Abstract
Purpose
This is an in-vitro experimental study to analyze the effect of Exo-HUVEC on endothelial cell (CD31), cell proliferation, matrix metalloproteinase 1 (MMP-1) and collagen type 1 on irradiated fibroblast with UVB as photo-aging model.
Patients and Methods
Fibroblast cultures were divided into 5 groups, namely without UVB exposure, UVB exposure 600mJ/cm2 for 80 seconds as photo-aging model, and UVB exposure +Exo-HUVEC exposure 0.1%, 0.5% and 1%. The endothelial cell was stained with a CD31 marker, MMP-1 were examined with ELISA, cell proliferation is detected using an MTT assay; meanwhile, collagen type 1 deposition and endothelial cell were measured using flowcytometry.
Results
This study found positive endothelial cell marker CD31. Significant difference was found in cell proliferation, MMP-1 and collagen type 1 level between the control group with UVB irradiation and the treatment group with Exo-HUVEC (p < 0.05).
Conclusion
Exo-HUVEC significantly increases cell proliferation and collagen type 1 level, while decrease MMP-1 levels on irradiated fibroblast; therefore, Exo-HUVEC ameliorate the photo-aging of skin fibroblast.
Keywords: photo-aging, Exo-HUVEC, endothelial cell, cell proliferation, MMP-1, collagen type 1
Graphical Abstract
Video abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
The linings of the whole blood circulatory system are endothelial cells.1 Human Umbilical Vein Endothelial Cell (HUVEC) is a source of endothelial cells.2–4 HUVEC has been recognized as a helpful model for human endothelium research.4 Conditioned media or secretomes are therapeutic modalities that have been shown to have effects equivalent to the paracrine effects of the stem cells. Stem cell therapy, especially those from mesenchymal origin, is widely used as anti-aging therapy because it can repair degenerated tissue. Cell-based therapies have a risk of rejection, and cells can grow excessively out of control. These conditions led to the development of cell-free therapies. Exo-HUVEC is one of the cell-free therapeutic modalities that has been shown to have a therapeutic effect equivalent to the paracrine effects of the originating cells, which has a less rejection reaction and is cancer-free.5,6 The exosomes of the HUVEC secretome have the same properties as endothelial cell. Exosomes have an important function in cell communication, both paracrine and autocrine. At the time, exosomes were in great demand as a therapy for various degenerative diseases and wound healing.7,8
The molecular and signaling pathways involved in angiogenesis have been studied using HUVEC models.3,4 Exo-HUVEC is rich in growth factor components, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and transforming growth factor-β (TGF-β). Exo-HUVEC can increase fibroblast migration, proliferation, and collagen synthesis.2,3
Photo-aging occurs in early skin aging process caused by UV light radiation, particularly UVB, in chronically photodamaged skin. The incidence of photo-aging is up to 80–90% in some studies and most common in men (72%) than in women (47%).9 UVB irradiation induces the production of reactive oxygen species (ROS) and direct DNA damage. ROS induces activation of mitogen-activated protein kinase (MAPK), nuclear factor kB (NF-kB), and activator protein-1 (AP-1). It leads to increased transcription of matrix metalloproteinase (MMP), especially MMP-1. An increase in MMP-1, the major MMP mediator of the collagen cycle, causes collagen fragmentation. Collagen type 1 is an important component of human skin, and a decrease in collagen type 1 leads to wrinkles. Molecular changes in the skin caused by photoaging lead to decreased collagen synthesis, fibroblast proliferation and migration.5,10–15 Photo-aging can decrease angiogenesis, blood vessels are reduced in persistently UV-damaged human skin.16 Angiogenesis is a critical therapeutic target since anti-angiogenic therapy has proven to be a successful treatment option in many diseases.17 Angiogenesis cannot begin without the assistance of endothelial cells, which are responsible for the formation of microvascular networks, besides creating the blood clots during the hemostasis phase of wound healing.3 Angiogenesis is linked to fibroblast proliferation, collagen synthesis, and wound re-epithelialization in addition to transporting oxygen and nutrients.5
Nowadays, there are still many demands in studies of the exosome. Exosome, a micro-vesicle with 30–100 nm in diameter, isolated in many materials, including secretome, is a metabolic product that carries messenger ribonucleic acid (mRNA) and micro-RNA (miRNA). The exosome is a critical paracrine secretion that acts as an information transfer mediator in cell communication through bioactive protein, mRNA, and miRNA inside of it. Nano-sized exosomes allow them to reach the whole body quickly, and it is non-immunogenic, so there will not be any rejection reaction.18–20
Therefore, the influence of Exo-HUVEC on endothelial cells, cell proliferation, MMP-1, and collagen type 1 level in photo-aging skin model was investigated in this study. UVB was utilized to expose fibroblast cells that were employed as photo-aging skin models.
Materials and Methods
Research Design and Data Collection
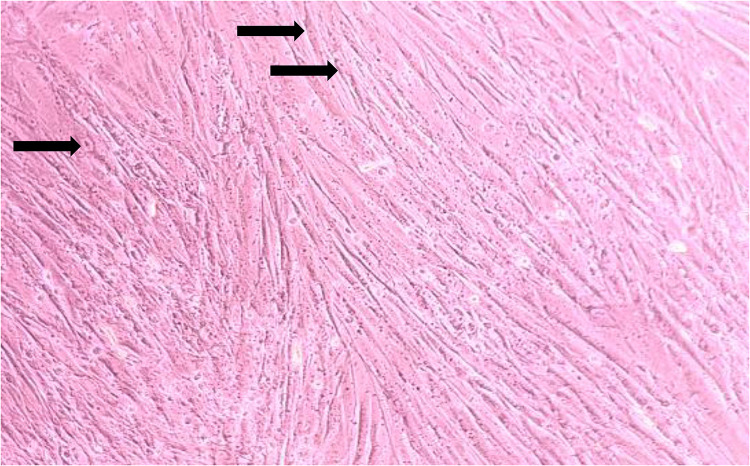
This research is an experimental post-test control group design study. Primary fibroblast cultures were taken from the preputium of post-circumcised children after the parents agreed and proved by informed consent. The preputium was used in this study because it was free from UV exposure and did not undergo photo aging. After being placed in transport media, Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) was added with 10% fetal bovine serum (Gibco, USA), 1% penicillin-streptomycin (Gibco, USA), and 1% fungizone (Gibsco, USA). The preputium was centrifuged with polypropylene and washed three times with PBS (Gibco, USA). The sample was given trypsin to separate the epidermis from the dermis. After separation, the models were incubated in 5% PBS. DMEM was given as culture medium, and then culture was checked every 3–4 days until confluent. Figure 1 shows the morphology of fibroblast cells ready for culture.
Figure 1.
Morphology of fibroblast cells.
Note: Black arrows ➔ Stellate fibroblast cells.
Fibroblast culture is recognized as the standard material in tissue engineering. UVB irradiated dermal fibroblast cultures showed changes in molecular composition, proving that human dermal fibroblasts exposed to UVB irradiation can be used as a photo-aging skin model to assess aging. This study was conducted at Dermama Biotechnology Laboratory of Surakarta and Pathology Clinic of Gajah Mada University. This study was approved by the Health Research Ethical Committee of Dr. Moewardi Hospital/Faculty of Medicine, Sebelas Maret University, Surakarta, Central Java, Indonesia (785/VIII/HREC/2021).
Exo-HUVEC Isolation
HUVEC was taken from the proximal part of the infant umbilical cord in cesarean section after the parents agreed and proved by informed consent. After being cut proximally, the umbilicus was cleaned from blood by venipuncture. After the venipuncture, within 24 hours the umbilicus should arrive at the laboratory. The umbilicus was cannulated and cleaned from the left blood with 0.2% buffer and collagenase, and then incubated for 15 minutes at 37°C. The endothelial and subendothelial layers were collected using a centrifuge and washed with PBS three times. The cultures were placed on discs and allowed to grow for three days. After fibroblastoid formation, the cells were subcultured by the warm trypsin method.
The HUVEC secretome was transferred into a sterile tube and Invitrogen ® exosome isolation reagent was added. Samples were incubated overnight at 2–80°C and centrifuged. After being centrifuged, the supernatant was removed, and the pellet at the bottom of the tube was left. The pellets were suspended with PBS and purified by the affinity method. The exosome was isolated at 2–80°C for a week
UVB Irradiation
Waldmann NB-UVB type 109 was used for UVB irradiation. The irradiation dose was 600mJ/cm2 and is used for 80 seconds to all well. Before, the cell was incubated for 24 hours in 37° Celsius and 5% CO2.
Detection for Endothelial Cell, Cell Proliferation, MMP-1 and Collagen Type 1 Level
The endothelial cell was stained with a CD31 marker (Thermo Fisher Scientific, USA). CD 31 as a microparticle biomarker is increased in endothelial dysfunction, which can be found from the endothelium when the endothelium undergoes activation or apoptosis.21 MMP-1 (Novus Biologicals, USA) were examined with ELISA. Meanwhile, cell proliferation is detected using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl-tetrazolium bromide] assay. MTT result was read with microplate reader (iMARKTM, BIO-RAD). Flow cytometry detected the Collagen type-1 level and the endothelial cell; we used the (BD FACS Canto II) flow cytometer. Collagen type 1 antibody (fluorescein-conjugated Rockland) was added as a primary antibody.
Experimental Design
The research design was a post-test-only control group design. The sample of this study was divided into five groups, each group consisting of 5×104 fibroblast cultures. Namely, K1: control group without UVB irradiation (normal skin model); K2: control group with UVB irradiation; P1: treatment group with 0.1% Exo-HUVEC; P2: treatment group with 0.5% Exo-HUVEC; P3: treatment group with 1% Exo-HUVEC. All the treatments were given once each. The group with UVB irradiation was given 600 mg/cm2 light dose UVB for 80 seconds and incubated at 37°C for a day.
Statistical Analysis
All data of endothelial cell, cell proliferation, MMP-1 and collagen type 1 level were analyzed using SPSS software for Windows. Average distribution data were analyzed with one-way ANOVA by a post-hoc test. While, if the data were not in a normal distribution, the analysis used were Kruskal Wallis followed by the Mann Whitney test. A P-value of <0.05 is considered statistically significant.
Results
Endothelial Cell of Exo-HUVEC
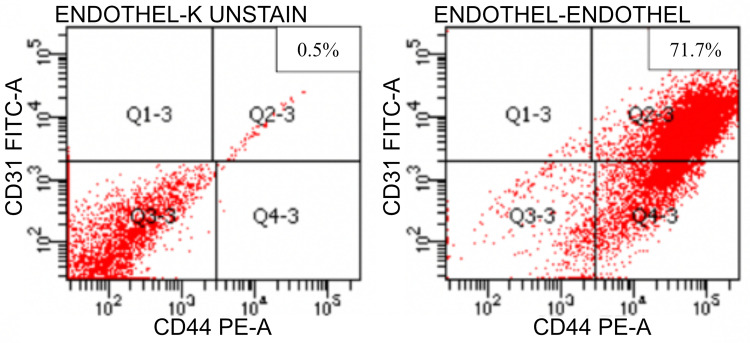
Flow cytometry analysis of CD31 expression, as seen in Figure 2, showed that endothelial cell adhesion molecule increased in the stained group by 71.7% compared to unstained 0.5%.
Figure 2.
Flow cytometry result in CD31 expression (left: unstained; right: stained).
Cell Proliferation
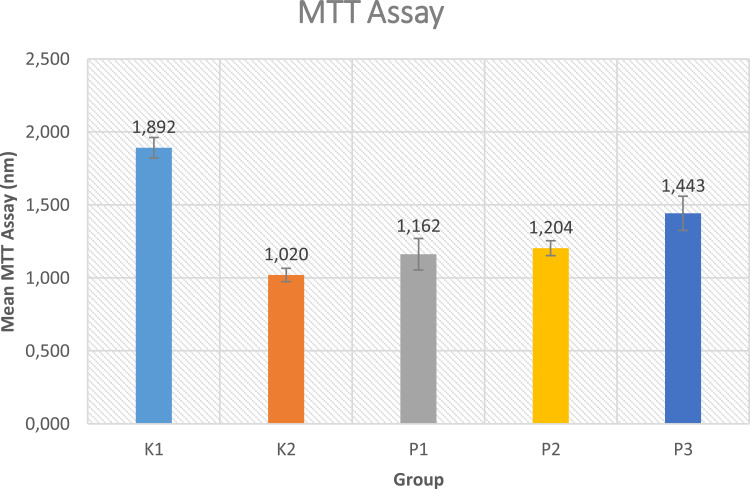
Cell proliferation rate was measured by using MTT assay. Figure 3 reports that the highest mean of cell proliferation seen from MTT assay is found in the control group without UVB irradiation (K1) (1.892±0.070). Meanwhile, in the treatment group, the highest mean of cell proliferation is located in the treatment group with 1% Exo-HUVEC (P3). The lowest mean among all the groups is found in the control group with UVB irradiation (K2). The data was followed with post-hoc Mann Whitney analysis. Even though 0.5% Exo-HUVEC (P2) do not have a significant result than 0.1% Exo-HUVEC (P1), there is still an increased cell proliferation.
Figure 3.
Mean MTT Assay. K1: control group without UVB irradiation, K2: Control group with UVB irradiation, P1: 0.1% Exo-HUVEC, P2: 0.5% Exo-HUVEC, P3: 1% EXO-HUVEC.
MMP-1 Level
The highest mean MMP-1 level is found in the control group with UVB irradiation. The results in Figure 4 show, among the treatment group, the highest level of MMP-1 is located in the group with the lowest Exo-HUVEC concentration (0.1%; 43481). The analysis was followed with the Mann Whitney test and there was significant difference between the control and treatment groups, respectively (P < 0.05). This study found that the lowest MMP-1 was in the control group without UVB irradiation (K1), while the highest MMP-1 level was in the control group with UVB irradiation (K2). MMP-1 had its lowest level among all of the treatment group in 1% Exo-HUVEC concentration (P3).
Figure 4.
Mean MMP-1 level. K1: control group without UVB irradiation, P1: 0.1% Exo-HUVEC, P2: 0.5% Exo-HUVEC, P3: 1% Exo-HUVEC.
Collagen Type-1 Level
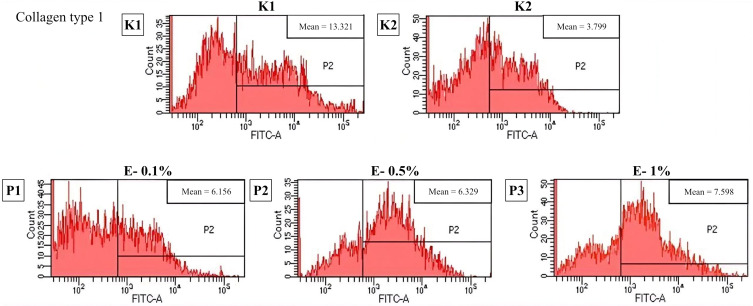
Based on Figure 5, the highest mean of collagen type 1 level is found in the control group without UVB irradiation (K1). Between treatment group, the highest level of collagen type 1 is found in group with 1% Exo-HUVEC (P3) (7.598±0.040). P-value is 0.001 (<0.05) means that there is a significant difference in effectivity in collagen type 1 level in photo-aging skin before Exo-HUVEC administration. A post-hoc test followed the analysis. There is a significant difference (p < 0.001) between all groups except the treatment group with 0.1% Exo-HUVEC (P1) compared to 0.5% Exo-HUVEC group (P2).
Figure 5.
Flowcytometry result of collagen type 1 level. K1: control group without UVB irradiation, K2: control group with UVB irradiation, P1: 0.1% Exo-HUVEC, P2: 0.5% Exo-HUVEC, P3: 1% Exo-HUVEC.
Discussion
Endothelial Cell of Exo-HUVEC
The findings obtained in Figure 2 indicate that HUVEC is an endothelial cell with an angiogenesis factor. This finding correlates with a previous study; Liang et al found that exosome secreted by mesenchymal stem cells promotes endothelial cells and Joo et al reported that HUVEC secretomes can increase angiogenic potential by upregulating tissue-derived Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2) signaling pathways.22,23 Another study by Gong et al using immunofluorescence staining also found that the number of CD31 positive cells in exosome-containing plugs was considerably higher than in non-exosome-containing pins in HUVEC.24
Endothelial cell proliferation drives capillary progress in the ECM during angiogenesis, while chemotaxis from the target location guides growth direction. Endothelial cells, angiogenesis factors, and surrounding ECM proteins interact in a time and space synchronized manner. Angiogenesis occurs as a sequence of molecular and cellular activities.25,26
Cell Proliferation
Result in Figure 3 found the highest concentration of Exo-HUVEC (1%) has the most increased cell proliferation seen in MTT assay. This finding is correlated with a previous study by Zhao et al,3 who reported the role of GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. This study found that GeIMA dressing combined with HUVEC-Exos improved wound healing by triggering re-epithelialization, collagen deposition, and angiogenesis. Besides that, the concentration of Exo-HUVEC that significantly increased proliferation in our study was 1% concentration. This finding is consistent with the study by Leszczynska et al,27 who investigated the role of human bone-derived endothelial cells (HBDC) in combination with or without HUVEC in the process of bone remodeling (osteoblast formation). This study found a significant increase in osteoblast proliferation with HBDC: HUVEC in a ratio of 1:4 compared to HBDC alone or HBDC: HUVEC, 1:1 and 4:1. Therefore, the concentration of HUVEC does affect the tissue remodeling process.3,27
Endothelial cells, as in Exo-HUVEC, are crucial even though the entire wound healing process after the wound has occurred, particularly in angiogenesis. Angiogenesis is a process in creating new blood vessels from the old vasculature. It supplies nutrients and oxygen to the wound site and is essential for fibroblast proliferation, collagen production, and re-epithelialization.8 Although endothelial cells have been shown to promote angiogenesis in the wound healing process, the exact mechanism is still uncertain.3 According to recent research, paracrine pathways are primarily responsible for cell-to-cell communication. Because of their ability to transmit RNAs and proteins to target cells, exosomes play an essential role in intercellular communication.28 Angiogenesis is known to be influenced by a variety of growth factors and signaling pathways, as well as the balance of pro- and anti-angiogenic factors.22
Exo-HUVEC contains many growth factors, including vascular endothelial growth factor (VEGF), keratinocyte growth factor (KGF), transforming growth factor (TGF)-β1, TGF-β2, basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), platelet-derived growth factor-AA (PDGF-AA), dan placental growth factor (PGF).17 These growth factors induced keratinocytes and fibroblasts to migrate from the wound’s perimeter to the center during the proliferative phase. Keratinocytes are responsible for wound re-epithelialization, while fibroblasts are responsible for extracellular matrix (ECM) formation.2,3
MMP-1 Level
MMP-1-related results obtained in Figure 4 was in line with Song et al, who studied that adipose-derived stem cells with HUVEC were seeded on top of the stem cells. It was reported that HUVEC lowering MMP level and subsequently inhibited sprouting of new blood vessels.29 Irradiation of UVB induces the production of reactive oxygen species (ROS) and direct DNA damage.30 ROS induce activation of mitogen-activated protein kinase (MAPK), nuclear factor-kB (NF-kB), and activator protein-1 (AP-1) that lead to increased transcription of matrix-metalloproteinase (MMP), especially MMP-1. Increased MMP-1 will promote collagen degradation.12,15
Collagen Type-1 Level
From the data presented in Figure 5, the finding is correlated with a previous study, Kim et al found that mesenchymal exosomes originating from the umbilical cord can absorb into human skin and promote collagen type 1 synthesis.8 Meanwhile, particularly for HUVEC matter, this study also has similar finding with Sgarioto et al, who reported the use of a double coating of collagen + fibronectin in HUVEC improves the capacity of vascular implants to become and stay endothelialized by providing a better support structure for endothelial cell development.31
The extracellular matrix consists of collagen, mainly collagen type 1 (85–90%), elastin fiber, and basic substance. UVB irradiation induces collagen fragmentation by activating MAPK, AP-1, and NF-kB pathways. Mesenchymal stem cells release cytokine and growth factors including EGF and bFGF that play an important role in skin rejuvenation and collagen synthesis. We suggest further research can use the senescence cellular examination, such as cellular senescence associated (SA)-β-galactosidase staining.
The limitation of this study is that there are several methods and raw materials for exosomes from other HUVEC secretomes, which can give different exosome results. The administration of exosomes from HUVEC secretomes has not been clinically implemented after this study, so it is necessary to conduct in vivo studies of other pathways and clinical studies in humans to determine the effect of administering exosomes from HUVEC secretomes on photoaging.
Conclusion
This study found that Exo-HUVEC significantly increases cell proliferation and collagen type-1 synthesis in human dermal fibroblast irradiated by UVB that reflect photo-aging skin model. Exo-HUVEC can also lower MMP-1 levels, so it proves the effects of Exo-HUVEC as pro-proliferative and promote collagen synthesis in photo-aging skin. Thus, these findings could imply potential efficacy of Exo-HUVEC as one of therapeutic modalities in photo-aging. Further research is needed to analyze the effect of Exo-HUVEC in vivo.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bodnar RJ. Chemokine regulation of angiogenesis during wound healing. Adv Wound Care. 2015;4(11):641–650. doi: 10.1089/wound.2014.0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fallah A, Sadeghinia A, Kahroba H, et al. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed Pharmacother. 2019;110:775–785. doi: 10.1016/j.biopha.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 3.Zhao D, Yu Z, Li Y, Wang Y, Li Q, Han D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J Mol Histol. 2020;51(3):251–263. doi: 10.1007/s10735-020-09877-6 [DOI] [PubMed] [Google Scholar]
- 4.Kant V, Gopal A, Kumar D, et al. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res. 2015;193(2):978–988. doi: 10.1016/j.jss.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi: 10.1177/0963689717725755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung JH, Eun HC. Angiogenesis in skin aging and photoaging. J Dermatol. 2007;34(9):593–600. doi: 10.1111/j.1346-8138.2007.00341.x [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917. doi: 10.2147/IJN.S264498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YJ, Mi Yoo S, Park HH, et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem Biophys Res Commun. 2017;493(2):1102–1108. doi: 10.1016/j.bbrc.2017.09.056 [DOI] [PubMed] [Google Scholar]
- 9.Ayer J, Griffiths CEM. CHAPTER 1. Photoaging in Caucasians. Royal Society of Chemistry; 2019:1–30. doi: 10.1039/9781788015981-00001 [DOI] [Google Scholar]
- 10.Lee C-H, Wu S-B, Hong C-H, Yu H-S, Wei Y-H. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: the implication in UV-based phototherapy. Int J Mol Sci. 2013;14(3):6414–6435. doi: 10.3390/ijms14036414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavinato M, Koziel R, Romani N, et al. UVB-induced senescence of human dermal fibroblasts involves impairment of proteasome and enhanced autophagic activity. J Gerontol Ser A Biol Sci Med Sci. 2016;glw150. doi: 10.1093/gerona/glw150 [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Asamitsu K, Uranishi H, et al. Protecting skin photoaging by NF-κB inhibitor. Curr Drug Metab. 2010;11(5):431–435. doi: 10.2174/138920010791526051 [DOI] [PubMed] [Google Scholar]
- 13.Wirohadidjojo YW, Budiyanto A, Soebono H. Platelet-rich fibrin lysate can ameliorate dysfunction of chronically UVA-irradiated human dermal fibroblasts. Yonsei Med J. 2016;57(5):1282. doi: 10.3349/ymj.2016.57.5.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu P, Xin Y, Zhang Z, et al. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res Ther. 2020;11(1):264. doi: 10.1186/s13287-020-01777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Wang L, Wen X, et al. NF-κB signaling in skin aging. Mech Ageing Dev. 2019;184:111160. doi: 10.1016/j.mad.2019.111160 [DOI] [PubMed] [Google Scholar]
- 16.Park H-J, Zhang Y, Georgescu SP, Johnson KL, Kong D, Galper JB. Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis. Stem Cell Rev. 2006;2(2):93–101. doi: 10.1007/s12015-006-0015-x [DOI] [PubMed] [Google Scholar]
- 17.Medina-Leyte DJ, Domínguez-Pérez M, Mercado I, Villarreal-Molina MT, Jacobo-Albavera L. Use of Human Umbilical Vein Endothelial Cells (HUVEC) as a model to study cardiovascular disease: a review. Appl Sci. 2020;10(3):938. doi: 10.3390/app10030938 [DOI] [Google Scholar]
- 18.Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A Protocol for Exosome Isolation and Characterization: Evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. Humana Press Inc; 2015:179–209. doi: 10.1007/978-1-4939-2550-6_15 [DOI] [PubMed] [Google Scholar]
- 19.Guo S-C, Tao S-C, Yin W-J, Qi X, Yuan T, Zhang C-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7(1):81–96. doi: 10.7150/thno.16803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao F, Lei B, Li X, et al. Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials. 2018;178:36–47. doi: 10.1016/j.biomaterials.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 21.Rahmawaty I, Garina LA. A literature review: biomarker CD 31+ as a sign of endothelial dysfunction in children and adults. Med Technol Environ Health. 2020;2020:123–127. [Google Scholar]
- 22.Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring mir-125a. J Cell Sci. 2016;129:2182–2189. doi: 10.1242/jcs.170373 [DOI] [PubMed] [Google Scholar]
- 23.Joo HJ, Song S, Seo HR, et al. Human endothelial colony forming cells from adult peripheral blood have enhanced sprouting angiogenic potential through up-regulating VEGFR2 signaling. Int J Cardiol. 2015;197:33–43. doi: 10.1016/j.ijcard.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 24.Gong M, Yu B, Wang J, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8(28):45200–45212. doi: 10.18632/oncotarget.16778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bang C, Thum T. Exosomes: new players in cell–cell communication. Int J Biochem Cell Biol. 2012;44(11):2060–2064. doi: 10.1016/j.biocel.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 26.Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–3885. doi: 10.1007/s00018-016-2268-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leszczynska J, Zyzynska-Granica B, Koziak K, Ruminski S, Lewandowska-Szumiel M. Contribution of endothelial cells to human bone-derived cells expansion in coculture. Tissue Eng Part A. 2013;19(3–4):393–402. doi: 10.1089/ten.TEA.2011.0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuel M, Brooke R, Hollis S, Griffiths CEM. Interventions for photodamaged skin. Cochrane Database Syst Rev. 2015;2015(6):CD001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song YH, Shon SH, Shan M, Stroock AD, Fischbach C. Adipose-derived stem cells increase angiogenesis through matrix metalloproteinase-dependent collagen remodeling. Integr Biol. 2016;8(2):205–215. doi: 10.1039/C5IB00277J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulms D, Schwarz T. Molecular mechanisms of UV-induced apoptosis. Photodermatol Photoimmunol Photomed. 2000;16(5):195–201. doi: 10.1034/j.1600-0781.2000.160501.x [DOI] [PubMed] [Google Scholar]
- 31.Sgarioto M, Vigneron P, Patterson J, Malherbe F, Nagel M-D, Egles C. Collagen type I together with fibronectin provide a better support for endothelialization. C R Biol. 2012;335(8):520–528. doi: 10.1016/j.crvi.2012.07.003 [DOI] [PubMed] [Google Scholar]