Abstract
Background
SARS-CoV-2 infection during pregnancy is associated with adverse pregnancy outcomes, including fetal death and preterm birth. It is not known whether that risk occurs only during the time of acute infection or whether the risk persists later in pregnancy.
Objective
This study aimed to evaluate whether the risk of SARS-CoV-2 infection during pregnancy persists after an acute maternal illness.
Study Design
A retrospective cohort study of pregnant patients with and without SARS-CoV-2 infection delivering at 17 hospitals in the United States between March 2020 and December 2020. Patients experiencing a SARS-CoV-2–positive test at or before 28 weeks of gestation with a subsequent delivery hospitalization were compared with those without a positive SAR-CoV-2 test at the same hospitals with randomly selected delivery days during the same period. Deliveries occurring at <20 weeks of gestation in both groups were excluded. The study outcomes included fetal or neonatal death, preterm birth at <37 weeks of gestation and <34 weeks of gestation, hypertensive disorders of pregnancy (HDP), any major congenital malformation, and size for gestational age of <5th or <10th percentiles at birth based on published standards. HDP that were collected included HDP and preeclampsia with severe features, both overall and with delivery at <37 weeks of gestation.
Results
Of 2326 patients who tested positive for SARS-CoV-2 during pregnancy and were at least 20 weeks of gestation at delivery from March 2020 to December 2020, 402 patients (delivering 414 fetuses or neonates) were SARS-CoV-2 positive before 28 weeks of gestation and before their admission for delivery; they were compared with 11,705 patients without a positive SARS-CoV-2 test. In adjusted analyses, those with SARS-CoV-2 before 28 weeks of gestation had a subsequent increased risk of fetal or neonatal death (2.9% vs 1.5%; adjusted relative risk, 1.97; 95% confidence interval, 1.01–3.85), preterm birth at <37 weeks of gestation (19.6% vs 13.8%; adjusted relative risk, 1.29; 95% confidence interval, 1.02–1.63), and HDP with delivery at <37 weeks of gestation (7.2% vs 4.1%; adjusted relative risk, 1.74; 95% confidence interval, 1.19–2.55). There was no difference in the rates of preterm birth at <34 weeks of gestation, any major congenital malformation, and size for gestational age of <5th or <10th percentiles. In addition, there was no significant difference in the rate of gestational hypertension overall or preeclampsia with severe features.
Conclusion
There was a modest increase in the risk of adverse pregnancy outcomes after SARS-CoV-2 infection.
Key words: COVID-19, fetal or neonatal death, hypertensive disorders of pregnancy, pregnancy, preterm birth, SARS-CoV-2 infection
Introduction
COVID-19 has been associated with severe maternal outcomes, including intensive care unit admission and death.1 Furthermore, several reports have suggested an increased risk of adverse pregnancy outcomes, including cesarean delivery, preterm birth (PTB), hypertensive disorders of pregnancy (HDP), and fetal or neonatal death.2, 3, 4, 5 However, most of these reports have included pregnancy outcomes at the time of a delivery admission in the setting of acute COVID-19, and adverse pregnancy outcomes would be expected as a complication of severe maternal disease.6, 7, 8 A report from the Centers for Disease Control and Prevention (CDC) indicated an increased risk of stillbirth among patients with COVID-19 diagnosis codes at the time of delivery hospitalization; however, the timing of stillbirth concerning maternal infection was unclear.4
AJOG at a Glance.
Why was this study conducted?
SARS-CoV-2 infection is associated with adverse pregnancy outcomes during acute illness. Data are lacking on whether SARS-CoV-2 infection in pregnancy is associated with adverse pregnancy outcomes beyond the time of acute infection.
Key findings
Patients with SARS-CoV-2 before 28 weeks of gestation had a subsequent increased risk of fetal or neonatal death (2.9% vs 1.5%; adjusted relative risk [aRR], 1.97; 95% confidence interval [CI], 1.01–3.85), preterm birth (19.6% vs 13.8%; aRR, 1.29; 95% CI, 1.02–1.63), and hypertensive disorders of pregnancy with delivery at <37 weeks of gestation (7.2% vs 4.1%; aRR, 1.74; 95% CI, 1.19–2.55).
What does this add to what is known?
This study demonstrated that there are risks from SARS-CoV-2 infection in pregnancy that seem to persist beyond the time of acute infection.
Based on existing data, it is difficult to determine whether there is an increased risk of adverse pregnancy outcomes among patients who experience COVID-19 in pregnancy and deliver at a later point in gestation. Current guidelines from the Society for Maternal-Fetal Medicine regarding antenatal evaluation after maternal infection have not changed since early in the pandemic because of a lack of clarity about whether infection in pregnancy is related to subsequent development of adverse pregnancy outcomes once a pregnant person has recovered.9 The presence of ongoing increased risk of adverse pregnancy outcomes after recovery from acute COVID-19 could alter subsequent pregnancy management.
This study aimed to evaluate whether patients who experience SARS-CoV-2 infection before 28 weeks of gestation experience adverse pregnancy outcomes later in gestation.
Materials and methods
This was a retrospective cohort study of pregnant patients with a singleton or twin pregnancy who delivered from March 2020 to December 2020 at 1 of 17 US hospitals participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network Gestational Research Assessments for COVID-19 (GRAVID) study.10 The group with a positive SARS-CoV-2 viral (antigen or polymerase chain reaction) test was composed of individuals at <28 weeks of gestation at the time of the positive test (either as an outpatient or as an inpatient) before their admission for delivery. Patients with a negative SARS-CoV-2 test or those with no documented SARS-CoV-2 infection at any time in pregnancy who delivered on randomly selected dates over the same period at the same hospital sites were the comparison group (no positive SARS-CoV-2 test). Of note, 6 weekdays and 2 weekend days per month were sampled from March 2020 to May 2020, and 3 weekdays and 1 weekend day per month were randomly selected from June 2020 to December 2020. Those with deliveries before 20 weeks of gestation were excluded from this analysis to ensure comparable groups given the lack of reliable delivery data before 20 weeks of gestation at most hospitals in the United States. Data were abstracted from the medical record by centrally trained and certified perinatal research staff.
Study outcomes included fetal or neonatal death, PTB at <37 weeks of gestation and <34 weeks of gestation, HDP, any major congenital malformation, and size for gestational age of <5th or <10th percentiles at birth based on published standards.11 HDP that were collected included gestational hypertension; preeclampsia (with or without severe features); hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome); or eclampsia.12
Patients in the SARS-CoV-2–positive group were compared with those without known infection. Descriptive summary statistics were calculated for baseline characteristics. The Wilcoxon rank-sum test for continuous variables and the chi-squared or Fisher exact test for categorical variables were used as appropriate. Race and ethnicity categories with low frequencies were collapsed to make statistical comparisons. Covariates for multivariable modeling were selected on the basis of clinical relevance and included maternal age, body mass index (BMI) at the first prenatal visit or (if that was not available) previous conception, and major medical comorbidity, including any of the following: asthma of any severity or chronic obstructive pulmonary disease, chronic hypertension, or pregestational diabetes mellitus. In addition, models for HDP included obstetrical history categorized as no previous delivery at >20 weeks of gestation, previous delivery with a hypertensive disorder or PTB, or previous delivery without a hypertensive disorder or PTB. To account for random sampling of individuals without a positive SARS-CoV-2 test, weighted analyses were performed. For maternal outcomes, modified Poisson regression models were used to estimate relative risks (RRs) and 95% confidence intervals (CIs). To account for patients with twin pregnancies, models based on a generalized estimating equations framework with an exchangeable correlation structure were used to estimate relative risks of neonatal outcomes. Multivariable modeling was not performed for outcomes with low frequencies. Frequencies by COVID-19 severity were summarized for significant neonatal outcomes.
A planned sensitivity analysis was performed in which those with no SARS-CoV-2 testing before delivery were excluded from the analysis. An additional sensitivity analysis was performed in which missing BMI values were imputed on the basis of a generalized linear model. The imputation modeled the natural-log scale BMI with linear, quadratic, and cubic natural-log scale BMI at delivery calculated from the most recent pregnancy weight before delivery. For patients with BMI at delivery and without prenatal (or preconceptional) BMI available, imputed BMI values were the back-transformed predicted values based on the model.
Nominal 2-sided P values are reported. A P value of <.05 was considered statistically significant. No adjustment was made for multiple comparisons. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute, Cary, NC).
Institutional review board approval was obtained at each site participating in the GRAVID study.
Results
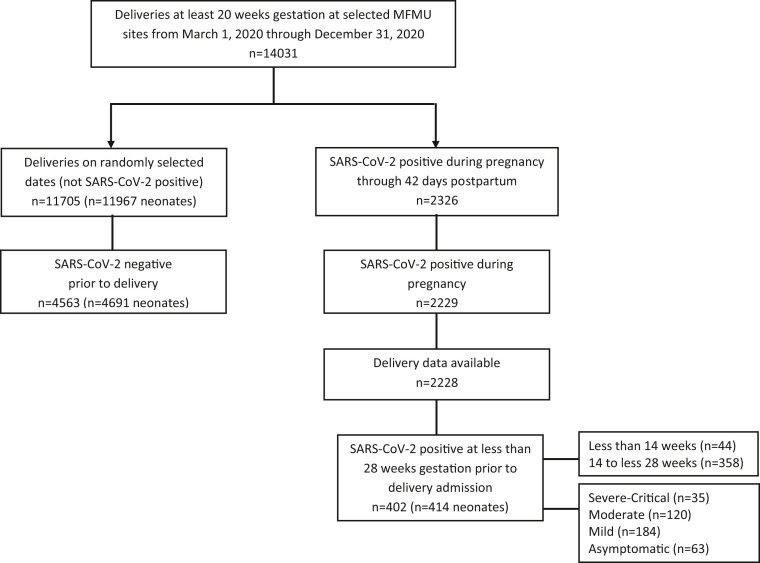
Of 2326 individuals who tested positive for SARS-CoV-2 during pregnancy and were at least 20 weeks of gestation at delivery from March 2020 to December 2020, 402 (delivering 414 fetuses or neonates) were SARS-CoV-2 positive before 28 weeks of gestation and before their admission for delivery (Figure ). During the study period, 11,705 individuals without known infection (delivering 11,967 fetuses or neonates) delivered on randomly selected delivery dates at the 17 hospitals. Among those who were SARS-CoV-2 positive at <28 weeks of gestation, most individuals (89%) tested positive in the second trimester of pregnancy (at least 14 to <28 weeks of gestation).
Figure.
Deliveries occurring at selected MFMU sites with and without SARS-CoV-2–positive results included in the analysis
MFMU, Maternal-Fetal Medicine Unit.
Hughes. Early SARS-CoV-2 infection and subsequent pregnancy outcomes. Am J Obstet Gynecol 2023.
Patients with SARS-CoV-2 were younger, had higher BMI, were more likely to have had a previous pregnancy, identify as Hispanic, have public or no insurance, and have a major medical comorbidity than patients without a positive SARS-CoV-2 test (Table 1 ). SARS-CoV-2 infection at <28 weeks of gestation was associated with subsequent fetal or neonatal death (2.9% vs 1.5%; adjusted RR [aRR], 1.97; 95% CI, 1.01–3.85). The median latency between a SARS-CoV-2–positive test and fetal death was 21 days (interquartile range, 16–89 days), with 4 of 7 cases (57%) occurring before 24 weeks of gestation. Fetal deaths occurred following asymptomatic (3.1%), mild (1.6%), moderate (0.8%), and severe and critical (2.7%) infections. Furthermore, SARS-CoV-2 infection at <28 weeks of gestation was associated with PTB at <37 weeks of gestation (19.6% vs 13.8%; aRR, 1.29; 95% CI, 1.02–1.63), with most cases (66·7%) being considered indicated PTB rather than spontaneous PTB. In addition, SARS-CoV-2 infection at <28 weeks of gestation was associated with HDP with delivery at <37 weeks of gestation (7.2% vs 4.1%; aRR, 1.74; 95% CI, 1.19–2.55). There was no association between SARS-CoV-2 infection and other outcomes evaluated, including small for gestational age (SGA) (Table 2 ).
Table 1.
Baseline demographic and clinical characteristics
| Characteristics | SARS-CoV-2 positive <28 wk (n=402) | Not SARS-CoV-2 positive (n=11,705) | P value |
|---|---|---|---|
| Age (y) | 29.0±5.9 | 30.0±5.8 | .002 |
| BMI (kg/m2) | 29.3 (24.5–35.5) | 26.6 (23.0–31.9) | <.001 |
| BMI≥30 | 173 (46.0) | 3426 (32.2) | <.001 |
| BMI≥40 | 53 (14.1) | 799 (7.5) | <.001 |
| Race and ethnicity | <.001 | ||
| American Indian or Alaska Native | 0 (0) | 43 (0.4) | |
| Asian | 6 (1.6) | 575 (5.2) | |
| Hispanic | 188 (48.5) | 2614 (23.5) | |
| Native Hawaiian or Pacific Islander | 4 (1.0) | 28 (0.3) | |
| Non-Hispanic Black | 117 (30.2) | 2519 (22.6) | |
| Non-Hispanic White | 72 (18.6) | 5297 (47.6) | |
| More than 1 race | 1 (0.3) | 59 (0.5) | |
| No previous pregnancy ≥20 wk | 115 (28.7) | 4773 (40.8) | <.001 |
| Previous preterm birth (20 to <37 wk) | 51 (12.7) | 1077 (9.2) | .02 |
| Previous cesarean delivery | 90 (22.4) | 2149 (18.4) | .04 |
| Previous hypertensive disorders of pregnancy | 48 (12.0) | 922 (7.9) | .003 |
| Private insurance | 138 (34.7) | 6368 (54.8) | <.001 |
| Smoked during this pregnancy | 16 (4.0) | 846 (7.2) | .01 |
| Any substance use during this pregnancy | 25 (6.2) | 967 (8.3) | .14 |
| Immunocompromising condition | 5 (1.2) | 160 (1.4) | .83 |
| Asthma or COPD | 66 (16.4) | 1552 (13.3) | .07 |
| Pregestational diabetes mellitus | 20 (5.0) | 267 (2.3) | <.001 |
| Thrombophilia | 3 (0.7) | 79 (0.7) | .75 |
| Chronic hypertension | 37 (9.2) | 640 (5.5) | .001 |
| Chronic cardiovascular disease | 7 (1.7) | 175 (1.5) | .69 |
| Chronic renal disease | 4 (1.0) | 59 (0.5) | .16 |
| Chronic liver disease | 1 (0.2) | 107 (0.9) | .27 |
| Thyroid disease | 18 (4.5) | 791 (6.8) | .07 |
| Neurocognitive disorder | 32 (8.0) | 1551 (13.3) | .002 |
| Neuromuscular disorder | 1 (0.2) | 52 (0.4) | 1.00 |
| Seizure disorder | 4 (1.0) | 147 (1.3) | .64 |
| Inflammatory bowel disease | 2 (0.5) | 129 (1.1) | .33 |
| Any comorbidity (asthma or COPD, pregestational diabetes mellitus, chronic hypertension) | 104 (25.9) | 2217 (18.9) | <.001 |
| Trimester of SARS-CoV-2 positive | |||
| <14 wk | 44 (10.9) | ||
| 14 to <28 wk | 358 (89.1) |
Data are presented as mean±standard deviation, median (interquartile range), or number (percentage), unless otherwise specified. Non-Hispanic Asian, Native Hawaiian or Pacific Islander, American Indian or Alaskan Native, or more than 1 race were collapsed for statistical comparisons. The following are the number of missing values: maternal age (n=3), BMI (n=1083), race and ethnicity (n=584), parity (n=13), and insurance status (n=81).
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Hughes. Early SARS-CoV-2 infection and subsequent pregnancy outcomes. Am J Obstet Gynecol 2023.
Table 2.
Association between adverse outcomes and SARS-CoV-2 infection before 28 weeks of gestation compared with randomly selected controls
| Variable | SARS-CoV-2 positive at <28 wk | Not SARS-CoV-2 positive | RR (95% CI) | aRR (95% CI) |
|---|---|---|---|---|
| Number of patients | 402 | 11,705 | ||
| Hypertensive disorders of pregnancy | 88 (21.9) | 2510 (21.4) | 1.01 (0.83–1.22) | 1.00 (0.82–1.21) |
| By COVID-19 severity | ||||
| Asymptomatic | 10/63 (15.9) | |||
| Mild | 41/184 (22.3) | |||
| Moderate | 23/120 (19.2) | |||
| Severe and critical | 14/35 (40.0) | |||
| Preeclampsia with severe features | 28 (7.0) | 609 (5.2) | ||
| Preeclampsia without severe features | 9 (2.2) | 349 (3.0) | ||
| Gestational hypertension | 51 (12.7) | 1552 (13.3) | ||
| Gestational age of <37 wk at delivery | 29 (7.2) | 477 (4.1) | 1.73 (1.21–2.49) | 1.74 (1.19–2.55) |
| By COVID-19 severity | ||||
| Asymptomatic | 5/63 (7.9) | |||
| Mild | 8/184 (4.3) | |||
| Moderate | 10/120 (8.3) | |||
| Severe and critical | 6/35 (17.1) | |||
| Preeclampsia with severe features | 28 (7.0) | 609 (5.2) | 1.34 (0.93–1.93) | 1.21 (0.81–1.81) |
| By COVID-19 severity | ||||
| Asymptomatic | 4/63 (6.3) | |||
| Mild | 13/184 (7.1) | |||
| Moderate | 6/120 (5.0) | |||
| Severe and critical | 5/35 (14.3) | |||
| Gestational age of <37 wk at delivery | 17 (4.2) | 274 (2.3) | 1.80 (1.11–2.90) | 1.68 (1.00–2.83) |
| By COVID-19 severity | ||||
| Asymptomatic | 2/63 (3.2) | |||
| Mild | 7/184 (3.8) | |||
| Moderate | 3/120 (2.5) | |||
| Severe and critical | 5/35 (14.3) | |||
| Number of fetuses or neonates | 414 | 11,967 | ||
| Fetal or neonatal death | 12 (2.9) | 174 (1.5) | 2.01 (1.10–3.67) | 1.97 (1.01–3.85) |
| By COVID-19 severity | ||||
| Asymptomatic | 3/64 (4.7) | |||
| Mild | 5/190 (2.6) | |||
| Moderate | 2/123 (1.6) | |||
| Severe and critical | 2/37 (5.4) | |||
| Gestational age of <24 wk | 6/12 (50.0) | 65/174 (37.4) | ||
| Gestational age of 24 wk | 6/12 (50.0) | 109/174 (62.6) | ||
| Fetal death | ||||
| By COVID-19 severity | 7 (1.7) | 114 (1.0) | ||
| Asymptomatic | 2/64 (3.1) | |||
| Mild | 3/190 (1.6) | |||
| Moderate | 1/123 (0.8) | |||
| Severe and critical | 1/37 (2.7) | |||
| Number of days from positive test to fetal death, median (interquartile range) | 21 (16–89) | |||
| Gestational age of <24 wk | 4/7 (57.1) | 42/114 (36.8) | ||
| Gestational age of 24 wk | 3/7 (42.9) | 72/114 (63.2) | ||
| Neonatal death | 5 (1.2) | 60 (0.5) | ||
| Gestational age of <24 wk | 2/5 (40.0) | 23/60 (38.3) | ||
| Gestational age of 24 wk | 3/5 (60.0) | 37/60 (61.7) | ||
| PTB at <37 wk | 81 (19.6) | 1654 (13.8) | 1.42 (1.14–1.76) | 1.29 (1.02–1.63) |
| By COVID-19 severity | ||||
| Asymptomatic | 11/64 (17.2) | |||
| Mild | 31/190 (16.3) | |||
| Moderate | 26/123 (21.1) | |||
| Severe and critical | 13/37 (35.1) | |||
| Spontaneous | 27 (33.3) | 795 (48.1) | ||
| Indicated | 54 (66.7) | 859 (51.9) | ||
| PTB at <34 wk | 33 (8.0) | 601 (5.0) | 1.52 (1.05–2.19) | 1.45 (0.97–2.16) |
| Spontaneous | 14 (42.4) | 332 (55.2) | ||
| Indicated | 19 (57.6) | 269 (44.8) | ||
| Live birth | 407 | 11,853 | ||
| Major congenital malformations | 14 (3.4) | 504 (4.3) | 0.83 (0.49–1.40) | 0.81 (0.47–1.39) |
| Size for gestational age of <10th percentile | 47 (11.8) | 1286 (10.9) | 1.10 (0.83–1.45) | 1.08 (0.82–1.44) |
| Size for gestational age of <5th percentile | 22 (5.5) | 647 (5.5) | 1.01 (0.66–1.55) | 0.97 (0.62–1.52) |
Data are presented as number (percentage) or number/total number (percentage), unless otherwise specified. Models for hypertensive disorders of pregnancy were adjusted for maternal age, BMI, any comorbidity (asthma or COPD, pregestational diabetes mellitus, or chronic hypertension), and obstetrical history (no previous pregnancy, previous pregnancy without PTB or preeclampsia, or previous pregnancy with PTB or preeclampsia). All other models were adjusted for maternal age, BMI, and any comorbidity (asthma or COPD, pregestational diabetes mellitus, or chronic hypertension).
aRR, adjusted relative risk; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; PTB, preterm birth; RR, relative risk.
Hughes. Early SARS-CoV-2 infection and subsequent pregnancy outcomes. Am J Obstet Gynecol 2023.
In the comparison group, there were 4563 patients (delivering 4691 neonates) with a documented negative SARS-CoV-2 test before delivery. The remainder did not have a documented test result (7142 patients delivering 7276 neonates). In the sensitivity analysis, excluding patients with no SARS-CoV-2 testing before delivery, the adjusted association between SARS-CoV-2 infection at <28 weeks of gestation and PTB at <37 weeks of gestation was of similar magnitude but no longer statistically significant (aRR, 1.22; 95% CI, 0.96–1.55). The results were similar for perinatal death when missing BMI was imputed (aRR, 1.78; 95% CI, 0.92–3.45). For other outcomes, the results remained the same in both sensitivity analyses (eg, HDP delivered at <37 weeks of gestation (aRR, 1.66 [95% CI, 1.12–2.47]; aRR, 1.50 [95% CI, 1.02–2.20]) and preeclampsia with severe features (aRR, 1.07 [95% CI, 0.71–1.62]; aRR, 1.09 [95% CI, 0.74–1.62]).
Comment
Principal findings
We found that among pregnant patients who experience SARS-CoV-2 infection before 28 weeks of gestation and who had a subsequent delivery hospitalization, there was a modest increase in adverse pregnancy outcomes, after adjusting for potential confounding variables, compared with a random sampling of patients with delivery hospitalizations during March 2020 to December 2020. Using the comparison group of a random sampling of deliveries during the COVID-19 pandemic, we found an increased risk of PTB, perinatal death, and HDP with delivery at <37 weeks of gestation among those who had experienced COVID-19 early in pregnancy and then went on to have a subsequent delivery hospitalization. In addition, this study demonstrated that some adverse outcomes were not restricted to those individuals who had experienced severe COVID-19.
Results in the context of what is known
The mechanism for potential increased risk of adverse outcomes later in pregnancy is unknown. The median number of days from a SARS-CoV-2–positive test to fetal death was 21, suggesting that there could be a persistent risk to the fetus after maternal recovery. The fact that fetal deaths occurred in 3.1% of asymptomatic patients and 2.7% of those with severe illness is concerning that the causes of stillbirth may not be limited to severe maternal illness. There have been several reports of “placentitis” among patients experiencing SARS-CoV-2 infection during pregnancy, but no large prospective cohort included placental assessment to assess the association between placental histologic abnormalities and stillbirth on a population level.13, 14, 15 Our study did not identify an increased risk of SGA birth, which might be expected if placental damage were the cause of adverse outcomes. Because of the nature of this study, which involved a detailed clinical record review under a waiver of consent and no biologic sample, we were unable to assess placental pathologic findings in a standardized fashion.
Our findings were somewhat consistent with a recent electronic health record study examining the risk of adverse outcomes in pregnancy with timing of COVID-19 in pregnancy.16 The study found an increased risk of stillbirth and PTB among those infected in the first or second trimester of pregnancy. They did not find an increased risk of HDP.
Clinical implications
Although the frequency of PTB was also modestly increased in this study, we found that this increased risk was related largely to indicated PTBs rather than spontaneous PTBs. This pattern differed somewhat from the control group in which the spontaneous and indicated PTB risks were nearly evenly split. In addition, other studies have reported an increased risk of PTB in the setting of COVID-19, but it has been difficult to assess the timing from maternal illness to delivery.17 Several reports have suggested that severe maternal illness is associated with PTB at the time of delivery hospitalization.6 , 7 By evaluating patients experiencing SARS-CoV-2 before 28 weeks of gestation and before the delivery hospitalization, our report demonstrated that the PTBs are occurring at hospitalization after the time a maternal infection is diagnosed.
Research implications
These data were from before the Delta variant wave, and they are not known if infection with the Delta or later variants would have further increased the risk beyond what we have demonstrated. A recent CDC report suggested an increased risk of stillbirth among those with Delta variant at the time of delivery hospitalization.4 Moreover, this study reported an increased risk of stillbirth before Delta (aRR, 1.47; 95% CI, 1.27–1.71) but could not evaluate the timing of occurrence of stillbirth concerning maternal infection, as all data were collected at the delivery hospitalization. Another recent study examined the association between COVID-19 and adverse pregnancy outcomes in the United Kingdom and found an increased risk of subsequent development of preeclampsia compared with the expected rate in a dose-response relationship, with the risk increasing in more severe disease.8 In that study, individuals may have been included if their infection occurred during the delivery hospitalization. Our study is unique in that the risk of adverse outcomes was evaluated among patients who experienced infection before their delivery hospitalization.
Strengths and limitations
The strength of this study included the multicenter nature of the NICHD MFMU Network that allows for sampling of deliveries across the country and minimizes bias related to the effect that care delivery at a single institution could have on outcomes. The data were collected by certified abstractors, and the independent biostatistical coordinating center performed routine data quality assessments and corrections. The limitations of this study included the retrospective nature of the data collection and the lack of data from the Delta or Omicron waves of the pandemic. It is possible that the patients who were included in the group with a SARS-CoV-2–positive test were tested because they presented to the hospital for evaluation of a condition that may have increased their risk of stillbirth or PTB. However, as we only included individuals who were not delivering at the time of their positive test, this potential bias was mitigated. Because the study period occurred before the availability of vaccination, we were also unable to assess whether vaccination might decrease the occurrence of adverse pregnancy outcomes among people who experience COVID-19 after vaccination. In addition, because of the lack of a standardized approach to antenatal fetal monitoring across the sites, we were unable to assess whether patients had antenatal fetal monitoring after their acute infection or whether such an approach would alter outcomes. The findings of increased risk of fetal or neonatal death and PTB were no longer statistically significant on sensitivity analyses. This loss of significance may be due to the inadequate sample size when restricting the analysis to those in the control group with a negative test at the time of delivery admission, as the direction and magnitude of the findings were unchanged.
Conclusions
We found that SARS-CoV-2 infection early in pregnancy was associated with a modest increase in the risk of subsequent adverse pregnancy outcomes. This report further highlighted the importance of vaccination to prevent COVID-19 in pregnancy.
Acknowledgments
Study sites and personnel
The members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network that participated in this study are as follows:
Clinical centers
The main centers are listed below. Personnel at subsites have the subsite listed in parentheses.
-
•
University of Pittsburgh, Pittsburgh, PA: H. Simhan, M. Bickus, and F. Facco
-
•
University of Alabama at Birmingham, Birmingham, AL: A. Tita, J. Grant, A. Leath, S. Longo (Ochsner Health), M. Hendricks (Ochsner Health), and K. Arias (Ochsner Health)
-
•
The Ohio State University, Columbus, OH: M. Costantine, A. Bartholomew, M. Landon, K. Rood, P. Schneider, H. Frey, D. McKenna (Miami Valley Hospital), S. Wiegand (Miami Valley Hospital), E.K. Snow (Miami Valley Hospital), K. Fennig (Miami Valley Hospital), M. Habli (Good Samaritan Hospital), D. Lambers (Good Samaritan Hospital), and M. McClellan (Good Samaritan Hospital)
-
•
The University of Utah Health Sciences Center, Salt Lake City, UT: T. Metz, A. Sowles, A. Nelsen (Utah Valley), M. Varner, and M.S. Esplin (Intermountain Medical Center)
-
•
Brown University, Providence RI: D, Rouse D, Allard C. Pettker (Yale), J. Leventhal (Yale), J. Rousseau, J. Milano, and L. Early.
-
•
Columbia University, New York, NY: C. Gyamfi-Bannerman, S. Bousleiman, R. Wapner, D. Sutton, H. Manchon, M. Hoffman (Christiana Care), C. Kitto (Christiana Care), K. Palomares (Saint Peters University Hospital), I. Beche (Saint Peters University Hospital), D. Skupski (NewYork-Presbyterian Queens), and R. Chan-Akeley (NewYork-Presbyterian Queens).
-
•
University of Texas Medical Branch, Galveston, TX: G. Saade, A. Salazar, L. Pacheco, S. Clark, H. Harirah, S. Jain, G. Olson, A. Saad, M. McDonnold (St. David’s Women’s Center of Texas), C. Brown (St. David’s Women’s Center of Texas), L. Allen, G. Carrington, J. Cornwell, J. DeVolder, L. Thibodeaux, and E. Welch
-
•
MetroHealth Medical Center-Case Western Reserve University, Cleveland, OH: J. Bailit, W. Dalton, A. Tyhulski, and A. Mayle (University Hospitals)
-
•
University of Texas Health Science Center at Houston-Children’s Memorial Hermann Hospital, Houston, TX: S. Chauhan, H. Mendez-Figueroa, and F. Ortiz
-
•
University of North Carolina at Chapel Hill, Chapel Hill, NC: J. Thorp, T. Manuck, K. Clark, B. Hughes (Duke), S. Timlin, L. Fried, H. Byers, C. Beamon, MD (WakeMed Health and Hospitals), J. Ferrara (Duke), A. Williams, K. Eichelberger (Greenville), and A. Moore (Greenville)
-
•
Northwestern University, Chicago, IL: W. Grobman, G. Mallett, M. Ramos-Brinson, B. Plunkett (NorthShore University Evanston Hospital), K. Kearns (NorthShore University Evanston Hospital), A. Palatnik (Froedhert Hospital and Medical College of Wisconsin), and S. Northey (Froedert Hospital and Medical College of Wisconsin)
-
•
University of Pennsylvania, Philadelphia, PA: S. Parry, H. Sehdev, M. McCabe, C. Fazio, A. Filipczak, J. Craig, and L. Muzzarelli
Data coordinating center
-
•
The George Washington University Biostatistics Center, Washington, DC: R. Clifton, G. Sandoval, E. Thom, C. Nwachuku, and V. L. Flowers-Fanomezantsoa
National Institutes of Health
-
•
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD: M. Longo, M. Miodovnik, and S. Archer
-
•
Maternal-Fetal Medicine Units Network Steering Committee Chair: George A. Macones
Footnotes
A list of the full members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network is available in the Acknowledgments.
This study was supported by the Eunice Kennedy ShriverNational Institute of Child Health and Human Development (grant numbers UG1HD087230, UG1HD027869, UG1HD027915, UG1HD034208, UG1HD040500, UG1HD040485, UG1HD053097, UG1HD040544, UG1HD040545, UG1HD040560, UG1HD040512, UG1HD087192, and U10HD036801) and the National Center for Advancing Translational Sciences (grant number UL1TR001873).
The authors report no conflict of interest.
Deidentified data and a data dictionary will be available to others through the NICHD Data and Specimen Hub (DASH) within 1 year of publication. Access to data will follow requirements in place through DASH.
Cite this article as: Hughes BL, Sandoval GJ, Metz TD, et al. First- or second-trimester SARS-CoV-2 infection and subsequent pregnancy outcomes. Am J Obstet Gynecol 2023;228:226.e1-9.
Supplementary Data
Hughes. Early SARS-CoV-2 infection and subsequent pregnancy outcomes. Am J Obstet Gynecol 2022.
References
- 1.Delahoy M.J., Whitaker M., O’Halloran A., et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 - COVID-NET, 13 states, March 1-August 22, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1347–1354. doi: 10.15585/mmwr.mm6938e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouachba A., Allias F., Nadaud B., et al. Placental lesions and SARS-Cov-2 infection: diffuse placenta damage associated to poor fetal outcome. Placenta. 2021;112:97–104. doi: 10.1016/j.placenta.2021.07.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSisto C.L., Wallace B., Simeone R.M., et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1640–1645. doi: 10.15585/mmwr.mm7047e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conde-Agudelo A., Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226:68–89.e3. doi: 10.1016/j.ajog.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metz T.D., Clifton R.G., Hughes B.L., et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodworth K.R., Olsen E.O., Neelam V., et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai J., Romero R., Tarca A.L., et al. SARS-CoV-2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose-response relationship supporting causality. Am J Obstet Gynecol. 2021;225:689–693.e1. doi: 10.1016/j.ajog.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Society for Maternal-Fetal Medicine (SMFM) COVID Task Force COVID-19 and pregnancy: what maternal-fetal medicine subspecialists need to know. 2021. https://s3.amazonaws.com/cdn.smfm.org/media/3238/PDF.pdf Available at:
- 10.Metz T.D., Clifton R.G., Hughes B.L., et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327:748–759. doi: 10.1001/jama.2022.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duryea E.L., Hawkins J.S., McIntire D.D., Casey B.M., Leveno K.J. A revised birth weight reference for the United States. Obstet Gynecol. 2014;124:16–22. doi: 10.1097/AOG.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 12.ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:1. doi: 10.1097/AOG.0000000000003018. [DOI] [PubMed] [Google Scholar]
- 13.Roberts D.J., Edlow A.G., Romero R.J., et al. A standardized definition of placental infection by SARS-CoV-2, a consensus statement from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development SARS-CoV-2 Placental Infection Workshop. Am J Obstet Gynecol. 2021;225:593.e1–593.e9. doi: 10.1016/j.ajog.2021.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz D.A., Morotti D. Placental pathology of COVID-19 with and without fetal and neonatal infection: trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS-CoV-2. Viruses. 2020;12:1308. doi: 10.3390/v12111308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunnell M.E., Koenigs K.J., Roberts D.J., Quade B.J., Hornick J.L., Goldfarb I.T. Third trimester stillbirth during the first wave of the SARS-CoV-2 pandemic: similar rates with increase in placental vasculopathic pathology. Placenta. 2021;109:72–74. doi: 10.1016/j.placenta.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piekos S.N., Roper R.T., Hwang Y.M., et al. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: a retrospective multicentre cohort study. Lancet Digit Health. 2022;4:e95–e104. doi: 10.1016/S2589-7500(21)00250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jafari M., Pormohammad A., Sheikh Neshin S.A., et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: a systematic review and meta-analysis. Rev Med Virol. 2021;31:1–16. doi: 10.1002/rmv.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hughes. Early SARS-CoV-2 infection and subsequent pregnancy outcomes. Am J Obstet Gynecol 2022.