Abstract
Background
Since the successful development of Coronavirus Disease (COVID-19) vaccine, COVID-19 vaccination has been actively advocated all over the world. As the key population for COVID-19 vaccination, the acceptance of Healthcare Workers (HCWs) is not only related to their risk of contracting COVID-19 infection at work, but also affects the decision of the general population on COVID-19 vaccination. Currently, a series of observational studies have been conducted on the acceptance of COVID-19 vaccines among HCWs in China, but there are presently no all-inclusive reviews. Therefore, this paper reviewed to identify a reliable estimate of acceptance rate of COVID-19 vaccine among HCWs in China.
Methods
We conducted a search on PubMed, EMbase, The Cochrane Library, Web of Science, CNKI (Chinese National Knowledge Infrastructure), Wanfang Database, CBM (Chinese Biomedical Literature Database) and VIP database (Chinese Scientific Journal Database) from January 2020 to June 2022. The quality of included articles was estimated using the Newcastle-Ottawa Quality Assessment tool suitable for cross-sectional studies and STATA 16 was used for analysis, A random-effects model was used to calculate acceptance rate for COVID-19 vaccine, as well as subgroup analysis and sensitivity analysis.
Result
This review included 18 studies involving 45,760 subjects, all of which were of medium or high quality. Meta-analysis results represented that, the pooled estimated acceptance rate of COVID-19 vaccine among HCWs in China was 78% (95%CI: 73–83%), and the pooled acceptance rate in 2021 (82%, 95%CI: 78–86%) was significantly higher than that in 2020 (73%, 95%CI: 65%-81%). Subgroup analysis showed different acceptance rates for COVID-19 vaccine among HCWs with different characteristics.
Conclusion
The result revealed that HCWs in China generally have a high acceptance rate of COVID-19 vaccines, but the acceptance rate varies with different characteristics of the population. Therefore, corresponding training should be carried out for HCWs with different characteristics, and they should play an exemplary and leading role in COVID-19 vaccination, so as to improve the vaccination rate of the whole population and form an immune barrier at an early date.
Introduction
Coronavirus disease-2019 (COVID-19), a highly transmissible ailment caused by the SARS-CoV-2 virus, had become a global public distress since it was first determined in Wuhan, China, in late December 2019. World Health Organization (WHO) officially proposed to name this infectious disease as coronavirus disease 2019 (COVID-19) on 12 February 2020, and finally made the judgment that COVID-19 to have the characteristics of a global pandemic on 11 March 2020 [1]. As of 8 June 2022, COVID-19 has officially spread to 230 countries and territories, with more than 500 million confirmed cottom and 6.32 million cumulative deaths reported [2], causing great loss of financial, manpower and material resources. It estimates that almost 15 million died directly and indirectly from SARS-COV-2 in 2020 and 2021, nearly three times the reported by governments around the world [3]. We are living through an unprecedented crisis, with the COVID-19 spreading rapidly around the world in a short period of time [4] and likely to continue to have a profound impact on healthcare systems [5].
Coronavirus is a positive single-stranded RNA viruses. SARSCoV-2 belongs to the b-coronavirus subgenus, same as other RNA viruses, and SARSCoV-2 undergoes a high degree of genomic mutation during host adaptation, which poses a significant challenge to existing treatment options and prevention [6]. For purpose of better preventing novel coronavirus infections and contain the spread of the outbreak, medical workers and scientific researchers all over the world have been searching for appropriate treatment strategies, including antiviral agents, immunotherapy and vaccine [7,8]. However, herd immunity against SARS-COV-2 cannot be achieved at present due to the absence of specific medicine for COVID-19, the inability of viral infection and vaccine-induced immunity to prevent the spread of the epidemic, and the occurrence of antigenically distinct variants [9,10]. In order to prevent repeated infection in the population, safe and effective vaccine is the most efficient and reliable means to establish an immune barrier in the population.
SARS-CoV-2 vaccines have reassuring safety and is effectively in reducing deaths, symptomatic cases, severe cases and infections caused by SARS-CoV-2 worldwide [11–13]. The Advisory Committee on Immunization Practices (ACIP) proposed prioritizing healthcare workers (HCWs) vaccination in December, 2020, since HCWs have easier access to populations of COVID-19 patients during routine diagnostic and treatment activities and are at much greater risk of contracting COVID-19 than other populations [14]. There have been review representing moderate acceptance of COVID-19 vaccination among HCWs [15]. Mandatory COVID-19 Vaccination for HCWs against is a sensitive and controversial topic, with different levels of support around the world [16]. Therefore, in order to prevent the spread of COVID-19, it is critical to adopt strategies to improve the acceptance and willingness of HCWs to be vaccinated against COVID-19.
The success of a vaccine depends not only on its effectiveness, but also on the coverage of vaccination [17]. Accordingly, the main intention of this meta-analysis was to pool the willingness and acceptance to vaccinate against COVID-19 among HCWs in China, study the acceptance characteristics of COVID-19 vaccine among different types of HCWs, and provide targeted strategies for improving the promotion of COVID-19 vaccine and evidence to improve willingness to receive COVID-19 vaccine among all types of health workers.
Materials and methods
Protocol registration and best practice
This systematic evaluation and meta-analysis was carried out in full accordance with the guidelines of the Preferred Reporting Items for Systematic Review and Meta Analysis (PRISMA) [18] (see S1 Table), and had been registered in the Prospective Register of Systematic Reviews (PROSPERO). (ID: CRD42022337627).
Eligibility criteria
The inclusion and exclusion criteria are developed according to the PIOT framework, which include the Population (P), Indicator (I), Outcome of interest (O), and Time (T).
Inclusion criteria
The PIOT criteria are: (1) Population: HCWs from China, included clinicians, nurses, paramedics, administrators, medical examiners, medical technicians, other medical personnel and full-time staff in Center for Disease Control and Prevention (CDC), including trainee medical personnel (2) Indicator: COVID-19 vaccine (3). Results: Acceptance rate (both vaccinated and unvaccinated but willing to be vaccinated) (4). Time: during the COVID-19 pandemic.
Original observational studies published in English and Chinese were included.
Exclusion criteria
Studies that reported only vaccination against COVID-19
Reviews, comments, case reports, editorials and letters were also excluded
Studies in which raw data cannot be transformed
Acceptance rates explicitly referring to participants in other regions besides China
Acceptance rates explicitly referring to participants other than HCWs
Studies where only abstracts are available and conference literature
Duplicate literature was excluded and only the most complete data of the population was retained.
Information sources and search strategies
Two investigators independently and comprehensive searched both Chinese- and English-language databases. The English-language databases were PubMed, EMbase, The Cochrane Library, and Web of Science, the Chinese- language databases were the CNKI (Chinese National Knowledge Infrastructure), Wanfang Database, CBM (Chinese Biomedical Literature Database) and VIP database (Chinese Scientific Journal Database). Omissions were prevented, there were no restrictions imposed on publication type and population, and all publication dates from January 2020 to June 2022 were suitable. Besides, the available references of included studies articles and relevant reviews were also tracked to identify gray literature. Medical Subject Headings (MeSH terms) and free text words were combined to conduct literature retrieval. The following search terms were used for each database: ‘COVID-19 Vaccines’, ‘COVID-19 Virus Vaccines’, ‘SARS-CoV-2 Vaccines’, ‘SARS2 Vaccines’, ‘2019-nCoV Vaccine’, ‘2019 Novel Coronavirus Vaccines’, ‘2019 Novel Coronavirus Vaccines’, ‘Willingness*’, ‘Hesitancy’, ‘Attitude*’, ‘Accept*’ and ‘China’. The full search string for the PubMed database is shown in the S2 Table.
Data screening and selection
After the duplicate articles were removed, the remaining titles and/or abstracts were screened by two independent reviewers in the Endnote software, and the articles that require full text were then imported into the Zetero software and screened against pre-determined criteria. If more than one study was published using the same dataset, only the studies with the largest sample sizes were included in our review. When conflicts arised, consensus was reached through consultation with a third researcher.
Data extraction and management
All data extraction was performed independently and simultaneously by two reviewers and then exchanged for checking to prevent data errors. If necessary, contacted the author by email for further information. We use standardized tables on the WPS Excel spreadsheet to extract data. The data extraction checklist included first author, publication year, participant group, sample size, survey period, study design, region (the area where studies were conducted), age, gender, and the outcome (acceptance rate).
Quality assessment
Two independent reviewers used the Agency of Healthcare Research and Quality (AHRQ) assessment tool(see S2 Table) [19] to evaluate risk of bias included original studies. It contains 11 items, the answer “yes” is scored 1 point, and “no” or “not clear” is scored 0 points. Moreover, studies were classified as “high quality” (8–11), “moderate quality” (4–7) and “low quality” (0–3) based on their total scores. During the time of quality assessment, discrepancies between two independent reviewers were resolved by a third reviewers after discussion.
Data synthesis
Pooled acceptance rate and 95% confidence intervals (CIs) were adopted to estimate the acceptance rate of Chinese HCWs and the statistical analysis STATA 16.0 was used to analyze quantitative data. The Cochrane Q test and the I2 value test were used to examine the heterogeneity of the studies; where <25%, 25–50%, and >50% indicated a low, moderate, and a high level heterogeneity, respectively [20]. Leave one out sensitivity analysis was performed to examine the robustness on the overall pooled estimate. Since the heterogeneity of all the Pooled results was greater than 50%, random effect model was used for analysis. Subgroup analysis were conducted based on the survey year, geographical region, gender, age, education level, occupation, monthly income, professional position, marital status, time of employment, whether to participated in quarantine or had contact with confirmed cases and whether to having chronic conditions. Potential publication bias was evaluated graphically by funnel plot and Egger test (when P < 0.05, publication bias was significant) [21].
Results
Search outcomes
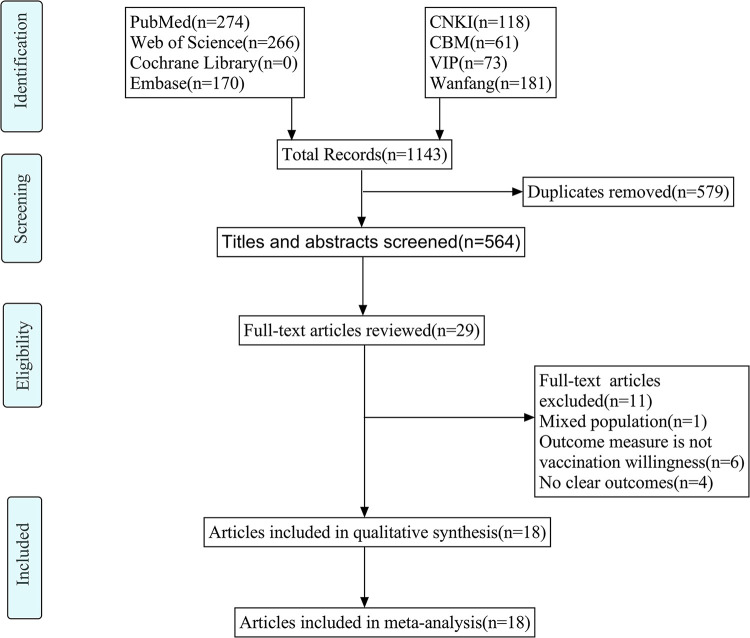
A total of 1,143 records were retrieved, and 579 records were excluded by duplicates. After excluding duplicates, 535 records were excluded by reviewing the title and abstract and 11 records were excluded based on full-text review. Finally, 18 studies were included in the final meta-analysis, including 45,760 participants [24,37]. The detailed process of selection according to the PRISMA guidelines is showed in Fig 1.
Fig 1. PRISMA flow diagram.
Quality assessment
S3 Table represents the quality assessment using the AHRQ appraisal tool and the level of evidence for each included 18 study. In general, two studies were considered high quality [13,34], while the remaining were stated as moderate quality [22–33,35–38]. All included studies were rated "yes" in items 1, 2, and 8, "unclear" in item 4, and "no" in item 9 and 11.
Characteristics of the studies
Cross-sectional surveys were employed in all studies. All studies used online surveys to measure acceptance rate of COVID-19 vaccines. Out of the 19 studies included, with sample sizes ranging from 416 to 11,951, included a total of 45,760 participants and were surveyed only in 2020 and 2021, with the first survey performing in February-March 2020 and the most recent in April 2021. Two studies were conducted on a scale spanning 5 provinces [13,30], two studies were conducted across 3 provinces [28,29], and four studies were conducted on whole national scale [31,32,35,38]. Two studies included nurses or practice nurses [24,37], and the remaining studies included different types of HCWs. Overall, the acceptance rate ranged from 37.62% to 95.85%. Table 1 summarises the characteristics of the included studies.
Table 1. Characteristics of the included studies.
| Author and Year | Region | Participants | Sample size | Survey period | Age/Years | Male(%) | Acceptance rate(%) | Quality evaluation |
|---|---|---|---|---|---|---|---|---|
| Zhang GF,2021 [22] | Beijing | Doctors, Nurses, Paramedics | 1658 | Jun,2020 | Majority;30-39(39.75%) | 21.29% | 68.40% | Moderate |
| Yu,2022 [23] | Hunan, Huaihua | Clinicians, Nurses, Technicians, Public health physicians, Administrators, Ancillary staff | 3958 | Not stated | Majority;20-29(37.49%) | 23.37% | 85.70% | Moderate |
| Liu,2022 [24] | Anhui | Nursing trainees | 551 | Dec,2020 | 18.34±1.31 | 11.07% | 57.53% | Moderate |
| Luo,2021 [25] | Sichuan | CDC staff | 551 | Jan,2021 | Majority;30-39(29.4%) | 41.70% | 84.57% | Moderate |
| Kong,2021 [13] | Shanxi, Beijing, Shandong, Hubei, Sichuan | Clinicians, Nurses, Technicians, Administrators, Medical examiners, CDC staff | 9345 | Nov,2020 | Majority;30-39(35.23%) | 27.33% | 70.82% | High |
| Cheng,2022 [26] | Yunnan | CDC staff | 416 | Dec,2020 | Majority;30-50(48.08%) | 41.35% | 83.65% | Moderate |
| Zhang HJ,2021 [27] | Zhejiang | Healthcare workers and CDC staff | 756 | Sept,2020 | Majority;31-40(36.11%) | 33.07% | 70.11% | Moderate |
| Shi,2022 [28] | Shanghai, Wuhan, Lanzhou | Healthcare workers | 627 | Jun,2020 | Majority;30-39(57.1%) | 49.76% | 95.85% | Moderate |
| Hao,2022 [29] | Inner Mongolia, Beijing, Hebei | Full-time healthcare workers | 621 | Apr,2021 | Majority;31-50(57.1%) | 28.50% | 68.28% | Moderate |
| Wang H,2022 [30] | Henan, Sichuan, Shandong, Guangdong, Inner Mongolia, Xinjiang, Liaoning | Healthcare workers, Administrators excluded | 2681 | Jan-May,2021 | Majority;25-34(37.22%) | 27.94% | 82.54% | Moderate |
| Wang MW,2021 [31] | 33 provinces | Healthcare workers | 1329 | Jan,2021 | Majority;18-24(29.0%) | 35.40% | 76.98% | Moderate |
| Ye,2021 [32] | 21 provinces | Doctors, Nurses | 2156 | Feb,2021 | 32.91±8.29 | 12.10% | 87.94% | Moderate |
| Li,2021 [33] | whole China | Doctors, Nurses, Ancillary staff, and others | 1779 | Jan-Feb,2021 | Majority;18-29(41.7%) | 11.80% | 93.87% | Moderate |
| Sun,2021 [34] | Sichuan, Chengdu | Healthcare workers | 505 | Jan,2021 | 32.35±8.98 | 22.57% | 76.63% | High |
| Wang C,2021 [35] | 31 provinces | Healthcare workers | 2386 | Jan,2021 | Majority;30-39(33.1%) | 37.05% | 80.85% | Moderate |
| Wang J,2021 [36] | Zhejiang | Doctors, Health technicians, Nurses and others | 3634 | Sept,2020 | Majority;<50(88.11%) | 22.56% | 79.09% | Moderate |
| Wang KL,2020 [37] | Hong Kong | Nurses | 856 | Feb-Mar,2020 | Majority;30-39(31.1%) | 11.80% | 37.62% | Moderate |
| Huang,2021 [38] | 30 provinces | doctors, nurses, and other medical professionals (medical and nursing trainees, technicians, and clinical pharmacists) working in ICUs | 11951 | Mar-Apr,2021 | Majority;31-40(45.8%) | 17.70% | 84.65% | Moderate |
Synthesis of results
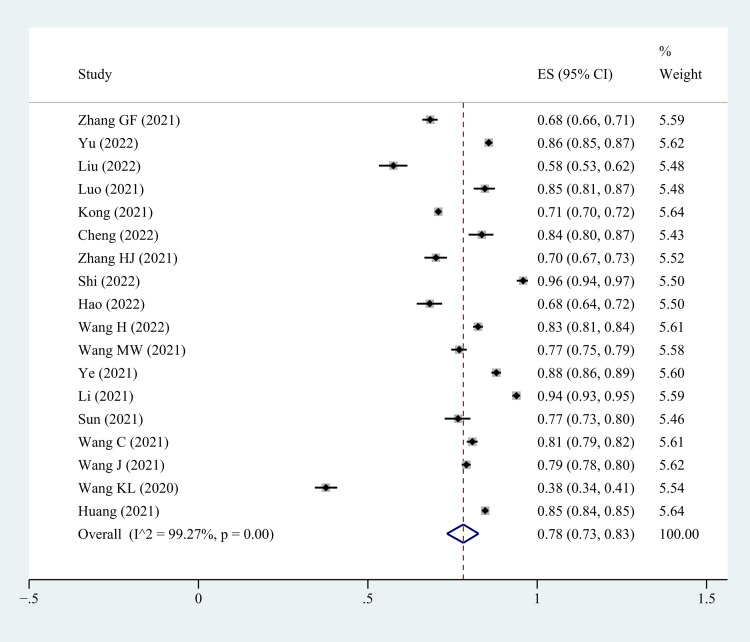
Based on the random-effects model, pooled COVID-19 vaccine acceptance rate and 95% confidence interval among HCWs in China was 78% (95% CI: 73%-83%). However, There was strong heterogeneity among these studies (I2 = 99.27%, p<0.001) (Fig 2).
Fig 2. Forest plot showing COVID -19 vaccine acceptance rate among HCWs in China.
Sensitivity analysis
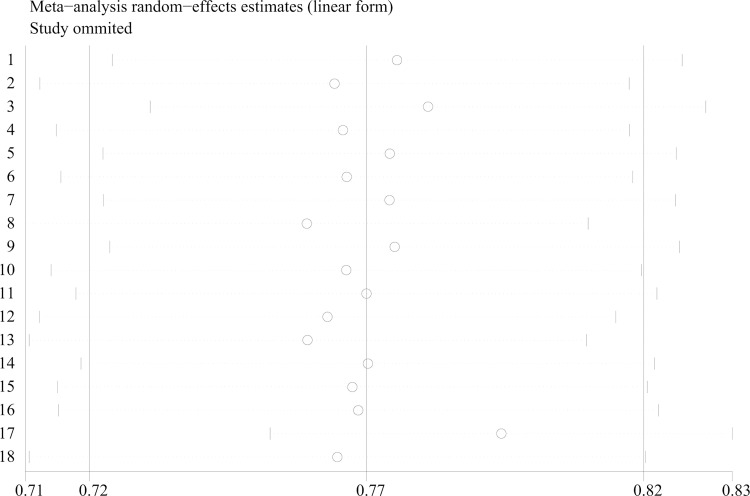
Sensitivity analysis of the total acceptance rate was performed by eliminating individual studies one by one. The results showed that no single study affected the overall estimated acceptance rate. After excluding any included studies, there was little change in estimated acceptance rate, suggesting that the robustness of this analysis. (S4 Table and Fig 3).
Fig 3. Sensitivity analysis for COVID-19 vaccine acceptance rate among HCWs in China.
Subgroup analysis
As shown in Table 2, subgroup analysis was based on survey year, the pooled estimated COVID-19 vaccine acceptance rate (73%, 95% CI: 65%-81%) in 2020 was significantly lower than that in 2021 (82%, CI: 78%-86%). Based on gender, the estimated pooled COVID-19 vaccine acceptance for male HCWs (83%, 95% CI: 78%-88%) was slightly higher than that for the female HCWs (80%, 95% CI: 74%-85%). Based on education level, the higher the education level, the lower the estimated pooled acceptance rate of COVID-19 vaccine. The acceptance rate of college degree or below was 84% (CI: 79%-89%), bachelor degree was 82% (CI: 78%-86%), and undergraduate degree or above was 79% (CI: 72%-86%). Based on occupation, the pooled estimated COVID-19 acceptance rate for nurses (76%, 95% CI: 68%-83%) was significantly lower than HCWs in other occupation. Based on Monthly income, The acceptance rate of HCWs with monthly income over 10000 yuan was the lowest (78%, 95% CI: 55%-94%). Based on region, the acceptance rate in Northern China was the lowest (79%, 95% CI: 77%-80%). the acceptance rate in Southern China was the highest (87%, 95% CI: 84%-90%). Based on professional position, the pooled estimated COVID-19 acceptance rate for HCWs with senior titles (85%, 95% CI: 78%-91%) was higher than HCWs with other professional titles. Based on marital status, widowed or divorced HCWs (70%, 95% CI: 38%-95%) had lower acceptance rate than married or unmarried HCWs. Moreover, acceptance of COVID-19 vaccine was low among HCWs with chronic conditions (75%, 95% CI: 57%-90%) and those who had not been participated in quarantine or contact with confirmed cases (78%, 95% CI: 69%-86%). Based on time of employment, HCWs with time of employment less than 5 years (83%, 95% CI: 78%-87%) and those with time of employment more than 20 years (83%, 95% CI: 79%-87%) had lower acceptance of COVID-19 vaccine.
Table 2. Subgroup analysis based on the characteristics of the included subjects.
| Study characteristics | No. of studies | Acceptance rate (%) (95% CI) | I2 | p-value |
|---|---|---|---|---|
| Survey year | ||||
| 2020 | 9 | 73(65–81) | 99.19% | <0.001 |
| 2021 | 8 | 82(78–86) | 98.17% | <0.001 |
| gender | ||||
| male | 16 | 83(78–88) | 97.37% | <0.001 |
| female | 16 | 80(74–85) | 99.19% | <0.001 |
| age(year) | ||||
| <30 | 11 | 81(75–86) | 97.92% | <0.001 |
| 30–39 | 8 | 73(60–85) | 99.17% | <0.001 |
| 40–49 | 8 | 81(69–90) | 98.38% | <0.001 |
| ≥50 | 11 | 86(76–93) | 96.68% | <0.001 |
| Education Level | ||||
| College degree or below | 11 | 84(79–89) | 97.54% | <0.001 |
| Bachelor degree | 11 | 82(78–86) | 98.25% | <0.001 |
| Postgraduate and above | 11 | 79(72–86) | 95.46% | <0.001 |
| Occupation | ||||
| doctors | 9 | 85(79–90) | 96.96% | <0.001 |
| Nurses | 10 | 76(68–83) | 99.24% | <0.001 |
| others | 10 | 84(82–87) | 70.57% | <0.001 |
| Monthly income(yuan) | ||||
| ≤5000 | 5 | 85(75–94) | 97.60% | <0.001 |
| 5001–1000 | 5 | 84(73–92) | 98.43% | <0.001 |
| >10000 | 4 | 78(55–94) | 98.79% | <0.001 |
| region | ||||
| Eastern | 6 | 82(69–92) | 99.31% | <0.001 |
| Southern | 4 | 87(84–90) | 89.10% | <0.001 |
| Western | 7 | 80(68–90) | 99.20% | <0.001 |
| Northern | 2 | 79(77–80) | 0% | <0.001 |
| Professional position | ||||
| Junior or no | 6 | 83(78–88) | 98.63% | <0.001 |
| Middle | 6 | 83(77–89) | 97.57% | <0.001 |
| Senior | 6 | 85(78–91) | 94.62% | <0.001 |
| Marital status | ||||
| Married | 5 | 87(82–91) | 95.33% | <0.001 |
| Unmarried | 5 | 87(80–93) | 94.54% | <0.001 |
| Others (Widowed or divorced) | 4 | 70(38–95) | 89.02% | <0.001 |
| Having chronic conditions | ||||
| Yes | 5 | 75(57–90) | 98.06% | <0.001 |
| No | 5 | 80(64–93) | 99.68% | <0.001 |
| Time of employment(year) | ||||
| ≤5 | 5 | 83(78–87) | 88.94% | <0.001 |
| 6–10 | 5 | 85(80–89) | 88.36% | <0.001 |
| 11–20 | 4 | 85(79–89) | 85.71% | <0.001 |
| >20 | 4 | 83(79–87) | 77.17% | <0.001 |
| Participated in quarantine or had contact with confirmed cases | ||||
| Yes | 8 | 83(75–90) | 98.82% | <0.001 |
| No | 8 | 78(69–86) | 99.31% | <0.001 |
Publication bias analysis
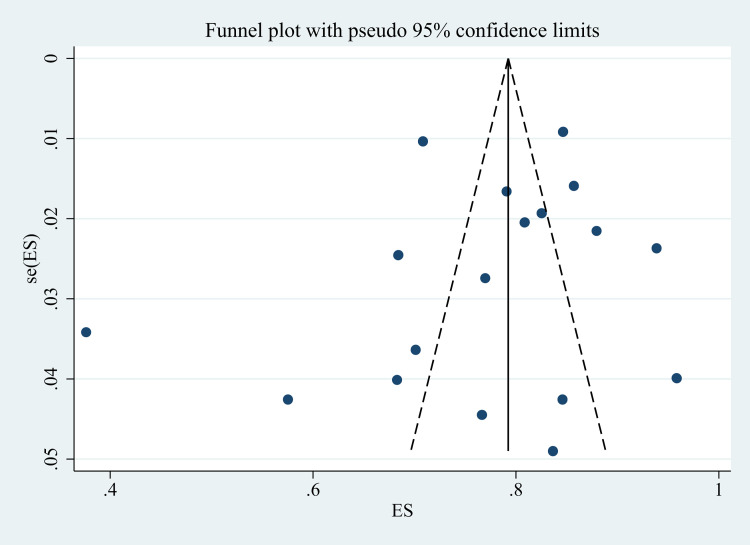
There was no obvious potential publication bias after symmetrical inspection using the funnel plot (Fig 4) and Egger’s regression test (t = -0.74, P = 0.469).
Fig 4. Funnel plot.
Discussion
This systematic review aimed to comprehensively pool the acceptance rate of COVID-19 vaccination among HCWs in China, to provide evidence for future improvements in vaccination implementation strategies. This systematic review and meta-analysis showed a pooled overall acceptance rate (78%) for COVID-19 vaccination among HCWs in China. But this result was lower than the acceptance rate in three previous national cross-sectional studies with more than 2,000 participants (87.94% [32], 80.85% [35], and 84.65% [38], respectively) and slightly higher than the result in a national study [31] with 1,329 participants, which indicated an overall acceptance rate of 76.98%. In addition to the small sample effect, another possible contributor to this finding was that in the latter study, the addition of attitudes of hesitation to the questionnaire led to a lower overall acceptance rate. However, this result was significantly higher than the pooled acceptance rate of COVID-19 vaccine among HCWs around the world (55%) reported in a meta-analysis [15].
Firstly, we found that vaccination intentions did vary by survey time, with vaccination intentions significantly higher in 2021 than in 2020 [39]. One possible explanation for this was that the amount of information related to COVID-19 and COVID-19 vaccines had been tremendous in recent years, and had increased at an unprecedented pace. As passage of time, scientific evidence about COVID-19 vaccine had become more comprehensive, and HCWs had become more aware of its safety and effectiveness [40]. In addition, The National Health Commission of China’s policy of voluntary and free vaccination with informed consent of all adults, issued in January 2021, also played a big role. Secondly, the subgroup analysis between gender indicated that male health workers were more likely than females to receive COVID-19 vaccines [41–48]. The likely reason was that COVID-19 severity and mortality were increased in male compared to female [49]. It may also be related to women’s special conditions, such as menstruation, pregnancy and lactation, which affect women’s willingness to get vaccinated against COVID-19. Moreover, it was worth noting that the results of this study showed that the willingness of HCWs to vaccinate against COVID-19 declined with the improvement of educational level, which was contrary to the results in Africa [50], suggesting that educational level played different roles in the willingness of different populations to vaccinate against COVID-19. Those with higher education levels were likely to receive more adverse information such as rumors about vaccines. We also found relatively low acceptance rate of COVID-19 vaccine among nurses compared to doctors and other HCWs, similar to a survey conducted in Kuwait [51] and in the India[44,46–48,52]. The fact that nurses have a negative attitude towards COVID-19 vaccine was of concern since they were the most vulnerable subgroup to be infected and it is important to improve the levels of trust in the efficacy and safety of COVID-19 vaccines in this subgroup. In this study, there were also differences in vaccination intentions among HCWs in different regions, with southern China having the highest vaccination rates (87%), and a global review of HCWs also confirmed differences in vaccination intentions among HCWs in different regions [15]. Better publicity and health education should be carried out for HCWs in regions with low vaccination intentions to improve their acceptance of COVID-19 vaccine. Finally, HCWs who participated in quarantine or had been in contact with confirmed cases had a stronger incentive to get vaccinated against COVID-19, because they believe they were more vulnerable to infection, which also affects the general population. A cross-sectional study in Iraqi showed that HCWs believe they were more vulnerable to COVID-19 and were more likely to be vaccinated against the disease than the general population. Targeted dissemination of COVID-19 knowledge was needed to improve the willingness of HCWs to receive COVID-19 vaccine and eliminate vaccine hesitancy among HCWs. At the same time, regarding adverse events in vaccine development and use, government departments and institutions should release authoritative, transparent and objective information in a timely manner to reduce vaccine hesitation caused by canard of Internet.
Strengths and limitations
The advantages of this study
First, in order to avoid repeated accidental duplication of reviews, reduce potential bias, and improve transparency, this study conducted a systematic review of prospective registries [53]. Therefore, to the authors’ knowledge, this is the first systematic review and meta-analysis of COVID-19 acceptance rates in China. Second, this review conducted a comprehensive literature search of eight databases, including published literature in Chinese and English, and tracked references to related studies to prevent omissions. In addition, the review used well-validated meta-analysis processes and models that fully conformed to international standards, and conducted sensitivity analyses to test the pooled results of the meta-analyses. Finally, the literature included in this review covered the majority of regions in China and was well representative.
Limitations of this study
First, there was significant heterogeneity among all articles, and only two articles were rated as high quality. First of all, the limited number of studies included in this meta-analysis may lead to bias in the inferred and summarized results, which reduced the reliability of research results. Secondly, all studies were conducted in the form of network survey, which may cause deviation in the selection of participants. Finally, although subgroup analysis was conducted according to population characteristics, the heterogeneity of each subgroup analysis was still high, which may be affected by a variety of factors such as survey tools. However, due to the limited available evidence, it could not be analyzed one by one.
Conclusion
In summary, pooled evidence showed that the estimated COVID-19 vaccine acceptance rate of HCWs in China is 78%; Among them, the acceptance rate of HCWs with different characteristics varies, and the pooled acceptance rate in 2021 was significantly higher than that in 2020. Therefore, based on these results, we should strengthen publicity on the safety and effectiveness of vaccines, improve HCWs ’ understanding of COVID-19 vaccines, and enhance their confidence in vaccines. The publicity of contraindications for COVID-19 vaccination should be strengthened to eliminate HCWs ’ concerns about COVID-19 vaccine and improve their willingness to be vaccinated.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the article and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Liu Y-C, Kuo R-L, Shih S-R. COVID-19: The first documented coronavirus pandemic in history. Biomed J. 2020;43: 328–333. doi: 10.1016/j.bj.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID Live—Coronavirus Statistics—Worldometer. [cited 8 Jun 2022]. Available: https://www.worldometers.info/coronavirus/.
- 3.Taylor L. Covid-19: True global death toll from pandemic is almost 15 million, says WHO. BMJ. 2022;377: o1144. doi: 10.1136/bmj.o1144 [DOI] [PubMed] [Google Scholar]
- 4.Mishra SK, Tripathi T. One year update on the COVID-19 pandemic: Where are we now? Acta Trop. 2021;214: 105778. doi: 10.1016/j.actatropica.2020.105778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umakanthan S, Sahu P, Ranade AV, Bukelo MM, Rao JS, Abrahao-Machado LF, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96: 753–758. doi: 10.1136/postgradmedj-2020-138234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z, Zhu Y, Chu M. Role of COVID-19 Vaccines in SARS-CoV-2 Variants. Front Immunol. 2022;13: 898192. doi: 10.3389/fimmu.2022.898192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Available: http://www.ncbi.nlm.nih.gov/books/NBK554776/. [PubMed] [Google Scholar]
- 8.Umakanthan S, Senthil S, John S, Madhavan MK, Das J, Patil S, et al. The protective role of statins in COVID-19 patients: a retrospective observational study. Transl Med Commun. 2021;6: 22. doi: 10.1186/s41231-021-00102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldblatt D. SARS-CoV-2: from herd immunity to hybrid immunity. Nat Rev Immunol. 2022;22: 333–334. doi: 10.1038/s41577-022-00725-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umakanthan S, Patil S, Subramaniam N, Sharma R. COVID-19 Vaccine Hesitancy and Resistance in India Explored through a Population-Based Longitudinal Survey. Vaccines (Basel). 2021;9: 1064. doi: 10.3390/vaccines9101064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Qin C, Liu M, Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10: 132. doi: 10.1186/s40249-021-00915-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umakanthan S, Lawrence S. Predictors of COVID-19 vaccine hesitancy in Germany: a cross-sectional, population-based study. Postgrad Med J. 2022; postgradmedj-2021-141365. doi: 10.1136/postgradmedj-2021-141365 [DOI] [PubMed] [Google Scholar]
- 13.Kong QF. Willingness to receive COVID-19 vaccine and influenza vaccination status among healthcare workers. M.Sc. Thesis, Chinese Center for Disease Control and Prevention. 2021. doi: 10.27511/d.cnki.gzyyy.2021.000038 [DOI]
- 14.Dooling K. The Advisory Committee on Immunization Practices’ Updated Interim Recommendation for Allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69. doi: 10.15585/mmwr.mm695152e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo C, Yang Y, Liu Y, Zheng D, Shao L, Jin J, et al. Intention to COVID-19 vaccination and associated factors among health care workers: A systematic review and meta-analysis of cross-sectional studies. Am J Infect Control. 2021;49: 1295–1304. doi: 10.1016/j.ajic.2021.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannakou K, Kyprianidou M, Christofi M, Kalatzis A, Fakonti G. Mandatory COVID-19 Vaccination for Healthcare Professionals and Its Association With General Vaccination Knowledge: A Nationwide Cross-Sectional Survey in Cyprus. Front Public Health. 2022;10: 897526. doi: 10.3389/fpubh.2022.897526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuño M, Chowell G, Gumel AB. Assessing the role of basic control measures, antivirals and vaccine in curtailing pandemic influenza: scenarios for the US, UK and the Netherlands. J R Soc Interface. 2007;4: 505–521. doi: 10.1098/rsif.2006.0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). 2021;74: 790–799. doi: 10.1016/j.rec.2021.07.010 [DOI] [PubMed] [Google Scholar]
- 19.Rostom A, Dubé C, Cranney A, Saloojee N, Sy R, Garritty C, et al. Appendix E. Summary ROC Curves. Celiac Disease. Agency for Healthcare Research and Quality (US); 2004. Available: https://www.ncbi.nlm.nih.gov/books/NBK35138/. [Google Scholar]
- 20.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21: 1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315: 629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang GF, Liu B, Wu J. Survey on willingness of medical staff to vaccinate and recommend novel coronavirus vaccine in Tongzhou district of Beijing. Capital Journal of Public Health., 2021,15(06):338–342. doi: 10.16760/j.cnki.sdggws.2021.06.012 [DOI] [Google Scholar]
- 23.Rong Yu, Ma DH, Liu YD, Ma L, He J, Deng ZH. Willingness to receive COVID−19 vaccination and its influencing factors among staff of medical institutions in Huaihua City. Practical Preventive Medicine. 2022,29(03):286–290. doi: 10.3969/j.issn.1006-3110.2022.03.008 [DOI] [Google Scholar]
- 24.Liu H, Xu ZJ, Wang CZ, Tao MF, Wang YY, Yuan T, et al. COVID-19vaccination intention and its influencing factors among intern nursing students. Journal of Shenyang Medical College. 2022,24(01):49–53. doi: 10.16753/j.cnki.1008-2344.2022.01.011 [DOI] [Google Scholar]
- 25.Luo X, Zhang H, Tan XX, Hu JW, Li MY, Huang T, et al. Investigation on Willingness to Inoculate COVID-19 Vaccine among 551 Workers of Municipal Centers for Disease Control and Prevention in Sichuan Province. Journal of Preventive Medicine Information. 2021,37(12):1674–1679. [Google Scholar]
- 26.Cheng JG, Zhang LP, Zhang CY, Yang YP, Gao TT, Zhu B. Analysis on situation of awareness and willingness to vaccinate and factors influencing 2019 − nCoV vaccine in CDC workers from Yunnan. Journal of Medical Pest Control. 2022,38(05):472–476+481. doi: 10.7629/yxdwfz202205016 [DOI] [Google Scholar]
- 27.Zhang HJ, Ding LL, Pan XJ, Sheng LZ, Zhu Y, Chen FX, et al. Willingness to receive novel coronavirus vaccine and factors influencing willingness among healthcare workers in Zhejiang province. Chinese Journal of Vaccines and Immunization.: 1–7[2022-06-10]. doi: 10.19914/j.CJVI.2021030 [DOI] [Google Scholar]
- 28.Shi HL, Wu QH, Chen ZY, Gong H, Yang J, Yu HJ, et al. Willingness to receive COVID−19 vaccination and its influencing factors among general population and medical staff in China. Practical Preventive Medicine. 2022,29(06):671–677. doi: 10.1969/j.issn.1006-3110.2022.06.007 [DOI] [Google Scholar]
- 29.Hao J, Liu H, Shi J, Wang Q, Su X, Shi Z, et al. A study on the willingness and influencing factors of novel coronavirus vaccination among medical personnel in North China. Hum Vaccin Immunother. 2022; 1–7. doi: 10.1080/21645515.2022.2031775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Huang Y-M, Su X-Y, Xiao W-J, Si M-Y, Wang W-J, et al. Acceptance of the COVID-19 vaccine based on the health belief model: a multicenter national survey among medical care workers in China. Hum Vaccin Immunother. 2022; 2076523. doi: 10.1080/21645515.2022.2076523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M-W, Wen W, Wang N, Zhou M-Y, Wang C-Y, Ni J, et al. COVID-19 Vaccination Acceptance Among Healthcare Workers and Non-healthcare Workers in China: A Survey. Front Public Health. 2021;9: 709056. doi: 10.3389/fpubh.2021.709056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye X, Ye W, Yu J, Gao Y, Ren Z, Chen L, et al. The landscape of COVID-19 vaccination among healthcare workers at the first round of COVID-19 vaccination in China: willingness, acceptance and self-reported adverse effects. Hum Vaccin Immunother. 2021;17: 4846–4856. doi: 10.1080/21645515.2021.1985354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X-H, Chen L, Pan Q-N, Liu J, Zhang X, Yi J-J, et al. Vaccination status, acceptance, and knowledge toward a COVID-19 vaccine among healthcare workers: a cross-sectional survey in China. Hum Vaccin Immunother. 2021;17: 4065–4073. doi: 10.1080/21645515.2021.1957415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Chen X, Cao M, Xiang T, Zhang J, Wang P, et al. Will Healthcare Workers Accept a COVID-19 Vaccine When It Becomes Available? A Cross-Sectional Study in China. Front Public Health. 2021;9: 664905. doi: 10.3389/fpubh.2021.664905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Wang Y, Han B, Zhao T-S, Liu B, Liu H, et al. Willingness and SARS-CoV-2 Vaccination Coverage among Healthcare Workers in China: A Nationwide Study. Vaccines (Basel). 2021;9: 993. doi: 10.3390/vaccines9090993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Feng Y, Hou Z, Lu Y, Chen H, Ouyang L, et al. Willingness to receive SARS-CoV-2 vaccine among healthcare workers in public institutions of Zhejiang Province, China. Hum Vaccin Immunother. 2021;17: 2926–2933. doi: 10.1080/21645515.2021.1909328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang K, Wong ELY, Ho KF, Cheung AWL, Chan EYY, Yeoh EK, et al. Intention of nurses to accept coronavirus disease 2019 vaccination and change of intention to accept seasonal influenza vaccination during the coronavirus disease 2019 pandemic: A cross-sectional survey. Vaccine. 2020;38: 7049–7056. doi: 10.1016/j.vaccine.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang W, Shao X, Wagner AL, Chen Y, Guan B, Boulton ML, et al. COVID-19 vaccine coverage, concerns, and preferences among Chinese ICU clinicians: a nationwide online survey. Expert Rev Vaccines. 2021;20: 1361–1367. doi: 10.1080/14760584.2021.1971523 [DOI] [PubMed] [Google Scholar]
- 39.Tan KWA, Wijaya L, Lim CT, Gan WH. COVID-19 vaccination acceptance of healthcare workers in Singapore. Ann Acad Med Singap. 2022;51: 304–308. [PubMed] [Google Scholar]
- 40.Umakanthan S, Bukelo MM, Gajula SS. The Commonwealth Caribbean COVID-19: Regions Resilient Pathway During Pandemic. Front Public Health. 2022;10: 844333. doi: 10.3389/fpubh.2022.844333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malik A, Malik J, Ishaq U. Acceptance of COVID-19 vaccine in Pakistan among health care workers. PLoS One. 2021;16: e0257237. doi: 10.1371/journal.pone.0257237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dzieciolowska S, Hamel D, Gadio S, Dionne M, Gagnon D, Robitaille L, et al. Covid-19 vaccine acceptance, hesitancy, and refusal among Canadian healthcare workers: A multicenter survey. Am J Infect Control. 2021;49: 1152–1157. doi: 10.1016/j.ajic.2021.04.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gadoth A, Halbrook M, Martin-Blais R, Gray A, Tobin NH, Ferbas KG, et al. Cross-sectional Assessment of COVID-19 Vaccine Acceptance Among Health Care Workers in Los Angeles. Ann Intern Med. 2021;174: 882–885. doi: 10.7326/M20-7580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrading WA, Trent SA, Paxton JH, Rodriguez RM, Swanson MB, Mohr NM, et al. Vaccination rates and acceptance of SARS-CoV-2 vaccination among U.S. emergency department health care personnel. Acad Emerg Med. 2021;28: 455–458. doi: 10.1111/acem.14236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youssef D, Abou-Abbas L, Berry A, Youssef J, Hassan H. Determinants of acceptance of Coronavirus disease-2019 (COVID-19) vaccine among Lebanese health care workers using health belief model. PLoS One. 2022;17: e0264128. doi: 10.1371/journal.pone.0264128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fotiadis K, Dadouli K, Avakian I, Bogogiannidou Z, Mouchtouri VA, Gogosis K, et al. Factors Associated with Healthcare Workers’ (HCWs) Acceptance of COVID-19 Vaccinations and Indications of a Role Model towards Population Vaccinations from a Cross-Sectional Survey in Greece, May 2021. Int J Environ Res Public Health. 2021;18: 10558. doi: 10.3390/ijerph181910558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briko NI, Korshunov VA, Mindlina AY, Polibin RV, Antipov MO, Brazhnikov AI, et al. Healthcare Workers’ Acceptance of COVID-19 Vaccination in Russia. Int J Environ Res Public Health. 2022;19: 4136. doi: 10.3390/ijerph19074136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan AK, Sahin MK, Parildar H, Adadan Guvenc I. The willingness to accept the COVID-19 vaccine and affecting factors among healthcare professionals: A cross-sectional study in Turkey. Int J Clin Pract. 2021;75: e14226. doi: 10.1111/ijcp.14226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fabião J, Sassi B, Pedrollo EF, Gerchman F, Kramer CK, Leitão CB, et al. Why do men have worse COVID-19-related outcomes? A systematic review and meta-analysis with sex adjusted for age. Braz J Med Biol Res. 2022;55: e11711. doi: 10.1590/1414-431X2021e11711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adeniyi OV, Stead D, Singata-Madliki M, Batting J, Wright M, Jelliman E, et al. Acceptance of COVID-19 Vaccine among the Healthcare Workers in the Eastern Cape, South Africa: A Cross Sectional Study. Vaccines (Basel). 2021;9: 666. doi: 10.3390/vaccines9060666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Sanafi M, Sallam M. Psychological Determinants of COVID-19 Vaccine Acceptance among Healthcare Workers in Kuwait: A Cross-Sectional Study Using the 5C and Vaccine Conspiracy Beliefs Scales. Vaccines (Basel). 2021;9: 701. doi: 10.3390/vaccines9070701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singhania N, Kathiravan S, Pannu AK. Acceptance of coronavirus disease 2019 vaccine among health-care personnel in India: a cross-sectional survey during the initial phase of vaccination. Clin Microbiol Infect. 2021;27: 1064–1066. doi: 10.1016/j.cmi.2021.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. 2012;1: 7. doi: 10.1186/2046-4053-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the article and its Supporting Information files.