Abstract
Background
Respiratory syncytial virus (RSV) surveillance is heavily dependent on the influenza-like illness (ILI) case definition from the World Health Organization (WHO). Because ILI includes fever in its syndromic case definition, its ability to accurately identify acute respiratory tract infections (ARTI) caused by RSV in older adults is uncertain.
Methods
The accuracy of the WHO ILI and a modified ILI (requiring only self-reported fever) case definitions in identifying patients with PCR-confirmed RSV-ARTI was evaluated in community-dwelling older adults (≥60 years) from the prospective European RESCEU cohort study.
Results
Among 1040 participants, 750 ARTI episodes were analyzed including 36 confirmed RSV-ARTI. Due to a general lack of fever, sensitivity for RSV-ARTI was 33% for modified ILI and 11% for ILI. The area under the curve for both ILI definitions was 0.52 indicating poor discrimination for RSV. RSV-ARTI could not be distinguished from all other ARTI based on clinical symptoms.
Conclusions
The use of ILI underestimated the occurrence of RSV-ARTI in community-dwelling older adults up to 9-fold (11% sensitivity). Because worldwide RSV surveillance depends largely on ILI, there is an urgent need for a better approach to measure the occurrence of RSV disease and the impact of future RSV vaccine introduction.
Clinical Trials Registration. NCT03621930.
Keywords: case definition, ILI, older adults, RESCEU, respiratory syncytial virus, RSV
Acute respiratory tract infections (ARTI) are the leading cause of disease worldwide with an estimated incidence of 17.2 billion upper ARTI annually [1]. Lower respiratory tract infections are estimated to be responsible for 4.4% of all deaths worldwide in people of all ages, with higher rates in both ends of the age spectrum [2]. Amongst others, respiratory syncytial virus (RSV) is responsible for part of this worldwide burden [3, 4]. Respiratory surveillance programs provide information for public health authorities, which is used to minimize the impact of the disease by planning appropriate control and intervention measures and allocate health resources. The World Health Organization (WHO) collects worldwide data about ARTI epidemiology by using standardized case definitions [5, 6]. Influenza-like illness (ILI) includes acute respiratory infection (ARI) with measured fever (≥38°C) and cough and is commonly used as a case definition for respiratory infection. Case definitions that include fever such as ILI and severe ARI show a wide range of sensitivities (24%–86%) in identifying confirmed RSV ARTI [7–11]. This wide range in sensitivity displays the high dependency on the studied population (children, adults, older adults) and clinical setting (community, outpatient, inpatient). Unfortunately, older adults living in the community were not well represented in these studies. Increasing age can alter symptomatology, which can result in a more atypical disease presentation in older adults compared to younger adults or children [12, 13]. Because RSV is known to cause an appreciable disease burden in the elderly population [14], we aimed to validate the performance of the WHO ILI case definition in identifying confirmed RSV ARTI in a population of community-dwelling older adults.
METHODS
Study Design
Participants from the RESCEU older adult study were studied. The design and data collection of the RESCEU older adult study has been described previously [14]. In summary, the RESCEU older adult study is a European multicentre, prospective, observational cohort study in community-dwelling adults aged 60 years or older. Before the start of the 2017–2018 and 2018–2019 RSV season (1 October–1 May), 1040 participants were recruited at 17 general practices in the Netherlands (Utrecht), Belgium (Antwerp), and the United Kingdom (Oxford). Participants with comorbidity were included as long as this was not life-threatening, caused immunodeficiency, or would hinder in completing the study procedures. More detailed information about the RESCEU older adult study and the complete inclusion and exclusion criteria can be found at Clinicaltrials.gov, identifier: NCT03621930.
This study was approved by the Ethical Review Authority in Belgium (reference No. B300201732907), The Netherlands (reference No. NL60910.041.17), and United Kingdom (Ethics reference 17/LO/1210, IRAS Ref: 224156). Participants gave informed consent before taking part in this study. The study was conducted according to the Declaration of Helsinki, as revised in 2013.
Study Procedures
During the RSV season, participants were followed up weekly by email or telephone by the local study teams. Home visits for viral testing were scheduled within 72 hours if 1 or more of the following ARTI symptoms were present for at least 1 day: nasal congestion or discharge, cough, wheezing, or shortness of breath. Nasopharyngeal flocked swabs collected during the home visit were tested for RSV and influenza with a molecular point-of-care test (POCT; Xpert Xpress Flu/RSV assay, Cepheid, Sunnyvale, CA, USA) [15]. All respiratory samples were validated for RSV after the study by in-house quantitative polymerase chain reaction (qPCR). During the home visit, vital signs, including temperature, saturation, heart rate, and respiratory rate, were measured by the local study teams. All participants were instructed to complete a daily log to score the presence and severity of various symptoms for as long as symptoms were present, for a maximum of 28 days (Supplementary Material). Participants had to indicate daily whether they measured their temperature or felt feverish, but they were not obliged to measure temperature daily. Participants from the RESCEU older adult study with an ARTI that was tested for RSV with POCT and/or qPCR and who completed a symptom diary during their illness were included in the current study.
Definitions
We tested the official ILI case definition from the WHO [5]. We also used a less stringent ILI-definition (modified ILI) based on the feeling of being feverish because participants were not obliged to measured temperature in our study (Box 1). Fever was defined as a recorded body temperature of ≥ 38°C during the home visit or as measured by patients during the respiratory episode. Measurement of temperature was either auricular, axillary, by infrared, or oral. Feeling feverish was defined as the feeling of being warm/feverish as reported in the daily diary. Symptoms were considered present if a symptom was reported for at least 1 day during the infectious episode. RSV-ARTI was defined as an ARTI with a positive RSV test result (POCT and/or qPCR positive for RSV). Those with a POCT-confirmed influenza infection were classified as influenza-ARTI, while all other PCR-negative ARTI were classified as other-ARTI.
Box 1. Influenza-Like Illness (ILI) Case Definitions.
| World Health Organization ILI [5] | An acute respiratory infection with: |
| • measured fever ≥ 38C° | |
| • and cough | |
| • with onset within the last 10 d | |
| Modified ILI | An acute respiratory infection with: |
| • feeling of being warm/feverish | |
| • and cough | |
| • with onset within the last 10 d |
Statistical Analysis
The presence of signs and symptoms was compared between those with RSV-ARTI, influenza-ARTI, or other-ARTI. We calculated the sensitivity, specificity, positive and negative predictive values, and likelihood ratios of the ILI and modified ILI case definitions for RSV-ARTI. The discriminative accuracy of these case definitions for RSV-ARTI was assessed as a point on the area under the receiver operating characteristic curve (AUC). The case definitions were subsequently tested in a subcohort of participants with medically attended (outpatient) RSV-ARTI. No imputation of missing values was performed.
Post hoc analyses were performed to explore 2 alternative case definitions. First, differences in symptomatology between RSV-ARTI, influenza-ARTI, and other-ARTI were used to develop a case definition with a high sensitivity for RSV that could distinguish RSV from all other infections. The second aim was to increase specificity to distinguish RSV from influenza. Alternative case definitions had to be concise and easy to use in order to make them applicable for community surveillance. These alterative case definitions were again tested in patients with RSV-ARTI and medically attended RSV-ARTI. Internal validation of these alternative case definitions was performed using bootstrapping (1000 random samples of 750 episodes with replacement). Last, we explored the performance of all tested case definitions to detect RSV using targeted sampling. From the proportion of RSV ARTI within a case definition we calculated the number patients that needed to be tested to identify 1 RSV case. By dividing this number of tested patients by the proportion of the case definition within all ARTI episodes we calculated the number of patients with ARTI that needed to be screened using a certain case definition to identify 1 RSV case. All analyses were performed in R version 4.0.1.
RESULTS
Patients and Infections
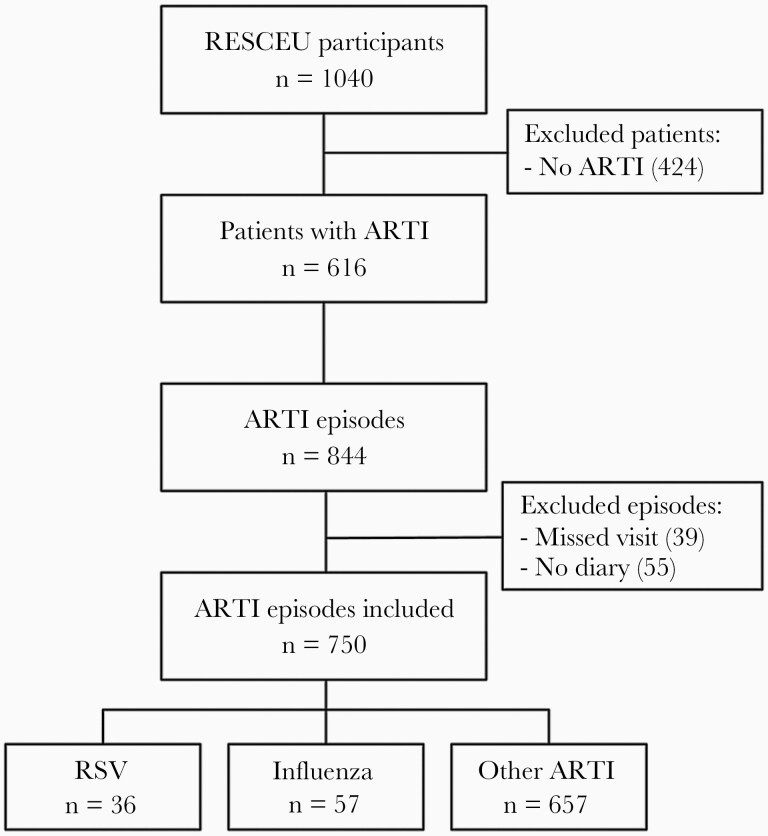
From the 1040 older adults that participated in the RESCEU study, 616 (59%) experienced at least 1 ARTI episode during study follow-up (median 1, range 1–5 ARTI). In total, 844 ARTI episodes occurred, of which 805 (95%) were sampled during a home visit. Diary information was available in 750/805 (93%) ARTI episodes from 583 patients. These 750 tested ARTI episodes with diary information were included for analysis (Figure 1). Thirty-six patients experienced an RSV-ARTI, while 56 patients experienced 57 influenza-positive ARTI. The remaining 657 ARTI episodes experienced by 583 patients were neither RSV nor influenza positive and were thus classified as other ARTI. Characteristics of the study population are described in Table 1.
Figure 1.
Flowchart of study participants and respiratory episodes. Abbreviations: ARTI, acute respiratory tract infection; RESCEU, Respiratory Syncytial Virus Consortium in Europe; RSV, respiratory syncytial virus.
Table 1.
Characteristics of Study Participants
| Characteristic | Patients With ARTI Included in Current Study (n = 616) |
Total RESCEU Study Population (n = 1040) |
||
|---|---|---|---|---|
| Patients With RSV (n = 36) |
Patients With Influenza (n = 56a) |
Patients With Other ARTI (n = 583) |
||
| Age | ||||
| Median, y (range) | 75 (63–89) | 71 (60–90) | 75 (60–100) | 75 (60–100) |
| Age older than 75 y | 20 (56) | 25 (44) | 302 (52) | 562 (54) |
| Female sex | 20 (56) | 28 (49) | 320 (55) | 554 (54) |
| Comorbidityb | ||||
| Cardiovascular | 7 (19) | 10 (18) | 121 (21) | 212 (21) |
| Lung | 5 (14) | 7 (12) | 76 (13) | 120 (12) |
| Diabetes | 2 (6) | 5 (9) | 59 (10) | 80 (8) |
| Allergies, anyc | 11 (29) | 15 (27) | 167 (29) | 276 (27) |
| Hay fever | 3 (9) | 2 (4) | 32 (6) | 59 (6) |
| House dust mite | 0 (0) | 4 (7) | 22 (4) | 32 (3) |
| Pneumococcal vaccinationd | 4 (12) | 10 (21) | 71 (14) | 118 (13) |
| Influenza vaccinatione | 30 (86 | 44 (79) | 444 (79) | 752 (76) |
| Smoking status | ||||
| Current smoker | 3 (8) | 5 (9) | 35 (6) | 80 (8) |
| Former smoker | 14 (39) | 17 (30) | 241 (41) | 409 (39) |
Data are No. (%) except where indicated. Numbers represent individual participants. Missing data < 1% is not shown; if more than 1% is missing, the percentages are given as footnote.
Abbreviations: ARTI, acute respiratory tract infection; RESCEU, Respiratory Syncytial Virus Consortium in Europe; RSV, respiratory syncytial virus.
aOne patient experienced 2 separate influenza B infections during follow-up.
bCardiovascular comorbidity included all arrhythmias, structural heart diseases, angina, and cardiac events such as infarction, percutaneous coronary intervention, and bypass surgery. Hypertension was not included in this definition. Lung disease included asthma, chronic obstructive pulmonary disease, chronic bronchitis, and emphysema. Diabetes was defined as either type.
cMissing n = 12 (2%).
dMissing n = 56 (10%).
eMissing n = 19 (3%).
Clinical Symptoms
Patient-reported symptoms and vital signs collected during the home visit are displayed in Table 2. Measured fever (≥38°C) was observed in 25% (14/57) of influenza-ARTI, 11% (4/36) of RSV-ARTI, and 5% (33/657) of other ARTI during the complete illness course. The feeling of being feverish was present in 65% (37/57) of influenza-ARTI, 33% (12/36) of RSV-ARTI, and 29% (191/657) of other ARTI. Patients with RSV-ARTI and those with influenza-ARTI more often experienced production of sputum (phlegm), dyspnea, and headache compared to other ARTI. Patients with RSV-ARTI as well as those with influenza-ARTI more often felt ill and indicated more disturbances in their daily activities compared to other ARTI. Vital signs collected during the home visit showed that patients with influenza-ARTI more often had a fever compared to other ARTI (16% vs 2%, P < .001) but not compared to RSV-ARTI (6%, P = .19). While an increased respiratory rate (>20/min) and lower saturation (<95% SaO2) were more often observed in those with RSV-ARTI and influenza-ARTI, these differences were not significant compared to other ARTI.
Table 2.
Clinical Symptoms of Respiratory Episodes
| Patient-Reported Symptoms | RSV-ARTI Episodes (n = 36) |
Influenza-ARTI Episodes (n = 57) |
Other ARTI Episodes (n = 657) |
|---|---|---|---|
| Rhinitis | 36 (100) | 55 (96) | 624 (95) |
| Cough | 35 (97) | 55 (96) | 572 (87) |
| Wheeze | 16 (44) | 26 (46) | 223 (34) |
| Phlegm | 34 (94) | 52 (91) | 466 (71)** |
| Dyspnea | 24 (67) | 42 (74) | 309 (47)* |
| Fever (≥38°C) | 2 (6) | 11 (19) | 26 (4) |
| Feeling feverish | 12 (33) | 37 (65)** | 191 (29) |
| Headache | 27 (75) | 45 (79) | 348 (53)* |
| Myalgia | 19 (53) | 41 (72) | 263 (40) |
| Disturbed sleep | 26 (72 | 51 (89)* | 440 (67) |
| Feeling unwell | 33 (91) | 56 (98) | 499 (76)* |
| Disturbance in daily activity | 27 (75) | 51 (89) | 348 (53)** |
| Vital signs from home visita | |||
| Temperature baseline, mean (SD) | 36.4°C (0.5) | 36.4°C (0.7) | 36.5°C (0.6) |
| Temperature ARTI, mean (SD) | 36.6°C (0.6) | 36.9°C (1.0)* | 36.5°C (0.6) |
| Temperature increase, mean (SD)b | 0.2°C (0.6) | 0.5°C (0.8) | 0°C (0.7) |
| Fever, ≥38°C | 2 (6) | 9 (16) | 13 (2) |
| Respiratory rate/min, mean (SD) | 17 (5) | 17 (5) | 17 (4) |
| Respiratory rate > 20/min | 6 (17) | 8 (14) | 63 (10) |
| Saturation, mean (SD) | 96% (2) | 96% (2) | 97% (2)* |
| Saturation, SaO2 < 95% | 5 (14) | 10 (18) | 39 (6) |
| Heart rate, bpm, mean (SD) | 74 (12) | 76 (12) | 71 (11) |
| Composite fever, ≥38°Cc | 4 (11) | 14 (25) | 33 (5) |
Data are No. (%) except where indicated. Numbers represent respiratory episodes unless stated otherwise.
Abbreviations: ARTI, acute respiratory tract infection; bpm, beats per minute; RSV, respiratory syncytial virus.
Statistical significance compared to RSV-ARTI: *P < .05, **P < .01, ***P < .001 (not indicated if nonsignificant).
aMeasured by the study team during the ARTI visit unless otherwise indicated.
bCompared to baseline measurement before the season.
cPatient reported or measured during ARTI home visit.
Performance of the ILI Case Definitions for RSV
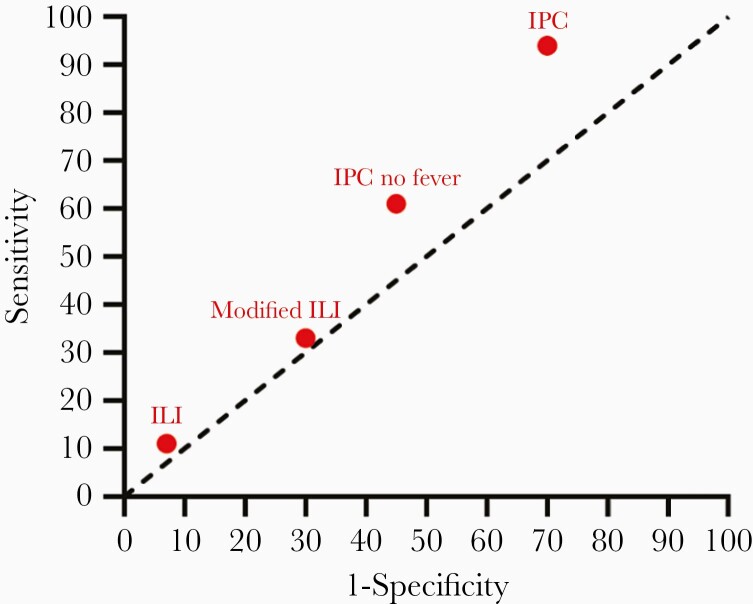
Sensitivity of the ILI and modified ILI case definitions was low for RSV-ARTI (11% for ILI, 33% for modified ILI; Table 3 and Figure 2). The AUC was 0.52 for both ILI case definitions. Performance of the case definitions in the subcohort of 178 medically attended patients (11 medically attended RSV-ARTI) showed similar results (Table 3). The sensitivity in identifying influenza was 25% for ILI and 65% for modified ILI with an AUC of 0.59 and 0.69, respectively (Supplementary Table 1).
Table 3.
Performance of ILI Case Definitions for RSV-ARTI
| RSV-ARTI (n = 36) |
MA-RSV-ARTI (n = 11) |
|
|---|---|---|
| ILIa | ||
| No. (%) | 4 (11) | 1 (9) |
| Sensitivity (95% CI) | 11 (3–26) | 9 (0–41) |
| Specificity (95% CI) | 93 (91–95) | 84 (77–89) |
| LR+ (95% CI) | 1.6 (.6–4.2) | 0.6 (.1–3.7) |
| LR− (95% CI) | 1.0 (.9–1.1) | 1.1 (.9–1.3) |
| PPV (95% CI) | 8 (2–18) | 4 (0–18) |
| NPV (95% CI) | 95 (94–97) | 93 (88–97) |
| AUC | 0.52 | 0.46 |
| Modified ILIa | ||
| No. (%) | 12 (33) | 4 (36) |
| Sensitivity (95% CI) | 33 (19–51) | 36 (11–69) |
| Specificity (95% CI) | 70 (67–74) | 58 (50–66) |
| LR+ (95% CI) | 1.1 (.7–1.8) | 0.9 (.9–1.0) |
| LR− (95% CI) | 1.0 (.8–1.2) | 1.1 (.7–1.8) |
| PPV (95% CI) | 5 (3–9) | 5 (2–13) |
| NPV (95% CI) | 95 (93–97) | 93 (86–97) |
| AUC | 0.52 | 0.47 |
Abbreviations: ARTI, acute respiratory tract infection; AUC, area under the receiver operating characteristic curve; CI, confidence interval; ILI, influenza-like illness; LR−, negative likelihood ratio; LR+, positive likelihood ratio; MA, medically attended; NPV, negative predictive value; PPV, positive predictive value; RSV, respiratory syncytial virus.
aILI includes measured temperature ≥ 38°C while the modified ILI includes also the feeling of being feverish.
Figure 2.
Area under the curve plot of performance of case definitions for respiratory syncytial virus. ILI includes measured temperature ≥ 38°C while modified ILI includes also the feeling of being feverish. Abbreviations: ILI, influenza-like illness; IPC, infectious productive cough.
Exploration of Alternative Case Definitions
Infectious productive cough (IPC) was defined as a respiratory infection with cough and production of sputum/phlegm as discriminating symptom irrespective of the presence of fever. IPC identified 94% of RSV cases (2 missed cases) but specificity was only 30%, resulting in an AUC of 0.62 (Supplementary Table 2). The second alternative, IPC without fever, specifically excluded cases with fever to discriminate RSV from influenza (Box 2). IPC without fever identified 61% of RSV cases with a specificity of 55% resulting in an AUC of 0.58 (Supplementary Table 2 and Figure 2). Bootstrapped results and performance in those with medical attendance showed similar results (Supplementary Tables 3 and 4).
Box 2. Alternative Case Definitions.
| Alternative 1: Infectious productive cough |
Acute respiratory infection with: |
| • cough with sputum/phlegm production | |
| Alternative 2: Infectious productive cough without fever |
Acute respiratory infection with: |
| • cough with sputum/phlegm production | |
| WITHOUT | |
| • measured fever or feeling feverish |
Targeted Testing for RSV
By testing everyone with an ARTI, 21 tested patients were required to identify 1 case of RSV. Targeted testing using ILI required just 13 tested ILI cases per case of RSV. But because the proportion of ILI within ARTI was low, 188 screened ARTI cases were needed to identify the 13 ILI cases required for 1 case of RSV. IPC required 23 ARTI cases to be screened to find 16 IPC patients that had to be tested to identify 1 case of RSV (Table 4).
Table 4.
Performance of Case Definitions for RSV Surveillance and Targeted Testing
| Case Definition | Proportion of Case Definition Within ARTI, % | Proportion of RSV Within Case Definition, % | Total Tested per RSV Case a | Total Screened per RSV Case b |
|---|---|---|---|---|
| ARTI | 100 | 4.8 | 21 | 21 |
| ILI | 7.1 | 7.5 | 13 | 188 |
| Modified ILI | 29.7 | 5.4 | 19 | 62 |
| IPC | 70.9 | 6.4 | 16 | 23 |
| IPC no fever | 45.5 | 6.5 | 15 | 33 |
Abbreviations: ARTI, acute respiratory tract infection; ILI, influenza-like illness; IPC, infectious productive cough.
a1/proportion RSV within case definition.
bTotal screened = total tested/proportion case definition within ARTI.
DISCUSSION
In this study we investigated the performance of the WHO ILI and a modified ILI case definition in identifying RSV infection in community-dwelling older adults. Sensitivity for RSV-ARTI was poor for ILI (11%) and modified ILI (33%) with an AUC of 0.52, indicating the inability of both case definitions to discriminate RSV-ARTI from other respiratory infections. Alternative case definitions formulated in this study were unable to substantially improve identification of RSV-ARTI.
Complementary to previous studies performed in children and adults [7–10], we confirm that RSV is not captured well by ILI in community-dwelling older adults. Fever is less frequently reported in RSV infections compared to influenza infections [7, 16–19]. Moreover, age might also negatively influence occurrence of fever as shown for newborns [8, 9, 20], but also for the elderly who may lack a robust febrile response in up to one-third of acute infections [21]. The latter can be caused by a lower baseline temperature (simply not reaching the fever threshold) or because of diminished febrile responses [21]. As observed in our study, occurrence of fever or even a significant increase in body temperature compared to baseline was low, which negatively impacts the sensitivity of ILI for RSV.
The strength of this study is the prospective design with a focus on the older adult community population. To our knowledge, this study represents the largest cohort of community-dwelling older adults to date in which the WHO ILI case definition was tested. Medical attendance was not required to trigger viral testing in those with respiratory symptoms, which limits the risk of selection bias for more severe disease. Because of intensive follow-up, we tested 95% of the ARTI episodes and had complete symptom diary data available in 93% of those episodes.
Limitations also deserve discussion. First, the number of RSV and influenza cases was low, which could have affected the performance of the case definitions. Bootstrapped results showed similar results and the observed symptomology was comparable to other studies [22, 23], which is reassuring. Second, the performance of case definitions is dependent on the clinical setting. Surveillance often takes place in a medical setting where disease is more severe and not in community-dwelling patients. We observed mainly mild disease, although results were similar in those with medical attendance. Generalizability to hospitalized patients is uncertain because RSV-related hospitalization did not occur in our study. Third, patients were not obliged to measure temperature when they felt feverish. This could have negatively affected ILI performance because ILI requires a measured fever to fulfil to the case definition. We therefore also used a modified ILI case definition requiring only the feeling of being feverish. Nevertheless, temperature was measured in 97% of the home visits and we showed that temperature was not substantially higher upon respiratory infection in those with RSV compared to baseline. The median timing of the home visits in those with RSV-ARTI was 1 day after the peak of symptoms. Additionally, while temperature was measured using different methods during the ARTI visits (tympanic membrane 50%, axillary 30%, infrared 10%, and oral 8%), 91% of patients were measured by the same method at baseline and during the study visit for an ARTI.
Worldwide RSV surveillance is largely dependent on the ILI and ARI case definitions [6]. ARI was not included in our analysis because we used ARI symptoms as criterion for sampling of ARTI episodes. RSV vaccines for older adults are currently in a late stage of clinical development. While clinical trials are able to show vaccine efficacy (performance of an intervention under ideal circumstances), we need good surveillance to measure vaccine effectiveness (real-world performance) after implementation. By using estimates of disease burden before and after implementation of a new vaccine in combination with vaccine coverage it is possible to calculate the impact of vaccine introduction. However, the impact of vaccination will be underestimated if estimates are obtained from a surveillance system in which the majority of the burden is not captured. The impact of future RSV vaccine introduction might go unnoticed if ILI is used for surveillance.
We show that in older adults the proportion of RSV in those who fulfil the ILI case definition is much lower (7.5%) (Supplementary Table 4) compared to 19%–45% for influenza [24, 25]. Consequently, even in the unrealistic scenario of a 100% effective vaccine, trends in case definition incidence would find at most a 7.5% reduction. These reductions might go unnoticed because of considerable variation in RSV seasonality [26], suboptimal vaccination coverage, and real-world vaccination procedures. Additionally, a reduction in RSV ARTI might be compensated by ARTI due to other respiratory pathogens. This phenomenon was seen for influenza when an unchanged incidence of ILI was observed despite a reduction in laboratory-confirmed influenza [24]. Unfortunately, we were not able to formulate an alternative RSV case definition that was able to increase the proportion of RSV and substantially increase discriminative performance (Supplementary Table 1). This suggests that there might not be a simple clinical case definition that can discriminate RSV from other respiratory infections. Additional diagnostic testing therefore seems inevitable to accurately determine the impact of RSV vaccination.
Vaccine effectiveness can be measured by determining the odds of vaccination between laboratory-confirmed cases and controls that fulfil a certain case definition using a test-negative design [27]. Large studies will be required to accurately determine RSV vaccine effectiveness because of the low proportion of RSV in currently available case definitions. The choice of which case definition to use for targeted sampling in these studies is crucial to decrease the costs and limit the risk of selection bias. The alternative case definitions from our study could improve this targeted sampling process as shown in Table 4. However, these alternative case definitions were developed with a relatively low number of cases and validation in other settings and larger groups of patients is still needed to confirm their performance.
CONCLUSION
In this study we showed that the syndromic WHO case definition for ILI underestimated the confirmed RSV occurrence by 9-fold in community-dwelling older adults. This is important because worldwide surveillance for RSV is largely dependent on the case definition for ILI. With the current surveillance programs, we risk being unable to measure the vaccine effectiveness of RSV vaccines. Because RSV vaccines are currently in a late stage of clinical development, there is an urgent need to determine the best approach to measure the impact of RSV vaccine introduction in older adults.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Study group members. The RESCEU investigators (including all authors of this article) are: Koos Korsten (University Medical Centre Utrecht); Niels Adriaenssens, Samuel Coenen (University of Antwerp); Christopher Butler (University of Oxford); Theo Verheij, Louis Bont, Joanne Wildenbeest (University Medical Centre Utrecht); Harish Nair, Harry Campbell, Steve Cunningham (University of Edinburgh); Philippe Beutels (University of Antwerp); Peter Openshaw, (Imperial College London); Andrew Pollard (University of Oxford); Eva Molero (Team-It Research); Adam Meijer (National Institute for Public Health and the Environment); Federico Martinon-Torres (Servicio Galego de Saude); Terho Heikkinen (University of Turku and Turku University Hospital); Thea K Fischer (Statens Serum Institut); Maarten van den Berge (Academisch Ziekenhuis Groningen); Carlo Giaquinto (Fondazione PENTA for the Treatment and Care of Children with HIV-ONLUS); Michael Abram (AstraZeneca); Deniz Öner, Jeroen Aerssens, (Janssen); Kena Swanson (Pfizer); Amanda Leach, Sonia Stoszek (GlaxoSmithKline); Clarisse Demont, Scott Gallichan, (Sanofi Pasteur); Veena Kumar (Novavax); and Ann Falsey (University of Rochester).
Acknowledgments. We gratefully acknowledge the support of volunteers who participated in this study. We thank all the general practitioners for their cooperation in the recruitment of participants from their offices. We thank the staff who was responsible for local patient recruitment and follow-up in Antwerp (Tita De Winter, Caroline Verschueren, Tine Maes, Ingrid Develter, Lizzy Winnepenninckx, and Lisbeth Minnen), Oxford (Behnaz Ravanfar, Heather Rutter, Julie Allen, Karen Madronal, Irene Noel, Bernadette Mundy, Belinda I’Anson, Samantha Squires, and Pippa Whitbread with the support with of the NIHR Clinical Research Network: Thames Valley and South Midlands research nurse team), and Utrecht (Brigitte Buiteman, Loes Nibbelke, Lieke Kam, Marin Bont, and Victor Kroon); staff of the Laboratory of Medical Microbiology of the University of Antwerp (Stalin Solomon Raj) and Oxford (Elisabeth Clutterbuck, Joseph McGinley, and Gu-Lung Lin) for sample processing and storage; epidemiologists Katrien Oude Rengerink and Marie Billard for their contributions in the statistical analysis; Cepheid for unconditionally providing us with the point-of-care test used in this study; Myra Widjojoatmodjo (Janssen, Leiden) for her contribution to overall setup of the project at the project initiation and Linong Zhang and Charlotte Vernhes (Sanofi Pasteur R&D Cambridge, MA) for their leadership on the RESCEU WP5 SP activities; and Hélène Bisceglia, Sandrine Montano, and Thibault Perret (Sanofi Pasteur R&D Marcy l’Etoile, France) for the hands-on work in the laboratories in France.
Disclaimer . This manuscript reflects only the author’s view. The European Commission is not responsible for any use that may be made of the information it contains.
Financial support. RESCEU has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 116019. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Supplement sponsorship . This article appears as part of the supplement “Results from RESCEU study: Evidence for Policy,” sponsored by RESCEU.
Contributor Information
Koos Korsten, Department of Pediatric Infectious Diseases and Immunology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht, the Netherlands.
Niels Adriaenssens, Vaccine and Infectious Disease Institute, Laboratory of Medical Microbiology, University of Antwerp, Faculty of Medicine and Health Sciences, Antwerp, Belgium; Department of Family Medicine and Population Medicine, Primary and Interdisciplinary Care, Centre for General Practice, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium.
Samuel Coenen, Vaccine and Infectious Disease Institute, Laboratory of Medical Microbiology, University of Antwerp, Faculty of Medicine and Health Sciences, Antwerp, Belgium; Department of Family Medicine and Population Medicine, Primary and Interdisciplinary Care, Centre for General Practice, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium.
Chris C Butler, Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, United Kingdom.
Theo J M Verheij, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands.
Louis J Bont, Department of Pediatric Infectious Diseases and Immunology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht, the Netherlands.
Joanne G Wildenbeest, Department of Pediatric Infectious Diseases and Immunology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht, the Netherlands.
RESCEU Investigators:
Koos Korsten, Niels Adriaenssens, Samuel Coenen, Christopher Butler, Theo Verheij, Louis Bont, Joanne Wildenbeest, Harish Nair, Harry Campbell, Steve Cunningham, Philippe Beutels, Peter Openshaw, Andrew Pollard, Eva Molero, Adam Meijer, Federico Martinon-Torres, Terho Heikkinen, Thea K Fischer, Maarten van den Berge, Carlo Giaquinto, Michael Abram, Deniz Öner, Jeroen Aerssens, Kena Swanson, Amanda Leach, Sonia Stoszek, Clarisse Demont, Scott Gallichan, Veena Kumar, and Ann Falsey
References
- 1. Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016; 388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis 2018; 18:1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the global burden of disease study 2017. Lancet Respir Med 2019; 7:69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fitzner J, Qasmieh S, Mounts AW, et al. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ 2018; 96:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World. Health Organization. RSV surveillance case definitions. https://www.who.int/teams/global-influenza-programme/global-respiratory-syncytial-virus-surveillance/case-definitions. Accessed 19 August 2019.
- 7. Hirve S, Crawford N, Palekar R, Zhang W; WHO RSV Surveillance Group. . Clinical characteristics, predictors, and performance of case definition—interim results from the WHO global respiratory syncytial virus surveillance pilot. Influenza Other Respir Viruses 2020; 14:647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saha S, Pandey BG, Choudekar A, et al. Evaluation of case definitions for estimation of respiratory syncytial virus associated hospitalizations among children in a rural community of northern India. J Glob Health 2015; 5:010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rha B, Dahl RM, Moyes J, et al. Performance of surveillance case definitions in detecting respiratory syncytial virus infection among young children hospitalized with severe respiratory illness—South Africa, 2009–2014. J Pediatric Infect Dis Soc 2019; 8:325–33. [DOI] [PubMed] [Google Scholar]
- 10. Nyawanda BO, Mott JA, Njuguna HN, et al. Evaluation of case definitions to detect respiratory syncytial virus infection in hospitalized children below 5 years in Rural Western Kenya, 2009–2013. BMC Infect Dis 2016; 16:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saez-Lopez E, Pechirra P, Costa I, et al. Performance of surveillance case definitions for respiratory syncytial virus infections through the sentinel influenza surveillance system, Portugal, 2010 to 2018. Euro Surveill 2019; 24:1900140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falsey AR, Baran A, Walsh EE. Should clinical case definitions of influenza in hospitalized older adults include fever? Influenza Other Respir Viruses 2015; 9 (Suppl 1):23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine 2005; 23:S10–25. [DOI] [PubMed] [Google Scholar]
- 14. Korsten K, Adriaenssens N, Coenen S, et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J 2021; 57:2002688. [DOI] [PubMed] [Google Scholar]
- 15. Cepheid. Xpert Xpress flu/RSV. http://www.cepheid.com/en/cepheid-solutions/clinical-ivd-tests/critical-infectious-diseases/xpert-xpress-flu-rsv. Accessed 31 July 2019.
- 16. Belongia EA, King JP, Kieke BA, et al. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥60 years old. Open Forum Infect Dis 2018; 5:ofy316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ 1997; 315:1060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walsh EE, Peterson DR, Falsey AR. Is clinical recognition of respiratory syncytial virus infection in hospitalized elderly and high-risk adults possible? J Infect Dis 2007; 195:1046–51. [DOI] [PubMed] [Google Scholar]
- 19. Bruyndonckx R, Coenen S, Butler C, et al. ; GRACE Project Group. . Respiratory syncytial virus and influenza virus infection in adult primary care patients: association of age with prevalence, diagnostic features and illness course. Int J Infect Dis 2020; 95:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawakami C, Sato A, Sumita H, et al. Fever responses are enhanced with advancing age during respiratory syncytial virus infection among children under 24 months old. Tohoku J Exp Med 2018; 245:217–22. [DOI] [PubMed] [Google Scholar]
- 21. Norman DC. Fever in the elderly. Clin Infect Dis 2000; 31:148–51. [DOI] [PubMed] [Google Scholar]
- 22. Falsey AR, McElhaney JE, Beran J, et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis 2014; 209:1873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sundaram ME, Meece JK, Sifakis F, Gasser RA Jr, Belongia EA. Medically attended respiratory syncytial virus infections in adults aged ≥ 50 years: clinical characteristics and outcomes. Clin Infect Dis 2014; 58:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Beek J, Veenhoven RH, Bruin JP, et al. Influenza-like illness incidence is not reduced by influenza vaccination in a cohort of older adults, despite effectively reducing laboratory-confirmed influenza virus infections. J Infect Dis 2017; 216:415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrew MK, McElhaney JE, McGeer AA, et al. ; Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance Network Investigators. . Influenza surveillance case definitions miss a substantial proportion of older adults hospitalized with laboratory-confirmed influenza: a report from the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS) Network. Infect Control Hosp Epidemiol 2020; 41:499–504. [DOI] [PubMed] [Google Scholar]
- 26. Broberg EK, Waris M, Johansen K, Snacken R, Penttinen P, European Influenza Surveillance Network. . Seasonality and geographical spread of respiratory syncytial virus epidemics in 15 European countries, 2010 to 2016. Euro Surveill 2018; 23:17-00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization (WHO). Evaluation of influenza vaccine effectiveness: a guide to the design and interpretation of observational studies. Geneva, Switzerland: WHO, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.