Abstract
The surgical treatment of occlusive acute mesenteric ischemia (AMI) without revascularization is associated with an 80% overall mortality. Early diagnosis is crucial, and revascularization may reduce overall mortality in AMI by up to 50%. A diagnosis of AMI requires a high index of clinical suspicion and the collaborative effort of emergency department physicians, general and vascular surgeons, and radiologists. This article provides an overview of the etiology, physiology, evaluation, and management of acute mesenteric ischemia.
Keywords: acute mesenteric ischemia, nonocclusive mesenteric ischemia, mesenteric venous thrombosis, endovascular procedures, endovascular therapy, angioplasty, embolectomy, mechanical thrombolysis, surgical treatment
Acute mesenteric ischemia (AMI) encompasses a group of diseases characterized by insufficient blood flow to varying parts of the small bowel, leading to ischemia and secondary inflammation. If left untreated, life-threatening intestinal necrosis develops. Despite advances in techniques used to treat problems within the mesenteric circulation, the most crucial factors influencing patient outcomes remain a high degree of clinical suspicion and swift intervention. 1 Mesenteric ischemia is rare, accounting for 0.09 to 0.2% of acute hospital admissions, with inaccurate or delayed diagnosis resulting in catastrophic outcomes with high mortality. 1 2
Pathophysiology and Diagnosis of Acute Mesenteric Ischemia
Incidence and Epidemiology
A 2011 population based case-control analysis from a national general practice database in the UK estimated the overall incidence of AMI as 0.63 (95% confidence interval [CI]: 0.47–0.86) per 100,000 person-years. 3 While a population-based study from Malmö, Sweden, reported an overall population-based incidence of AMI, diagnosed at either autopsy or operation, of 12.9 (95% CI: 11.6–14.1) per 100,000 person-years. 4 The varying etiologies among the Swedish population were thromboembolic occlusive mesenteric ischemia (67%), non-occlusive mesenteric ischemia (NOMI; 15%), mesenteric venous thrombosis (MVT; 16%), and 2% had an indeterminate cause. The most common cause of AMI was acute occlusion of the superior mesenteric artery (SMA), with a reported incidence of 8.6/100 000/year. Causes of SMA occlusion were embolism (70%) and thrombosis (30%). 5 In the Swedish population, the incidence of AMI increased exponentially with age, with equal distribution in both men and women. 5 The mean age at presentation in most studies was 70 years. 6
Etiology and Risk Factors
Acute mesenteric ischemia can be divided into four specific types based on etiology: arterial embolism, arterial thrombosis, NOMI, and MVT. 1 Each type is associated with different characteristics and risk factors ( Table 1 ).
Table 1. Characteristics and risk factors associated with acute mesenteric ischemia.
| Cause | Incidence | Presentation | Risk factors |
|---|---|---|---|
| Arterial embolism | 40–50% | Triad–sudden onset of pain, spontaneous gastric emptying (vomiting and diarrhea) with absence of physical signs. | Atrial fibrillation, myocardial ischemia or infarction, atrial tachyarrhythmia's, endocarditis, cardiomyopathies, ventricular aneurysm, valvular disorders incl. rheumatic fever |
| Arterial thrombosis | 25–30% | Post-prandial pain, weight loss, nausea, insidious onset with progression to chronic pain | Atherosclerosis, hypercholesterolemia, estrogen, hypercoagulable state. |
| NOMI | 20% | Acute or subacute | Low cardiac output state, shock, hypovolemia, hypotension, digoxin. |
| Venous thrombosis | 10–15% | Acute or subacute, nausea and vomiting | Factor V Leiden, prothrombin mutation, protein S deficiency, protein C deficiency, antithrombin deficiency, antiphospholipid syndrome, DVT, malignancies, pancreatitis, diverticulitis. |
Arterial Embolism
Arterial embolism is the most frequent cause of AMI. 7 Superior mesenteric artery (SMA) embolism is characterized by the acute onset of symptoms and severe abdominal pain, without localization or “ pain out of proportion to clinical signs.” The most mesenteric emboli originate from a cardiac source secondary to mural thrombus. Risk factors that predispose to development of arterial thrombi may be present in the patient's history. These include atrial fibrillation, rheumatic fever, recent myocardial infarction, prosthetic valve, and atrial tachyarrhythmia. 7 8 A history of embolism is present in 33% of patients and an absence of therapeutic anticoagulation should raise clinical suspicion. 9 The vast majority of visceral arterial emboli lodge preferentially in the proximal SMA (just beyond the first few jejunal branches) due to the oblique angle at which it emerges from the aorta; 15% occur at its origin, while 50% lodge distal to the origin of the middle colic artery (MCA). 9 This results in a classic pattern of ischemia that spares the first portion of the small bowel and ascending colon.
Arterial Thrombosis
Arterial thrombosis accounts for 25 to 30% of all ischemic events. 7 Patients typically present with symptoms of mesenteric angina, including post-prandial pain, food aversion, nausea, and weight loss. Risk factors include severe atherosclerotic occlusive disease and hypercholesterolemia. 7 The chronic nature of this condition results in the development of collateral vascular beds. Consequently, intestinal ischemia develops when the last remaining visceral artery or critical collateral vessel is compromised. The extent of ischemia is generally worse compared with arterial embolism, as occlusion often occurs at the origin of the SMA. 9
Non-occlusive Mesenteric Ischemia
Non-occlusive mesenteric ischemia accounts for 20% of cases and results from a low cardiac output state, causing diffuse mesenteric vasoconstriction. 8 The compromised blood flow affects the SMA and consequently the proximal colon due to involvement of the ileocolic artery. 2 Vasoactive drugs, particularly vasopressin and digoxin, have been implicated in the pathogenesis of NOMI. 9 Patient outcomes tend to be extremely poor in this population as it commonly affects the critically ill. A rare form of NOMI has been described in intensive care patients receiving enteral nutrition, who have undergone major surgical stress. The proposed mechanism of ischemia is an imbalance between demand (created by enteral feeding) and supply (reduced due to systemic hypoperfusion and mesenteric vasoconstriction). 10
Mesenteric Venous Thrombosis
Mesenteric venous thrombosis is the least common cause of AMI and accounts for 10 to 15% of cases. 8 This variant is characterized by acute thrombosis of the superior mesenteric vein (SMV) and its branches with or without extension to the portal vein. 6 Pathogenesis is attributed to Virchow's triad (stagnated blood flow, hypercoagulability, and vascular endothelial inflammation). 2 Etiology can be primary or idiopathic; however, 90% of cases are related to thrombophilia, trauma, or local inflammatory states. Up to 50% of patients may report a history of previous deep venous thrombosis or pulmonary embolism; hypercoagulability may be secondary to inherited disorders. 2 Thrombophilia may be secondary to underlying malignancy, hematological disorders, or oral contraceptive use. 11
Additional factors altering blood flow include portal hypertension, pancreatitis, inflammatory bowel disease, sepsis (including toxic colitis), and blunt abdominal trauma. 2 In these situations, bowel wall edema and increased vascular resistance secondary to venous thrombosis, results in reduced arterial blood flow and bowel ischemia. 2
Ischemic Colitis
Acute mesenteric ischemia can often be confused with ischemic colitis as symptoms may overlap. While the two conditions are similar in nature, there are several important differences.
Ischemic colitis is generally considered to be non-occlusive in nature as angiograms are frequently normal. 12 Despite this, most cases of ischemic colitis are not preceded by shocked states and the majority either have a thrombogenic disorders or cardiac or vascular emboli. Most intensive care patients with ischemic colitis have a history of recent aortic surgery with loss of the inferior mesenteric artery (IMA). 12 The incidence of ischemic colitis following aortic surgery ranges from 1 to 2% following elective surgery and up to 20% in emergency aortic surgery and is associated with hypovolemic shock or sigmoid colon ischemia. 12 Therefore, ischemic colitis is not associated with major vessel occlusion, but with transient hypoperfusion or occlusion of small vessels.
Anatomically, the watershed areas of the colon are most affected. Griffiths point is at the splenic flexure or the separation point of distribution between the SMA and IMA. This occurs between the ascending left colic artery and the marginal artery of Drummond. Sudeck point refers to a specific location at the rectosigmoid junction where the origin of the last sigmoid arterial branch from the IMA forms an anastomosis with a branch of the superior rectal artery.
In most cases of ischemic colitis, the disease resolves spontaneously with intravenous fluid resuscitation, antibiotics, and bowel decompression when indicated. Angiography is rarely needed. Surgery is reserved for patients with complications. Acute SMA occlusion in contrast to low flow states and left-sided colonic ischemia, causes a more significant reduction in blood flow with rapid progression to intestinal infarction and therefore requires immediate treatment. 13
Pathophysiology
Prolonged ischemia, whatever the pathophysiologic cause, leads to the disruption of the intestinal mucosal barrier, primarily due to reactive oxygen metabolites and polymorphonuclear neutrophils. 8 12 14 Once reperfusion is achieved, inflammatory cytokines interleukin (IL)-6 and tumor necrosis factor (TNF) are released. 15 High levels of IL-6 and TNF in the systemic circulation induce severe systemic adverse reactions, which cause multi-organ dysfunction syndrome. 15 Tumor necrosis factor causes a shock response, with reduced blood pressure and peripheral perfusion. 15 In the pulmonary vascular bed, IL-6 and TNF lead to sequestration of leucocytes and macromolecules into the lungs, leading to adult respiratory distress syndrome. 15 Once ischemia has progressed transmurally, signs of peritonitis and septicemia develop.
Investigations
Early diagnosis in AMI is necessary to commence appropriate treatment and avoid poor patient outcomes. A 24-hour delay in diagnosis decreases survival by 20%. 16
Laboratory Tests
Patients with AMI classically present with leukocytosis, metabolic acidosis, raised D-dimer, and raised lactate. 6 The diagnostic challenge in AMI is that conventional laboratory tests are nonspecific and cannot be used to rule in a diagnosis of AMI due to their insufficient likelihood ratios. 17
Arterial lactate may be negative in the early phase of bowel ischemia, with a predominant metabolic alkalosis secondary to profound vomiting. 18 Elevated lactate is typically associated with late-stage mesenteric ischemia due to extensive transmural intestinal infarction, tissue hypoperfusion, anaerobic metabolism, and cell death. Despite this, normal serum lactate values may be observed in cases of extensive intestinal ischemia due to the liver's ability to clear large quantities of lactate from the portosystemic circulation. 17 A 2017 meta-analysis found a pooled sensitivity and specificity of D-lactate for diagnosing AMI were 0.72 (95% CI: 0.59–0.82) and 0.74 (95% CI: 0.69–0.79), respectively. 19
Imaging
Computed tomography angiography (CTA) has replaced conventional angiography as the gold standard in diagnosing AMI, with a sensitivity and specificity of 96% and 94%, respectively. 17 Ultrasound (US) is not recommended in AMI as it is time consuming and of limited diagnostic value. Plain abdominal radiographs are not useful, as gas within the portal veins is a sign of extensive intestinal pneumatosis and indicates intestinal necrosis. 6 Radiographic findings of thumbprinting or thickened bowel loops are observed in <40% of patients. 9
If AMI is suspected, CT should be performed with contrast enhancement in the arterial and venous phases. The arterial phase allows for accurate detection of vascular pathology with detailed vascular reconstruction, while the venous phase provides assessment of intestinal perfusion ( Fig. 1 ). Multi-planar reconstruction permits assessment of the origin of the mesenteric arteries. 20 Computed tomography findings in AMI can be either specific or nonspecific for intestinal ischemia. The most specific finding is reduced or absent bowel wall enhancement, which has 96% specificity, but very low sensitivity (16–62%). 21 Pneumatosis intestinalis is reported in 6 to 28% of cases of AMI and portomesenteric venous gas in 3 to 14%. 21 While the presence of pneumatosis is specific for bowel ischemia, its low sensitivity does not indicate irreversible bowel ischemia. Non-specific CT findings in AMI include bowel wall thickening, mesenteric fat stranding, ascites, and luminal dilatation. 6
Fig. 1.
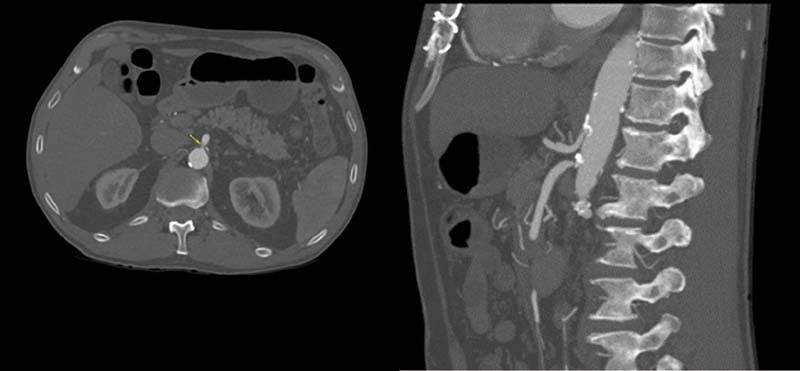
SMA occlusion. Figures courtesy of Dr. Benjamin W. Starnes, University of Washington, Seattle, USA.
More important than the CTA itself is clinical suspicion, which is a major factor in the correct interpretation of CT findings in AMI. Where the clinical suspicion of AMI is raised in the CT referral, the initial report is correct in 97% of cases, compared with a corresponding rate of 81% when it is not ( p = 0.04). 22 Therefore, direct inquiry for mesenteric ischemia should be clearly stated in the CT referral.
Treatment of SMA Occlusion
Principles of Treatment
Fluid resuscitation should begin immediately with intravenous fluids (isotonic crystalloid) and correction of electrolyte imbalances. 23 Invasive monitoring, including hourly urine output and arterial pressure monitoring is advisable to ensure that all parameters are optimized prior to definitive surgical management.
The aim of management in AMI is restoration of the mesenteric circulation by endovascular or surgical means, prior to the development of irreversible intestinal damage. When transmural bowel necrosis has already occurred, intestinal revascularization reduces the extent of tissue damage and may facilitate healing. Optimal treatment may include open or endovascular surgery and patients are best managed in a tertiary referral center with a hybrid operating room. 24 In patients with peritonitis, elevated lactate, metabolic acidosis, and CT findings of advanced bowel ischemia, the primary treatment option is exploratory laparotomy. The goals of surgical intervention include, 2
(1) Restoration of mesenteric blood flow,
(2) Resection of non-viable bowel, and
(3) Preservation of viable bowel.
In stable patients with transmural intestinal necrosis, revascularization is preferentially performed prior to definitive bowel surgery. This is because the extent of irreversible bowel injury can be difficult to assess prior to restoration of blood flow. 25
Intraoperatively, the surgeon can quickly resect frankly necrotic bowel prior to vascular intervention. 26 However, at this stage, bowel resection must be done sparingly. The authors advocate use of a temporary abdominal closure (TAC) system, such as ABTHERA, KCI, St Paul, MN, USA if further re-look laparotomy is required. If preoperative CTA is not performed, or is non-diagnostic, there are two options for intraoperative vascular examination. 27 The first is to perform on table angiography, while the second is to explore the SMA surgically and assess the vessel intraoperatively with intraoperative duplex US. If a pulse is palpable at the base of the mesentery this suggests an embolic event since the embolus usually lodges at the first branch of the SMA, the MCA. 26 If SMA embolism is found, open embolectomy is usually the quickest way to achieve bowel reperfusion. 26
If the artery feels diseased and there is no pulse in the mesentery this is most likely secondary to in situ thrombosis and endovascular revascularization may be considered; this can be done via a percutaneous approach or open retrograde recanalization. If endovascular treatment fails or the lesion is not suitable, surgical bypass may be performed. Assessment of bowel viability can be difficult, and the surgeon should wait 20 to 30 minutes following revascularization to adequately assess this. 23 The following factors are considered: intestinal peristalsis, bowel sheen and color, bleeding from cut ends and visible or palpable pulsations in the mesenteric arcade. A low threshold for relook laparotomy within 24 hours is recommended, with up to 50% of patients requiring further resection at relook surgery. 23 26 Occasionally, a third look may be required before a final bowel anastomosis is confidently performed. A mandatory return aims to protect the patient from ongoing bowel necrosis, before irreversible physiologic changes develop or perforation. 23
Vascular Therapeutic Options
Embolectomy
When laparotomy has been performed, access to the SMA is achieved via a horizontal incision in the peritoneum at the base of the transverse mesocolon. 23 The SMA lies to the left of the SMV and divides into several branches, which need to be isolated with vessel loops or microvascular clamps. 27 A transverse arteriotomy is made across the SMA distal to the MCA, and embolectomy is performed using a 3- or 4F balloon catheter. 24 Following embolus extraction, torrential and pulsatile inflow should be observed.
Balloon embolectomy should be performed carefully as the SMA is fragile and arterial dissection occurs easily if excess traction is applied or if the balloon is over dilated. The arteriotomy is closed with interrupted prolene sutures. Intraoperative angiography follows to assess for proximal SMA stenosis, distal embolization, or dissection although a palpable pulse in the mesenteric arcade is a useful compromise. 24
Endovascular Aspiration Thrombectomy
Endovascular aspiration thrombectomy (EVT) is an option for patients without features of peritonitis. 23 The SMA is reached via brachial or femoral access routes; however, the angle to the SMA is more favorable via a brachial approach. 24 27 The SMA is catheterized and a hydrophilic 0.035 guidewire is passed deep into the SMA. Once the wire is in place, a 6- or 7F 60-cm introducer sheath with removable hub or an 8F-9F guiding catheter is placed in the SMA. The catheter is used to aspirate the blood clot using vacuum aspiration with a 20 mL locking syringe manually, or by employing an electric vacuum aspiration device such as the Penumbra System. 27 28 Several passes of the aspiration sheath may be required to achieve adequate clot removal. Completion angiography is performed.
Risks associated with EVT include propagation of the thrombus distally to peripheral, smaller arterial branches of the mesentery. 27 In a retrospective study of 18 patients with SMA embolism treated with EVT, there was one failure, although distal emboli or residual thrombus was present in six patients; due to the presence of a rich mesenteric collateral network this rarely caused complications. 29 Careful examination of the patient for any clinical deterioration is mandatory following successful embolus aspiration.
Catheter-directed Thrombolysis
Successful local intra-arterial thrombolysis for acute SMA occlusion was first described in Sweden in 1997. 30 Local SMA thrombolysis is a minimally invasive treatment (MIT) option for elderly patients with multiple comorbidities. This technique can be used as an adjunct in the setting of incomplete aspiration embolectomy following EVT or as a primary treatment modality in patients without peritonitis or advanced intestinal ischemia. 31
With an introducer in the proximal SMA, a multi-side hole infusion catheter is introduced into the main ileocolic trunk of the SMA and a thrombolytic agent is administered as a continuous infusion. Common thrombolytic agents include recombinant tissue plasminogen activator (tPA) infused at a rate of 0.5 to 1 mg/h or urokinase (120,000 IU/h); progress is assessed angiographically every 12 hours or otherwise depending on the patient's clinical status. 24 27
Antegrade Recanalization and Stenting of the SMA
Endovascular treatment (EVT) of atherosclerotic occlusion of the SMA is often performed following clot removal by aspiration or thrombolysis. 9 Percutaneous transluminal angioplasty with stenting can be performed via femoral or brachial access points. Once the surgeon gains access to the ileocolic artery with a stable 0.035-inch wire, an introducer sheath is advanced beyond the atherosclerotic lesion. 24 28 A balloon-expandable stent is deployed at the site of occlusion, followed by retraction of the sheath to expose the stent. The lesion is then treated with a 7–8 mm diameter stent. 24 A balloon-expandable stent is preferred over a self-expanding stent to maintain vessel patency. 27 The result is confirmed with repeat angiography ( Fig. 2 ).
Fig. 2.

Antegrade recanalization and stenting of the SMA. ( A ) Catheter-based recanalization and stenting. ( B ) Confirmation of SMA patency and stent placement via CT imaging. Figures courtesy of Dr. Benjamin W. Starnes, University of Washington, Seattle, USA.
The most feared complication of stenting is dissection from the SMA ostium into its peripheral branches, thus further worsening the intestinal circulation. Additional stents can be applied distally if there is significant dissection at the distal end of the first stent. 27
Open Retrograde Recanalization and Stenting of the SMA
Open retrograde recanalization may be used as a primary revascularization technique in cases where laparotomy is mandatory due to high clinical suspicion of intestinal necrosis or when the antegrade approach fails. The SMA is exposed in a similar fashion to the open embolectomy technique described above.
Retrograde SMA access is established using a micropuncture needle. 28 32 Retrograde angiography is performed, and the SMA occlusion or stenosis is crossed, pre-dilated, and stented with a balloon-expandable stent. Prior to restoring antegrade flow to the SMA, the sheath is flushed to prevent distal embolization. The puncture site is closed with interrupted sutures or opened longitudinally and closed over a patch if severely diseased. 32 Restenosis rates appear to be high following this approach mandating close patient follow up. 33
SMA Bypass
When an endovascular approach is not possible in the setting of atherosclerotic occlusion, or if embolectomy fails to restore inflow, revascularization by surgical bypass may be performed. Options include retrograde bypass to the SMA from the external or common iliac artery or from the infrarenal aorta. 23 Bypass to the SMA alone is preferred in AMI as opposed to multi-vessel bypass grafting. Most authors prefer an autologous vein graft in AMI due to the fear of peritoneal contamination and subsequent graft infection. 23 27 All patients undergoing laparotomy for presumed AMI should be prepped to include both lower extremities to the knee. This allows for possible saphenous or femoral vein harvest if required. 23
Endovascular versus Open Therapy
To date there are no randomized controlled trials comparing the different treatment modalities available for AMI. This makes it difficult to draw any valid conclusions as to whether the primary treatment approach should be open or endovascular revascularization.
A retrospective cohort study of 72 patients who underwent various open surgical interventions for AMI, reported perioperative morbidity and 30-day mortality rates of 39% and 31%, respectively. 34 The authors found that advanced age (>70 years; p = 0.03) and prolonged symptom duration ( p = 0.02) were predictive of mortality. 34 Similarly, Edwards et al. reviewed 41 patients with AMI undergoing traditional revascularization, including two patients treated with visceral angioplasty and stenting. 35 They reported a perioperative mortality of 62% and found long term parenteral nutrition was required in 31% of survivors. 35
A common theme amongst patients with AMI, is that advanced age and visceral ischemia with a requirement for bowel resection are predictors of poor outcome. The lack of significant superiority of endovascular treatment in severe AMI is likely due to the often-delayed presentation in combination with a prolonged ischemic time. 36 The need for urgent assessment of intestinal viability and resection often renders endoluminal therapy alone superfluous. It is undeniable however that the number of endovascular interventions for AMI will continue to increase as techniques improve over time. 36
Evidence for this is highlighted by Arthurs et al. who reported outcomes in 56 patients with AMI receiving endovascular therapy. 37 The primary mode of endovascular therapy was thrombolysis infusion, comprising 48% of the population; tPA was used in all cases. Overall technical success for endovascular therapy was 87%. Patients who were successfully treated with endovascular therapy experienced significant reduction in mortality compared with traditional therapy (36 vs 50% respectively; p = < 0.05).
Management of Ischemic Bowel
Techniques available for diagnosing intestinal ischemia intraoperatively are limited, particularly when the bowel appears 'dusky' or threatened without obvious features of ischemia. In this case, temporary abdominal closure allows for 'second-look' surgery, at which time, the bowel is often more clearly demarcated as either viable or non-viable.
Standard Clinical Approach
Traditional inspection of the bowel is suboptimal for the accurate diagnosis of intestinal ischemia. 38 Initial clinical evaluation of the bowel consists of noting the normal color and appearance of bowel serosa, the presence or absence of peristalsis, arterial pulsations within mesenteric arcades, and bleeding from cut surfaces. Each of these is subjective and prone to inaccuracy. The use of clinical criteria alone, to predict bowel viability has a sensitivity of 82% and specificity of 91%. 23 Frankly necrotic bowel should be resected.
When a significant portion of bowel has already been removed, the surgeon may be reluctant to resect additional bowel due to the future risk of short gut syndrome. In this setting, the surgeon may employ the principles of damage control surgery. This involves temporary abdominal closure systems and admission to intensive care for ongoing invasive monitoring and fluid resuscitation. Planned, repeat laparotomy or 'second-look' laparotomy, is a recognized strategy employed AMI management.
The timing of second-look laparotomy is often 24 to 48 hours following the index surgery. 39 Evidence supporting the assertion that this interval is adequate to allow for physiological optimization is largely drawn from animal models. 40 In these studies, severe mucosal injury begins at one hour, reaches maximal severity at three hours and then subsides at 24 hours. Both serum and histopathological markers of intestinal ischemic injury are well past their peak 24 hours following reperfusion and by 48 hours, have returned to baseline. 40 This is consistent with other reports that morphological intestinal repair begins after three hours of reperfusion and is complete by 24 hours. 41 42 43
Despite this, the incidence of bowel resection is routinely higher during second-look surgery (53%) compared with the initial procedure (31%). 23 It is for this reason that the authors advocate use of second-look surgery over anastomosis in the unstable and under resuscitated patient. Whatever the duration, the goal of this interval is to perform repeat surgery when the patient's physiology is optimized. This ensures that any stunned bowel has been reperfused to the extent that it no longer exhibits questionable viability, in addition to decreasing the risk of anastomotic leak and fistula formation. 44
If the adequacy of intestinal perfusion is in question or the patient is nutritionally depleted, the ends of the bowel may be brought out as stoma. 8 The stoma can also be used as an access point for endoscopic procedures if required. Diagnostic and therapeutic options are summarized in Fig. 3 .
Fig. 3.

Flow chart for diagnostic and therapeutic options in AMI. These options may depend on local access to vascular and interventional radiology services.
Techniques to Assess Intestinal Viability
The technique of second-look laparotomy in AMI is focused on repeat inspection of the bowel. While US, fluorescein, and angiography all have roles in diagnosing AMI, their reliability in predicting intestinal viability is unacceptably low.
Doppler Ultrasonography
Doppler ultrasonography (DUS) is a safe and non-invasive technique that measures blood flow using reflected sound waves. Several cohort and animal models find DUS to be an effective, feasible, and cost-efficient adjunct to the standard clinical approach. 45 Despite encouraging results however, several publications have highlighted the shortcomings and inefficacies of DUS for intraoperative assessment in AMI. 45
Fluorescein
In 1981, Bulkley et al published their seminal paper on the intraoperative assessment of small intestinal viability compared with standard clinical judgment, doppler-detected pulsatile mural blood flow and fluorescein ultraviolet (UV) fluorescence pattern. 46 The use of fluorescein was correct in all 54 cases and proved to be more sensitive, specific, predictive, and significantly more accurate than standard clinical judgment or doppler. Since then, fluorescein has been used as an accepted technique for intraoperative assessment of bowel viability. 45
Sodium fluorescein delivered intravenously, is a fluorophore with excitation and emission peaks of 490 nm and 513 nm at a physiological pH in 1% saline. 45 Fluorescein is used to visualize perfusion in open laparotomies using a Woods Lamp or laparoscopically using an endoscope with the correct filters and UV excitation light. Perfusion assessment with fluorescein in animal models with AMI has been shown to improve accuracy of surgical resection compared with standard clinical assessment alone. 45
While comparative studies in animal models have consistently demonstrated the superiority of dye-based perfusion monitoring for intraoperative bowel assessment compared with standard clinical criteria and DUS, results are not universal. 45 Some large animal models have demonstrated no difference between these techniques, and an additional study found that DUS outperforms fluorescein when assessing bowel viability. 47 48
Indocyanine Green
Indocyanine green (ICG) is a near infrared fluorophore with an emission peak of 832 nm in whole blood. 45 Indocyanine green is large molecule which binds to plasma proteins such as albumin and globulins, thus increasing retention compared with fluorescein. 49 The use of ICG in AMI, has not been well investigated to date, although early animal models and isolated case and cohort studies show some promise.
In animal models, ICG predicts survival of ischemic bowel with greater accuracy than clinical judgement alone. Its efficacy has been demonstrated in individual case reports, where ICG detected ischemia not diagnosed on pre-op CT, predicted delayed intestinal ischemic complications, and confirmed resection margins. Single-center patient experiences performing intraoperative intestinal viability assessment with ICG lead to a clinically significant benefit in 11–32% of cases, preserving bowel length and defining resection margins better than clinical judgement alone. 50 51
Hybrid Management
There are no RCTs comparing laparotomy with endovascular treatment as a first line strategy in AMI. The most important argument in favor of early laparotomy is the ability to assess bowel viability directly thus minimizing delays in restoring mesenteric blood flow. However, on occasion, the patient's clinical status may be less alarming, presenting a window of opportunity for initial endovascular intervention. In one retrospective series, the authors found that 14.4% of patients undergoing endovascular procedures required bowel resection compared with 33.4% for open revascularization ( p ≤0.001). While endovascular repair was also less commonly associated with requirement for TPN (13.7% vs. 24.4%; p = 0.025). 52
New Technologies
Visual evaluation of the viability of the intestine is subjective and therefore, associated with a certain degree of error. Use of diagnostic laparoscopy is increasingly described in the literature. In one series, routine use of explorative laparoscopy avoided non-therapeutic laparotomies in 45% of patients presenting with clinical and radiological features of AMI. 53 Similarly, several case reports have been published describing diagnostic multi-port laparoscopy with ICG fluorescence angiography and UV light to assess intestinal viability in AMI with good outcomes. 54 55 While not described in the literature, once intestinal ischemia has been identified (and the patient remains stable), a complete MIS may be performed. Using this hybrid technique, intestinal blood flow can be evaluated in real time, except in patients who are allergic to ICG.
Conclusions
Acute mesenteric ischemia is a surgical emergency that requires a high index of clinical suspicion and prompt diagnosis. A multidisciplinary approach should be used in the assessment and treatment of AMI where there is a clear role for vascular intervention. Following assessment of the CTA, surgery aims to assess viability of the bowel, re-establish vascular flow, and resect non-viable bowel. The potential to combine varying modalities for the intraoperative assessment of bowel viability has been demonstrated. However, the evidence base for current practices is outdated and inconclusive and requires further high-quality research.
Footnotes
Conflict of Interest None declared.
References
- 1.Clair D G, Beach J M. Mesenteric ischemia. N Engl J Med. 2016;374(10):959–968. doi: 10.1056/NEJMra1503884. [DOI] [PubMed] [Google Scholar]
- 2.Bala M, Kashuk J, Moore E E. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2017;12:38. doi: 10.1186/s13017-017-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huerta C, Rivero E, Montoro M A, García-Rodriguez L A. Risk factors for intestinal ischaemia among patients registered in a UK primary care database: a nested case-control study. Aliment Pharmacol Ther. 2011;33(08):969–978. doi: 10.1111/j.1365-2036.2011.04614.x. [DOI] [PubMed] [Google Scholar]
- 4.Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010;23(01):4–8. doi: 10.1053/j.semvascsurg.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Acosta S, Ogren M, Sternby N H, Bergqvist D, Björck M. Incidence of acute thrombo-embolic occlusion of the superior mesenteric artery–a population-based study. Eur J Vasc Endovasc Surg. 2004;27(02):145–150. doi: 10.1016/j.ejvs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Kärkkäinen J M, Acosta S. Acute mesenteric ischemia (part I) - Incidence, etiologies, and how to improve early diagnosis. Best Pract Res Clin Gastroenterol. 2017;31(01):15–25. doi: 10.1016/j.bpg.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Tilsed J V, Casamassima A, Kurihara H. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(02):253–270. doi: 10.1007/s00068-016-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldenburg W A, Lau L L, Rodenberg T J, Edmonds H J, Burger C D. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164(10):1054–1062. doi: 10.1001/archinte.164.10.1054. [DOI] [PubMed] [Google Scholar]
- 9.Berland T, Oldenburg W A. Acute mesenteric ischemia. Curr Gastroenterol Rep. 2008;10(03):341–346. doi: 10.1007/s11894-008-0065-0. [DOI] [PubMed] [Google Scholar]
- 10.Wyers M C, Zwolak R M.The management of splanchnic vascular disordersEdited by Seeger JM.Philadelphia: Rutherford-Elsevier-Saunders; 20051707–1717. [Google Scholar]
- 11.Cohn D M, Roshani S, Middeldorp S. Thrombophilia and venous thromboembolism: implications for testing. Semin Thromb Hemost. 2007;33(06):573–581. doi: 10.1055/s-2007-985753. [DOI] [PubMed] [Google Scholar]
- 12.Kolkman J J, Mensink P B. Non-occlusive mesenteric ischaemia: a common disorder in gastroenterology and intensive care. Best Pract Res Clin Gastroenterol. 2003;17(03):457–473. doi: 10.1016/s1521-6918(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 13.Granger D N, Seifert H, Senchenkova E. Springer; Berlin, Heidelberg: 2014. Intestinal Ischemia and Reperfusion: Consequences and Mechanisms. [Google Scholar]
- 14.Freeman B A, Crapo J D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47(05):412–426. [PubMed] [Google Scholar]
- 15.Syk I, Brunkwall J, Ivancev K. Postoperative fever, bowel ischaemia and cytokine response to abdominal aortic aneurysm repair–a comparison between endovascular and open surgery. Eur J Vasc Endovasc Surg. 1998;15(05):398–405. doi: 10.1016/s1078-5884(98)80200-1. [DOI] [PubMed] [Google Scholar]
- 16.Boley S J, Feinstein F R, Sammartano R, Brandt L J, Sprayregen S. New concepts in the management of emboli of the superior mesenteric artery. Surg Gynecol Obstet. 1981;153(04):561–569. [PubMed] [Google Scholar]
- 17.van den Heijkant T C, Aerts B AC, Teijink J A, Buurman W A, Luyer M DP. Challenges in diagnosing mesenteric ischemia. World J Gastroenterol. 2013;19(09):1338–1341. doi: 10.3748/wjg.v19.i9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acosta S, Björck M. Acute thrombo-embolic occlusion of the superior mesenteric artery: a prospective study in a well defined population. Eur J Vasc Endovasc Surg. 2003;26(02):179–183. doi: 10.1053/ejvs.2002.1893. [DOI] [PubMed] [Google Scholar]
- 19.Treskes N, Persoon A M, van Zanten A RH. Diagnostic accuracy of novel serological biomarkers to detect acute mesenteric ischemia: a systematic review and meta-analysis. Intern Emerg Med. 2017;12(06):821–836. doi: 10.1007/s11739-017-1668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furukawa A, Kanasaki S, Kono N. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192(02):408–416. doi: 10.2214/AJR.08.1138. [DOI] [PubMed] [Google Scholar]
- 21.Wiesner W, Khurana B, Ji H, Ros P R. CT of acute bowel ischemia. Radiology. 2003;226(03):635–650. doi: 10.1148/radiol.2263011540. [DOI] [PubMed] [Google Scholar]
- 22.Lehtimäki T T, Kärkkäinen J M, Saari P, Manninen H, Paajanen H, Vanninen R. Detecting acute mesenteric ischemia in CT of the acute abdomen is dependent on clinical suspicion: Review of 95 consecutive patients. Eur J Radiol. 2015;84(12):2444–2453. doi: 10.1016/j.ejrad.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Wyers M C. Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23(01):9–20. doi: 10.1053/j.semvascsurg.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg. 2014;101(01):e100–e108. doi: 10.1002/bjs.9330. [DOI] [PubMed] [Google Scholar]
- 25.Acosta S. Surgical management of peritonitis secondary to acute superior mesenteric artery occlusion. World J Gastroenterol. 2014;20(29):9936–9941. doi: 10.3748/wjg.v20.i29.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darwood R, Smith F CT. Mesenteric ischaemia. Journal of Vascular Surgery. 2012;30(08):420–426. [Google Scholar]
- 27.Kärkkäinen J M, Acosta S. Acute mesenteric ischemia (Part II) - Vascular and endovascular surgical approaches. Best Pract Res Clin Gastroenterol. 2017;31(01):27–38. doi: 10.1016/j.bpg.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Acosta S, Sonesson B, Resch T. Endovascular therapeutic approaches for acute superior mesenteric artery occlusion. Cardiovasc Intervent Radiol. 2009;32(05):896–905. doi: 10.1007/s00270-009-9559-x. [DOI] [PubMed] [Google Scholar]
- 29.Kärkkäinen J M, Lehtimäki T T, Saari P. Endovascular therapy as a primary revascularization modality in acute mesenteric ischemia. Cardiovasc Intervent Radiol. 2015;38(05):1119–1129. doi: 10.1007/s00270-015-1064-9. [DOI] [PubMed] [Google Scholar]
- 30.Mellander S, Hellberg R, Karlqvist P A, Svahn M. Local fibrinolysis in acute thromboembolism of the superior mesenteric artery. Eur J Surg. 2001;167(04):308–311. doi: 10.1080/110241501300091589. [DOI] [PubMed] [Google Scholar]
- 31.Björnsson S, Björck M, Block T, Resch T, Acosta S. Thrombolysis for acute occlusion of the superior mesenteric artery. J Vasc Surg. 2011;54(06):1734–1742. doi: 10.1016/j.jvs.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 32.Oderich G S, de Souza L R. Springer; 2015. Techniques of endovascular mesenteric revascularization; pp. 165–86. [Google Scholar]
- 33.Wyers M C, Powell R J, Nolan B W, Cronenwett J L. Retrograde mesenteric stenting during laparotomy for acute occlusive mesenteric ischemia. J Vasc Surg. 2007;45(02):269–275. doi: 10.1016/j.jvs.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 34.Kougias P, Lau D, El Sayed H F, Zhou W, Huynh T T, Lin P H. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg. 2007;46(03):467–474. doi: 10.1016/j.jvs.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 35.Edwards M S, Cherr G S, Craven T E. Acute occlusive mesenteric ischemia: surgical management and outcomes. Ann Vasc Surg. 2003;17(01):72–79. doi: 10.1007/s10016-001-0329-8. [DOI] [PubMed] [Google Scholar]
- 36.David R A, Kalra M. Springer; New York: 2015. Results of Open and Endovascular Revascularization for Acute Mesenteric Ischemia; pp. 265–276. [Google Scholar]
- 37.Arthurs Z M, Titus J, Bannazadeh M.A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia J Vasc Surg 20115303698–704., discussion 704–705 [DOI] [PubMed] [Google Scholar]
- 38.Semmlow J L, Orland P J, Reddell M T, Brolin R E. Evaluation of quantitative approaches to assessment of bowel viability. Biomed Instrum Technol. 1997;31(06):591–599. [PubMed] [Google Scholar]
- 39.Mulholland J H, Ellison E H, Friesen S R. Philadelphia: Saunders; 1957. Current surgical management; a book of alternative viewpoints on controversial surgical problems; pp. 1957–1965. [Google Scholar]
- 40.Guzmán-de la Garza F J, Ibarra-Hernández J M, Cordero-Pérez P. Temporal relationship of serum markers and tissue damage during acute intestinal ischemia/reperfusion. Clinics (São Paulo) 2013;68(07):1034–1038. doi: 10.6061/clinics/2013(07)23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang J X, Chen S, Ma L P. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol. 2005;11(35):5485–5491. doi: 10.3748/wjg.v11.i35.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone W C, Bjorling D E, Southard J H, Galbreath E J, Lindsay W A. Evaluation of intestinal villus height in rats after ischemia and reperfusion by administration of superoxide dismutase, polyethylene glycol-conjugated superoxide dismutase, and two 21-aminosteroids. Am J Vet Res. 1992;53(11):2153–2156. [PubMed] [Google Scholar]
- 43.Illyés G, Hamar J. Sequence of morphological alterations in a small intestinal ischaemia/reperfusion model of the anesthetized rat. A light microscopy study. Int J Exp Pathol. 1992;73(02):161–172. [PMC free article] [PubMed] [Google Scholar]
- 44.Kundi R, Rasmussen T E. Springer; New York, NY: 2015. Second-Look Laparotomy, the Open Abdomen, and Temporary Abdominal Closure in Acute Mesenteric Ischemia; pp. 253–263. [Google Scholar]
- 45.Bryski M G, Frenzel Sulyok L G, Kaplan L, Singhal S, Keating J J. Techniques for intraoperative evaluation of bowel viability in mesenteric ischemia: a review. Am J Surg. 2020;220(02):309–315. doi: 10.1016/j.amjsurg.2020.01.042. [DOI] [PubMed] [Google Scholar]
- 46.Bulkley G B, Zuidema G D, Hamilton S R, O'Mara C S, Klacsmann P G, Horn S D. Intraoperative determination of small intestinal viability following ischemic injury: a prospective, controlled trial of two adjuvant methods (Doppler and fluorescein) compared with standard clinical judgment. Ann Surg. 1981;193(05):628–637. doi: 10.1097/00000658-198105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tollefson D F, Wright D J, Reddy D J, Kintanar E B. Intraoperative determination of intestinal viability by pulse oximetry. Ann Vasc Surg. 1995;9(04):357–360. doi: 10.1007/BF02139407. [DOI] [PubMed] [Google Scholar]
- 48.Freeman D E, Gentile D G, Richardson D W. Comparison of clinical judgment, Doppler ultrasound, and fluorescein fluorescence as methods for predicting intestinal viability in the pony. Am J Vet Res. 1988;49(06):895–900. [PubMed] [Google Scholar]
- 49.Mordon S, Devoisselle J M, Soulie-Begu S, Desmettre T. Indocyanine green: physicochemical factors affecting its fluorescence in vivo. Microvasc Res. 1998;55(02):146–152. doi: 10.1006/mvre.1998.2068. [DOI] [PubMed] [Google Scholar]
- 50.Liot E, Assalino M, Buchs N C. Does near-infrared (NIR) fluorescence angiography modify operative strategy during emergency procedures? Surg Endosc. 2018;32(10):4351–4356. doi: 10.1007/s00464-018-6226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karampinis I, Keese M, Jakob J. Indocyanine green tissue angiography can reduce extended bowel resections in acute mesenteric ischemia. J Gastrointest Surg. 2018;22(12):2117–2124. doi: 10.1007/s11605-018-3855-1. [DOI] [PubMed] [Google Scholar]
- 52.Beaulieu R J, Arnaoutakis K D, Abularrage C J, Efron D T, Schneider E, Black J H., III Comparison of open and endovascular treatment of acute mesenteric ischemia. J Vasc Surg. 2014;59(01):159–164. doi: 10.1016/j.jvs.2013.06.084. [DOI] [PubMed] [Google Scholar]
- 53.Cocorullo G, Mirabella A, Falco N. An investigation of bedside laparoscopy in the ICU for cases of non-occlusive mesenteric ischemia. World J Emerg Surg. 2017;12:4. doi: 10.1186/s13017-017-0118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alemanno G, Somigli R, Prosperi P. Combination of diagnostic laparoscopy and intraoperative indocyanine green fluorescence angiography for the early detection of intestinal ischemia not detectable at CT scan. Int J Surg Case Rep. 2016;26:77–80. doi: 10.1016/j.ijscr.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paral J, Cecka F, Chobola M. Fluorescein dye and ultraviolet light technique in diagnosis of small bowel ischaemia. ANZ J Surg. 2010;80(10):762–763. doi: 10.1111/j.1445-2197.2010.05475.x. [DOI] [PubMed] [Google Scholar]


