Abstract
The COVID-19 pandemic disrupted the regular injections of anti-vascular endothelial growth factor (anti-VEGF) in patients with various retinal diseases globally. It is unclear to what extent delayed anti-VEGF injections have worsened patients’ visual acuity. We performed a meta-analysis to assess the impact of delayed anti-VEGF injections on the best-corrected visual acuity (BCVA) in patients with neovascular age-related macular degeneration (nAMD), retinal vein occlusion (RVO), and diabetic macular edema (DME). We searched four computer databases (EMBASE, MEDLINE, Web of Science, Scopus) from inception to January 5, 2022. Data were pooled using the random-effects model. Results were reported by less than 4 months and 4 months or longer for the time period between the first injection during the pandemic and the last pre-pandemic injection. All BCVA measures were converted to the logarithm of the minimum angle of resolution (logMAR) for analyses. Among patients who received injections 4 months or longer apart, the mean difference in BCVA was 0.10 logMAR (or 5 ETDRS letters) (95% confidence interval [CI] 0.06∼0.14) for nAMD patients, 0.01 logMAR (or∼ 1 ETDRS letter) (95% CI -0.25∼0.27) for RVO patients, and 0.03 logMAR (or ∼1 ETDRS letters) (95% CI -0.06∼0.11) for DME patients. These results suggest that patients with nAMD needing scheduled anti-VEGF injections may require priority treatment over those with RVO and DME in the event of disturbed anti-VEGF injections from COVID-19 lockdowns or similar scenarios.
Keywords: Systematic review and meta-analysis, COVID-19, Neovascular age-related macular degeneration, Retinal vein occlusion, Diabetic macular edema
1. Introduction
The coronavirus disease 2019 (COVID-19) was declared a global pandemic in March, 2020.6 ,A As of February 1, 2022, the disease has affected over 370 million people and caused 5.6 million deaths worldwide.B To curb the viral transmission, large-scale public health policies and measures (e.g., limiting hospital activities, social distancing mandates and city wide lockdowns) were implemented in many countries. These measures, specifically lockdowns and decreased clinical activities, have significantly limited patients’ access to healthcare services, including eye care. As a result, both outpatient and emergency eye care service attendance have been reduced by as much as 50% or more at some time point during the pandemic.41
Neovascular age-related macular degeneration (nAMD), retinal vein occlusion (RVO), and diabetic macular edema (DME) are retinal disorders that are among the most common causes of vision loss and comprise a major healthcare burden.7 , 11 , 15 , 42 Anti-vascular endothelial growth factor (anti-VEGF) therapy is the standard of care for individuals with these conditions.39 Regular intravitreal injections of anti-VEGF, either based on treat-and-extend or as-needed regimens, are necessary for the greatest improvements in the visual acuity of these patients;3 however, with lockdowns, reduced hospital activities, and the unfeasibility of receiving anti-VEGF treatment by virtual care, the schedules of anti-VEGF treatment regimens for many patients have inevitably been delayed or canceled and are suspected to have severe consequences for vision outcomes among those with retinal diseases.22 Solid evidence is needed, however, to confirm this suspicion and assess whether patients with various retinal diseases are equally impacted. We hypothesized that vision loss would be greater among nAMD patients due to its more severe nature relative to RVO and DME. 39
Several individual studies have evaluated the impact of delays in anti-VEGF treatment due to the COVID-19 pandemic on visual acuity, but a meta-analysis to summarize quantitatively prior findings has not yet been conducted. We systematically reviewed and meta-analyzed studies that examined the impact of delayed anti-VEGF injections by the COVID-19 pandemic on the best-corrected visual acuity (BCVA) among patients with nAMD, RVO, or DME.
2. Methods
2.1. Quality appraisal and data extraction
The quality of studies included in this review was evaluated by 2 independent reviewers (J.H.B.I., R.C.) using the Joanna Briggs Institute (JBI) Checklist for Case Series.23 Studies were considered case series due to the nature of observations in the studies as well as lacking a distinct comparative arm (control group).21 Disagreements on study quality were resolved through discussions or consulting with a senior author (Y.P.J.) when consensus between the 2 reviewers could not be reached. For studies that were included after full-text screening, background data on studies (authors, publication year, study design), participant characteristics (sample size, mean age, percentage female), and outcome-related information (mean BCVA and standard deviations between pre-pandemic and during-pandemic, length of treatment delay, and patient condition) were extracted into a standardized Excel spreadsheet by two independent reviewers (J.H.B.I., R.C.). Study authors of extracted studies were contacted when more detailed data were required.
2.2. Data synthesis and analysis
All measures of BCVA were converted to logarithm of the minimum angle of resolution (logMAR) to calculate the mean BCVA and associated standard deviation for each individual study. Two studies presented median and range or interquartile range of BCVA.33 , 45 The mean BCVA and standard deviation were imputed for these 2 studies using the methods presented by Wan and coworkers and Weir and coworkers.38 , 40 Based on commonly agreed upon treatment regimens by retinal specialists for patients with nAMD, RVO or DME, we stratified patients by less than 4 months and 4 months or longer from the last regular pre-pandemic injection to the first subsequent injection during the pandemic. As most patients are treated in up to 12-week intervals using the treat-and-extend regimen, we chose a cut point of 4 months to identify patients whose treatment was significantly delayed.
The mean difference (MD) in BCVA between two consecutive injections (during-pandemic vs pre-pandemic) and corresponding 95% confidence intervals (CI) were calculated and presented. The mean differences were also presented in Early Treatment Diabetic Retinopathy Study (ETDRS) letters to assist comparisons.18 If the overall BCVA estimate from individual studies included patients with retinal diseases other than nAMD, RVO or DME, subgroup-specific BCVAs pertaining to nAMD, RVO or DME were used in the overall quantitative synthesis. Heterogeneity was assessed using the I2 statistic, with cut-off values of <25%, 25%-50%, and >50% corresponding to low, medium, and high levels of heterogeneity, respectively.14 As we expected population-based heterogeneity between studies, we used the random-effects model, rather than a fixed-effects model, to pool data.9 Weights given to each study were calculated using the inverse of the variance of the effect estimates (i.e., the inverse-variance method). Subgroup analyses were conducted by type of disease (nAMD, RVO, DME). Visual inspection of funnel plots and the Egger's test were used to assess publication bias. A P-value <0.05 was used as the cut-off for statistical significance. All analyses were performed using R, version 4.0.2 (R Foundation for Statistical Computing; Vienna, Austria).
3. Results
A total of 98 studies were identified through the database search. Two additional studies were found through forward and backward citation searching. After removing duplicates, 35 studies remained and underwent title and abstract screening. At this stage, 4 studies were deemed irrelevant and the full texts of 31 studies were collected and further examined. 18 studies met the inclusion criteria and were incorporated into the qualitative synthesis. Four of these 18 studies were excluded from the meta-analysis due to lack of sufficient information required to quantitatively pool the results.2 , 27 , 28 , 36 In the end, 14 studies (including 1,931 patients) were included in the quantitative synthesis.4 , 5 , 8 , 24 , 29, 30, 31 , 33, 34, 35 , 37 , 44, 45, 46 The PRISMA diagram detailing the study screening process is shown in Supplementary File, Figure S1.
3.1. Study characteristics
Study characteristics are displayed in Table 1 . All included studies were observational in nature. All studies measured changes in patients with nAMD while 10 studies measured changes in patient with DME5 , 8 , 24 , 27 , 28 , 29 , 33 , 34 , 36 , 44 and 9 studies measured changes in those with RVO.5 , 8 , 24 , 28 , 29 , 33 , 34 , 36 , 44 The mean length of time between the last pre-pandemic visit and the first visit during the pandemic was 4 months or greater for 8 studies.5 , 30 , 33 , 35 , 37 , 44 , 45 , 46 BCVA was measured using logMAR in 10 studies, ETDRS letters in seven studies, and decimal Snellen in one study. The majority of studies reported worsening of vision due to delays caused by the COVID-19 pandemic with the exception of patients with DME in studies by Bulut and coworkers, Sindal and coworkers, and Elfalah and coworkers,5 , 8 , 33 as well as patients with RVO in studies by Naravane and coworkers and Sindal and coworkers .24 , 33 These studies reported either no change or minor improvements in vision.
Table 1.
Characteristics of included studies.
| Study | Study Location | Study Design | Conditions | Sample Size* | Patient Characteristics | Mean Length Between Appointments | BCVA Measure | Pre-Pandemic BCVA | During-Pandemic BCVA | Change in BCVA | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Arruabarrena et al.2 | Europe | Retrospective observational study | nAMD | 546 patients (546 eyes) | Mean age: 79.4 years % female: 44.9% |
2.45 months | ETDRS letters | 59.02 | 55.61 | -3.41 |
| 2 | Borrelli et al.4 | Italy | Case series | nAMD | 100 patients (112 eyes) | Mean age: 79.1 years % female: 49.0% |
110.7 days | logMAR | 0.45 | 0.50 | 0.05 |
| 3 | Bulut et al.5 | Turkey | Retrospective observational study | nAMD, DME, RVO | 46 patients (55 eyes) | Mean age: 61.0 years % female: 47.8% |
5 months | logMAR | 0.72 (all) 1.11 (nAMD) 0.60 (DME) 0.85 (RVO) |
0.76 (all) 1.27 (nAMD) 0.58 (DME) 1.02 (RVO) |
0.04 (all) 0.16 (nAMD) -0.02 (DME) 0.17 (RVO) |
| 4 | Elfalah et al.8 | Jordan | Retrospective observational study | nAMD, DME, RVO | 145 eyes | Mean age: 64.8 years % female: 45.6% |
60.97 days | Snellen (decimal) | NA | NA | -0.04 (nAMD) 0.02 (DME) -0.03 (BRVO) -0.09 (CRVO) |
| 5 | Naravane et al.24 | USA | Retrospective review | nAMD, DME, RVO | 57 patients (77 eyes) | Mean age: 73 years % female: 61.4% |
>2 weeks† | logMAR | 0.63 (all) 0.73 (nAMD) 0.54 (DME) 0.56 (RVO) |
0.77 (all) 0.85 (nAMD) 0.72 (DME) 0.55 (RVO) |
0.14 (all) 0.12 (nAMD) 0.18 (DME) -0.01 (RVO) |
| 6 | Rush et al.28 | USA | Case series | nAMD, DME, RVO | 129 patients (129 eyes) | Mean age: 73.4 years % female: 57.4% |
11.8 weeks | logMAR | 0.38 (all) 0.44 (nAMD) 0.34 (DME) 0.31 (RVO) |
0.65 (all) 0.75 (nAMD) 0.53 (DME) 0.63 (RVO) |
0.27 (all) 0.31 (nAMD) 0.19 (DME) 0.32 (RVO) |
| 7 | Rahimzadeh et al.27 | United Kingdom | Retrospective observational study | nAMD, DME | 80 patients (86 eyes) | Mean age: NA % female: NA |
>47 days |
ETDRS letters | NA | NA | 1.67 (nAMD) 1.63 (DME) |
| 8 | Saleh et al.29 | Jordan | Retrospective observational study | nAMD, DME, RVO | 119 patients | Mean age: 59.9 years % female: 46.3% |
6.2 weeks | logMAR | 0.48 (nAMD) 0.46 (DME) 0.44 (RVO) |
0.66 (nAMD) 0.58 (DME) 0.6 (RVO) |
0.18 (nAMD) 0.12 (DME) 0.16 (RVO) |
| 9 | Sekeroglu et al.30 | Turkey | Retrospective observational study | nAMD | 140 patients (140 eyes) | Mean age: 72.0 years % female: 55.7% |
29.4 weeks | ETDRS letters | 50.2 | 38.8 | -11.4 |
| 10 | Sevik et al.31‡ | Turkey | Retrospective observational study | nAMD | 31 patients (33 eyes) | Mean age: 70.7 years % female: 53.7% |
>10 weeks | logMAR | 0.52 | 0.70 | 0.08 |
| 11 | Sindal et al.33‡ | India | Retrospective observational study | nAMD, DME, RVO | 131 eyes | Mean age: 58.3 years % female: 30.4% |
>15.8 weeks | logMAR | 0.4 (nAMD) 0.3 (DME 0.5 (RVO) |
0.5 (nAMD) 0.3 (DME) 0.5 (RVO) |
0.1 (nAMD) 0.0 (DME) 0.0 (RVO) |
| 12 | Song et al.34 | USA | Retrospective chart review | nAMD, DME, RVO | 421 patients | Mean age: 77.5 years % female: 59.9% |
11.95 weeks | ETDRS letters | NA | NA | -1.23 (nAMD) -3.48 (DME) -3.22 (RVO) |
| 13 | Stattin et al.35 | Austria | Retrospective observational study | nAMD | 142 patients (142 eyes) | Mean age: 78.1 years % female: 56.3% |
120 days | ETDRS letters | 70 | 67.8 | -2.3 |
| 14 | Stone et al.36 | United Kingdom | Retrospective observational study | nAMD, DME, RVO | 261 patients (298 eyes) | Mean age: 78.8 years % female: 57.6% |
13.1 weeks | ETDRS letters | 60.4 (all) 60.1 (nAMD) 63.0 (DME) 59.7 (RVO) |
55.7 (all) 55.2 (nAMD) 61.1 (DME) 54.5 (RVO) |
-4.7 (all) -4.9 (nAMD) -1.9 (DME) -5.2 (RVO) |
| 15 | Valverde-Megias et al.37 | Spain | Case series | nAMD | 242 patients (270 eyes) | Mean age: 82.8 years % female: NA |
184.2 days | ETDRS letters | 60.2 | 55.9 | -4.3 |
| 16 | Yang et al.44 | China | Retrospective chart review | nAMD, DME, RVO | 46 patients (59 eyes) | Mean age: 62.4 years % female: 45.8% |
5.3 months | logMAR | 0.57 | 0.98 | 0.41 |
| 17 | Yeter et al.45c | Turkey | Retrospective observational study | nAMD | 106 patients (116 eyes) | Mean age: 73.2 years % female: 51.9% |
5.08 months | logMAR | 0.67 | 0.78 | 0.11 |
| 18 | Zhao et al.46 | China | Retrospective observational study | nAMD | 82 patients (96 eyes) | Mean age: 74.4 years % female: 51.2% |
4.37 months | logMAR | 0.80 | 0.95 | 0.15 |
Where possible, sample size only includes delayed patients
Authors provided length of delay rather than length of time between two visits
BCVA measurements provided in medians
3.2. Methodological quality
Most studies were of fairly high quality, although none satisfied all measured domains of the quality assessment tool (Supplementary File, Table S1). In particular, answers were “No” for all 18 studies for the question, “Was there clear reporting of the presenting site(s) and/or clinic(s) demographic information?” Answers were “Unclear” or “No” in 7 of 18 studies for questions related to consecutive and complete inclusion of participants. Other problematic domains included lack of clear reporting of outcomes (n = 8).
3.3. Meta-analysis of change in BCVA for the 3 conditions combined
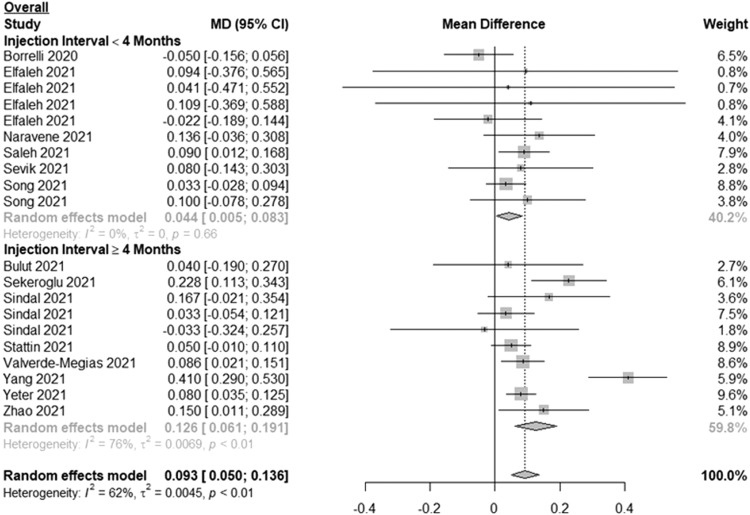
For the overall change in BCVA, 6 studies included patients with less than 4 months between injections and 8 studies included patients with 4 months or longer between injections (Fig. 1 ). Among patients with less than 4 months between injections, the MD in BCVA between 2 subsequent visits (pre-pandemic and during-pandemic) was 0.044 logMAR (or ∼2 ETDRS letters) (95% CI: 0.005, 0.083; I2=0%). Among patients with 4 months or longer between injections, the MD in BCVA was 0.126 logMAR (or ∼6 ETDRS letters) (95% CI: 0.061, 0.191; I2=76%). Overall, the MD in BCVA among all patients was 0.093 logMAR (or ∼5 ETDRS letters) (95% CI: 0.050, 0.136; I2=62%). Visual inspection of the funnel plot for overall change in BCVA (Supplementary File, Figure S2) as well as Egger's test (P = 0.5182) showed no evidence of publication bias.
Fig. 1.
Forest plot of mean difference in the best-corrected visual acuity between the first visit during the pandemic and the last pre-pandemic visit among patients with neovascular age-related macular degeneration, retinal vein occlusion and diabetic macular edema stratified by length of time between injections.
3.4. Subgroup analyses by condition
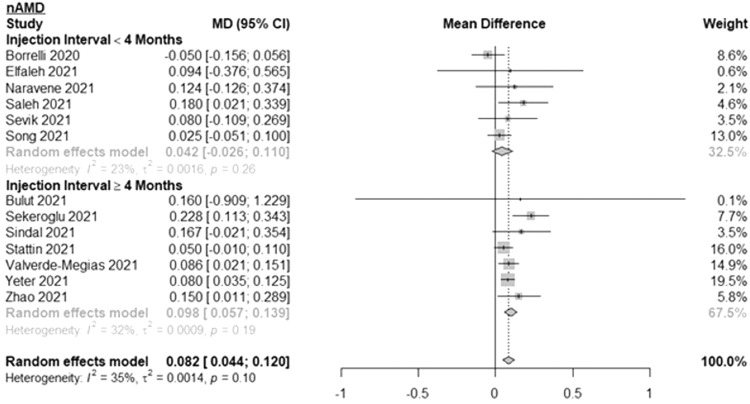
Results of subgroup analyses of nAMD patients are shown in Fig. 2 . The MD in BCVA between 2 subsequent visits was 0.042 logMAR (or ∼2 ETDRS letters) (95% CI: -0.026, 0.110; I2=23%) for nAMD patients with less than 4 months between injections and 0.098 logMAR (or ∼5 ETDRS letters) (95% CI: 0.057, 0.139; I2=32%) for nAMD patients with 4 months or longer between injections. Overall, the MD in BCVA among all nAMD patients was 0.082 logMAR (or ∼4 ETDRS letters) (95% CI: 0.044, 0.120; I2=35%). The mean time between subsequent injections was 5.1 months for the 4 months or longer group and 2.4 months for the less than 4 months group. The number of patients ranged from 7 to 270 for the 4 months or longer group and 22 to 264 for the less than 4 months group.
Fig. 2.
Forest plot of mean difference in the best-corrected visual acuity between the first visit during the pandemic and the last pre-pandemic visit among patients with neovascular age-related macular degeneration stratified by length of time between injections.
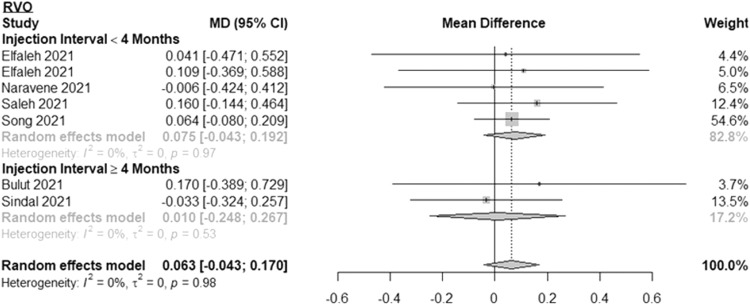
Results of subgroup analyses of RVO patients are shown in Fig. 3 . The MD in BCVA between 2 subsequent pre-pandemic and during-pandemic visits was 0.075 logMAR (or ∼4 ETDRS letters) (95% CI: -0.043, 0.192; I2=0%) for RVO patients with less than 4 months between injections and 0.010 logMAR (or <1 ETDRS letter) (95% CI: -0.248, 0.267; I2=0%) for RVO patients with 4 months or longer between injections. Overall, the MD in BCVA among all RVO was 0.063 logMAR (or ∼3 ETDRS letters) (95% CI: -0.043, 0.170; I2=0%). The mean time between subsequent injections was 4.8 months for the 4 months or longer group and 2.1 months for the less than 4 months group. The number of patients ranged from 11 to 25 for the 4 months or longer group and 4 to 89 for the less than 4 months group.
Fig. 3.
Forest plot of mean difference in the best-corrected visual acuity between the first visit during the pandemic and the last pre-pandemic visit among patients with retinal vein occlusion stratified by length of time between injections.
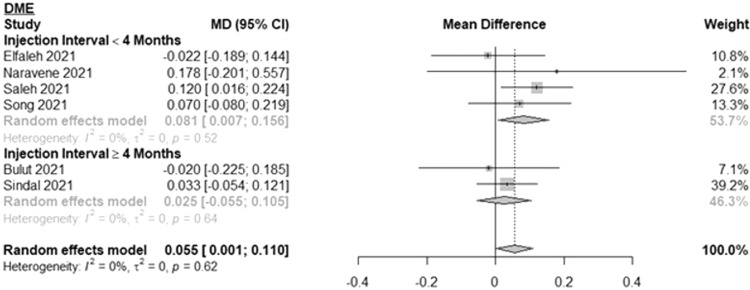
Fig. 4 shows the results for DME patients. The MD in BCVA between 2 subsequent pre-pandemic and during-pandemic visits was 0.081 logMAR (or ∼4 ETDRS letters) (95% CI: 0.007, 0.156; I2=0%) for DME patients with less than 4 months between injections and 0.025 logMAR (or ∼1 ETDRS letter) (95% CI: -0.055, 0.105; I2=0%) for DME patients with 4 months or longer between injections. Overall, the MD among all DME patients was 0.055 logMAR (or ∼3 ETDRS letters) (95% CI: 0.001, 0.110; I2=0%). The mean time between subsequent injections was 4.8 months for the 4 months or longer group and 2.1 months for the less than 4 months group. The number of patients ranged from 37 to 72 for the 4 months or longer group and 34 to 109 for the less than 4 months group.
Fig. 4.
Forest plot of mean difference in the best-corrected visual acuity between the first visit during the pandemic and the last pre-pandemic visit among patients with diabetic macular edema stratified by length of time between injections.
4. Discussion
To our knowledge, this is the first meta-analysis that has investigated the impact of delayed intravitreal anti-VEGF injections due to the COVID-19 pandemic on patients with 3 common retinal diseases. We found that nAMD patients with 4 months or longer between subsequent injections (mean 5.1 months) experienced significant worsening of vision (0.098 logMAR [95% CI: 0.057, 0.139], about 5 ETDRS letters or 1 line of vision deterioration). Although the BCVA of DME patients with an injection interval less than 4 months seems to have worsened statistically speaking (0.081 logMAR [95% CI: 0.007, 0.156]), the marginally significant MD raises caution when interpreting it. There was no significant evidence of worsening of vision for patients with RVO, regardless of length of time between injections. nAMD is a leading cause of severe and irreversible vision loss and blindness among older adults.26 Regular anti-VEGF intravitreal injections at scheduled intervals have demonstrated superior efficacy in reducing chances of blindness and vision loss associated with nAMD.1 Consequently, it is believed that delays in injections may result in significant, permanent vision loss.10 Regularly scheduled anti-VEGF intravitreal injections are also necessary for patients with RVO and DME, but studies suggest that patients with RVO or DME seem less susceptible to short-term vision loss as a result of delayed injections.19 , 43 Reports suggest that vision loss associated with RVO and DME progresses more gradually than nAMD.39 Our results corroborated these observations. We showed patients with nAMD sustained greater vision loss than patients with DME or RVO due to delayed injections during the pandemic. Alternatively, the lack of vision loss seen in RVO and DME patients in this meta-analysis may suggest that the decisions of physicians and patients with RVO or DME during the COVID-19 pandemic to delay treatment were made appropriately. This delayed care did not lead to harm to the patients.
One lingering question surrounding the COVID-19 pandemic is the long-term implications of delayed visits. Among patients who experienced vision loss, Rush and coworkers found that the visual acuity of patients with nAMD, RVO or DME who were delayed for 10 or more weeks did not recover to baseline levels after 6 months of retreatment.28 Similarly, Greenlee and coworkers found that patients with nAMD who delayed their anti-VEGF treatment by 3 months or more lost a full line of vision that did not recover even after 12 months of retreatment.13 Findings from these studies suggest that many patients with lengthy delays in injections may suffer from permanent vision loss; however, as the overall magnitude of vision loss reported in this study was an approximate 1-line drop in Snellen acuity, in conjunction with a mean time between subsequent injections of 5.1 months for nAMD patients in the 4 months or longer group, the potential cannot be ruled out that some nAMD patients may be able to regain some visual acuity after retreatment. Further empirical studies quantifying the long-term vision consequences associated with pandemic-related delays would be beneficial for understanding the impact of the COVID-19 pandemic on patients with nAMD, RVO or DME.
Various studies have investigated the specific barriers encountered by patients during the COVID-19 pandemic. Even with precautionary measures implemented to reduce the risk of COVID-19 transmission, outpatient clinics have seen decreased patient attendance rates.12 , 25 In a study by Fung and coworkers, 85% of patients with retinal diseases who did not attend their appointments reported fear of COVID-19 infection as the primary reason for non-attendance.12 About 62% of surveyed patients in the same study indicated that they were unaware of the adaptations made by the clinic and would benefit from better and clearer communications.12 Similarly, Shields and coworkers and Lindeke-Myers and coworkers also found that fear of COVID-19 infection was the largest barrier to seeking care among patients with retinal diseases and that appointment reminders and clearer communications would be most helpful in improving clinic attendance.20 , 32 In our own retinal clinic, some patients returned for treatment only when they noticed a significant worsening of their vision. Health administrators and clinics should therefore aim to address patients’ fears and concerns regarding COVID-19 infection, the health and safety measures adapted by the clinics and hospitals, as well as the necessity of regular injections to maintain their vision, even when they do not see a decrease in their visual acuity.
One limitation of this review is that the number of studies and number of patients examining RVO or DME was smaller than those investigating nAMD. Subgroup analyses of patients with RVO or DME are thus likely to be more imprecise. This was reflected by their wider confidence intervals. Results for these subgroups should therefore be interpreted with caution. Secondly, while we were able to obtain missing or required information from most study authors, we imputed mean BCVAs and standard deviations for 2 studies that therefore may not represent the true values. In 4 other studies, we could not reach the study authors and these studies had to be excluded. Thirdly, the individual studies included in our meta-analysis did not provide the distributions of changes in visual acuity. Therefore, we are unable to assess whether their reported visual acuity changes were driven by a small subset of patients. Lastly, as COVID-related literature is still rapidly evolving, larger studies may be published in the future that could be used to update the results reported. Study strengths include a timely meta-analysis that examines the collateral impact of the COVID-19 pandemic on the visual acuity of patients with 3 common retinal diseases. Furthermore, methodological rigor in this study was promoted through the utilization of two independent reviewers at each stage of this review.
In conclusion, patients with nAMD with an anti-VEGF injection interval 4 months or longer during the pandemic experienced significant, likely irreversible deterioration of their vision. Compared to patients with nAMD, vision loss in patients with RVO or DME seems to be less sensitive to short-term delayed injections. Emerging anti-VEGF therapies such as faricimab and the Port Delivery System may help decrease treatment burden by extending treatment duration for patients with retinal diseases. This will mitigate the potential impacts of short-term interruptions in care.16 , 17 In the absence of these, ophthalmologists may consider prioritizing the treatment of nAMD patients in the event of future emergencies and lockdowns.
5. Literature Search
We systematic searched four computer databases (EMBASE, MEDLINE, Web of Science, Scopus) to identify articles related to changes in BCVA due to pandemic-related delays in anti-VEGF treatment among patients with nAMD, RVO or DME. Databases were initially searched on July 26, 2021 and an updated search was performed on January 5, 2022. All English-language articles published from inception until January 5, 2022 were included for screening. Reference lists of extracted articles were also manually searched to identify additional relevant studies. Key search terms included “wet macular degeneration,” “retinal vein occlusion,” “diabetic macular edema,” “coronavirus disease 2019,” “delay,” and “anti-VEGF.” The search strategies for each database are included in Supplementary File, Appendix S1.
5.1. Study Screening
Titles and abstracts of extracted studies were initially screened to identify potentially relevant studies that reported on changes in BCVA due to pandemic-related treatment delays among patients with either nAMD, RVO or DME. Information from full-text articles was then used to confirm screening results and determine if a study was included or excluded. Studies were excluded if: (a) they reported on diseases other than nAMD, RVO, and DME (with or without proliferative diabetic retinopathy); (b) full-length texts were not available; (c) pre-pandemic and during-pandemic BCVA and related dispersion information (range, interquartile range or standard deviation) were not reported or (d) they did not pertain to the COVID-19 pandemic. All studies were screened by two independent reviewers (J.H.B.I., R.C.) and any disagreements were resolved through discussions. Where consensus on the inclusion or exclusion of an article could not be reached, a senior author (P.Y.) was consulted.
Disclosures
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Other Cited Material
AWorld Health Organization. Listing of WHO's response to COVID-19. https://www.who.int/news/item/29-06-2020-covidtimeline. Accessed February 1, 2022.
BWorld Health Organization. Weekly epidemiological update on COVID-19 - 1 February 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—1-february-2022. Accessed February 1, 2022.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.survophthal.2022.08.002.
Appendix. Supplementary materials
References
- 1.Adrean SD, Chaili S, Ramkumar H, et al. Consistent Long-Term Therapy of Neovascular Age-Related Macular Degeneration Managed by 50 or More Anti-VEGF Injections Using a Treat-Extend-Stop Protocol. Ophthalmology. 2018;125:1047–1053. doi: 10.1016/j.ophtha.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Arruabarrena C, Toro MD, Onen M, et al. Impact on Visual Acuity in Neovascular Age Related Macular Degeneration (nAMD) in Europe Due to COVID-19 Pandemic Lockdown. J Clin Med. 2021;10:3281. doi: 10.3390/jcm10153281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augsburger M, Sarra G-M, Imesch P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol. 2019;257:1889–1895. doi: 10.1007/s00417-019-04404-0. [DOI] [PubMed] [Google Scholar]
- 4.Borrelli E, Grosso D, Vella G, et al. Short-term outcomes of patients with neovascular exudative AMD: the effect of COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258:2621–2628. doi: 10.1007/s00417-020-04955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulut MN, Sönmez HS, Gökçe G, et al. The impact of delayed anti-vascular endothelial growth factor treatment for retinal diseases during the COVID-19 lockdown. Photodiagnosis Photodyn Ther. 2021;35 doi: 10.1016/j.pdpdt.2021.102449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciotti M, Ciccozzi M, Terrinoni A, et al. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57:365–388. doi: 10.1080/10408363.2020.1783198. [DOI] [PubMed] [Google Scholar]
- 7.Ciulla TA, Amador AG, Zinman B. Diabetic Retinopathy and Diabetic Macular Edema. Diabetes Care. 2003;26:2653–2664. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- 8.Elfalah M, AlRyalat SA, Toro MD, et al. Delayed Intravitreal Anti-VEGF Therapy for Patients During the COVID-19 Lockdown: An Ethical Endeavor. Clin Ophthalmol. 2021;15:661–669. doi: 10.2147/OPTH.S289068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Field AP, Gillett R. How to do a meta-analysis. Br J Math Stat Psychol. 2010;63:665–694. doi: 10.1348/000711010X502733. [DOI] [PubMed] [Google Scholar]
- 10.Finger RP, Daien V, Eldem BM, et al. Anti-vascular endothelial growth factor in neovascular age-related macular degeneration – a systematic review of the impact of anti-VEGF on patient outcomes and healthcare systems. BMC Ophthalmol. 2020;20:294. doi: 10.1186/s12886-020-01554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freund KB, Korobelnik J-F, Devenyi R, et al. Treat-and-extend regimens with anti-vegf agents in retinal diseases: a literature review and Consensus Recommendations. Retina. 2015;35:1489–1506. doi: 10.1097/IAE.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 12.Fung THM, Kuet M-L, Patel MK, Puri P. Addressing COVID-19 fear to improve clinic attendance for patients with wet age-related macular degeneration. Acta Ophthalmol. 2021;99:e285. doi: 10.1111/aos.14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenlee TE, Wang VY, Kang H, et al. Consequences of lapses in treatment with vascular endothelial growth factor inhibitors in neovascular age-related macular degeneration in routine clinical practice. Retina. 2021;41:581–587. doi: 10.1097/IAE.0000000000002888. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jager RD, Mieler WF, Miller JW. Age-Related Macular Degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 16.Khanani AM, Aziz AA, Weng CY, et al. Port delivery system: a novel drug delivery platform to treat retinal diseases. Expert Opin Drug Deliv. 2021;18:1571–1576. doi: 10.1080/17425247.2021.1968826. [DOI] [PubMed] [Google Scholar]
- 17.Khanani AM, Patel SS, Ferrone PJ, et al. Efficacy of Every Four Monthly and Quarterly Dosing of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: The STAIRWAY Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2020;138:964–972. doi: 10.1001/jamaophthalmol.2020.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoshnood B, Mesbah M, Jeanbat V, Lafuma A, Berdeaux G. Transforming scales of measurement of visual acuity at the group level. Ophthalmic Physiol Opt. 2010;30:816–823. doi: 10.1111/j.1475-1313.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- 19.Korobelnik J-F, Loewenstein A, Eldem B, et al. Guidance for anti-VEGF intravitreal injections during the COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258:1149–1156. doi: 10.1007/s00417-020-04703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindeke-Myers A, Zhao PYC, Meyer BI, et al. Patient Perceptions of SARS-CoV-2 Exposure Risk and Association With Continuity of Ophthalmic Care. JAMA Ophthalmol. 2021;139:508–515. doi: 10.1001/jamaophthalmol.2021.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathes T, Pieper D. Clarifying the distinction between case series and cohort studies in systematic reviews of comparative studies: potential impact on body of evidence and workload. BMC Med Res Methodol. 2017;17:107. doi: 10.1186/s12874-017-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muether PS, Hermann MM, Koch K, Fauser S. Delay between medical indication to anti-VEGF treatment in age-related macular degeneration can result in a loss of visual acuity. Graefes Arch Clin Exp Ophthalmol. 2011;249:633–637. doi: 10.1007/s00417-010-1520-9. [DOI] [PubMed] [Google Scholar]
- 23.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18:2127–2133. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 24.Naravane AV, Mundae R, Zhou Y, et al. Short term visual and structural outcomes of anti-vascular endothelial growth factor (anti-VEGF) treatment delay during the first COVID-19 wave: A pilot study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellegrini M, Roda M, Lupardi E, et al. The impact of COVID-19 pandemic on ophthalmological emergency department visits. Acta Ophthalmol. 2020;98:e1058–e1059. doi: 10.1111/aos.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelletier AL, Rojas-Roldan L, Coffin J. Vision Loss in Older Adults. Am Fam Physician. 2016;94:219–226. [PubMed] [Google Scholar]
- 27.Rahimzadeh M, Muniraju R, Izadi S. Effect of COVID-19 Pandemic on Anti-VEGF Treatment of Medical Retinal Conditions. The Physician. 2020;6:1–9. [Google Scholar]
- 28.Rush RB, Rush SW. Outcomes in Patients Resuming Intravitreal Anti-Vascular Endothelial Growth Factory Therapy Following Treatment Delay During the Coronavirus-19 Pandemic. Retina. 2021;41:2456–2461. doi: 10.1097/IAE.0000000000003276. [DOI] [PubMed] [Google Scholar]
- 29.Saleh OA, Jammal H, Alqudah N, et al. Clinical Experience in the Administration of Intravitreal Injection Therapy at a Tertiary University Hospital in Jordan During the COVID-19 Lockdown. Clin Ophthalmol. 2020;14:2473–2480. doi: 10.2147/OPTH.S269179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekeroglu MA, Hekimsoy HK, Ceran TH, Doguizi S. Treatment of neovascular age related macular degeneration during COVID-19 pandemic: The short term consequences of unintended lapses. Eur J Ophthalmol. 2021;32(2):1064–1072. doi: 10.1177/11206721211010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sevik MO, Aykut A, Özkan G, Dericioğlu V, Şahin Ö. The effect of COVID-19 pandemic restrictions on neovascular AMD patients treated with treat-and-extend protocol. Int Ophthalmol. 2021;41:2951–2961. doi: 10.1007/s10792-021-01854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shields CN, Cherkas EG, Mokhashi N, et al. Barriers to Follow-Up Retinal Care During the COVID-19 Pandemic: A Survey Study. Ophthalmic Surg Lasers Imaging Retina. 2021;52:526–533. doi: 10.3928/23258160-20210904-01. [DOI] [PubMed] [Google Scholar]
- 33.Sindal MD, Chhabra K, Khanna V. Profile of patients receiving intravitreal anti-vascular endothelial growth factor injections during COVID-19-related lockdown. Indian J Ophthalmol. 2021;69:730–733. doi: 10.4103/ijo.IJO_2807_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song W, Singh RP, Rachitskaya AV. The Effect of Delay in Care among Patients Requiring Intravitreal Injections. Ophthalmol Retina. 2021;5:975–980. doi: 10.1016/j.oret.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Stattin M, Haas A-M, Ahmed D, et al. Evaluation of a calculation model to estimate the impact of the COVID-19 pandemic lockdown on visual acuity in neovascular AMD. Eur J Ophthalmol. 2022;32(4):2312–2318. doi: 10.1177/11206721211052389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stone LG, Grinton ME, Talks JS. Delayed follow-up of medical retina patients due to COVID-19: impact on disease activity and visual acuity. Graefes Arch Clin Exp Ophthalmol. 2021;259:1773–1780. doi: 10.1007/s00417-021-05174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valverde-Megías A, Rego-Lorca D, Fernández-Vigo JI, et al. Effect of COVID-19 Lockdown in Spain on Structural and Functional Outcomes of Neovascular AMD Patients. J Clin Med. 2021;10:3551. doi: 10.3390/jcm10163551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wecker T, Ehlken C, Bühler A, et al. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol. 2016;101:353–359. doi: 10.1136/bjophthalmol-2016-308668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weir CJ, Butcher I, Assi V, et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol. 2018;18:25. doi: 10.1186/s12874-018-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wickham L, Hay G, Hamilton R, et al. The impact of COVID policies on acute ophthalmology services—experiences from Moorfields Eye Hospital NHS Foundation Trust. Eye (Lond) 2020;34:1189–1192. doi: 10.1038/s41433-020-0957-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong TY, Scott IU. Retinal-Vein Occlusion. N Engl J Med. 2010;363:2135–2144. doi: 10.1056/NEJMcp1003934. [DOI] [PubMed] [Google Scholar]
- 43.Yalamanchili SP, Maatouk CM, Enwere DU, et al. The Short-term Effect of a Single Lapse in Anti–Vascular Endothelial Growth Factor Treatment for Diabetic Macular Edema Within Routine Clinical Practice. Am J Ophthalmol. 2020;219:215–221. doi: 10.1016/j.ajo.2020.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang K-B, Feng H, Zhang H. Effects of the COVID-19 Pandemic on Anti-vascular Endothelial Growth Factor Treatment in China. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.576275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeter DY, Dursun D, Bozali E, Ozec AV, Erdogan H. Effects of the COVID-19 pandemic on neovascular age-related macular degeneration and response to delayed Anti-VEGF treatment. J Fr Ophtalmol. 2021;44:299–306. doi: 10.1016/j.jfo.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao X, Meng L, Luo M, et al. The influence of delayed treatment due to COVID-19 on patients with neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Ther Adv Chronic Dis. 2021;12 doi: 10.1177/20406223211026389. 20406223211026389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.