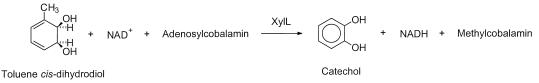
Abstract
We identified and characterized a methyl transfer activity of the toluate cis-dihydrodiol (4-methyl-3,5-cyclohexadiene-cis-1,2-diol-1-carboxylic acid) dehydrogenase of the TOL plasmid pWW0 towards toluene cis-dihydrodiol (3-methyl-4,5-cyclohexadiene-cis-1,2-diol). When the purified enzyme from the recombinant Escherichia coli containing the xylL gene was incubated with toluene cis-dihydrodiol in the presence of NAD+, the end products differed depending on the presence of adenosylcobalamin (coenzyme B12). The enzyme yielded catechol in the presence of adenosylcobalamin, while it gave 3-methylcatechol in the absence of the cofactor. Adenosylcobalamin was transformed to methylcobalamin as a result of the enzyme reaction, which indicates that the methyl group of the substrate was transferred to adenosylcobalamin. Other derivatives of the cobalamin such as aquo (hydroxy)- and cyanocobalamin did not mediate the methyl transfer reaction. The dehydrogenation and methyl transfer reactions were assumed to occur concomitantly, and the methyl transfer reaction seemed to depend on the dehydrogenation. To our knowledge, the enzyme is the first dehydrogenase that shows a methyl transfer activity as well.
Many metabolic pathways have been reported regarding aerobic microbial degradation of aromatic hydrocarbons (1–3, 13, 14). The TOD and the TOL pathways are among those most studied so far. In the TOD pathway, the aromatic ring is first attacked, and cis-dihydrodiol intermediates are formed (2, 3). On the contrary, in the TOL pathway, the methyl group of the substrate is converted to a carboxyl group through several reaction steps leading to the formation of cis-dihydrodiol carboxylates (13, 14). The resulting cis-dihydrodiols in both pathways are transformed to catechol or its derivatives, and these catechol compounds are further degraded after the cleavage of the aromatic ring (4, 15). The conversion of the aromatic substrate to the cis-dihydrodiols is catalyzed by oxygenase (toluene dioxygenase in the TOD pathway and benzoate dioxygenase in the TOL pathway). The breakage of the aromatic ring of the catechol intermediate is also catalyzed by oxygenase (catechol 2,3-dioxygenase in both pathways). cis-Dihydrodiol dehydrogenase, which converts cis-dihydrodiols to catechol or its derivatives, lies between the former oxygenase and the latter.
Many attempts have been made to enlarge the substrate range of microorganisms for degradation of pollutants (8, 10, 12). Microorganisms with a broad substrate range often facilitate the environmental cleanup process because a single organism can mineralize several pollutants at the same time. Thus, overcoming the specificity barrier of enzymes involved in the catabolic pathways has been one of the most actively sought goals in environmental microbiology.
We previously reported a recombinant Pseudomonas strain with an enlarged substrate range by which the total degradation of a benzene, toluene, and p-xylene mixture could be achieved (5). The strain was constructed by the combination of the TOD and the TOL pathways. The toluate cis-dihydrodiol dehydrogenase (XylL) of the TOL plasmid pWW0 encoded by the xylL gene was found to have a relaxed substrate specificity so that it could oxidize not only the original substrates but also dihydrodiols formed in the TOD pathway (benzene cis-dihydrodiol, toluene cis-dihydrodiol, and p-xylene cis-dihydrodiol). The end products of metabolism of the three alien dihydrodiols were identified as catechols which are then routed into the TOL pathway and completely mineralized. Given the structure of toluene cis-dihydrodiol (4-methyl-3,5-cyclohexadiene-cis-1,2-diol), one of the alien dihydrodiols, we assumed that the formation of catechol might require that XylL possess a demethylation, or similar, activity. In this study, we purified XylL and identified and characterized the methyl transfer activity of the enzyme in detail.
Identification of the methyl transfer activity from the crude cell extract.
Cell extracts of the recombinant Escherichia coli strain harboring the xylL (5) gene catalyzed a conversion of benzene cis-dihydrodiol and toluene cis-dihydrodiol to catechol in the presence of NAD+. No other cofactor was required. The E. coli JM109 host cells did not show the activity. The formation of catechol from toluene cis-dihydrodiol led us to assume that the XylL of the TOL plasmid pWW0 is able to catalyze a methyl transfer or demethylation reaction as well as dehydrogenation. Given the general knowledge that the methyl group constitutes a stable tetrahedron structure and shows very low reactivity, the observation seemed very interesting. No dehydrogenase among those reported to date has a methyl transfer activity.
Purification of XylL.
XylL was purified to homogeneity by monitoring the p-toluate cis-dihydrodiol-dependent conversion of NAD+ to NADH following each purification step. The recombinant E. coli cells suspended in 20 mM Tris-HCl (Tris) buffer (pH 8.0) with 0.5 mM phenylmethylsulfonyl fluoride were subjected to sonication. The resulting crude extract was centrifuged at 15,000 × g for 10 min, and the supernatant was saved. A solution of 2% protamine sulfate in 100 mM Tris buffer (pH 8.0) was added to the supernatant to a final concentration of 0.05% with constant stirring. After 5 min, the mixture was centrifuged at 15,000 × g for 10 min, and the supernatant was saved. Solid ammonium sulfate was added to the supernatant to 30% saturation with constant stirring. After 15 min of stirring, the mixture was centrifuged at 15,000 × g for 10 min. The precipitate was discarded. Additional ammonium sulfate was added to the supernatant to 70% saturation with constant stirring. After 15 min of stirring, the mixture was centrifuged at 15,000 × g for 15 min, and the precipitate was saved. The precipitate was dissolved in 15 ml of 20 mM Tris buffer (pH 8.0). The protein solution was concentrated to less than 1 ml by Centriprep-10 (Amicon Inc., Beverly, Mass.). The volume was adjusted again to 15 ml by adding the same buffer, and the solution was concentrated by Centriprep-10. The sample was injected onto a Resource Q column (Pharmacia) previously equilibrated with 20 mM Tris buffer (pH 8.0). Protein was eluted with a step and linear gradient of NaCl (percentage of 1 M NaCl in the same buffer: 0%, 5 ml; 0 to 100%, 20 ml [linear gradient]; 100%, 10 ml; 0%, 5 ml) by fast protein liquid chromatography (FPLC) (Pharmacia). The enzyme was eluted at around 300 to 400 mM NaCl. Fractions containing the enzyme activity were pooled and concentrated to less than 1 ml by Centriprep-10. The buffer was changed to 20 mM potassium phosphate buffer (pH 7.0) by Centriprep-10. The concentrated protein solution was loaded onto another Resource Q column previously equilibrated with 20 mM potassium phosphate buffer (pH 7.0). Proteins were eluted with the same gradient of NaCl as described above. The proteins were eluted at around 220 to 300 mM NaCl. Fractions containing the enzyme activity were pooled. The buffer was changed to a fresh 20 mM potassium phosphate buffer (pH 7.0) by Centriprep-10 for desalting. The proteins from the Resource Q column were injected onto a 10-ml Cibacron blue 3GA agarose (Sigma) column (10 by 1.5 cm) previously equilibrated with 20 mM potassium phosphate buffer (pH 7.0). After the sample was loaded, the column was washed with 30 ml of the equilibrating buffer and then washed with 30 ml of 1 M NaCl in the same buffer. The enzyme activity was found in bound fractions. The fractions were washed with 1 M NaCl and concentrated by Centriprep-10, and then the buffer was changed to 20 mM Tris buffer (pH 8.0). The above procedure resulted in an 86-fold purification of the enzyme relative to the crude extract. After dye chromatography, a single band was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1). The molecular mass of the enzyme was estimated to be 28 kDa by SDS-PAGE, which is consistent with the previously reported XylL of the TOL plasmid pWW0 (8).
FIG. 1.
SDS-PAGE gel containing purified XylL. Lane a, low-range molecular weight standard (Bio-Rad); lane b, about 4 μg of XylL.
Reconstitution of the methyl transfer activity.
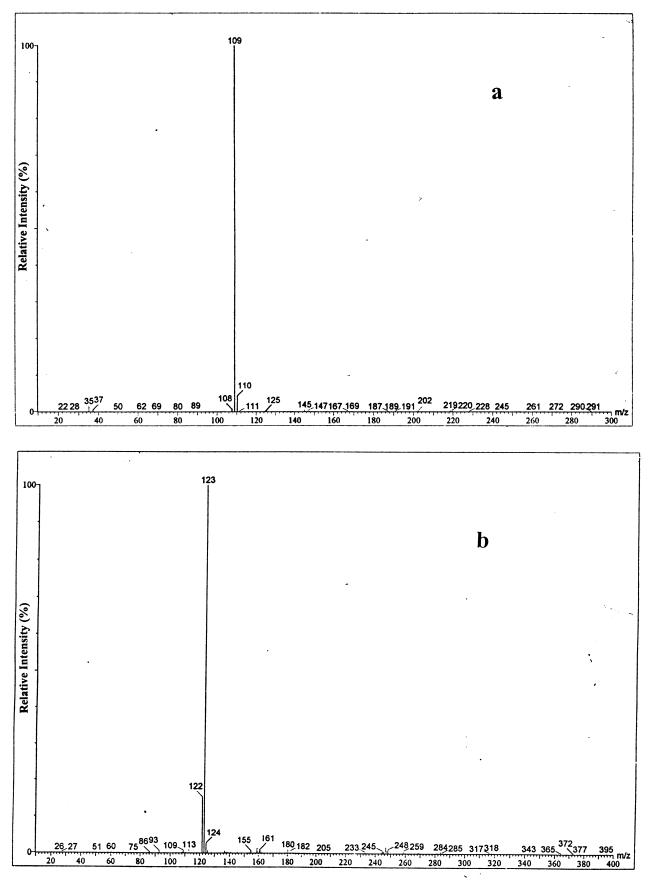
On incubation of the purified XylL with toluene cis-dihydrodiol in the presence of NAD+, 3-methylcatechol was formed as an end product. This is contrary to the observation that the cell extract yielded catechol. The result suggested that the methyl transfer activity of the enzyme was lost upon purification, while the dehydrogenation activity was retained. The enzyme reaction with benzene cis-dihydrodiol still gave catechol as an end product, which supports the above assumption. It has been reported that enzymes involved in the methyl transfer reaction in the biosynthesis of l-methionine in E. coli require a cofactor such as cobalamin, S-adenosylmethionine, or tetrahydrofolate (6, 11). We made a hypothesis that one of these cofactors might be able to restore the methyl transfer activity of the purified XylL. We added each of these cofactors to the reaction mixture and analyzed the end product. In the presence of adenosylcobalamin, high-performance liquid chromatographic analyses of the reaction mixture showed that the product was eluted at the same retention time as that of authentic catechol. To confirm the formation of catechol, the reaction mixture was further analyzed by liquid chromatography-mass spectrometry (MS) according to a method previously reported (5). Figure 2 shows the typical mass spectra of the reaction products in the presence and absence of adenosylcobalamin. The molecular ion peak of the reaction product obtained in the presence of adenosylcobalamin was detected at an m/z value of 109 (Fig. 2a), which confirms the formation of catechol. On the other hand, in the absence of adenosylcobalamin, the molecular ion peak was observed at an m/z value of 123 (Fig. 2b), which indicates the formation of 3-methylcatechol. The mass analysis used was based on the hydrogen abstraction method so that the molecular ion peaks appeared at the M-1 position in the spectrum, where M denotes molecular weight. The addition of other cofactors (tetrahydrofolate and S-adenosylmethionine) could not restore the methyl transfer activity. Cyano- and aquo (hydroxy)-cobalamins, derivatives of adenosylcobalamin, were also tested, but only adenosylcobalamin was able to revive the activity. From these results, it can be concluded that adenosylcobalamin is an absolute cofactor in the methyl transfer from toluene cis-dihydrodiol by XylL.
FIG. 2.
Mass spectra of the reaction product in the presence (a) and in the absence (b) of adenosylcobalamin.
It is interesting that XylL catalyzes the transfer of the methyl group only in the presence of adenosylcobalamin as a methyl group acceptor. Cyano- and aquo (hydroxy)-cobalamin have structures very similar to that of adenosylcobalamin except that the carbon molecules attached to the cobalt of the corrin ring are different. The amino acid sequence of XylL has previously been revealed by Niedle et al. (7). No sequence similarities were found between XylL and the adenosylcobalamin-binding domain of the methionine synthase of E. coli. One possible explanation for the participation of the adenosyl group in the reaction is that the bond energy of the cobalt and carbon bond between the adenosyl group and the corrin ring supplies the energy needed for demethylation of toluene cis-dihydrodiol when it is broken during the reaction. Considering that the methyl group constitutes a very stable tetrahedron structure and the reactivity is low, external energy would need to be provided for demethylation. More evidence is needed to confirm the exact role of adenosylcobalamin. We tested toluate cis-dihydrodiol, one of the original substrates of XylL, to see whether the methyl group of the original substrate is also transferred. In this case, however, only 4-methylcatechol was detected, regardless of the presence of adenosylcobalamin.
Fate of the methyl moiety of toluene cis-dihydrodiol during the reaction.
The fate of the methyl moiety from toluene cis-dihydrodiol was traced. We made a hypothesis that the methyl moiety of toluene cis-dihydrodiol is transferred to adenosylcobalamin, producing methylcobalamin. The reaction mixture was analyzed by MS to detect whether a molecular ion peak corresponding to methylcobalamin was present. Unlike the case in catechol analysis, the MS system used generates a positively charged molecular ion by a hydrogen addition mechanism. The control spectrum with the authentic methylcobalamin gave an ion peak at an m/z value of 673 (Fig. 3a), half the molecular weight of the methylcobalamin (1344). This implies that the molecular ion of methylcobalamin is doubly charged by chemical ionization, and thus the molecular ion peak appears at the M/2e + 1 position, where e denotes charge density. Figure 3b shows the mass spectrum of the sample, and the molecular ion peak was observed at an m/z value of 673, which is identical to that of authentic methylcobalamin. Control experiments in which the enzyme, adenosylcobalamin, or toluene cis-dihydrodiol was omitted gave no methylcobalamin. The reaction mixture was heat treated (65°C, 30 min) to deactivate the enzyme and was also analyzed as a control, but no methylcobalamin was detected. These results indicate that the methyl moiety of toluene cis-dihydrodiol is transferred to the adenosylcobalamin, resulting in the formation of methylcobalamin. The best way to trace the transfer of the methyl group is thought to be to use an isotope-labeled toluene cis-dihydrodiol at the methyl moiety, and this experiment is in progress.
FIG. 3.
Mass spectra of the authentic methylcobalamin (a) and of the reaction mixture (b).
Several cobalamin-dependent methyl transferases have been reported from E. coli and animal cells (6, 11). To our knowledge, the XylL of the TOL plasmid pWW0 is the only enzyme that exhibits both the methyl transfer and the dehydrogenation activities. In addition, while other methyl transferases require multiple steps in the course of the methyl transfer reaction, the XylL could directly transfer the methyl group of the substrate to adenosylcobalamin.
Dehydrogenation and methyl transfer reaction of XylL.
In order to determine whether the methyl transfer reaction and dehydrogenation occur concomitantly or sequentially, we designed the following set of experiments. If the methyl transfer reaction followed the dehydrogenation of toluene cis-dihydrodiol to 3-methylcatechol, the enzyme should be able to transform 3-methylcatechol to catechol under appropriate conditions. Our experimental results showed that XylL was not able to attack 3-methylcatechol. Conversely, if the methyl transfer reaction were to occur before dehydrogenation, the enzyme should produce benzene cis-dihydrodiol as an intermediate. The enzyme reaction was carried out in the absence of NAD+ to prevent dehydrogenation, but benzene cis-dihydrodiol was not detected. These results strongly imply that the two reactions occur concomitantly. This conclusion was also supported by the observation that no conversion of toluene cis-dihydrodiol took place in the absence of NAD+ even when adenosylcobalamin was provided in an excess amount.
Based on the above results, a schematic depiction of the methyl transfer reaction catalyzed by XylL in the presence of adenosylcobalamin is shown in Fig. 4.
FIG. 4.
Proposed mechanism of the methyl transfer reaction catalyzed by XylL in the presence of adenosylcobalamin.
Acknowledgments
We greatly thank Harry P. C. Hogenkamp, L. Nicholas Ornston, and Tetsuo Toraya for helpful comments and suggestions.
REFERENCES
- 1.Burlage R S, Hooper S W, Sayler G S. The TOL (pWW0) catabolic plasmid. Appl Environ Microbiol. 1989;55:1323–1328. doi: 10.1128/aem.55.6.1323-1328.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibson D T, Koch J R, Kallio R E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968;7:2653–2662. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- 3.Gibson D T, Mahadevan V, Davey J F. Bacterial metabolism of para- and meta-xylene: oxidation of the aromatic ring. J Bacteriol. 1974;119:930–936. doi: 10.1128/jb.119.3.930-936.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harayama S, Lehrbach P R, Timmis K N. Transposon mutagenesis analysis of meta-cleavage pathway operon genes of the TOL plasmid of Pseudomonas putida mt-2. J Bacteriol. 1984;160:251–255. doi: 10.1128/jb.160.1.251-255.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J Y, Jung K H, Choi S H, Kim H S. Combination of the tod and the tol pathways in redesigning a metabolic route of Pseudomonas putida for the mineralization of a benzene, toluene, and p-xylene mixture. Appl Environ Microbiol. 1995;61:2211–2217. doi: 10.1128/aem.61.6.2211-2217.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig M L, Matthews R G. Structure-based perspectives on B12-dependent enzymes. Annu Rev Biochem. 1997;66:269–313. doi: 10.1146/annurev.biochem.66.1.269. [DOI] [PubMed] [Google Scholar]
- 7.Niedle E, Hartnett C, Ornston L N, Bairoch A, Rekik M, Harayama S. cis-Diol dehydrogenases encoded by the TOL pWWO plasmid xylL gene and the Acinetobacter calcoaceticus chromosomal benD gene are members of the short-chain alcohol dehydrogenase superfamily. Eur J Biochem. 1992;204:113–120. doi: 10.1111/j.1432-1033.1992.tb16612.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramos J J, Wasserfallen A, Rose K, Timmis K N. Redesigning metabolic route: manipulation of the TOL plasmid pathway for catabolism of alkylbenzoates. Science. 1987;235:593–596. doi: 10.1126/science.3468623. [DOI] [PubMed] [Google Scholar]
- 9.Reiner A M. Metabolism of aromatic compounds in bacteria. J Biol Chem. 1972;247:4960–4965. [PubMed] [Google Scholar]
- 10.Rojo F, Pieper D H, Engesser K H, Knackmuss H J, Timmis K N. Assemblage of ortho cleavage route for simultaneous degradation of chloro- and methylaromatics. Science. 1987;238:1395–1398. doi: 10.1126/science.3479842. [DOI] [PubMed] [Google Scholar]
- 11.Stryer L. Biochemistry. New York, N.Y: W. H. Freeman & Company; 1988. pp. 582–583. [Google Scholar]
- 12.Wackett L P, Sadowsky M J, Newman L M, Hur H G, Li S. Metabolism of polyhalogenated compounds by a genetically engineered bacterium. Nature. 1994;368:627–629. doi: 10.1038/368627a0. [DOI] [PubMed] [Google Scholar]
- 13.Worsey M J, Williams P A. Metabolism of toluene and xylenes by Pseudomonas putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975;124:7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeyer J, Lehrbach P R, Timmis K N. Use of cloned genes of Pseudomonas TOL plasmid to effect biotransformation of benzoate to cis-dihydrodiols and catechols by Escherichia coli cells. Appl Environ Microbiol. 1985;50:1409–1413. doi: 10.1128/aem.50.6.1409-1413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zylstra G J, McCombie W R, Gibson D T, Finette B A. Toluene degradation by Pseudomonas putida F1: genetic organization of the TOD operon. Appl Environ Microbiol. 1988;54:1489–1503. doi: 10.1128/aem.54.6.1498-1503.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]