nephrol Dial Transplant 2009;24:519–25. [DOI] [PubMed] [Google Scholar]
- 45. Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matllana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017;377: 613–22. [DOI] [PubMed] [Google Scholar]
- 46. Chakravarty EF, Colón I, Langen ES, Nix DA, El‐Sayed YY, Genovese MC, et al. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am J Obstet Gynecol 2005;192:1897–904. [DOI] [PubMed] [Google Scholar]
- 47. Al Arfaj AS, Khalil N. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus 2010;19:1665–73. [DOI] [PubMed] [Google Scholar]
- 48. Kaplowitz ET, Ferguson S, Guerra M, Laskin CA, Buyon JP, Petri M, et al. Contribution of socioeconomic status to racial/ethnic disparities in adverse pregnancy outcomes among women with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2018;70:230–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosureform:
Supplementary Table 1 Delivery‐Related Billing Codes.
Supplementary Table 2 List of autoimmune diseases in controls.
Supplementary Table 3 Adverse pregnancy outcomes in patients with systemic lupus erythematosus including first pregnancy only.
Supplemental Table 4. Adverse pregnancy outcomes in controls including first pregnancy only.
Supplemental Table 5. Comparison of adverse pregnancy outcomes.
Supplemental Table 6. Outcomes in deliveries after SLE diagnosis.
Supplemental Table 7. Ever medication use during SLE pregnancies.
Supplemental Table 8. Different modeling methods for outcomes in SLE and control deliveries.


