Summary
A fundamental task of visual perception is to group visual features – sometimes spatially separated and partially occluded – into coherent, unified representations of objects. Perceptual grouping can vastly simplify the description of a visual scene and is critical for our visual system to understand the three-dimensional visual world. Numerous neurophysiological and brain imaging studies have demonstrated that neural mechanisms of perceptual grouping are characterized by the enhancement of neural responses throughout the visual processing hierarchy, from lower visual areas processing grouped features to higher visual areas representing objects/shapes from grouping [1–3]. In a series of psychophysical adaptation experiments, we made the counterintuitive observation that perceptual grouping amplified the shape aftereffect (SAE), but meanwhile, reduced the tilt aftereffect (TAE) and the threshold elevation aftereffect (TEAE). Furthermore, the modulation of perceptual grouping on the TEAE showed a partial interocular transfer. This finding suggests a two-fold effect of perceptual grouping – enhancing the high-level shape representation and attenuating the low-level feature representation even at a monocular level. We propose that this effect is a functional manifestation of a predictive coding scheme [4–8] and reflects an efficient code of visual information across lower and higher visual cortical areas.
Results
We used adaptation to explore the effect of perceptual grouping on visual pattern representation in the human visual system. Adaptation is a general property of almost all neural systems. Due to its power to isolate and temporarily reduce the contribution of specific neural populations, measuring the aftereffects of adaptation has been a powerful tool of psychophysics to study the representation of various visual patterns, from low-level features to high-level shapes, objects and faces [9–11].
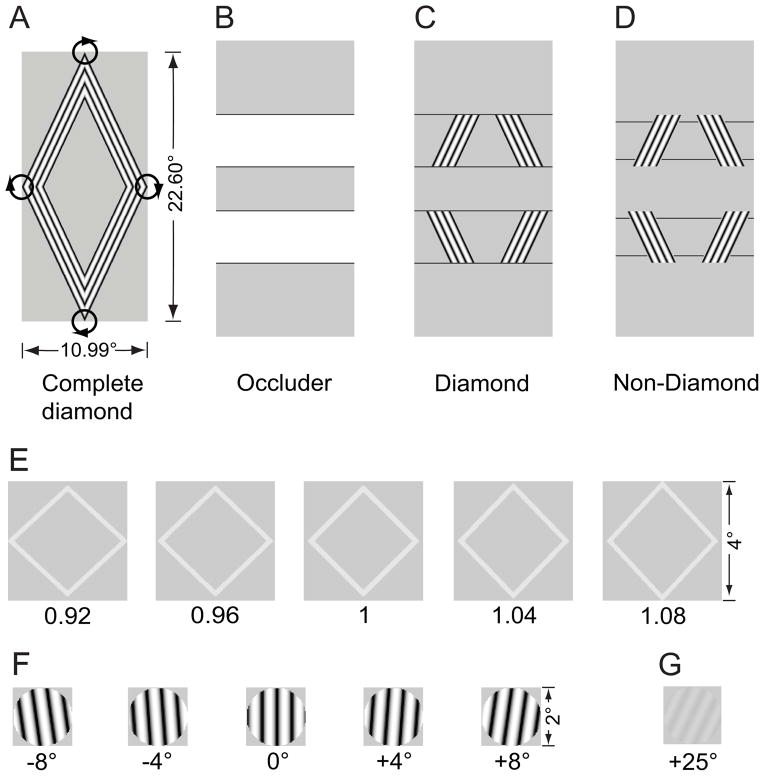
In the current study, adapting stimuli were a partially occluded diamond (the diamond stimulus) and its variant (the non-diamond stimulus) (Figure 1). The diamond stimulus was constructed by masking a complete thin diamond (Figure 1A) with three horizontal occluders (Figure 1B). Only the tilted bars were visible to subjects. The thin diamond translated with a circular trajectory, thereby maintaining the bars at a constant orientation. Its direction (clockwise or counter-clockwise) reversed every 5 sec. By ‘thin’, it means that the vertical/horizontal aspect ratio of a diamond is larger than one. The aspect ratio of a normal or fat diamond is equal to or less than one. The occluders were rendered with the background color except part of their borders (i.e. the four horizontal lines). Although the four corners of the thin diamond were hidden by the occluders, the T-junctions formed by the horizontal lines and the visible part of the diamond facilitated the grouping of the four bars and helped to generate a vivid percept of a coherently translating diamond (Figure 1C). A similar stimulus was used by Lorenceau and Alais [12]. For the non-diamond stimulus, the four horizontal lines were slightly displaced by 0.6° either downward or upward, which eliminated the T-junctions and broke the non-diamond stimulus into four separate moving bars (Figure 1D). Although the physical difference between the diamond and the non-diamond stimuli was very small, it led to a dramatic change in the extent of perceptual grouping. When subjects viewed the diamond stimulus, they perceived a translating diamond during 93% of the viewing time. However, for the non-diamond stimulus, they could only see a diamond occasionally (3% of the viewing time). This observation is consistent with a previous report [13]. Using a similar stimulus, our previous fMRI studies [14, 15] show that perceptual grouping increased response in a higher object-selective area, but reduced response in V1. However, the interpretation of the phenomenon is still equivocal and its function and behavioral significance is still unclear (see Discussion).
Figure 1.
Visual stimuli. (A) A complete thin diamond translated with a circular trajectory. Its direction (clockwise or counter-clockwise) reversed every 5 sec. (B) Three horizontal occluders were rendered with the background color except part of their borders – the four horizontal lines. (C) The diamond stimulus as adaptor was generated by masking the complete diamond with the occluders. T-junctions formed by the horizontal lines and the visible parts of the complete diamond made subjects see a coherently translating diamond. (D) The non-diamond stimulus as a second adaptor was generated by displacing the horizontal lines vertically. The absence of the T-junctions broke the stimulus into four separate moving bars. (E) Diamond test stimuli for measuring shape aftereffect. (F) High-contrast grating test stimuli for measuring tilt aftereffect. (G) A sample low-contrast grating test stimulus for measuring threshold elevation aftereffect.
Effect of perceptual grouping on shape aftereffect and tilt aftereffect
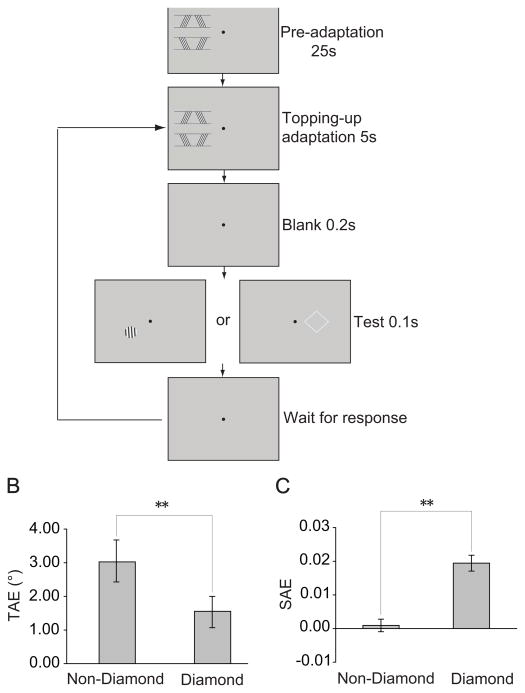
In Experiment 1, to examine the effect of perceptual grouping on the representation of the diamond shape and its constituent bars, we measured the shape aftereffect (SAE) and the tilt aftereffect (TAE) from adapting to the diamond and the non-diamond stimuli respectively. An adaptation block had only one adapting stimulus presented in the left visual field. It began with a pre-adaptation (Figure 2A). In a trial, after a topping-up adaptation and a blank interval, a test stimulus was presented briefly and subjects were asked to make a 2-alternative-forced-choice (2-AFC) judgment. The test stimulus could be one of the five normal or close-to-normal diamonds (Figure 1E) presented in the right visual field for measuring the SAE and subjects judged whether the diamond was thin or fat. Or the test stimulus could be one of the five vertical or close-to-vertical gratings (Figure 1F) presented in the left visual field for measuring the TAE and subjects needed to indicate that the grating was left or right tilted. Note that the center of the gratings was coincident with that of the area covered by the moving lower-right bar. Based on psychometric functions constructed from subjects’ responses, we calculated the perceived vertical and the perceived normal diamond with and without adaptation. The orientation change of the perceived vertical and the aspect ratio change of the perceived normal diamond caused by adaption were taken as the magnitude of the TAE and the SAE respectively. If adaptation could generate a significant TAE and/or SAE, a vertical grating would be perceived to be left tilted and/or a normal diamond be fat.
Figure 2.
Procedure and results of Experiment 1. (A) Experimental procedure. An adaptation block had only one adapting stimulus (the diamond stimulus or the non-diamond stimulus) presented in the left visual field. It began with a 25 sec pre-adaptation. In a trial, after a 5 sec topping-up adaptation and a 0.2 sec blank interval, a test stimulus was presented for 0.1 sec and subjects were asked to make a 2-alternative-forced-choice (2-AFC) judgment. The test stimulus could be one of the five grating test stimuli presented in the left visual field for measuring TAE and subjects needed to indicate that the grating was left or right tilted. Or the test stimulus could be one of the five diamond test stimuli presented in the right visual field for measuring SAE and subjects judged whether the diamond was thin or fat. (B) TAE magnitudes from adapting to the diamond and the non-diamond stimuli. (C) SAE magnitudes from adapting to the diamond and the non-diamond stimuli. Asterisks indicate a statistically significant difference between two stimulus conditions (*p<0.05; **p<0.01). Error bars denote 1 SEM calculated across subjects for each condition.
For three experimental conditions - adapting to the diamond stimulus, adapting to the non-diamond stimulus and baseline (without adaptation), the perceived verticals (mean±sem) were 1.84±0.76°, 3.31±0.90° and 0.29±0.72° respectively. TAEs were significant after adapting to both the non-diamond stimulus (t=4.90, p<0.01) and the diamond stimulus (t=3.31, p<0.05). The TAE from the non-diamond stimulus was significantly larger than that from the diamond stimulus (t=6.85, p<0.01) (Figure 2B). However, SAE measurements had a distinctive pattern. The aspect ratios of the perceived normal diamonds were 0.9937±0.009, 0.9752±0.01 and 0.9743±0.01 for the three conditions. A significant SAE was found after adapting to the diamond stimulus (t=8.21, p<0.01), but not the non-diamond stimulus (t=0.49, p>0.05). The difference between the two adapting stimuli was significant (t=5.07, p<0.01) (Figure 2C). These results demonstrate that perceptual grouping could enhance the representation of the diamond shape, but attenuate the representation of the bar orientation. The shape adaptation should take place in high-level visual areas because the SAE was evident even when the adapting and test stimuli were presented in the left and right visual fields respectively. A possible area is the lateral occipital area (LO) because the LO in either hemisphere is responsive to shape images presented in both the left and right visual fields [16], although it still has a contralateral preference [17].
Effect of perceptual grouping on threshold elevation aftereffect
It could be argued that, in Experiment 1, the TAE reduction by perceptual grouping is due to different spatial distributions of attention when subjects viewed the diamond and the non-diamond stimuli. For the non-diamond stimulus, subjects’ spatial attention might be more focused on the four moving bars. For the diamond stimulus, attention might even spread to the occluders, the hidden corners and the area bound by the four bars because they are intrinsically related to each other and construct a representational entity [18]. To confirm that the representation attenuation of the bar orientation is due to perceptual grouping rather than a pure attentional effect, we performed Experiment 2 with five other subjects to measure the effect of perceptual grouping on contrast threshold elevation aftereffect (TEAE). Although TAE could be modulated by attention, TEAE has been demonstrated to be independent of attention, especially when adapting stimuli have a high contrast [19–21].
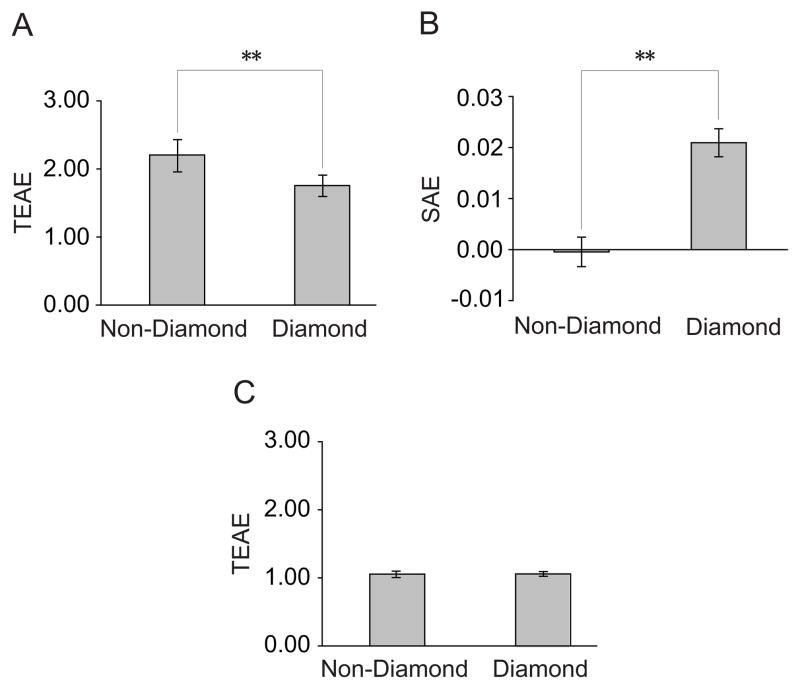
Experiment 2 measured the TEAE and the SAE from adapting to the diamond and the non-diamond stimulus. For the TEAE measurement, we used a temporal 2-AFC QUEST staircase procedure (82% correct) [22] to measure subjects’ contrast detection thresholds with and without adaptation. The ratio of the threshold with adaption to that without adaptation was taken as the TEAE magnitude. The orientation of test stimuli was identical to that of the adapting stimulus (i.e. the lower right bar) (Figure 1G). We found a significant TEAE after adapting to both the non-diamond stimulus (t=9.31, p<0.01) and the diamond stimulus (t=11.21, p<0.01). The TEAE from the non-diamond stimulus was significantly larger than that from the diamond stimulus (t=5.02, p<0.01) (Figure 3A). The SAE measurement was performed in the same way as that in Experiment 1 and it replicated the finding (Figure 3B). In addition, we also measured the TEAE when the orientation of test stimuli was orthogonal to that of the adapting stimulus. No significant effect was found, which confirmed that this local adaptation was orientation-specific (Figure 3C). These results provide further evidence that perceptual grouping could attenuate the representation of the bar orientation.
Figure 3.
Results of Experiment 2. (A) TEAE magnitudes from adapting to the diamond and the non-diamond stimuli when the adapted and test orientations were identical. (B) SAE magnitudes from adapting to the diamond and the non-diamond stimuli. (C) TEAE magnitudes from adapting to the diamond and the non-diamond stimuli when the test orientation was orthogonal to the adapted orientation. Asterisks indicate a statistically significant difference between two stimulus conditions (*p<0.05; **p<0.01). Error bars denote 1 SEM calculated across subjects for each condition.
Interocular transfer of perceptual grouping effect
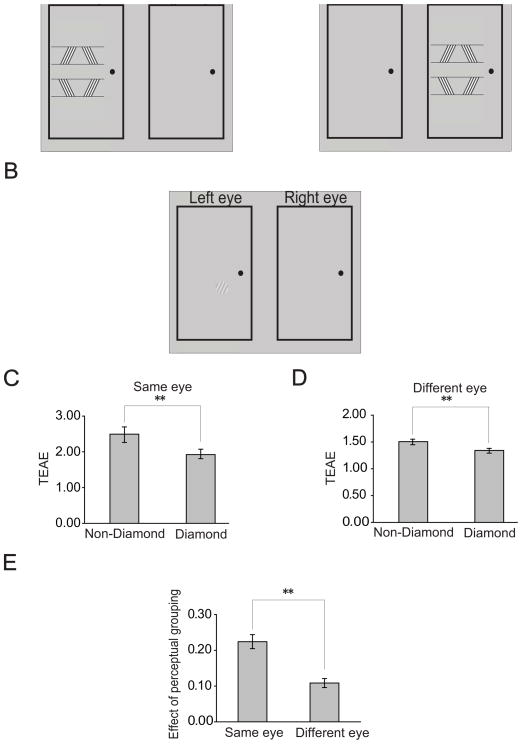
Since TAE and TEAE are believed to be generated in early visual cortex, even as early as in V1 (especially for TEAE) [20, 23], the results above suggest that perceptual grouping could attenuate the representation of the bar orientation in early visual cortical areas. To further examine whether some monocular mechanism in V1 contributes to the perceptual grouping effect in Experiment 2 (i.e. the TEAE reduction), we performed Experiment 3 to measure the amount of the interocular transfer of the TEAE reduction. The TEAE measurement was similar to that in Experiment 2 except that adapting and test stimuli were presented in either the same eye or different eye. Subjects viewed the stimuli through a mirror stereoscope. Adapting stimuli were presented in either the left or the right eye (Figure 4A). Test stimuli were always presented in the left eye (Figure 4B). We quantified the effect of perceptual grouping on TEAE with an index (1- TEAE from adapting to the diamond/TEAE from adapting to the non-diamond). A large index means a strong effect of perceptual grouping. Interocular transfer refers to the relative size of the grouping effect when the adapting and test stimuli are presented to different eyes compared to when presented to the same eye. A 100% transfer means that the different-eye and same-eye effects are of the same magnitude, and indicates a purely binocular process. A small transfer suggests that the mechanism involved is primarily monocular. Intermediate levels of transfer are best explained in terms of a mixture of monocular and binocular mechanisms [24] (but see also [25]).
Figure 4.
Stimuli and results of Experiment 3. (A) Adapting stimuli were presented in either the left or the right eye. (B) Test stimuli were always presented in the left eye for measuring TEAE. (C) TEAE magnitudes from adapting to the diamond and the non-diamond stimuli when adapting and test stimuli were presented in the same eye. (D) TEAE magnitudes from adapting to the diamond and the non-diamond stimuli when adapting and test stimuli were presented in different eyes. (E) Effects of perceptual grouping on TEAE when adapting and test stimuli were presented in the same or different eyes. Asterisks indicate a statistically significant difference between two stimulus/eye conditions (*p<0.05; **p<0.01). Error bars denote 1 SEM calculated across subjects for each condition.
Regardless of whether the adapting and test stimuli were presented in the same eye or different eyes, we found a significant TEAE after adapting to both the non-diamond stimulus (same eye: t=11.46, p<0.01; different eye: t=28.60, p<0.01) and the diamond stimulus (same eye: t=14.43, p<0.01; different eye: t=29.46, p<0.01). The TEAE from the non-diamond stimulus was significantly larger than that from the diamond stimulus (same eye: t=6.46, p<0.01; different eye: t=8.01, p<0.01) (Figure 4C and 4D). These results are consistent with the finding in Experiment 2. An interesting finding in this experiment is that the effect of perceptual grouping on TEAE in the same eye condition was significantly larger than that in the different eye condition (t=7.79, p<0.01). The indices of the perceptual grouping effect for the two conditions are 0.22 and 0.11 respectively (Figure 4E). Thus, the interocular transfer of the effect was 48.4%, suggesting a mixture of monocular and binocular mechanisms underlying the grouping effect. A caveat should be noted that the extent of eye specificity inferred from the above analysis might be overestimated because of a higher level of measurement noise in the same eye condition, as indicated by the larger error bars in Figure 4C than those in Figure 4D.
Finally, we carried out experiments demonstrating that the observed effects in Experiments 1–3 were not due to the physical difference between the diamond and the non-diamond stimuli. Their difference was the tiny position changes of the four horizontal lines. We had subjects adapt to four horizontal lines whose positions were identical to those in the diamond stimulus or the non-diamond stimulus. No detectable TAE, TEAE or SAE was observed after adaptation.
Discussion
Our experiments provide clear evidence that perceptual grouping could magnify the high-level SAE, but reduce the low-level TAE and TEAE. These results demonstrate that a functional role of perceptual grouping is enhancing the high-level shape representation and meanwhile weakening the representation of the constituent elements (i.e. bar orientations) of the shape. Moreover, the effect of perceptual grouping on the TEAE showed a partial interocular transfer - it was significantly reduced when the adapting and test stimuli are presented to different eyes compared to when presented to the same eye. This finding indicates that the grouping might have exerted influence on monocular neurons encoding the bar orientations.
In the past decade, many of single-unit and brain imaging studies showed that perceptual grouping increases neural activities not only in higher occipito-temporal areas selective for shapes, but also in early retinotopic areas analyzing local features [1–3, 26–28]. The SAE magnification in our study is consistent with these findings, as well as other psychophysical studies [11, 29]. However, the attenuation of the TAE and TEAE is surprising and is contradictory to the prediction from these studies. There are two notable differences between previous studies and ours. One is that they often used much more cluttered images as stimuli than ours. For example, they employed a closed contour (i.e. foreground) consisted of similarly oriented local elements and embedded in a background of randomly oriented elements. Successful grouping necessitates an effortful segmentation process that distinguishes the foreground elements and the background ones. The other is that the grouping in their studies is guided by the Gestalt rule of good continuation, which does not necessarily require perceiving a shape (like our study). The finding of the TAE and TEAE attenuation is reminiscent of two psychophysical studies demonstrating that global perception impairs the perception of local elements. Verghese and Stone found that manipulations that cause multiple stimuli to appear as parts of a single patch degrade speed discrimination, whereas manipulations that perceptually divide a single large stimulus into parts improve discrimination [30]. Using a visual search paradigm, Suzuki and Cavanagh showed that face perception blocks the access of our visual system to face components [31].
Our results suggest that perceptual grouping involves increases in activity in higher visual areas that code for shapes along with decreases in activity in lower visual areas that code for local, individual elements. However, an unsolved question to answer is – what is the theoretical implication of this inverse relationship in neural activity between higher and lower visual areas? We propose that the relationship reflects an efficient code of visual information across lower and higher visual areas. As higher visual areas converge on a single, global hypothesis (i.e. a diamond) for the individual elements (i.e. bars) in a visual scene, lower visual areas no longer need to represent the individual elements. A variety of computational models propose mechanisms including interactions between high- and low-level representations of image feature that result in inverse activity patterns. Predictive coding models [4–8], for example, are one class of models that suggest that feedback may operate to reduce activity in lower areas. These models posit that higher areas are actively attempting to “explain” activity patterns in lower areas via feedback projections. Because most predictive coding models include a subtractive comparison between the hypotheses formed in higher areas and the incoming sensory input represented in lower areas, the overall effect of feedback may be to reduce activity in lower areas. Specifically, reduced activity in lower visual areas would occur whenever the predictions of higher level areas match incoming sensory information. In the case of our stimuli, when high visual areas (e.g. LO) maintains a representation of a grouped shape, this “expectation” or “understanding” of the image features is sent back to lower visual areas (e.g. V1) and removed, resulting in less activity. When higher areas are unable to form such an understanding (i.e., when the bars are perceived as ungrouped), these feedback processes are not occurring and there is consequently more activity in lower areas. Predictive coding models have strong intuitive appeal— why bother signaling what you already know? [32]. The reduced activity that would result from such a process would also have substantial biological benefits. There are clear efficiency constraints placed on the visual system—both because of inherent capacity limitations in neural pathways and because spikes are metabolically expensive [33]. The visual system would do well to use a representational strategy that maximizes biologically efficiency by utilizing a code that minimizes spike rate.
Recent fMRI and MEG studies have provided evidence for predictive coding models [34–37]. We also performed fMRI experiments to test the models. We observed a BOLD signal increase in the LO and a concurrent signal decrease in V1 when visual elements were assembled into a coherent shape [14, 15]. However, it should be pointed out that, due to the complicated nature of BOLD signal and the limit of its spatial resolution [38], BOLD signal reductions in lower areas cannot be unequivocally explained as a decrease in neural activity representing low-level elements [32]. The reductions may be a manifestation of representation sharpening or noise removal [39]. They could also be attributed to other factors, including changes in visual stimulus, perceived context and attentional state. More critically, behavioral significance of predictive coding has rarely been verified. By showing that shape perception from perceptual grouping affects not only high-level vision, but also low-level vision, the current adaptation study provides the first piece of behavioral evidence for a predictive coding scheme.
Our study suggests that feedback from higher visual areas serves to reduce activity in lower visual areas during perceptual grouping. The feedback could even penetrate back to monocular neurons in V1. It should be noted that a major challenge for the predictive coding view is how a higher visual area predicts the precise metrics of a stimulus. A dominant functional interpretation of the feedforward ventral pathway is increased selectivity at the expense of insensitivity to variables such as translation, illumination, scale; but if information about position and size is gradually lost, then how could a feedback signal be spatially precise? One possibility is that transformation information might be retained in the visual processing hierarchy, as suggested by a recent study [40]. The predictive coding view is an alternative proposal to the conventional wisdom that favors enhancement and attention in the conceptualization of the role of feedback in visual processing. Understanding how the feedback is implemented in the visual cortex will be a scientific challenge in the future.
Supplementary Material
Perceptual grouping amplifies the shape aftereffect
Perceptual grouping reduces the tilt aftereffect
Perceptual grouping reduces the threshold elevation aftereffect
The effects reflect an efficient code of visual information across cortical areas
Acknowledgments
This work was supported by the Ministry of Science and Technology of China (2011CBA00405 and 2010CB833903), the National Natural Science Foundation of China (Project 30925014, 30870762 and 90920012), and the Fundamental Research Funds for the Central Universities. D.K. was partially supported by the WCU (World Class University) program funded by the Ministry of Education, Science and Technology through the National Research Foundation of Korea (R31-10008) and by NIH R01 EY015261.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kourtzi Z, Tolias AS, Altmann CF, Augath M, Logothetis NK. Integration of local features into global shapes: Monkay and human fMRI studies. Neuron. 2003;37:333–346. doi: 10.1016/s0896-6273(02)01174-1. [DOI] [PubMed] [Google Scholar]
- 2.Roelfsema PR. Cortical algorithms for perceptual grouping. Annu Rev Neurosci. 2006;29:203–227. doi: 10.1146/annurev.neuro.29.051605.112939. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert CD, Sigman M. Brain states: Top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Mumford D. On the computational architecture of the neo-cortex: II. The role of the cortico-cortical loops. Biol Cybern. 1992;66:241–251. doi: 10.1007/BF00198477. [DOI] [PubMed] [Google Scholar]
- 5.Barlow HB. What is the computational goal of the neocortex? In: Koch C, Davis JL, editors. Large-scale neuronal theories of the brain. Cambridge, MA: MIT; 1994. pp. 1–22. [Google Scholar]
- 6.Rao RP, Ballard DH. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- 7.Kersten D, Mamassian P, Yuille A. Object perception as Bayesian inference. Annu Rev Psychol. 2004;55:271–304. doi: 10.1146/annurev.psych.55.090902.142005. [DOI] [PubMed] [Google Scholar]
- 8.Friston K. The free-energy principle: a unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 9.Blakemore C, Campbell FW. On the existence of neurons in the human viusal system selectively sensitive to the orientation and size of retinal image. J Physiol. 1969;203:237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webster MA, Kaping D, Mizokami Y, Duhamel P. Adaptation to natural facial categories. Nature. 2004;428:557–561. doi: 10.1038/nature02420. [DOI] [PubMed] [Google Scholar]
- 11.Fang F, He S. Viewer-centered object representation in the human visual system revealedd by viewpoint aftereffects. Neuron. 2005;45:793–800. doi: 10.1016/j.neuron.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Lorenceau J, Alais D. Form constraints in motion binding. Nat Neurosci. 2001;4:745–751. doi: 10.1038/89543. [DOI] [PubMed] [Google Scholar]
- 13.McDermott J, Weiss Y, Adelson EH. Beyond junctions: nonlocal form constraints on motion interpretation. Perception. 2001;30:905–923. doi: 10.1068/p3219. [DOI] [PubMed] [Google Scholar]
- 14.Murray SO, Kersten D, Olshausen BA, Schrater P, Woods DL. Shape perception reduces activity in human primary visual cortex. Proc Nati Acad Sci USA. 2002;99:15164–15169. doi: 10.1073/pnas.192579399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang F, Kersten D, Murray SO. Perceptual grouping and inverse fMRI activity patterns in human visual cortex. J Vis. 2008;8:1–9. doi: 10.1167/8.7.2. [DOI] [PubMed] [Google Scholar]
- 16.Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- 17.Niemeier M, Goltz HC, Kuchinad A, Tweed DB, Vilis T. A contralateral preference in the lateral occipital area: sensory and attentional mechanisms. Cereb Cortex. 2005;15:325–331. doi: 10.1093/cercor/bhh134. [DOI] [PubMed] [Google Scholar]
- 18.Naber M, Carlson TA, Verstraten FAJ, Einhauser W. Perceptual benefit of objecthood. J Vis. 2011;11:1–9. doi: 10.1167/11.4.8. [DOI] [PubMed] [Google Scholar]
- 19.Festman Y, Ahissar M. Attentional states and the degree of visual adaptation to gratings. Neural Networks. 2004;17:849–860. doi: 10.1016/j.neunet.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Blake R, Tadin D, Sobel KV, Raissian TA, Chong SC. Strength of early visual adaptation depends on visual awareness. Proc Nati Acad Sci USA. 2006;103:4783–4788. doi: 10.1073/pnas.0509634103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi T, Cai P, Zhou T, Fang F. The effect of crowding on orientation-selective adaptation in human early visual cortex. J Vis. 2009;9:1–10. doi: 10.1167/9.11.13. [DOI] [PubMed] [Google Scholar]
- 22.Watson AB, Pelli DG. QUEST: A Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- 23.Movshon JA, Lennie P. Pattern selective adaptation in striate cortical neurones. Nature. 1979;278:850–852. doi: 10.1038/278850a0. [DOI] [PubMed] [Google Scholar]
- 24.Blake R, Overton R, Lema-Stern S. Interocular transfer of visual aftereffects. J Exp Psychol Hum Percept Perform. 1981;213:157–174. doi: 10.1037//0096-1523.7.2.367. [DOI] [PubMed] [Google Scholar]
- 25.Howarth CM, Vorobyov V, Sengpiel F. Interocular Transfer of Adaptation in the Primary Visual Cortex. Cereb Cortex. 2009;19:1835–1843. doi: 10.1093/cercor/bhn211. [DOI] [PubMed] [Google Scholar]
- 26.Altmann CF, Bulthoff HH, Kourtzi Z. Perceptual organization of local elements into global shapes in the human visual cortex. Curr Biol. 2003;13:342–349. doi: 10.1016/s0960-9822(03)00052-6. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Piech V, Gilbert CD. Learning to link contours. Neuron. 2008;57:442–551. doi: 10.1016/j.neuron.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wannig A, Stanisor L, Roelfsema PR. Automatic spread of attentional response modulation along Gestalt criteria in primary visual cortex. Nat Neurosci. 2011;14:1243–1244. doi: 10.1038/nn.2910. [DOI] [PubMed] [Google Scholar]
- 29.Sekuler AB, Palmer SE. Perception of partly occluded objects: A microgenetic analysis. J Exp Psychol Gen. 1992;121:95–111. [Google Scholar]
- 30.Verghese P, Stone LS. Perceived visual speed constrained by image segmentation. Nature. 1996;381:161–163. doi: 10.1038/381161a0. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki S, Cavanagh P. Facial organization blocks access to low-level features: An object inferiority effect. J Exp Psychol Hum Percept Perform. 1995;21:901–913. [Google Scholar]
- 32.Murray SO, Schrater P, Kersten D. Perceptual grouping and the interaction between visual cortcial areas. Neural Networks. 2004;17:695–705. doi: 10.1016/j.neunet.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Lennie P. The cost of cortical computation. Curr Biol. 2003;13:493–497. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 34.Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J. Predictive codes for forthcoming perception in the frontal cortex. Science. 2006;314:1311–1314. doi: 10.1126/science.1132028. [DOI] [PubMed] [Google Scholar]
- 35.Furl N, van Rijsbergen NJ, Treves A, Friston KJ, Dolan RJ. Experience-dependent coding of facial expression in superior temporal sulcus. Proc Nati Acad Sci USA. 2007;104:13485–13489. doi: 10.1073/pnas.0702548104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison LM, Stephan KE, Rees G, Friston KJ. Extra-classical receptive field effects measured in striate cortex with fMRI. NeuroImage. 2007;34:1199–1208. doi: 10.1016/j.neuroimage.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Summerfield C, Trittschuh EH, Monti JM, Mesulam M, Egner T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci. 2008;11:1004–1006. doi: 10.1038/nn.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 39.Vinje WE, Gallant JL. Natural stimulation of the nonclassical receptive field increases information transmission efficiency in V1. J Neurosci. 2002;22:2904–2915. doi: 10.1523/JNEUROSCI.22-07-02904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox DD, Meier P, Oertelt N, DiCarlo JJ. ‘Breaking’ position-invariant object recognition. Nat Neurosci. 2005;8:1145–1147. doi: 10.1038/nn1519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.