Abstract
Background
Snakebite envenomation exerts a heavy toll in sub-Saharan Africa. The design and production of effective polyspecific antivenoms for this region demand a better understanding of the immunological characteristics of the different venoms from the most medically important snakes, to select the most appropriate venom combinations for generating antivenoms of wide neutralizing scope. Bitis spp. and Echis spp. represent the most important viperid snake genera in Africa.
Methodology/Principal findings
Eight rabbit-derived monospecific antisera were raised against the venoms of four species of Bitis spp. and four species of Echis spp. The effects of immunization in the rabbits were assessed, as well as the development of antibody titers, as judged by immunochemical assays and neutralization of lethal, hemorrhagic, and in vitro coagulant effects. At the end of immunizations, local and pulmonary hemorrhage, together with slight increments in the plasma activity of creatine kinase (CK), were observed owing to the action of hemorrhagic and myotoxic venom components. Immunologic analyses revealed a considerable extent of cross-reactivity of monospecific antisera against heterologous venoms within each genus, although some antisera provided a more extensive cross-reactivity than others. The venoms that generated antisera with the broadest coverage were those of Bitis gabonica and B. rhinoceros within Bitis spp. and Echis leucogaster within Echis spp.
Conclusions/Significance
The methodology followed in this study provides a rational basis for the selection of the best combination of venoms for generating antivenoms of high cross-reactivity against viperid venoms in sub-Saharan Africa. Results suggest that the venoms of B. gabonica, B. rhinoceros, and E. leucogaster generate antisera with the broadest cross-reactivity within their genera. These experimental results in rabbits need to be translated to large animals used in antivenom production to assess whether these predictions are reproduced in horses or sheep.
Author summary
Snakebite envenomation exerts a heavy toll in sub-Saharan Africa. Antivenom is the only valid therapy for these envenomations. However, owing to the wide variety of snake species and the large variation in venom composition, the selection of the best combination of venoms to generate effective polyclonal antivenoms of wider neutralizing coverage is challenging. In this study, an experimental protocol was developed based on the generation of monospecific antisera in rabbits immunized with venoms of Bitis spp. or Echis spp. from Africa. Cross-reactivity of monospecific antisera was assessed by immunochemical analyses and by neutralization of toxic effects. Results revealed a large extent of intrageneric cross-reactivity by all antisera, with few exceptions. The venoms that generated antisera with the highest cross-reactivity and neutralizing ability among Bitis spp. were those of Bitis gabonica and B. rhinoceros, and of Echis leucogaster in Echis spp. These results provide evidence for the selection of the best combination of venoms for preparing antivenoms of high neutralizing scope within these two medically relevant genera of African viperid species. These results in rabbits must be translated to large animals used in antivenom production to assess whether these predictions are confirmed.
Introduction
Snakebite envenomation is an important public health problem in tropical and sub-tropical countries around the world, especially in impoverished rural agricultural and pastoral communities in sub-Saharan Africa, Asia, Latin America, and some regions of Oceania [1, 2]. Therefore, the World Health Organization considers it as a highest impact (i.e., Category A) neglected tropical disease [1, 3].
It has been estimated that 1.2–5.5 million of snakebites occurred worldwide in 2007, from which 421,000–1,841,000 cases resulted in envenomation, and 20,000–94,000 in death. Approximately 27% of these incidents occurred in Africa [4]. According to the reptile database (https://reptile-database.reptarium.cz/), there are 61 species of snakes of the family Viperidae in Africa, which are grouped in five genera (i.e., Atheris, Bitis, Causus, Cerastes, and Echis). The species with major potential to induce envenomations of high incidence and severity belong to the Bitis and Echis genera.
Among the 14 African Bitis species, only B. arietans (Puff adder), B. gabonica (East African Gaboon viper), B. nasicornis (Rhinoceros viper), and B. rhinoceros (West African Gaboon viper) are considered by WHO as highly venomous snakes whose bites result in high levels of morbidity, disability, or mortality (i.e., Category 1 species) [1]. On the other hand, all five species of African Echis, i.e., E. coloratus (Palestine saw-scaled viper), E. jogeri (Mali carpet viper), E. leucogaster (White-bellied carpet viper), E. ocellatus/romani (West African carpet viper), and E. pyramidum (North-East African carpet viper), are included in the Category 1 in the WHO classification [1].
Venoms of Bitis spp. and Echis spp. are composed of different proportions of proteins belonging to the same families, such as Zn2+-dependent snake venom metalloproteinases (SVMPs), snake venom serine proteinases (SVSPs), phospholipases A2 (PLA2s), cysteine-rich secretory proteins (CRISPs), C-type lectin-like proteins, disintegrins, L-amino acid oxidases (LAAOs), Kunitz-type inhibitors, and other less abundant components [5–9].
Envenomations by species of Bitis and Echis can be differentiated according to their clinical manifestations: cases produced by Bitis spp. are characterized by marked local swelling with coagulable blood, with the exceptions of some populations of B. arietans which induce coagulopathies [10, 11]. On the other hand, the syndrome induced by Echis spp. is characterized by marked local swelling, with incoagulable blood and/or spontaneous systemic bleeding [11]. In addition, and depending on the severity of the envenomation, clinical manifestations could include local necrosis, blistering, and hemodynamic disturbances, among other effects [12].
Intravenous administration of animal-derived antivenoms is the only validated therapy for snakebite envenomations [1]. Antivenoms are formulations of whole immunoglobulins, or their Fab or F(ab´)2 fragments, purified from plasma of animals immunized towards snake venoms [1, 13]. Depending on the number of venoms used for immunization, antivenoms can be either monospecific, bispecific or polyspecific.
The anti-venom immunoglobulins bind and neutralize venom toxins in the circulation or tissues and contribute to their elimination. However, their efficacy is limited to venoms antigenically similar to those used as immunogens to stimulate the immune response in animals. Therefore, to ensure a wide coverage of neutralization without requiring the identification of the offending snake species, the use of polyspecific formulations is generally preferred [1, 14]. This is the case of antivenoms designed for use in sub-Saharan Africa, most of which are polyspecific, although some monospecific products are also available [11].
To produce polyspecific antivenoms with wide neutralization scope, venoms used as immunogens should be selected considering the intra- and inter-specific variation in their composition, and the intrageneric conservation of the antigenic characteristics of venoms of the medically most important snakes in the region where the antivenom is intended to be used [1]. The selection of the most appropriate venoms for immunization should therefore be based on a detailed knowledge of the antigenic relatedness of venoms and the medical relevance of the species. This represents a particular challenge in the case of Africa, where abundant venomous snakes are widely distributed [1].
In this work, we inferred the antigenic similarity between venoms of Bitis spp. and Echis spp. classified by the WHO as African Category 1 snakes on the basis of the intrageneric cross-reactivity between monospecific rabbit sera raised against individual venoms. This information is of value for the rational, knowledge-based design of the most appropriate venom mixtures for the generation of pan-African antivenoms.
Materials and methods
Ethics statement
This manuscript presents an experimental study performed following the standard procedures of scientific ethics, including those related to the use and care of animals. All procedures carried out in this study meet the International Guiding Principles for Biomedical Research Involving Animals [15]. All procedures involving animals were approved by the Institutional Committee for the Care and Use of Laboratory Animals of Universidad de Costa Rica (approval code CICUA 202–2020). Mice and rabbits were obtained from the Bioterium of Instituto Clodomiro Picado. Mice were handled in Tecniplast Eurostandard Type II 1264C cages (L25.0 x W40.0 x H 14.0 cm), five mice per cage, while rabbits were managed in Scanbur type EC3 cages (L 823 x W 660 x H 110 mm), one rabbit per cage. In both cases, animals were maintained at 18–24°C, 60–65% relative humidity and 12:12 light-dark cycle. Human plasma for coagulation experiments was collected from healthy donors who were over 18 years old and provided written informed consent, according to the normative of the Institutional Bioethics Committee of Universidad de Costa Rica (approval VI-2925-2013).
Snake venoms
Venoms of adult specimens of B. arietans (unspecified origin, batch #322.061), B. gabonica (unspecified origin, batch #725.031), B. nasicornis (unspecified origin, batch #500.102), B. rhinoceros (from Ghana, batch #701.070), E. coloratus (from Egypt, batch #512.191), E. leucogaster (from Mali, batch #623.070), E. ocellatus (unspecified origin, batch #216.031), and E. pyramidum (from Egypt, batch #523.070) were purchased from Latoxan (Portes-dès Valence, France). After collection, venoms were stabilized by lyophilization and stored at -40°C. Solutions of venoms were prepared immediately before use.
Reverse-phase HPLC profiling
Five milligrams of each venom were dissolved in 200 μL of 0.1% trifluoroacetic acid (TFA) and 5% acetonitrile buffer (buffer A). Insoluble material was removed by centrifugation and the proteins in the supernatant were separated by reverse-phase HPLC (RP-HPLC, HPLC system: Agilent 1100 series; Agilent Technologies), equipped with a C18 column (250 x 4.6 mm, 5 μm particle size; Agilent Technologies). The flow rate was set to 1 mL/min and the protein separation was performed with the following buffer gradient: 0% buffer B (buffer B: 95% acetonitrile, 0.1% TFA) for 5 min, followed by 0–15% B over 10 min, 15–45% B over 60 min, 45–70% B over 10 min and 70% B for 9 min [16]. Protein peaks were detected at 215 nm. HPLC fractions were identified by comparing the chromatograms with those previously published for B. arietans) [17], B. gabonica [6], B. nasicornis and B. rhinoceros [7], and E. ocellatus [5]. We did not find published proteomic analysis of venoms of E. coloratus, E. leucogaster and E. pyramidum that could be used as a reference.
Determination of toxic activities of venoms
Lethal activity
Groups of five mice (16–18 g; CD-1 strain; both sexes) received a subcutaneous (SC) injection of the analgesic Tramadol, at a dose of 50 mg/kg, to reduce pain during the tests [18]. Fifteen minutes afterwards, mice received an intraperitoneal (IP) injection of 0.5 mL of 0.12 M NaCl, 0.04 M phosphate, pH 7.2 solution (PBS) containing different amounts of venoms (12.0–91.1 μg/mouse for venoms of Bitis spp, or 10.0–113.9 μg/mouse for venoms of Echis spp). The number of deaths during the following 6 h was recorded [19] and used to estimate the median lethal dose (LD50, i.e., the amount of venom that results in the death of 50% of the injected mice) by Probits [20]. Surviving mice were euthanized by CO2 inhalation. Results were reported as LD50 and the corresponding 95% confidence interval (95% CI).
Hemorrhagic activity
Groups of three mice (18–20 g; CD-1 strain; both sexes) were treated by the SC route with the analgesic Tramadol, at a dose of 50 mg/kg. Fifteen minutes afterwards, mice received an intradermal (ID) injection of various doses of venoms (0.05–0.80 μg/mouse for venoms of Bitis spp, or 0.10–3.20 μg/mouse for venoms of Echis spp), dissolved in 100 μL PBS. After 2 h, mice were sacrificed by CO2 inhalation. Then, by using the Inkscape 0.91 program (https://inkscape.org/download/), the area and intensity of the hemorrhagic lesion induced by the venom were measured in the inner side of the skin and expressed in hemorrhagic units (HaU). The minimum hemorrhagic dose (MHD) was defined as the amount of venom that generates 100 HaU [21]. Results were reported as the mean ± SD (n = 3).
Coagulant activity in vitro
Coagulant activity of venoms was determined based on a turbidimetric assay [22, 23]. Different amounts of venoms, dissolved in 100 μL of 25 mM Tris-HCl, 137 mM NaCl, 3.4 mM KCl, pH 7.4 (TBS) were added in triplicate to wells in a 96-well plate and incubated for 5 min at 37°C in a microplate reader (Cytation 3 Imaging Reader, Bio- Tek). Then, 4 μL of 0.4 M CaCl2 was added to 100 μL of human citrated plasma previously incubated at 37°C, and this mixture was added immediately to each venom-containing well using a multichannel pipette. Samples were mixed for 5 s by shaking, and the absorbance at 340 nm was monitored every 30 s over 15 min. The increase in absorbance reflects the formation of a clot. The minimum coagulant dose (MCD) was calculated as the amount of venom that induce a change in absorbance of 0.01 units within 1 min. Results were reported as the mean ± SD of triplicate determinations.
Immunization of rabbits
Groups of four rabbits (New Zealand, 2.5–3.0 kg body weight) were immunized with the venoms of either B. arietans, B. gabonica, B. nasicornis, B. rhinoceros, E. coloratus, E. leucogaster, E. ocellatus, or E. pyramidum. Immunization was carried out by five SC injections, applied at two-week intervals. The total volume of injections was 2 mL and each one of them contained 1 mg of venom. In the first injection, the venoms were emulsified in Complete Freund’s Adjuvant (CFA). In the second one, Incomplete Freund’s Adjuvant (IFA) was used, and in the following injections venoms were dissolved in PBS. Samples of blood were collected from the ear marginal vein, at the end of the immunization, for hematological, serum chemistry, and immunological analyses. Rabbits were then euthanized by an overdose of anesthetic (i.e., a dose of 100 mg/kg of sodium pentobarbital, administered by the IP route). A group of four non-immunized rabbits was included in parallel with immunized animals, and used as control for the hematological, serum chemistry, necropsy, and immunological analyses.
Hematological, serum chemistry and necropsy analysis
Hematological, serum chemistry, and necropsy analyses were applied to each individual rabbit sample. Hematological analyses (i.e., erythrocytes, leukocytes and platelets count, hematocrit, and hemoglobin concentration) were carried out in a Veterinary Hematology Analyzer (Exigo Eos Hematology System; Boule Diagnostics AB, Stockholm, Sweden). The following analytes were quantified in a clinical chemistry analyzer (Spin200E Automatic biochemistry analyzer; Spinreact, Barcelona, España): creatine kinase (CK), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), and were determined by the corresponding International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) methods. Urea was quantified by a modification of the Talke and Schubert method [24]; creatinine by a kinetic modification of the Jaffe colorimetric method [25]; albumin by the bromocresol green colorimetric method [26]; and total protein by the Biuret method [27]. Immediately after euthanasia, tissue samples of skin, liver, lungs, kidneys, and heart, were collected and added to 3.7% formalin solution. Tissues were processed routinely and embedded in paraffin. Sections of 4 μm thickness were obtained and stained with hematoxylin-eosin for histological analysis.
Reactivity of rabbit sera by enzyme-linked immunosorbent assay (ELISA)
Polystyrene plates were coated overnight at room temperature with 100 μL of PBS containing 3 μg of venom. After washing the plates five times with distilled water, 100 μL of several dilutions of each rabbit serum sample, in PBS-2% bovine serum albumin (BSA), were added. Plates were incubated for 1 h at room temperature and washed five times. Afterwards, 100 μL of goat anti-rabbit IgG conjugated with peroxidase, diluted 1:5000 with PBS-2% BSA, were added to each well. Microplates were incubated for 1 h at room temperature. After a final washing step, color was developed by the addition of H2O2 and o-phenylenediamine, during 20 min. Color development was stopped by the addition of 1.0 M HCl. Absorbances at 492 nm were recorded. The relative concentration of anti-venom antibodies in the samples was calculated by interpolation of their absorbances in a calibration curve. Relative concentration was expressed as percentage, 100% corresponding to the titer of the serum raised against the homologous venom of each species. Results were expressed as mean ± SD of all rabbits in each group. Heterologous sera with content of specific antibodies higher than 33% of the corresponding homologous serum were arbitrarily considered as “ELISA cross-reactive sera” and were selected for the assessment of in vivo neutralization assays.
Electrophoretic analysis and western blot
SDS-PAGE was run under reducing conditions using an acrylamide concentration of 12% [28]. Gels were stained with Coomassie Brilliant Blue R-250 or transferred to a nitrocellulose membrane at 30 mAmp overnight. Then, the membranes were blocked with PBS-0.1% casein for 30 min. Next, membranes were incubated for 1 h with a pool of serum samples of all rabbits of each monospecific antiserum, diluted 1/500 with PBS-0.1% casein. After washing the membranes three times with PBS-0.1% casein, they were incubated for 1 h with goat anti-rabbit IgG conjugated with alkaline phosphatase, diluted 1:2000 with PBS-0.1% casein. Finally, after the last washing step, 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium (BCIP/NBT) color development substrate was added, and the reaction was stopped, after 20 min, with distilled water.
Neutralization of the lethal, hemorrhagic, and coagulant activities
To reduce the number of mice used in the in vivo neutralization assays, only the antisera that gave higher than 33% cross-reactivity by ELISA were evaluated in the in vivo assays. The ability of pools of serum samples from all rabbits in each group to neutralize the activities of the venoms was assessed by mixing a constant challenge dose of each venom with different dilutions of the pool of each antiserum. Mixtures were incubated at 37°C for 30 min before determining the residual activity of venom by using the experimental systems described above. Five and three mice per group were used in the neutralization assays of lethality and hemorrhage, respectively. The challenge doses utilized were: 2 LD50s for lethal activity, 5 MHDs for hemorrhagic activity and 2 MCDs for coagulant activity. In all cases, venom-only controls were included, in which venoms were incubated with PBS instead of antiserum. The use of 2 LD50s as a challenge dose in the neutralization of lethality studies, instead of the usual 4–5 LD50s, is justified to increase the sensitivity of the assay, i.e., to optimize the detection of cross-reactivity of antisera against venoms. For lethal and hemorrhagic activities, neutralization was expressed as the median effective dose (ED50), defined as the ratio mg venom/mL antiserum at which the activity of venom was reduced to 50% [29]. For coagulant activity, neutralization was expressed as Effective Dose (ED), defined as the ratio of venom/antivenom in which the change in absorbance is prolonged three times as compared to plasma incubated with venom alone [23].
Statistical analysis
In the case of lethality and its neutralization, groups having non-overlapping values of 95% CI were considered significantly different. For the other toxic effects and ELISA, the significance of the differences between mean values of groups was assessed by one-way ANOVA, followed by a Ryan-Einot-Gabriel-Welsch Range (R-E-G-W Q) and Dunnett’s t post-hoc test, when applicable. Regarding specific comparisons between antisera and venoms in hemorrhagic and coagulant activities, a t test was performed. Linearity and homogeneity of variances were tested, and a p-value <0.05 was considered significant.
Results and discussion
General characterization of the Bitis spp. and Echis spp. venoms
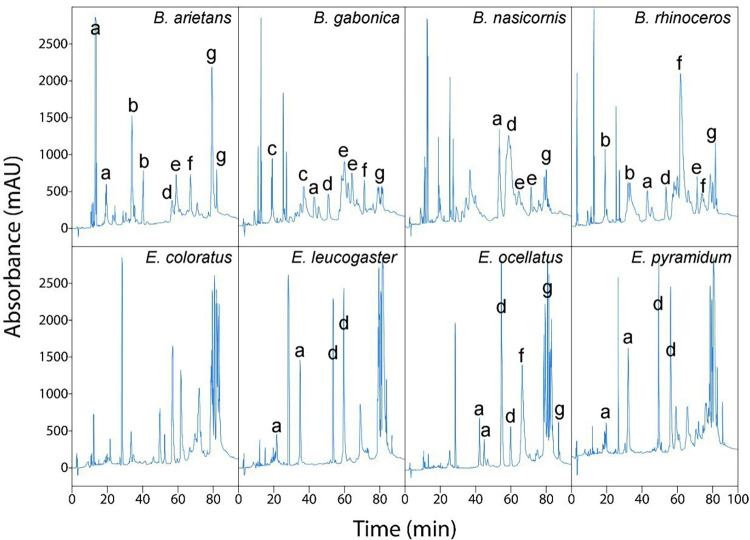
The proteomic analyses of venoms of B. arietans [17], B. gabonica [6], B. nasicornis [7], B. rhinoceros [7], and E. ocellatus [5] have been previously described. To the best of our knowledge, the complete proteomic analysis of venoms of E. coloratus, E. leucogaster and E. pyramidum has not been done, although partial analyses (i.e., identification of fractions not immunodepleted by an antivenom) of the latter two species are available [8]. The HPLC profiles of the venoms used in this study are shown in Figs 1 and S1. As expected, there are intra-species variations in the relative abundance of various fractions compared to previously published data.
Fig 1. RP-HPLC chromatograms of venoms.
Families of the most abundant toxins in the venom fractions were identified according to previously published proteomic analyses: a) disintegrins, b) Kunitz-type inhibitors, c) bradykinin potentiating peptides, d) PLA2s, e) SVSPs, f) C-type lectin-like proteins and g) SVMPs.
In terms of toxicity, the venoms of Bitis spp. had similar LD50s, except for those of B. arietans and B. nasicornis, whose 95% CIs do not overlap (Table 1). In the case of venoms of Echis spp., LD50s were also similar, since only those of E. coloratus and E. leucogaster showed a significant difference (Table 1).
Table 1. Toxic activities of venoms of African Bitis and Echis species.
| Venom | Lethality (LD50)a |
Hemorrhage (MHD)b |
Coagulant (MCD)c |
|---|---|---|---|
| Bitis arietans | 22.0 (12.9–31.6) | 0.10 ± 0.01 | NAD |
| Bitis gabonica | 29.4 (22.1–38.5) | 0.18 ± 0.02 | NAD |
| Bitis nasicornis | 47.4 (33.2–67.9) | 0.24 ± 0.09 | NAD |
| Bitis rhinoceros | 31.7 (24.9–42.0) | 0.05 ± 0.01 | NAD |
| Echis coloratus | 22.1 (14.8–30.6) | 0.31 ± 0.09 | 0.01 ± 0.00 |
| Echis leucogaster | 45.5 (33.0–62.0) | 0.56 ± 0.13 | 0.19 ± 0.02 |
| Echis ocellatus | 31.2 (21.1–49.1) | 0.51 ± 0.06 | 0.20 ± 0.05 |
| Echis pyramidum | 39.2 (26.4–52.2) | 0.50 ± 0.08 | 0.13 ± 0.01 |
a Lethality is expressed as LD50 (95% CI) by the i.p. route, i.e., the Median Lethal Dose, defined as the amount of venom (μg) that results in the death of 50% of the injected mice.
b Hemorrhage is expressed as MHD, i.e., the Minimum Hemorrhagic Dose, defined as the amount of venom (μg) that generates 100 HaU (see Materials and Methods for details).
c Coagulant activity is expressed as MCD, i.e., the Minimum Coagulant Dose, defined as the amount of venom (μg) that induces a change in absorbance of 0.01 units within 1 min. NAD means that no activity was detected. In the cases of hemorrhagic and coagulant activities, results are expressed as mean ± SD (n = 3).
Bitis spp. venoms had higher hemorrhagic activity than Echis spp. venoms (F = 21.621 (7; 16), p< 0.0001; Table 1). The most hemorrhagic Bitis venom was B. rhinoceros (R-E-G-W Q post-hoc test p = 0.504), while the Echis venom having the highest hemorrhagic activity was E. coloratus (R-E-G-W Q post-hoc test p = 0.079). The hemorrhagic activity of snake venoms is due to the action of SVMPs [30, 31]. Hemorrhagic SVMPs have been isolated from the venoms of African Bitis spp. [32] and Echis spp. [33–35]. Interestingly, even though SVMPs are less abundant in venoms of Bitis spp. (22.9–40.9% of total venom proteins) [7] than in venoms of Echis spp. (60–70% of total venom proteins) [5], venoms of Bitis spp. are more hemorrhagic than those of Echis spp. (Table 1). This might be explained by the presence of procoagulant, non-hemorrhagic SVMPs in Echis spp. venoms [33].
Only Echis spp. venoms induced plasma-clotting activity in vitro. The venom with the highest procoagulant activity is that of E. coloratus (F = 31.925 (3; 8), p< 0.0001; R-E-G-W Q post-hoc test p = 1.0; Table 1). These toxicological profiles generally agree with previous studies of African snake venoms [36–38]. Coagulant activity is due to the action of SVMPs on the coagulation cascade as prothrombin activators [39], since clotting activity of E. ocellatus venom is abrogated by SVMP inhibitors and cation chelating agents [40, 41].
In the clinical setting, coagulopathy has been reported in envenomations caused by Echis spp. and in some cases of envenomation caused by Bitis spp. in East and South Africa. In contrast, reports of envenomations by Bitis spp. in West Africa do not describe coagulation disorders [1, 11]. Intraspecies variation in the composition and action of venoms of B. arietans from various geographical locations has been reported [42, 43].
Tissue damage induced by Bitis spp. and Echis spp. venoms in the immunized rabbits
Groups of four rabbits were immunized with venoms of Bitis spp. or Echis spp. The first two venom injections were administered in a water/oil (w/o) emulsion, i.e., FCA and FIA, from which venoms were slowly released, thus reducing the tissue damage caused by the venom, and enhancing the antibody response of rabbits. Assuming that the antibody response developed during the first immunizations can reduce the toxic effects of the additional venom boosters, and to minimize the inflammation caused by Freund’s adjuvants, the rest of the venom boosters were dissolved in PBS. It was therefore of interest to assess the venom-induced toxic effects in these animals.
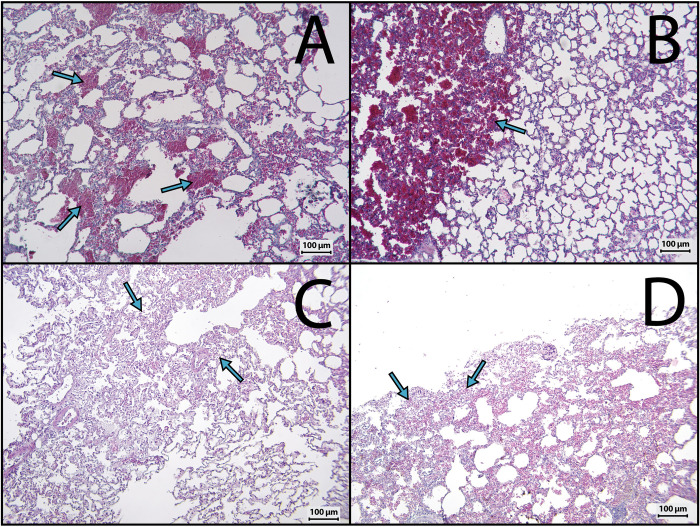
The necropsies at the end of the immunization revealed hemorrhagic lesions of varying extent at the site of venom injection and in the lungs of rabbits immunized with all Bitis spp. and Echis spp. venoms. Histologically, sections from pulmonary tissue of rabbits immunized with Bitis sp venoms showed more extensive hemorrhagic areas than those injected with Echis spp venoms (Fig 2), although extravasation in the lungs was observed for all venoms. These pathological findings agree with the higher hemorrhagic activity of Bitis spp venoms in mice, as described above. In contrast, no evident histological alterations were detected in skin, liver, kidneys, and heart. Hemorrhage in lungs is likely induced by PIII-SVMPs after being released from the site of injection and reached the systemic circulation. If similar damage were caused in animals used for the industrial production of snake antivenoms, these could be prevented by SVMPs inhibitors. However, this may affect epitopes and limit the neutralizing ability of antibodies, depending on the inhibitors. Another alternative to reduce toxicity would be the use of venoms detoxified by physicochemical methods, but this also has the potential risk of affecting relevant epitopes, hence reducing the ability of antibodies to neutralize the native venom [14]. Other alternatives to decrease venom toxicity during immunization would be to use low doses of venoms in more prolonged immunization schemes and to employ depot-forming adjuvants instead of PBS or saline solution to generate a stronger immune response.
Fig 2. Histological alterations in lungs induced by Bitis spp and Echis spp venoms in rabbits.
Immediately after euthanasia, lung samples were collected from rabbits immunized with venoms, and added to 3.7% formalin solution. After embedding the samples in paraffin, sections of 4 μm of thickness were obtained and stained with hematoxylin-eosin. Light micrographs correspond to sections obtained from rabbits immunized with the venoms of B. arietans (A), B. gabonica (B), E. coloratus (C) and E. ocellatus (D). Arrows depict areas with extravasated erythrocytes. Bar corresponds to 100 μm.
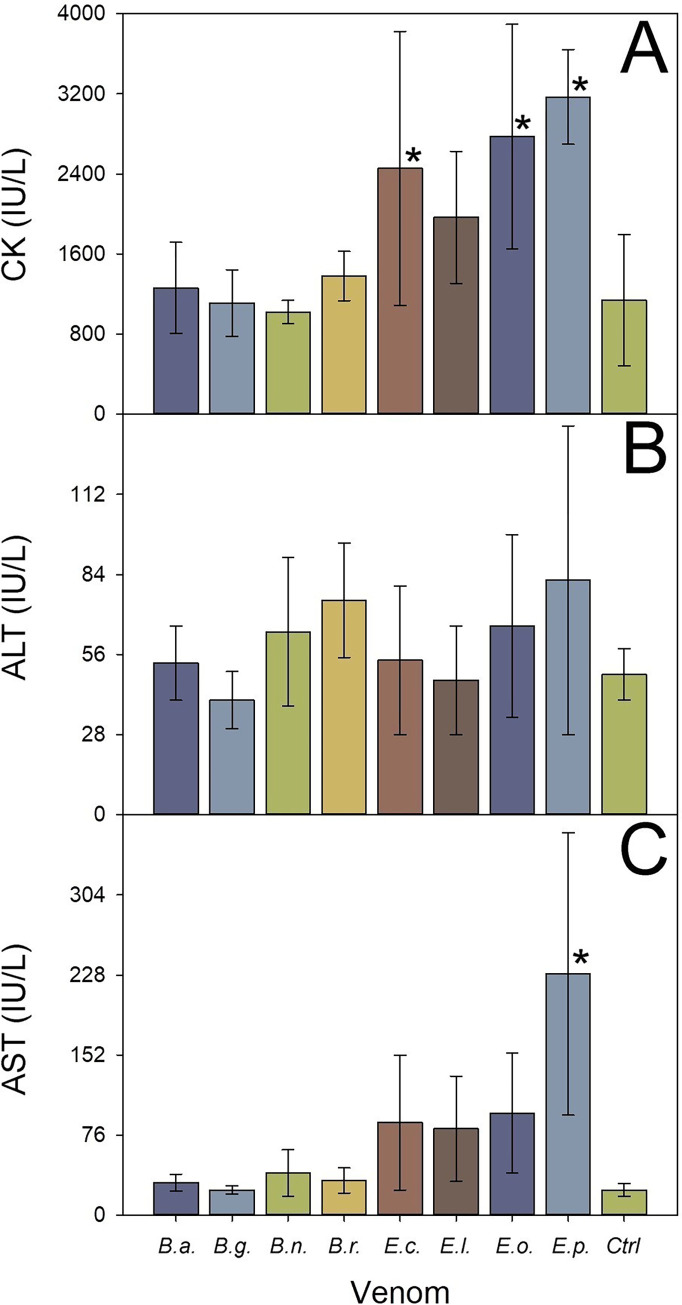
Muscle tissue damage during immunization was evidenced by a significant increase of the plasma CK activity, as compared to non-immunized controls (F = 4.004 (8; 25), p = 0.004), in rabbits immunized with the venoms of three species of Echis spp. (Dunnett’s t-test: E. coloratus p = 0.048, E. ocellatus p = 0.013, E. pyramidum p = 0.011; Fig 3A). Since myotoxicity is generally caused by the action of PLA2s on muscle fibers, the use of PLA2 inhibitors during immunization would be an option to reduce the extent of muscle damage, a hypothesis that needs to be tested in large animals used in antivenom production. No differences were found between rabbits immunized either with Bitis spp. (F = 2.629 (4; 15), p = 0.076) or Echis spp. (F = 0.799 (4; 15), p = 0.545) venoms and the control group regarding the plasma activity of ALT (Fig 3B). Moreover, no apparent alterations on the plasma activity of AST in rabbits immunized with Bitis spp. venoms were observed (F = 1.211 (4; 15), p = 0.347), when compared to the control group. In contrast, elevated plasma activity of AST was observed in rabbits immunized with Echis spp. venoms (F = 4.090 (4; 15), p = 0.019), particularly in those immunized with the venom of E. pyramidum (Dunnett’s t-test p< 0.005; Fig 3C), suggesting that a degree of hepatic damage was induced by this venom during the immunization. However, histopathological analysis of liver did not reveal lesions.
Fig 3. Plasma activity of enzymes in rabbits immunized with venoms.
Plasma activity of A) creatine kinase (CK), B) alanine aminotransferase (ALT), and C) aspartate aminotransferase (AST) in plasma of rabbits immunized with venoms of B. arietans (B.a.), B. gabonica (B.g.), B. nasicornis (B.n.), B. rhinoceros (B.r.), E. coloratus (E.c.), E. leucogaster (E.l.), E. ocellatus (E.o.), and E. pyramidum (E.p.). Results are expressed in International Units (IU) / L and correspond to the mean ± SD (n = 4). *P < 0.05 when compared to the control group of non-immunized rabbits (Ctrl).
At the end of the immunization, immunized rabbits showed normal values of all hematological parameters analyzed (Table A in S1 Text) [44]. Also, normal values of creatinine (F = 1.320 (8; 27), p = 0.276) and urea (F = 2.034 (8; 27), p = 0.08) in serum were found in all rabbits (Table B in S1 Text) [44], indicating that no significant renal injury was induced during immunization, in agreement with necropsy results. Our findings should prompt the analysis of local myonecrosis and pulmonary hemorrhage when venoms of Bitis spp. and Echis spp. are used in large animals for antivenom production. On this basis, the addition of SVMP and PLA2 inhibitors to the immunizing mixtures can be considered in order to limit the extent of these pathophysiological alterations [45]. However, care should be taken as to ensure that inhibitors do not alter epitopes in relevant toxins that would affect the immune response.
Cross-reactivity and neutralization between anti-Bitis spp. sera
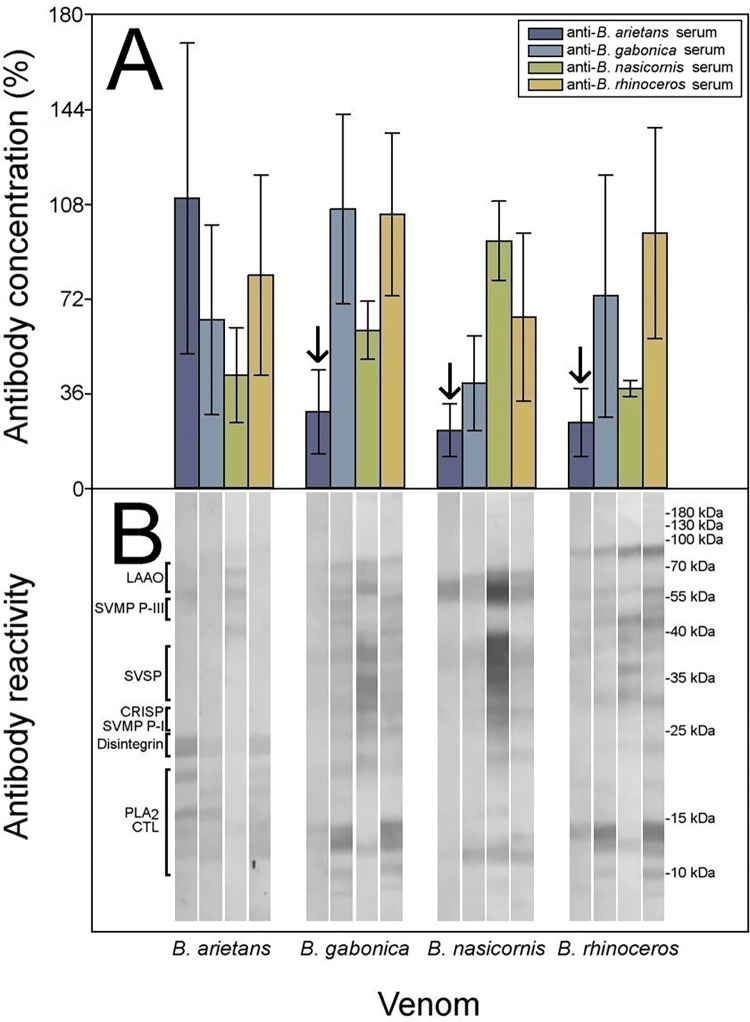
Our experimental protocol, which combines antibody titers by ELISA, Western blot, and neutralization of toxic activities, allowed the assessment of the intrageneric cross-reactivity between monospecific rabbit sera against homologous and heterologous venoms. Cross-reactivity was observed between the four anti-Bitis antisera against the four Bitis spp. venoms, revealing a clear antigenic similarity of the venoms, although there were quantitative differences between them (Fig 4A and 4B).
Fig 4. Immunochemical cross-reactivity between anti-Bitis spp. sera.
Cross-reactivity between anti-Bitis spp. monospecific antisera and Bitis spp. venoms was determined by A) ELISA and B) Western blot. ELISA results are expressed as percentage, considering as 100% the titer of sera raised against the homologous venom of each species and correspond to the mean ± SD (n = 4). ↓ Heterologous sera with less than 33% content of specific antibodies of the corresponding homologous serum. LAAO: L-amino acid oxidases, SVMP: Zn2+-dependent snake venom metalloproteinases, SVSP: snake venom serine proteinases, CRISP: cysteine-rich secretory proteins, PLA2: phospholipases A2, CTL: C-type lectin-like proteins.
As expected, the strongest antibody responses were observed against the homologous venoms. The monospecific anti-B. gabonica (F = 8.485 (3; 12), p = 0.003), and anti-B. nasicornis (F = 9.402 (3; 12), p = 0.002) antisera showed a strong cross-reactivity by ELISA against heterologous venoms, while the anti-B. rhinoceros antiserum showed the strongest cross-reactivity (F = 4.434 (3; 12), p = 0.026; Fig 4A). In contrast, the anti-B. arietans serum showed the lowest extent of cross-reactivity (F = 1.982 (3; 12), p = 0.170). This antiserum presented reactivities lower than the arbitrarily pre-established limit of 33%, selected as our threshold value, against heterologous venoms (R-E-G-W Q post-hoc test p = 0.139), and therefore was not assessed in the in vivo neutralization assays to avoid the unnecessary use of mice.
Cross-reactivity was also evident in Western blot. Presumptive identification of the immunoreactive protein bands, based on the molecular masses, is presented in Fig 4B, according to Mackessy [46]. In agreement with ELISA results, stronger reactions were observed by Western blot when antisera were tested against homologous venoms, and a weak reaction was seen in the case of B. arietans antiserum when confronted with the other venoms (Fig 4B).
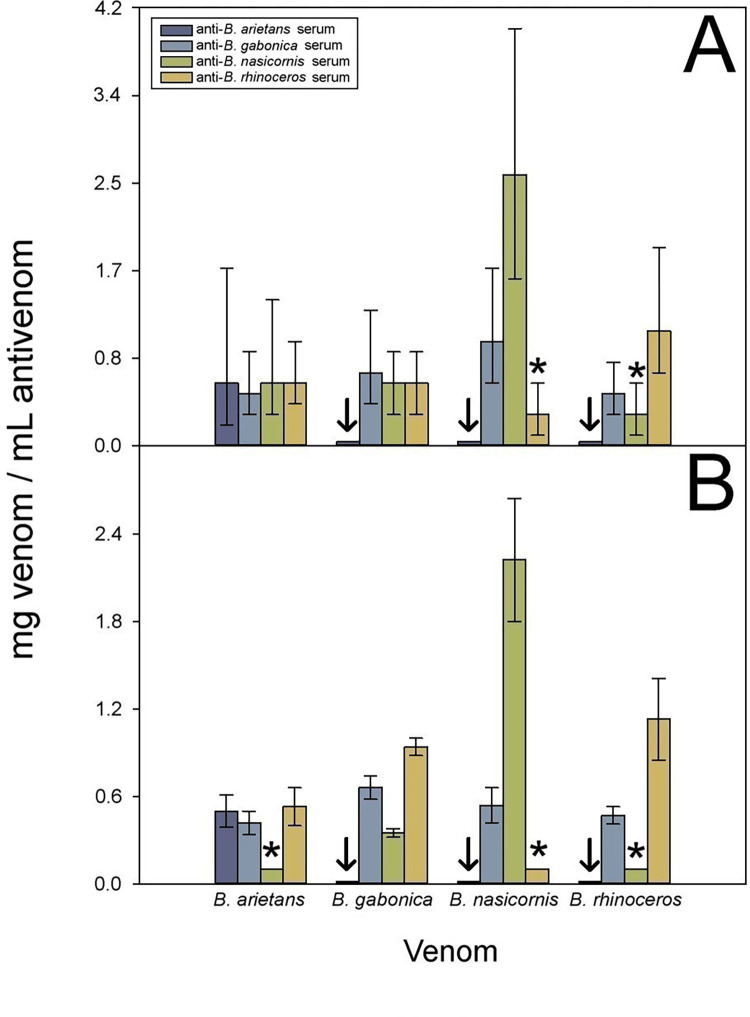
When cross-neutralization of toxic effects was analyzed, it was found that anti-B. gabonica, anti-B. nasicornis, and anti-B. rhinoceros neutralized lethality of the four venoms, with varying values of ED50s, but with a high extent of overlap in the 95% CI (Fig 5A). Exceptions were the anti-B. rhinoceros antiserum against the venom of B. nasicornis, and the anti-B. nasicornis antiserum against the venom of B. rhinoceros, whose 95% CIs did not overlap with those of the corresponding homologous systems.
Fig 5. Cross-neutralization of toxic activities of Bitis spp. venoms by monospecific antisera.
Cross-neutralization of A) lethal and B) hemorrhagic activities of Bitis spp. venoms. Only the antisera that gave higher than 33% cross-reactivity by ELISA were tested in these assays. Neutralization is expressed as ED50, i.e., mg venom neutralized per mL antivenom. In the case of lethality, error bars represent the 95% confidence intervals, while in the case of hemorrhage, error bars represent SD (n = 4). *Non-overlapping values with the anti-lethal 95% CI of the homologous serum, or no detectable ability to neutralize the hemorrhagic activity. ↓ heterologous sera that were no tested as they have less than 33% content of specific antibodies of the corresponding homologous serum.
Regarding neutralization of hemorrhagic activity, the antisera neutralized the homologous and most of the heterologous venoms assessed, with different ED50s (F B. arietans = 5.269 (3; 8), p = 0.027; F B. gabonica = 103.724 (3; 8), p< 0.0001; F B. nasicornis = 56.695 (3; 8), p = 0.0001; F B. rhinoceros = 23.020 (3; 8), p = 0.0001). This result highlights structural conservation of SVMPs, which is expected in snakes of close phylogenetic relationships. When compared to the corresponding homologous antisera, the anti-B. gabonica antiserum showed lower ability to neutralize the hemorrhagic activity of the venoms of B. nasicornis (t = 6.121(4), p = 0.004) and B. rhinoceros (t = -4.828(4), p = 0.008). A similar case was that of anti-B. nasicornis antiserum against the venom of B. gabonica (t = 6.679(4), p = 0.003). In contrast, no neutralization was detected in the case of the anti-B. nasicornis antiserum against venoms of B. arietans (t = 8.161(4), p = 0.001) and B. rhinoceros (t = 8.161(4), p = 0.001) venoms; and anti-B. rhinoceros serum failed to neutralize the activity of B. nasicornis (t = 5.147(4), p = 0.007; Fig 5B). In these cases, the difference/similarity balance between SVMPs suggest that several venoms should be used as immunogens to generate an antivenom with the ability to neutralize hemorrhagic activity of venoms of several Bitis species. All mean values and SD used for Figs 4 and 5 are included in the Tables C, D and E in S1 Text.
Taken together, the data on ELISA and neutralization of lethality and hemorrhage indicate that the anti-Bitis antisera can be arranged from highest to lowest cross-reactivity as follows: anti-B. gabonica, anti-B. rhinoceros, anti-B. nasicornis, and anti-B. arietans.
Cross-reactivity and neutralization between anti-Echis spp. sera
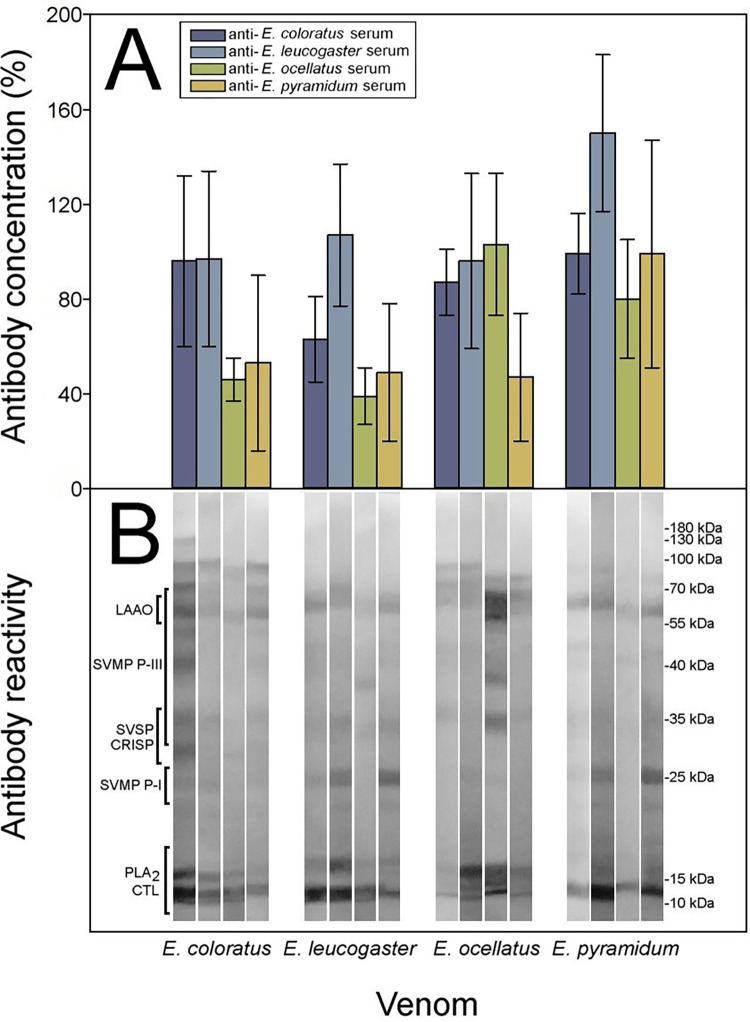
As in the case of Bitis spp. venoms, an extensive cross-reactivity was observed between anti-Echis spp. antisera when confronted with the four Echis spp. venoms, as judged both by ELISA and Western blot, revealing a close antigenic similarity between these venoms (Fig 6A and 6B). A range of immunoreactive bands of various molecular masses, corresponding to different venom components, was observed in Western blot analysis (Fig 6B). As with Bitis spp. venoms, the number and intensity of the immunoreactive bands were higher in homologous venom-antiserum systems, as compared to heterologous systems. Since ELISA results showed cross-reactivities higher than 33% for all antisera against heterologous venoms when compared to homologous antisera, especially with cross-reactivities shown by anti-E. leucogaster (F = 6.461 (3; 12), p = 0.008) antiserum, all venoms and antisera were then subjected to experiments of neutralization of toxic activities.
Fig 6. Immunochemical cross-reactivity between anti-Echis spp. sera.
Cross-reactivity between anti-Echis spp. monospecific antisera and Echis spp. venoms determined by A) ELISA and B) Western blot. ELISA results are expressed as a percentage, considering as 100% the titer of serum raised against the homologous venom of each species and correspond to the mean ± SD (n = 4). All heterologous antisera have > 33% content of specific antibodies of the corresponding homologous antiserum. LAAO: L-amino acid oxidases, SVMP: Zn2+-dependent snake venom metalloproteinases, SVSP: snake venom serine proteinases, CRISP: cysteine-rich secretory proteins, PLA2: phospholipases A2, CTL: C-type lectin-like proteins.
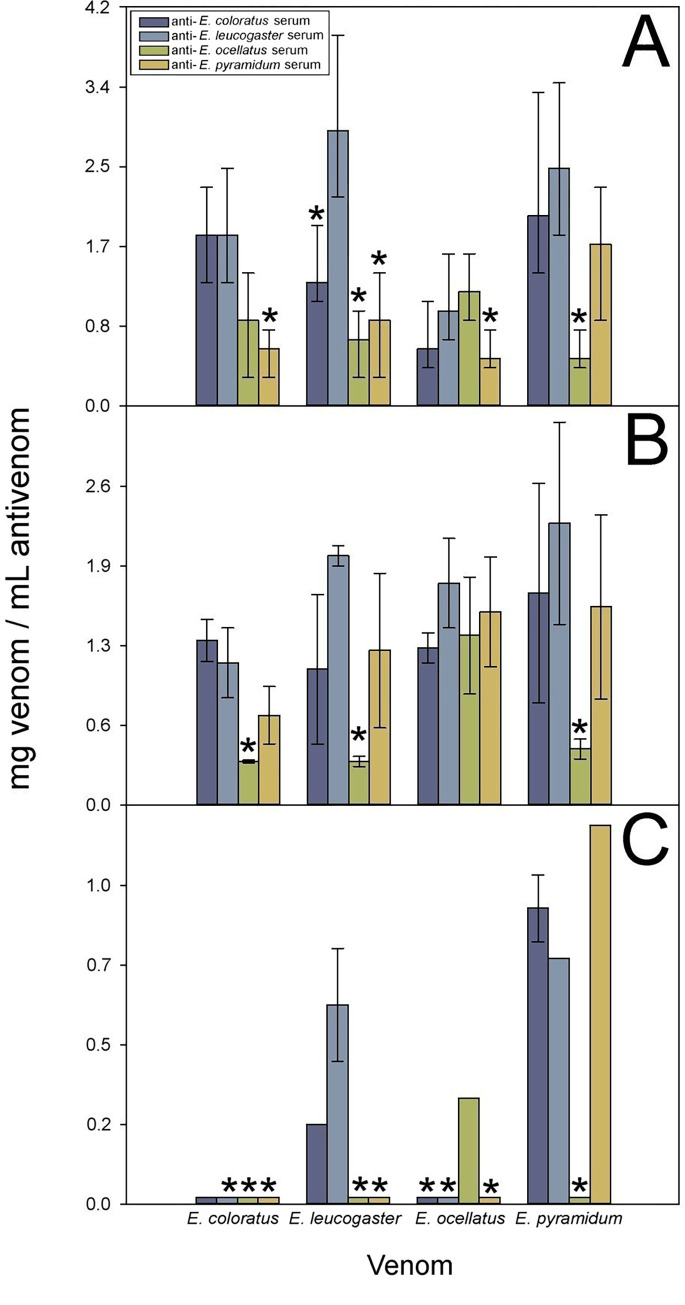
Regarding the neutralization of lethality, all antisera were capable of neutralizing homologous and heterologous venoms, albeit with significant variations in the values of ED50 (Fig 7A). This agrees with a previous study in which a high extent of cross reactivity and neutralization of a monospecific anti-E. ocellatus serum against the venoms of E. coloratus and E. pyramidum was demonstrated [38].
Fig 7. Cross-neutralization of toxic activities of Echis spp. venoms.
Cross-neutralization of A) lethal, B) hemorrhagic, and C) coagulant activities of the Echis spp. venoms. Neutralization is expressed as ED50 (lethality and hemorrhage) and ED (coagulant effect), i.e., mg venom neutralized per mL antivenom. In the case of lethality, error bars represent the 95% confidence intervals, while in the case of hemorrhage and coagulation, error bars represent SD (n = 4). * Non-overlapping values with the anti-lethal 95% CI of the homologous serum, or no detectable or poor ability to neutralize hemorrhagic or coagulant activities.
The 95% CI did not overlap with those of the corresponding homologous systems in the case of the anti-E. pyramidum antiserum against the venoms of E. coloratus, E. leucogaster and E. ocellatus, the anti-E. ocellatus antiserum against the venoms of E. leucogaster and E. pyramidum, and the anti-E. coloratus antiserum against the venom of E. leucogaster.
A similar trend was observed in the neutralization of hemorrhagic activity, where an extensive cross-neutralization was observed, although with variable ED50s (F E. coloratus = 14.356 (3; 8), p = 0.001; F E. leucogaster = 7.349 (3; 8), p< 0.011; F E. ocellatus = 1.104 (3; 8), p = 0.402; F E. pyramidum = 3.462 (3; 8), p = 0.071). An exception was the anti-E. ocellatus antiserum which showed low efficacy against the venoms of E. coloratus (t = 0.320(4), p = 0.765), E. leucogaster (t = -1.239(4), p = 0.283), and E. pyramidum (t = -0.516(4), p = 0.633; Fig 7B).
The coagulant activity of venom of E. coloratus was not neutralized by any of the antisera, including the homologous one (Fig 7C). Moreover, the coagulant activity of the venom of E. leucogaster was neutralized by the homologous antisera and the anti-E. coloratus antiserum (t = 3.600(4), p = 0.023; Fig 7C). The coagulant activity of the venom of E. ocellatus was neutralized only by the homologous antiserum (Fig 7C). Finally, the coagulant activity of E. pyramidum was neutralized by the anti-E. coloratus (t = 4.750(4), p = 0.009) and anti-E. leucogaster (t = 4.000(4), p = 0.016) sera and the homologous antiserum. These findings underscore a complex pattern of antigenic variation in the coagulant toxins present in these venoms. Taken together, our data indicate that the anti-Echis antisera can be ordered from most to least cross-reactive as follows: anti-E. leucogaster, anti-E. coloratus, anti-E. pyramidum, and anti-E. ocellatus. All mean values and SD used for Figs 6 and 7 are included in the Tables F, G, H and I in S1 Text.
The extensive cross-reactivity of the monospecific antisera generated in this work agrees with previous studies where antivenoms generated by using different mixtures of venoms neutralized lethal, hemorrhagic, and coagulant effects of Bitis spp. and Echis spp. venoms [29, 36, 47, 48]. According to our findings, however, the venom of B. arietans generated a relatively limited immune response in rabbits when tested against venoms of the other three Bitis species. Likewise, our results underscore the limited extent of cross-neutralization of coagulant activity of Echis spp. venoms by heterologous antisera, in agreement with previous findings showing a narrow taxonomic range of efficacy of these antisera to neutralize in vitro coagulant effect of Echis spp. venoms [49].
Conclusions
Our results underscored the toxic effects induced by venoms in immunized rabbits, especially regarding myonecrosis and systemic hemorrhage. This raises the possibility of introducing the use of inhibitors of venom enzymes, i.e., SVMPs and PLA2s, which would reduce toxicity, but ensuring that this does not affect immunogenicity in antivenom production, as previously shown in the case of a chelating agent [45]. The main goal of this study was to infer the intrageneric antigenic relatedness between venoms of species of African Bitis spp and Echis spp based on the analysis of the cross-reactivity of monospecific sera generated in rabbits. The protocol can be used for inferring antigenic similarities of venoms of clinically relevant snake species for other venoms in sub-Saharan Africa and other regions as well. This information can be complemented by other approaches to determine antigenic similarities between venoms, such as antivenomics [8, 48].
Our observations demonstrate a remarkable cross-reactivity between monospecific antisera and the most important venoms of Bitis and Echis species in sub-Saharan Africa. Cross-reactivity between monospecific anti-Bitis spp. antisera suggests antigenic similarities between lethal and hemorrhagic toxins of venoms of B. arietans, B. gabonica, B. nasicornis, and B. rhinoceros. In a similar way, but with some exceptions, cross-reactivity between monospecific anti-Echis spp antisera suggests antigenic similarity between lethal and hemorrhagic toxins of venoms of E. coloratus, E. leucogaster, E. ocellatus, and E. pyramidum, but not in their procoagulant toxins. As a broad conclusion, the highest immune coverage within each genus was observed with the venoms of B. gabonica, B. rhinoceros, and E. leucogaster. However, it is likely that the production of antivenoms with the widest neutralizing scope will require the use of venoms from additional species of Bitis and Echis, a hypothesis that will be explored in future studies.
The rabbit immunization models are pragmatic alternatives to generate preliminary results that allow the formulation of rational hypotheses regarding the design of the immunization strategy to produce broad neutralizing antivenoms. However, as immunogenicity depends on the nature of the immune system of the animal selected as source of immunoglobulins, the conclusions of our experiments in rabbits cannot be directly extrapolated to the species used in industrial production of antivenoms. Experimental evaluation of these hypotheses in horses or sheep is required.
Supporting information
(PDF)
Table A: Hematological parameters of rabbits at the end of the immunization with venoms of African Bitis or Echis species. Table B: Plasma biochemical parameters of rabbits at the end of the immunization with venoms of African Bitis or Echis species. Table C: Cross-reactivity by ELISA between anti-Bitis spp. sera. Table D: Cross-neutralization of lethality of venoms of African Bitis species. Table E: Cross-neutralization of hemorrhagic activity of venoms of African Bitis species. Table F: Cross-reactivity by ELISA between anti-Echis spp. sera. Table G: Cross-neutralization of lethality of venoms of African Echis species. Table H: Cross-neutralization of hemorrhagic activity of venoms of African Echis species. Table I: Cross-neutralization of coagulant activity of venoms of African Echis species.
(DOCX)
Acknowledgments
The authors thank Jorge Gómez, Christhian Vargas, Orlando Morales, and other colleagues at Instituto Clodomiro Picado for their collaboration in the animal tests. This work was performed in partial fulfillment of the doctoral degree of Aarón Gómez at Universidad de Costa Rica.
Data Availability
Data are available in the Supplementary files of the submission.
Funding Statement
This study was supported by a Wellcome Trust grant [Reference 220517/Z/20/Z] awarded to GL and JMG, and by Vicerrectoría de Investigación, Universidad de Costa Rica [projects 741-A0-804 and 741-C0-523] awarded to GL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All the authors received salary from Universidad de Costa Rica.
References
- 1.WHO (World Health Organization). Guidelines for the production, control and regulation of snake antivenom immunoglobulins. WHO, Geneva; 2016. [Google Scholar]
- 2.Gutiérrez JM. Snakebite envenomation as a neglected tropical disease: new impetus for confronting an old scourge. In Handbook of Venoms and Toxins of Reptiles, Mackessy SP (Ed.). CRC Press, Boca Raton, FL, USA. 2021; pp. 471–483. [Google Scholar]
- 3.Minghui R, Malecela MN, Cooke E, Abela-Ridder B. WHO’s Snakebite envenoming strategy for prevention and control. Lancet Glob Health. 2019; 7(7):e837–e838. doi: 10.1016/S2214-109X(19)30225-6 . [DOI] [PubMed] [Google Scholar]
- 4.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008; 5(11):e218. doi: 10.1371/journal.pmed.0050218 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagstaff SC, Sanz L, Juárez P, Harrison RA, Calvete JJ. Combined snake venomics and venom gland transcriptomic analysis of the ocellated carpet viper, Echis ocellatus. J Proteomics. 2009; 71(6):609–23. doi: 10.1016/j.jprot.2008.10.003 . [DOI] [PubMed] [Google Scholar]
- 6.Calvete JJ, Marcinkiewicz C, Sanz L. Snake venomics of Bitis gabonica gabonica. Protein family composition, subunit organization of venom toxins, and characterization of dimeric disintegrins bitisgabonin-1 and bitisgabonin-2. J Proteome Res. 20017; 6(1):326–36. doi: 10.1021/pr060494k . [DOI] [PubMed] [Google Scholar]
- 7.Calvete JJ, Escolano J, Sanz L. Snake venomics of Bitis species reveals large intragenus venom toxin composition variation: application to taxonomy of congeneric taxa. J Proteome Res. 2007; 6(7):2732–45. doi: 10.1021/pr0701714 . [DOI] [PubMed] [Google Scholar]
- 8.Calvete JJ, Cid P, Sanz L, Segura Á, Villalta M, Herrera M, et al. Antivenomic assessment of the immunological reactivity of EchiTAb-Plus-ICP, an antivenom for the treatment of snakebite envenoming in sub-Saharan Africa. Am J Trop Med Hyg. 2010; 82(6):1194–201. doi: 10.4269/ajtmh.2010.09-0733 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingwoke EJ, Adamude FA, Mohamed G, Klein A, Salihu A, Abubakar MS, et al. Venom proteomic analysis of medically important Nigerian viper Echis ocellatus and Bitis arietans snake species. Biochem Biophys Rep. 2021; 28:101164. doi: 10.1016/j.bbrep.2021.101164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavonas EJ, Tomaszewski ChA, Ford MD, Rpuse AM, Kerns WP. Severe puff adder (Bitis arietans) envenomation with coagulopathy. J Toxicol Clin Toxicol. 2002; 40:911–18. doi: 10.1081/clt-120016963 . [DOI] [PubMed] [Google Scholar]
- 11.WHO (World Health Organization). Guidelines for the prevention and clinical management of snakebite in Africa. WHO, Brazzaville; 2010. [Google Scholar]
- 12.Warrell DA. Clinical toxicology of snakebite in Africa and the Middle East (Arabian Peninsula. In Handbook of Clinical Toxicology of Animal Venoms and Poisons, Meier J, White J (Eds). CRC Press, Boca Raton, FL, USA. 1995; pp. 433–492. [Google Scholar]
- 13.León G, Vargas M, Segura Á, Herrera M, Villalta M, Sánchez A, et al. Current technology for the industrial manufacture of snake antivenoms. Toxicon. 2018; 151:63–73. doi: 10.1016/j.toxicon.2018.06.084 . [DOI] [PubMed] [Google Scholar]
- 14.León G, Sánchez L, Hernández A, Villalta M, Herrera M, Segura A, et al. Immune response towards snake venoms. Inflamm Allergy Drug Targets. 2011; 10:381–98. doi: 10.2174/187152811797200605 . [DOI] [PubMed] [Google Scholar]
- 15.CIOMS (Council of International Organizations of Medical Sciences). The international guiding principles for biomedical research involving animals. Bankowski Z, Howard-Jones N, Geneva; 1986. doi: 10.1080/01652176.1986.9694068 [DOI] [PubMed] [Google Scholar]
- 16.Lomonte B, Calvete JJ. Strategies in ’snake venomics’ aiming at an integrative view of compositional, functional, and immunological characteristics of venoms. J Venom Anim Toxins Incl Trop Dis. 2017; 28:23–6. doi: 10.1186/s40409-017-0117-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juárez P, Wagstaff SC, Oliver J, Sanz L, Harrison RA, Calvete JJ. Molecular cloning of disintegrin-like transcript BA-5A from a Bitis arietans venom gland cDNA library: a putative intermediate in the evolution of the long-chain disintegrin bitistatin. J Mol Evol. 2006; 63(1):142–52. doi: 10.1007/s00239-005-0268-z . [DOI] [PubMed] [Google Scholar]
- 18.Herrera C, Bolton F, Arias AS, Harrison RA, Gutiérrez JM. Analgesic effect of morphine and tramadol in standard toxicity assays in mice injected with venom of the snake Bothrops asper. Toxicon. 2018; 154:35–41. doi: 10.1016/j.toxicon.2018.09.012 . [DOI] [PubMed] [Google Scholar]
- 19.Durán G, Solano G, Gómez A, Cordero D, Sánchez A, Villalta M, et al. Assessing a 6-h endpoint observation time in the lethality neutralization assay used to evaluate the preclinical efficacy of snake antivenoms. Toxicon X. 2021; 12:100087. doi: 10.1016/j.toxcx.2021.100087 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finney DJ. Probit Analysis. Cambridge University Press, Cambridge; 1971. [Google Scholar]
- 21.Jenkins TP, Sánchez A, Segura Á, Vargas M, Herrera M, Stewart TK, et al. An improved technique for the assessment of venom-induced haemorrhage in a murine model. Toxicon. 2017; 139:87–93. doi: 10.1016/j.toxicon.2017.10.005 . [DOI] [PubMed] [Google Scholar]
- 22.O’Leary MA, Isbister GK. A turbidimetric assay for the measurement of clotting times of procoagulant venoms in plasma. J Pharmacol Toxicol Methods. 2009; 61(1):27–31. doi: 10.1016/j.vascn.2009.06.004 . [DOI] [PubMed] [Google Scholar]
- 23.Sánchez A, Herrera M, Villalta M, Solano D, Segura Á, Lomonte B, et al. Proteomic and toxinological characterization of the venom of the South African Ringhals cobra Hemachatus haemachatus. J Proteomics. 2018; 181:104–117. doi: 10.1016/j.jprot.2018.04.007 . [DOI] [PubMed] [Google Scholar]
- 24.Talke H, Schubert GE. Enzymatic urea determination in the blood and serum in the Warburg optical test. Klin Wochenschr. 1965; 43:174–5. doi: 10.1007/BF01484513 . [DOI] [PubMed] [Google Scholar]
- 25.Mazzachi BC, Peake MJ, Ehrhardt V. Reference range and method comparison studies for enzymatic and Jaffé creatinine assays in plasma and serum and early morning urine. Clin Lab. 2000; 46(1–2):53–5. . [PubMed] [Google Scholar]
- 26.Rodkey FL. Direct spectrophotometric determination of albumin in human serum. Clin Chem. 1965; 11:478–87. . [PubMed] [Google Scholar]
- 27.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the Biüret reaction. J. Biol. Chem. 1949; 177:751–66. . [PubMed] [Google Scholar]
- 28.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227:680–685. doi: 10.1038/227680a0 . [DOI] [PubMed] [Google Scholar]
- 29.Segura A, Castillo MC, Núñez V, Yarlequé A, Gonçalves LR, Villalta M, et al. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically relevant Bothrops snake venoms. Toxicon. 2010; 56(6):980–9. doi: 10.1016/j.toxicon.2010.07.001 . [DOI] [PubMed] [Google Scholar]
- 30.Fox JW, Serrano SM. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon. 2005; 45(8):969–85. doi: 10.1016/j.toxicon.2005.02.012 . [DOI] [PubMed] [Google Scholar]
- 31.Escalante T, Rucavado A, Fox JW, Gutiérrez JM. Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J Proteomics. 2001; 74(9):1781–94. doi: 10.1016/j.jprot.2011.03.026 . [DOI] [PubMed] [Google Scholar]
- 32.Yamakawa Y, Omori-Satoh T, Mebs D. Hemorrhagic principles in the venom of Bitis arietans, a viperous snake. II. Enzymatic properties with special reference to substrate specificity. Biochim Biophys Acta. 1995; 1247(1):17–23. doi: 10.1016/0167-4838(94)00171-c . [DOI] [PubMed] [Google Scholar]
- 33.Howes JM, Wilkinson MC, Theakston RD, Laing GD. The purification and partial characterisation of two novel metalloproteinases from the venom of the West African carpet viper, Echis ocellatus. Toxicon. 2003; 42(1):21–7. doi: 10.1016/s0041-0101(03)00096-5 . [DOI] [PubMed] [Google Scholar]
- 34.Wahby AF, Abdel-Aty AM, El-Kady EM. Purification of hemorrhagic SVMPs from venoms of three vipers of Egypt. Toxicon. 2012; 59(2):329–37. doi: 10.1016/j.toxicon.2011.11.014 . [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Aty AM, Wahby AF. Purification and characterization of five snake venom metalloproteinases from Egyptian Echis pyramidum pyramidum venom. J Toxicol Sci. 2014; 39(4):523–36. doi: 10.2131/jts.39.523 . [DOI] [PubMed] [Google Scholar]
- 36.Ramos-Cerrillo B, de Roodt AR, Chippaux JP, Olguín L, Casasola A, Guzmán G, et al. Characterization of a new polyvalent antivenom (Antivipmyn Africa) against African vipers and elapids. Toxicon. 2008; 52(8):881–8. doi: 10.1016/j.toxicon.2008.09.002 . [DOI] [PubMed] [Google Scholar]
- 37.Segura Á, Villalta M, Herrera M, León G, Harrison R, Durfa N, et al. Preclinical assessment of the efficacy of a new antivenom (EchiTAb-Plus-ICP) for the treatment of viper envenoming in sub-Saharan Africa. Toxicon. 2010; 55(2–3):369–74. doi: 10.1016/j.toxicon.2009.08.010 . [DOI] [PubMed] [Google Scholar]
- 38.Casewell NR, Cook DA, Wagstaff SC, Nasidi A, Durfa N, Wüster W, et al. Pre-clinical assays predict pan-African Echis viper efficacy for a species-specific antivenom. PLoS Negl Trop Dis. 2010; 4(10):e851. doi: 10.1371/journal.pntd.0000851 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howes JM, Kamiguti AS, Theakston RD, Wilkinson MC, Laing GD. Effects of three novel metalloproteinases from the venom of the West African saw-scaled viper, Echis ocellatus on blood coagulation and platelets. Biochim Biophys Acta. 2005; 1724(1–2):194–202. doi: 10.1016/j.bbagen.2005.03.011 . [DOI] [PubMed] [Google Scholar]
- 40.Arias AS, Rucavado A, Gutiérrez JM. Peptidomimetic hydroxamate metalloproteinase inhibitors abrogate local and systemic toxicity induced by Echis ocellatus (saw-scaled) snake venom. Toxicon. 2017; 132:40–49. doi: 10.1016/j.toxicon.2017.04.001 . [DOI] [PubMed] [Google Scholar]
- 41.Albulescu LO, Hale MS, Ainsworth S, Alsolaiss J, Crittenden E, Calvete JJ, et al. Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite. Sci Transl Med. 2020; 12(542):eaay8314. doi: 10.1126/scitranslmed.aay8314 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Currier RB, Harrison RA, Rowley PD, Laing GD, Wagstaff SC. Intra-specific variation in venom of the African Puff Adder (Bitis arietans): Differential expression and activity of snake venom metalloproteinases (SVMPs). Toxicon. 2010; 55(4):864–73. doi: 10.1016/j.toxicon.2009.12.009 . [DOI] [PubMed] [Google Scholar]
- 43.de Oliveira PRS, França FS, Villas Boas IM, Rocha MMTD, Sant’Anna SS, Bastos ML, et al. Snake venoms from Angola: Intra-specific variations and immunogenicity. Toxicon. 2018; 148:85–94. doi: 10.1016/j.toxicon.2018.04.013 . [DOI] [PubMed] [Google Scholar]
- 44.Hewitt CD, Innes DJ, Savory J, Wills MR. Normal biochemical and hematological values in New Zealand white rabbits. Clin Chem. 1989; 35(8):1777–9. . [PubMed] [Google Scholar]
- 45.León G, Estrada R, Chaves F, Rojas G, Ovadia M, Gutiérrez JM. Inhibition by CaNa2EDTA of local tissue damage induced by Bothrops asper (terciopelo) venom: application in horse immunization for antivenom production. Toxicon. 1998; 36(2):321–31. doi: 10.1016/s0041-0101(97)00114-1 . [DOI] [PubMed] [Google Scholar]
- 46.Mackessy SP. The field of reptile toxinology. Snakes, lizards, and their venoms. In Handbook of Venoms and Toxins of Reptiles, Mackessy SP (Ed). CRC Press, Boca Raton, FL, USA. 2010; pp 3–23. [Google Scholar]
- 47.Sánchez LV, Pla D, Herrera M, Chippaux JP, Calvete JJ, Gutiérrez JM. Evaluation of the preclinical efficacy of four antivenoms, distributed in sub-Saharan Africa, to neutralize the venom of the carpet viper, Echis ocellatus, from Mali, Cameroon, and Nigeria. Toxicon. 2015; 106:97–107. doi: 10.1016/j.toxicon.2015.09.027 . [DOI] [PubMed] [Google Scholar]
- 48.Calvete JJ, Arias AS, Rodríguez Y, Quesada-Bernat S, Sánchez LV, Chippaux JP, et al. Preclinical evaluation of three polyspecific antivenoms against the venom of Echis ocellatus: Neutralization of toxic activities and antivenomics. Toxicon. 2016; 119:280–8. doi: 10.1016/j.toxicon.2016.06.022 . [DOI] [PubMed] [Google Scholar]
- 49.Rogalski A, Soerensen C, Op den Brouw B, Lister C, Dashevsky D, Arbuckle K, et al. Differential procoagulant effects of saw-scaled viper (Serpentes: Viperidae: Echis) snake venoms on human plasma and the narrow taxonomic ranges of antivenom efficacies. Toxicol Lett. 2017; 280:159–170. doi: 10.1016/j.toxlet.2017.08.020 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Table A: Hematological parameters of rabbits at the end of the immunization with venoms of African Bitis or Echis species. Table B: Plasma biochemical parameters of rabbits at the end of the immunization with venoms of African Bitis or Echis species. Table C: Cross-reactivity by ELISA between anti-Bitis spp. sera. Table D: Cross-neutralization of lethality of venoms of African Bitis species. Table E: Cross-neutralization of hemorrhagic activity of venoms of African Bitis species. Table F: Cross-reactivity by ELISA between anti-Echis spp. sera. Table G: Cross-neutralization of lethality of venoms of African Echis species. Table H: Cross-neutralization of hemorrhagic activity of venoms of African Echis species. Table I: Cross-neutralization of coagulant activity of venoms of African Echis species.
(DOCX)
Data Availability Statement
Data are available in the Supplementary files of the submission.