Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 269 million people and killed more than 5.3 million people worldwide. Although fomite transmission of SARS-CoV-2 has been continuously reported, few studies have been conducted on food contact surfaces. Therefore, this study aimed to investigate the viability of coronaviruses on food contact surfaces and to remove SARS-CoV-2 contaminated on food contact surfaces with disinfectants. At 20 °C, SARS-CoV-2 was inactivated within 48 h on all food contact surfaces. At 4 °C, it was inactivated at 48 h on kraft paper and 96 h on parchment paper, but it was viable up to 5 days in low-density polyethylene (LDPE). At −20 °C, SARS-CoV-2 did not decrease by even 1 log on all food contact surfaces until 5 days. Treatment with 70% ethanol or 1000 ppm sodium hypochlorite for 5 min was sufficient to completely remove SARS-CoV-2 from 6 food contact surfaces. Similarly, UV-C irradiation at 60 mJ/cm2 eliminated SARS-CoV-2 contaminated on food contact surfaces. Also, the wiping test showed that even wiping an area contaminated with SARS-CoV-2 with a cloth moistened with 70% ethanol or 1000 ppm sodium hypochlorite, it took 5 min to inactivate the virus. Our findings suggested that SARS-CoV-2 contaminated on food contact surfaces in local retail may be viable enough to be transported home. However, if the type and method of use of the disinfectant suggested in this study are followed, it is possible to sufficiently control the fomite transmission of SARS-CoV-2 through food contact surfaces at home.
Keywords: COVID-19, Food contact surface, Viability, Quantitative carrier test, Wiping test
1. Introduction
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first isolated and reported in Wuhan, China (Gorbalenya et al., 2020). It causes pneumonia and is now termed coronavirus disease 2019 (COVID-19) (Lai et al., 2020). The World Health Organization (WHO) has declared COVID-19 a pandemic, and 269 million confirmed cases and 5.3 million deaths have been reported worldwide by December 10, 2021 (WHO, 2021b). The SARS-CoV, SARS-CoV-2, and Middle East respiratory syndrome coronavirus (MERS-CoV) are more recently discovered than human coronavirus 229E, NL63, OC43, and HKU1 and cause more severe diseases. SARS-CoV-2 is primarily transmitted via close contact and respiratory droplets, and airborne transmission is probable through aerosols (WHO, 2020b, p. 27; WHO, 2020c). Currently, the global mortality rate for SARS-CoV-2 is approximately 2.09% that is lower than SARS-CoV (approximately 11%) and MERS-CoV (approximately 36%); however, the basic reproduction number is highest at 2.2, which is an increased threat to humans (Chan‐Yeung & Xu, 2003; Li et al., 2020; WHO, 2019). Therefore, quarantine and disinfection are crucial to prevent the SARS-CoV-2 community transmission.
Although vaccines are already available, the role of disinfection plays remains important until immunization of the global population is achieved. In addition, as cases of breakthrough infection are continuously being reported in fully vaccinated people, it is necessary to continue quarantine and disinfection (Birhane et al., 2021). SARS-CoV-2 is viable for hours in aerosols and fomites, which can contaminate various surfaces (Van Doremalen et al., 2020). Depending on the surface type, SARS-CoV-2 can survive from several hours to up to 3 days, suggesting that surface contamination is associated with subsequent transmission of infection (CDC, 2021; Cheng et al., 2020). Therefore, we must reduce contamination of surfaces that may contribute to the transmission of SARS-CoV-2 in healthcare and non-healthcare settings through disinfection. Several previous studies have reported the control of coronavirus using disinfectants (Kampf et al., 2020). However, to date, few studies have been conducted with SARS-CoV-2.
Transmission through food intake is not considered a major source of transmission because SARS-CoV-2 cannot occur via consumption of contaminated food (FDA, 2021). However, there were concerns about the possibility of SARS-CoV-2 infection in food handlers in close proximity to contaminated food packaging. For example, viable SARS-CoV-2 and its RNA were found in imported frozen seafood and seafood packaging during the SARS-CoV-2 outbreak in Qingdao, China, on September 24, 2019 (Bai et al., 2021). In the United States, there was a simultaneous SARS-CoV-2 outbreak among workers in several meat and poultry processing facility (Birhane et al., 2020). These issues may also occur in local retail, and there is a risk that SARS-CoV-2 can be transmitted to homes through contaminated food packaging. To discuss the possibility of retail-to-home transmission, it is necessary to evaluate the viability of food packaging-contaminated SARS-CoV-2, and potential methods for eliminating SARS-CoV-2 contaminated on food contact surfaces at home.
The WHO and European Centre for Disease Prevention and Control (ECDC) have provided interim guidance on selection and use of disinfectants for coronavirus on environmental surfaces (ECDC, 2020; WHO, 2020a, p. 19). However, there are no definitive scientific data on SARS-CoV-2, only previous studies outcomes on other coronaviruses. Therefore, this study aimed to investigate the viability of coronaviruses including two types of SARS-CoV-2 and human coronavirus 229E (229E) at three different temperatures (20, 4, and −20 °C) on food contact surfaces and to evaluate the decontamination of surfaces using common disinfectants and UV-C. In addition, the appropriateness of interim guidelines was also evaluated.
2. Materials and methods
2.1. Viruses and cells
Vero E6 (ATCC CL-1586) and MRC-5 (ATCC CL-171) cells were grown in DMEM (Gibco, NY, USA) with 10% FBS and 1% antibiotics-antimycotics (Gibco). These cells were then cultured at 37 °C with 5% CO2 in a humidified incubator. Confluent Vero E6 cells were inoculated with two types of SARS-CoV-2 (L type, KOR/KCDC03-NCCP43326/2020, accession number: MW466791.1; S type, KOR/KCDC12-NCCP43330/2020, accession number: MW466795.1) at 0.1 multiplicities of infection (MOI) in DMEM with 2% FBS, and MRC-5 cells were inoculated with hCoV 229E under the same conditions. SARS-CoV-2 and hCoV 229E were incubated at 37 °C and 33 °C, respectively, with 5% CO2 for 4 d. After two freeze/thaw cycles, the viral titer was assessed using 50% tissue culture infectious doses (TCID50). All experiments related to SARS-CoV-2 were performed at a biosafety level 3 facility of the Zoological Infectious Disease Research Institute, Chonbuk National University, South Korea.
2.2. TCID50 assay
For virus titration using TCID50, 2 × 104 Vero E6 and MRC-5 cells were inoculated per well in 96-well plates one day prior to the assay. SARS-CoV-2 and hCoV 229E were serially diluted 10-fold and infected 90% confluent cells in quadruplicate samples. Plates infected with SARS-CoV-2 and hCoV 229E were incubated for 4 d at 37 °C and 33 °C, respectively. The wells showing cytopathic effects were then counted under a microscope, and the virus titer was calculated according to the Sperman-Kaerber method (Ramakrishnan, 2016).
2.3. Preparation of food contact surfaces
Kraft paper, parchment paper, and low-density polyethylene (LDPE) were purchased from an online market. Each surface was made into a carrier with a diameter of 8 mm using a punch. Stainless steel (SS), glass, and polypropylene (PP) were additionally selected as the most frequently used food contact surfaces in food processing. SS, glass, and PP were processed to a thickness of 1 mm and a diameter of 1 cm. All surfaces except LDPE were sterilized by autoclave, and LDPE was degreased with acetone and then sterilized by immersion in 70% EtOH and dried aseptically in class Ⅱ biosafety cabinet (BSCⅡ).
2.4. Viability test on food packaging surfaces
On each carrier, 10 μL of 6 log TCID50/mL of the viruses were inoculated and dried for 1 h in BSCⅡ. The carrier was maintained at 40% relative humidity (RH) under three temperature conditions (room temperature: 20 °C, refrigerated temperature: 4 °C, and frozen temperature: −20 °C). Viruses were recovered at 0, 2, 4, 8, 24, 48, 96, and 120 h post-inoculation for the room temperature and refrigerated samples, whereas the frozen samples were recovered at 0, 24, 96, and 120 h post-inoculation. Viruses were eluted and vortexed in 1 mL DMEM with 5% FBS for at least 1 min, and the eluates were immediately diluted 10-fold to determine viral infectivity by TCID50.
2.5. Quantitative carrier test
The quantitative carrier test for evaluating the inactivation effect of disinfectants and UV-C on three CoVs was measured by following the Organization for Economic Co-operation and Development (OECD) guidelines (OECD, 2013), with slight modification. Briefly, 340 μL of viral suspension was added to 160 μL of soil load (0.25% BSA, 0.35% yeast extract, and 0.08% mucin) considered to mimic the components of body secretions. Thereafter, 10 μL of the mixture was dropped into each carrier and dried for 1 h in a BSCⅡ. The carrier was treated with 50 μL of 50% and 70% EtOH (Sigma-Aldrich, St. Louis, MO, USA) or 100, 200, 500, and 1000 ppm NaClO (Sigma-Aldrich) for 1 and 5 min, respectively. Thereafter, the carrier was immediately transferred to 950 mL of DMEM with 10% FBS to neutralize the disinfectant. All test substances were diluted with hard water, prepared according to the OECD guidelines. UV-C (254 nm, 500 μW/cm2) was used to irradiate the carrier for 2, 5, and 10 min corresponding to 60, 150, and 300 mJ/cm2, respectively, and transferred to 1 mL of DMEM with 10% FBS. The virus was eluted by vortexing for at least 1 min, and the eluate was immediately diluted 10-fold to determine viral infectivity as TCID50.
2.6. Wiping test
The wiping test to verify the interim guidelines was performed on three hard surfaces (SS, glass, and PP) (ECDC, 2020; WHO, 2020a, p. 19). Each carrier was contaminated with virus under the same conditions as described for the quantitative carrier test. A sterile cotton swab moistened with 70% EtOH, 500 or 1000 ppm NaClO was used to wipe the virus-contaminated hard surface 1–3 times, until the dry stains disappeared. Immediately after exposure to the disinfectant for a specified time, the carrier was transferred to 1 mL of DMEM containing 10% FBS to neutralize the disinfectant. All test substances were diluted with hard water and the same test was performed with a sterile cotton swab moistened with hard water to evaluate the effect of the wiping action itself. Virus elution and infectivity evaluation were performed using the same method as described for the quantitative carrier test.
2.7. Statistical analysis
Virus viability was denoted as the mean and standard error. To assess the intrinsic stability of the viruses, a Bayesian regression model was used to estimate the decay rate of viable virus titers (Van Doremalen et al., 2020). This modeling approach could account for the differences in initial inoculation levels across replicates and interval censoring in titer data and other experimental noise sources (Gelman et al., 1995). Posterior samples were run using Stan, which implements a No-U-Turn Sampler (a form of Markov Chain Monte Carlo) (Van Doremalen et al., 2020). As a warm-up/adaptation period, four replicate chains were run at random initial conditions for 2000 iterations, storing the final 1000 iterations in each chain, giving a total of 4000 posterior samples. One-way ANOVA was used to analyze differences between strains. p-values <0.05 were considered statistically significant. All experiments were performed in triplicate and statistical analysis was performed using R version 4.1.0.
3. Results
3.1. Viability test on food packaging surface
At 20 °C, all coronaviruses rapidly decreased regardless of the surface type (Fig. 1 A). The titers of the coronaviruses on kraft paper decreased by > 1.5 log TCID50/mL after 2 h (SARS-CoV-2 L from 4.77 ± 0.04 to 2.81 ± 0.15 log TCID50/mL, SARS-CoV-2 S from 4.82 ± 0.05 to 3.24 ± 0.17 log TCID50/mL, and 229E from 4.84 ± 0.05 to 3.27 ± 0.16 log TCID50/mL), and SARS-CoV-2 L was less viable than other coronaviruses (p = 0.022). Similarly, on parchment paper, the titers of coronaviruses decreased by > 1.5 log after 2 h (SARS-CoV-2 L from 4.77 ± 0.04 to 2.81 ± 0.15 log TCID50/mL, SARS-CoV-2 S from 4.82 ± 0.05 to 3.24 ± 0.17 log TCID50/mL, and 229E from 4.84 ± 0.05 to 3.27 ± 0.16 log TCID50/mL). The titers of coronaviruses on LDPE decreased by > 1.5 log after 8 h (SARS-CoV-2 L from 4.77 ± 0.04 to 2.87 ± 0.09 log TCID50/mL, SARS-CoV-2 S from 4.82 ± 0.05 to 3.20 ± 0.37 log TCID50/mL, and 229E from 4.84 ± 0.05 to 3.21 ± 0.36 log TCID50/mL), making them more stable than on other surfaces. During experiments, infectious coronaviruses were not detected on kraft paper after 24 h; while they were detected on parchment paper and LDPE after 48 h. Virus titers decreased exponentially on all surfaces, as indicated by a log-linear decrease over time (Fig. 1B). The decay model data were noisier than LDPE due to many variations of the experiment on parchment paper and especially on kraft paper. The half-lives of the three viruses were similar on all surfaces (Fig. 1C and Table S1). On kraft paper, the median estimates for SARS-CoV-2 L were 1.05 h and 1.07 h for SARS-CoV-2 S and 229E. The half-lives on parchment paper were also similar for all three viruses and were 2.25, 2.27 and 2.25 h, respectively. On LDPE, SARS-CoV-2 L was 2.06 h, slightly shorter than the other two viruses (2.22 h for SARS-CoV-2 S and 2.20 h for 229E).
Fig. 1.
Viability of coronaviruses on food packaging surface at 20 °C. (A) Titer of virus recovered from the surface by timepoint. (B) Bayesian regression plots showing the predicted decay of virus titers over time. The dots are slightly jittered to avoid overlapping. Lines show exponential decay rates and were randomly drawn 150 per panel from the joint posterior distribution. (C) Violin plot representing the half-life of viruses. The dot are the median estimates, and the lines are the 95% confidence intervals. The dashed line in (A) and (B) indicates the limit of detection.
At 4 °C, the viability of coronaviruses was different for each surface (Fig. 2 A). The viability of coronaviruses on kraft paper was slightly longer than at 20 °C, decreasing by > 3 log after 24 h (SARS-CoV-2 L from 4.77 ± 0.04 to 1.53 ± 0.13 log TCID50/mL, SARS-CoV-2 S from 4.82 ± 0.05 to 1.02 ± 0.39 log TCID50/mL, and 229E from 4.84 ± 0.04 to 1.03 ± 0.40 log TCID50/mL) and below the detection limit at 48 h. On parchment paper, the titer of coronaviruses decreased by > 3 log after 72 h (SARS-CoV-2 L from 4.77 ± 0.04 to 1.07 ± 0.40 log TCID50/mL, SARS-CoV-2 S from 4.82 ± 0.05 to 1.49 ± 0.08 log TCID50/mL, and 229E from 4.86 ± 0.05 to 1.52 ± 0.08 log TCID50/mL), and was not detected from 96 h. SARS-CoV-2 L decreased faster than the other two coronaviruses at 8, 24, and 48 h (p < 0.001, p = 0.042, p < 0.001, respectively). On LDPE, the coronaviruses decreased by > 3 log after 120 h (SARS-CoV-2 L from 4.77 ± 0.04 to 1.39 ± 0.63 log TCID50/mL, SARS-CoV-2 S from 4.82 ± 0.05 to 1.53 ± 0.18 log TCID50/mL, and 229E from 4.85 ± 0.05 to 1.53 ± 0.18 log TCID50/mL) but were still detected. The decay model data on the surfaces except for kraft paper were substantially slower compared to 20 °C, but the kraft paper was similar to that of parchment paper or LDPE at 20 °C (Fig. 2B). Similarly, the half-life of the virus on kraft paper was as short as 2.73 h for SARS-CoV-2 L, 2.04 h for SARS-CoV-2 S, and 2.09 h for 229E (Fig. 2C and Table S1). The half-lives of the virus on parchment paper were 5.82, 6.68, and 6.71 h, respectively, which increased more than double that at 20 °C, and exceeded 10 h on LDPE (10.6 h for SARS-CoV-2 L, 11.1 h for SARS-CoV-2 S, and 10.9 h for 229E).
Fig. 2.
Viability of coronaviruses on food packaging surface at 4 °C. (A) Titer of virus recovered from the surface by timepoint. (B) Bayesian regression plots showing the predicted decay of virus titers over time. The dots are slightly jittered to avoid overlapping. Lines show exponential decay rates and were randomly drawn 150 per panel from the joint posterior distribution. (C) Violin plot representing the half-life of viruses. The dot are the median estimates, and the lines are the 95% confidence intervals. The dashed line in (A) and (B) indicates the limit of detection.
At −20 °C, the infectivity of the coronavirus was hardly reduced (Fig. 3 A). On kraft paper, the titers of the coronaviruses decreased by about 1 log after 120 h (SARS-CoV-2 L from 4.77 ± 0.04 to 3.73 ± 0.12 log TCID50/mL, SARS-CoV-2 S from 4.82 ± 0.05 to 3.74 ± 0.11 log TCID50/mL, and 229E from 4.83 ± 0.05 to 3.76 ± 0.10 log TCID50/mL), but hardly decreased on the other two surfaces (SARS-CoV-2 L, SARS-CoV-2 S and 229E from 4.77 ± 0.04 to 4.10 ± 0.29 log TCID50/mL, 4.82 ± 0.05 to 3.99 ± 0.29 log TCID50/mL, and 4.83 ± 0.06 to 4.02 ± 0.29 log TCID50/mL, respectively, on parchment; from 4.77 ± 0.04 to 4.42 ± 0.30 log TCID50/mL, from 4.82 ± 0.05 to 4.29 ± 0.30 log TCID50/mL, from 4.84 ± 0.04 to as 4.32 ± 0.31 log TCID50/mL, respectively, on LDPE). There were no differences between coronavirus strains. The decay model data were flat on all surfaces until the end of the experiment (Fig. 3B). Accordingly, the half-life of the virus on each surface was significantly increased (Fig. 3C and Table S1). On kraft paper, the half-life exceeded 1 day with 41.8 h for SARS-CoV-2 L, 40.5 h for SARS-CoV-2 S and 229E and exceeded 2 days on parchment paper (56.8, 5.76, and 57.4 h respectively). Coronaviruses infectivity decreased most slowly on LDPE. The half-lives of SARS-CoV-2 S were 91.2 h, close to 4 days, and the half-lives of SARS-CoV-2 L and 229E were 106 and 111 h, respectively, exceeding 100 h.
Fig. 3.
Viability of coronaviruses on food packaging surface at −20°C. (A) Titer of virus recovered from the surface by timepoint. (B) Bayesian regression plots showing the predicted decay of virus titers over time. The dots are slightly jittered to avoid overlapping. Lines show exponential decay rates and were randomly drawn 150 per panel from the joint posterior distribution. (C) Violin plot representing the half-life of viruses. The dot are the median estimates, and the lines are the 95% confidence intervals. The dashed line in (A) and (B) indicates the limit of detection.
3.2. Virucidal effect of disinfectants and UV on food contact surfaces
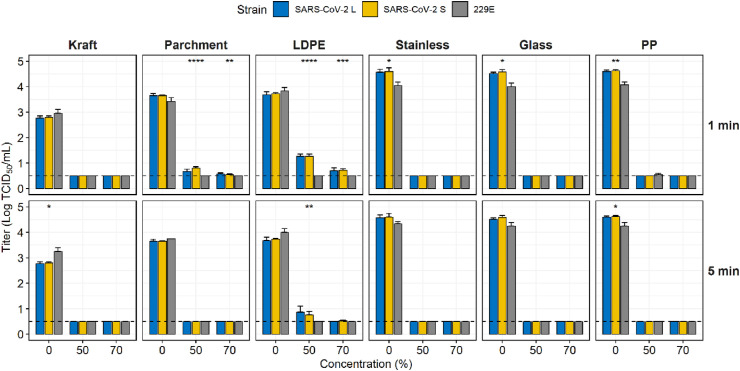
EtOH and NaClO were used to evaluate the disinfection ability of surface-contaminated coronaviruses as they are the most widely used disinfectants. EtOH effectively reduced the infectivity of contaminated coronaviruses on six surfaces (Fig. 4 ). Infectious coronaviruses were not detected on kraft paper, SS, and glass treated with 50% and 70% EtOH for 1 min. On parchment, although 229E was completely inactivated at all concentrations, SARS-CoV-2 L and SARS-CoV-2 S were reduced by 2.98 ± 0.13 and 2.85 ± 0.08 log TCID50/mL at 50% and 3.08 ± 0.06 and 3.10 ± 0.03 log TCID50/mL at 70% respectively for 1 min. In the EtOH treatment for 5 min, no viruses were detected on all experimental surfaces except for LDPE treated with 50% EtOH. On LDPE, SARS-CoV-2 L and SARS-CoV-2 S were reduced by 2.96 ± 0.32 and 3.50 ± 0.18 log TCID50/mL at 50%, even 229E was completely inactivated.
Fig. 4.
Virucidal effect of ethanol against coronaviruses that contaminated food contact surfaces. The dashed line indicates the limit of detection. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001).
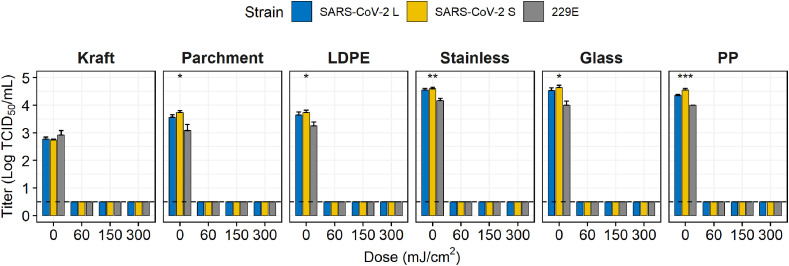
Routine concentrations (not exceeding 1000 ppm) of NaClO, rather than high concentrations (5000 ppm) were used to decontaminate surfaces (Fig. 5 ). Treatment with 1000 ppm NaClO for 1 min showed a complete reduction in viruses on SS only and >3 log reductions on parchment paper, glass, and PP. However, on LDPE, SARS-CoV-2 S and 229E were almost eliminated (they decreased by 3.06 and 3.54 log, respectively); However, the SARS-CoV-2 L was reduced by 2.21 log. which was significantly different (p < 0.001). When treated for 5 min at 500 ppm, complete reduction of viruses was observed on kraft paper and PP with a decrease of >3 log on glass. In contrast, at 1000 ppm, no viruses were detected on any surface; however, trace amounts of two SARS-CoV-2 strains were present on LDPE (0.55 TCID50/mL for S and L types). Consequently, 229E was more sensitive to 500 ppm NaClO than the two SARS-CoV-2 on LDPE, SS, and glass. In addition, all coronaviruses in this study were extremely sensitive to UV-C, as they were completely eliminated on all food contact surfaces upon UV-C exposure at 60 mJ/cm2 (Fig. 6 ).
Fig. 5.
Virucidal effect of sodium hypochlorite against coronaviruses that contaminated food contact surfaces. The dashed line indicates the limit of detection. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001).
Fig. 6.
Virucidal effect of UV-C against coronaviruses that contaminated food contact surfaces. The dashed line indicates the limit of detection. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, ***p < 0.005, *****p < 0.001).
3.3. Verification the effectiveness of interim guidelines for cleaning and disinfecting of SARS-CoV-2
The interim guidelines provided by the WHO and ECDC detail the disinfectant selection and their concentrations; however, their exposure time is somewhat overlooked. The wiping test addressed this issue (Fig. 7 ). Following exposure for 1 min, 70% EtOH and 1000 ppm NaClO were effective in the quantitative carrier test; however, complete reduction of SARS-CoV-2 was difficult with the wiping test. For complete reduction, surfaces were exposed to 1000 ppm NaClO and 70% EtOH for at least 5 min after wiping, whereas 500 ppm NaClO required 10 min. Meanwhile, 229E was reduced more rapidly compared to SARS-CoV-2 after a short exposure time.
Fig. 7.
Wiping test for inactivation of coronaviruses that contaminated food contact surfaces. The dashed line indicates the limit of detection. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001).
4. Discussion
The vaccine, which will be a game-changer for COVID-19, became widely available on December 2, 2020, with the Medicines and Healthcare products Regulatory Agency (MHRA) granting interim regulatory approval for the Pfizer-BioNTech vaccine (MHRA, 2021; WHO, 2020a). By November 29, 2021, 3.3 billion people (42.9% of the world's population) had completed their vaccinations (Mathieu et al., 2021). However, the coronavirus has also evolved rapidly and various variants of SARS-CoV-2 emerged until 2020 continue to threaten humanity, with increased transmissibility, severe clinical symptoms, and breakthrough infections caused by reduced vaccine effectiveness (Callaway, 2021; WHO, 2021a). Providing clear guidelines for surface disinfection could be the basis for consumers to avoid cross-contamination from handling surfaces contaminated with SARS-CoV-2. Therefore, this study investigated SARS-CoV-2 viability on various surfaces and their inactivation.
Although previous studies have been conducted SARS-CoV-2 viability on surfaces, there have been no studies on food contact packaging materials (Chin et al., 2020; Kratzel et al., 2020; Van Doremalen et al., 2020). This study evaluated SARS-CoV-2 viability on food packaging surfaces while simultaneously comparing the differences in survival characteristics between the two variants and evaluating the potential of 229E as a surrogate for human coronavirus. The viability of coronaviruses on porous surfaces including kraft paper and parchment paper was lower than that on LDPE, which is a non-porous surface and viability of coronaviruses rising sharply as the storage temperature decreased. No significant differences were found in surface viability between the three coronaviruses at all storage temperatures. However, even on the same surface, results differed depending on factors such as temperature, humidity, presence of organic matter as a mimic of body composition, and initial titer. Van Doremalen et al. (2020) reported that SARS-CoV-2 survived for 4, 24, 24, and 48 h in copper, cardboard, SS, and PP, respectively, which was similar to our study design and results. In another study by Chin et al. (2020), SARS-CoV-2 was stable from 30 min to 48 h on porous surfaces (paper, wood, cloth, etc.) and up to 96 h on non-porous surfaces (glass, SS, plastic), which indicated longer viability than our room temperature study. This longer viability may be associated with the use of a higher initial titer (1 log TCID50/mL) and RH (25%) compared to that in our study. However, our findings showed that SARS-CoV-2 viability differs markedly with temperature. In contrast, Kratzel, et al. (2020) observed that SARS-CoV-2 stability was temperature independent. In their study, an interfering substance (0.3% BSA) was used together with virus inoculation on surface, which may have helped the SARS-CoV-2 survivability; conversely, BSA was not used in our study. Similarly, Kasloff, et al. (2021) demonstrated the viability of coronavirus for 14 and 21 d on SS and plastic, respectively; however, they used soil load according to the American Society for Testing and Materials standards and the initial titer was 1 log higher than our study.
A few previous studies used disinfectants, fixatives, and lysis buffers to inactivate SARS-CoV-2 (Chan et al., 2020; Chin et al., 2020). However, as they were only performed against virus suspensions, the virucidal effect may be exaggerated when compared to use on environmental surfaces (Sattar et al., 2003). The results obtained in this study for the quantitative carrier test were expected to be more similar to the virucidal effect when using actual disinfectants when compared to the suspension test because the disinfectant must penetrate the dried virus on the environmental surface and subsequently inactivate the virus. Therefore, disinfection by exposure to 70% EtOH for 1 min or 1000 ppm NaClO for 5 min can efficiently reduce the SARS-CoV-2 contamination on surfaces. Notably, we expected a difference in virucidal effect between porous and non-porous surfaces; however, the results also showed a difference between non-porous surfaces. Within non-porous surfaces, LDPE showed a difference with hard surfaces (SS, glass, and PP) during virus inactivation. Meanwhile, LDPE and PP are generally called plastics but in chemical structure LDPE has many branches and PP does not; thus, their properties of elasticity, transparency, and strength are different. The difference in virucidal effect on the two surfaces may be related to the interaction between the virus and the branching structure (Achilias et al., 2007). Therefore, when researching the virucidal effect on surfaces, it is necessary to consider the name of the compound more accurately. In addition, a UV-C emission of 1048 mJ/cm2 was required to complete reduction of SARS-CoV-2 in aqueous solution (Heilingloh et al., 2020); whereas only 60 mJ/cm2 was sufficient in our carrier study. This result demonstrated that UV-C was more effective in a dry environment.
As seen above, the application of additional disinfectants to SARS-CoV-2 contaminated surfaces may be limited dependent on type of surface or environment. Therefore, the interim guidelines recommend wiping with a disinfectant (ECDC, 2020; WHO, 2020a, p. 19). However, the reported guidelines provided approximate outcomes according to disinfectant usage (disinfectant type, concentration, and exposure time) based on the type of coronavirus studies including murine hepatitis virus, SARS-CoV, and MERS-CoV. Therefore, the recommended guidelines may be insufficient for SARS-CoV-2 reduction, indicating a lack of scientific evidence for disinfectants against SARS-CoV-2. Accordingly, the current study suggested that contaminated surfaces must have sufficient exposure time to reach complete reduction of SARS-CoV-2 after wiped with a disinfectant. In addition, although only one strain of SARS-CoV-2 was used in a similar previous study, two types of SARS-CoV-2 and 229E were comparatively evaluated in this study. No significant differences were noted between SARS-CoV-2 L and S for viability and treatment (disinfectants and UV-C) on all examined surfaces. Considering that the S type (before mutation) is an early strain discovered in Wuhan and the L type is a mutant strain with two single nucleotide polymorphisms (Tang et al., 2020). Even though mutations persist, there may not be much of a difference regarding sensitivity to disinfectants, according to our finding. However, SARS-CoV-2 and 229E displayed different sensitive patterns to disinfectants; therefore, targeting SARS-CoV-2 directly is strongly recommended when researching an effective virucidal agents.
Only two types of disinfectants and UV-C were used in this study, therefore it is necessary to evaluate various virucides that may effectively inactivate SARS-CoV-2. Viability evaluation and virucidal evaluation were performed for two types of SARS-CoV-2, and it shows that even if mutated, similar results are shown. However, it could not be performed on variants such as Omicron, which are currently rapidly spreading and prevalent.
5. Conclusions
SARS-CoV-2 contaminated with food-contact surfaces at local retail stores is viable enough for home delivery and refrigerated or frozen at home has a much greater chance of survival. In particular, the viability of SARS-CoV-2 is maintained longer on vinyl than on paper surface. We found that SARS-CoV-2 can be effectively reduced from contaminated food contact surfaces treated with 70% EtOH, 1000 ppm NaClO, and 60 mJ/cm2 of UV-C regardless of the type of surface used in this study. In addition, the wiping test results recommend an exposure time of the disinfectant of at least 5 min after wiping. These are conditions that can be easily used at home, and even if they are transferred to the home with SARS-CoV-2 contaminated on the surface, consumers can remove them. Moreover, the food contact surfaces used in the experiments are used in a variety of ways, not limited to the food industry; thus, it provides more precise disinfection standards than the existing interim guidelines in healthcare and non-healthcare settings that may be contaminated with SARS-CoV-2.
CRediT authorship contribution statement
Soontag Jung: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. Dong-Hwi Kim: Formal analysis, Investigation, Visualization, Writing – original draft. Hee-Seop Ahn: Investigation. Hyeon-Jeong Go: Investigation. Zhaoqi Wang: Investigation. Daseul Yeo: Investigation. Seoyoung Woo: Validation. Yeeun Seo: Validation. Md Iqbal Hossain: Writing – review & editing. In-Soo Choi: Supervision, Funding acquisition. Sang-Do Ha: Supervision, Funding acquisition. Changsun Choi: Conceptualization, Project administration, Supervision, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by a grant (20162MFDS039) from the Ministry of Food and Drug Safety in 2020.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.foodcont.2022.109306.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- Achilias D., Roupakias C., Megalokonomos P., Lappas A., Antonakou Ε. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP) Journal of Hazardous Materials. 2007;149(3):536–542. doi: 10.1016/j.jhazmat.2007.06.076. [DOI] [PubMed] [Google Scholar]
- Bai L., Wang Y., Wang Y., Wu Y., Li N., Liu Z. Controlling COVID-19 transmission due to contaminated imported frozen food and food packaging. China CDC Weekly. 2021;3(2):30–33. doi: 10.46234/ccdcw2021.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birhane M., Bressler S., Chang G., Clark T., Dorough L., Fischer M., Watkins L., Goldstein J.M., Kugeler K., Langley G., Lecy K., Martin S., Medalla F., Mitruka K., Nolen L., Sadigh K., Spratling R., Thompson G., Trujillo A. Update: COVID-19 among workers in meat and poultry processing facilities―United States, April–may 2020. Morbidity and Mortality Weekly Report. 2020;69(27):887. doi: 10.15585/mmwr.mm6927e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birhane M., Bressler S., Chang G., Clark T., Dorough L., Fischer M., Watkins L.F., Goldstein J.M.K.K., Langley G., Lecy K., Martin S., Medalla F., Mitruka K., Nolen L., Sadigh K., Spratling R., Thompson G., Trujillo A. COVID-19 vaccine breakthrough infections reported to CDC—United States, January 1–April 30, 2021. Morbidity and Mortality Weekly Report. 2021;70(21):792. doi: 10.15585/mmwr.mm7021e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. Fast-spreading COVID variant can elude immune responses. Nature. 2021:500–501. doi: 10.1038/d41586-021-00121-z. [DOI] [PubMed] [Google Scholar]
- CDC Cleaning and disinfecting your facility | CDC. 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/disinfecting-building-facility.html
- Chan K.-H., Sridhar S., Zhang R.R., Chu H., Fung A.-F., Chan G., Chan J.-W., To K.-W., Hung I.-N., Cheng V.-C. Factors affecting stability and infectivity of SARS-CoV-2. Journal of Hospital Infection. 2020;106(2):226–231. doi: 10.1016/j.jhin.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan‐Yeung M., Xu R.H. SARS: Epidemiology. Respirology. 2003;8:S9–S14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C., Wong S.-C., Chen J.H., Yip C.C., Chuang V.W., Tsang O.T., Sridhar S., Chan J.F., Ho P.-L., Yuen K.-Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infection Control and Hospital Epidemiology. 2020;41(5):493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W., Chu J.T., Perera M.R., Hui K.P., Yen H.-L., Chan M.C., Peiris M., Poon L.L. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC . 2020. Interim guidance for environmental cleaning in non-healthcare facilities exposed to SARS-CoV-2.https://www.ecdc.europa.eu/sites/default/files/documents/novel-coronavirus-guidance-environmental-cleaning-non-healthcare-facilities.pdf [Google Scholar]
- FDA U.S. 2021. COVID-19 update.https://www.fda.gov/news-events/press-announcements/covid-19-update-usda-fda-underscore-current-epidemiologic-and-scientific-information-indicating-no USDA, FDA Underscore Current Epidemiologic and Scientific Information Indicating No Transmission of COVID-19 Through Food or Food Packaging | FDA. [Google Scholar]
- Gelman A., Carlin J.B., Stern H.S., Rubin D.B. 1st ed. Chapman and Hall/CRC; 1995. Bayesian data analysis. [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., amp; Coronaviridae Study Group of the International Committee on Taxonomy of, V The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilingloh C.S., Aufderhorst U.W., Schipper L., Dittmer U., Witzke O., Yang D., Zheng X., Sutter K., Trilling M., Alt M. Susceptibility of SARS-CoV-2 to UV irradiation. American Journal of Infection Control. 2020;48(10):1273–1275. doi: 10.1016/j.ajic.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. Journal of Hospital Infection. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasloff S.B., Leung A., Strong J.E., Funk D., Cutts T. Stability of SARS-CoV-2 on critical personal protective equipment. Scientific Reports. 2021;11(1):984. doi: 10.1038/s41598-020-80098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzel A., Steiner S., Todt D., V'Kovski P., Brueggemann Y., Steinmann J., Steinmann E., Thiel V., Pfaender S. Temperature-dependent surface stability of SARS-CoV-2. Journal of Infection. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International Journal of Antimicrobial Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S., Lau E.H., Wong J.Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England Journal of Medicine. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., Giattino C., Rodés-Guirao L. A global database of COVID-19 vaccinations. Nature Human Behaviour. 2021;5(7):947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- MHRA . 2021. Conditions of authorisation for COVID-19 vaccine pfizer/BioNTech.https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/conditions-of-authorisation-for-pfizerbiontech-covid-19-vaccine (Regulation 174) [Google Scholar]
- OECD . 2013. OECD guidance document on quantitative methods for evaluating the activity of microbicides used on hard non-porous surfaces - OECD.https://www.oecd.org/chemicalsafety/testing/evaluating-the-activity-of-microbicides-used-on-hard-non-porous-surfaces.htm [Google Scholar]
- Ramakrishnan M.A. Determination of 50% endpoint titer using a simple formula. World Journal of Virology. 2016;5(2):85–86. doi: 10.5501/wjv.v5.i2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S.A., Springthorpe V.S., Adegbunrin O., Zafer A.A., Busa M. A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. Journal of Virological Methods. 2003;112(1–2):3–12. doi: 10.1016/s0166-0934(03)00192-7. [DOI] [PubMed] [Google Scholar]
- Tang X., Wu C., Li X., Song Y., Yao X., Wu X., Duan Y., Zhang H., Wang Y., Qian Z. On the origin and continuing evolution of SARS-CoV-2. National Science Review. 2020;7(6):1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2019. Middle East respiratory syndrome coronavirus (MERS-CoV)https://covid-19.conacyt.mx/jspui/bitstream/1000/1335/1/107881.pdf [Google Scholar]
- WHO . 2020. Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected Interim guidance; p. 19.https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125 March 2020. [Google Scholar]
- WHO . 2020. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations: Scientific brief; p. 27.https://apps.who.int/iris/bitstream/handle/10665/331601/WHO-2019-nCoV-Sci_Brief-Transmission_modes-2020.1-eng.pdf March 2020. [Google Scholar]
- WHO . 2020. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Google Scholar]
- WHO . 2021. Tracking SARS-CoV-2 variants.https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ [Google Scholar]
- WHO WHO coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.