Abstract
Gap junction channels, once clustered into gap junction plaques, allow communication of essential metabolites between cells. Gap junction plaques have been reported to be lost from the cell surface during cell division. The mechanism involved in this loss of gap junction plaques during mitosis is unclear but we hypothesize that an endoexocytotic mechanism that results in cytoplasmic double-membraned annular gap junction vesicles is involved. In this study, gap junction plaque changes in dividing cells were examined in SW-13 adrenocortical tumor cells. Endogenous gap junction protein, connexin 43 (Cx43), was detected with immunofluorescence, and live-cell imaging was used to monitor green fluorescent protein-tagged Cx43 (Cx43-GFP). Mitotic stages were identified by Hoechst chromosomal staining. During interphase, large gap junction plaques were detected, however the presence of these plaques decreased while cytoplasmic puncta increased beginning with prophase. The cytoplasmic puncta were demonstrated with immuno-electron microscopy to be Cx43- positive annular gap junction vesicles. As gap junction plaques reformed at cleavage furrows between daughter cells, and the number of annular gap junctions decreased during cytokinesis. The data is consistent with the mechanism of gap junction plaque loss during mitosis relying on an endoexocytotic process that results in annular gap junction vesicles formation. The rapid formation of gap junction plaques during cytokinesis points to the intriguing possibility of a connexin recycling from annular gap junction vesicles to form gap junction plaques as mitosis is completed.
Keywords: Annular Gap Junction Vesicles, Mitosis
1. INTRODUCTION
Gap junction intercellular communication involves the movement of signaling molecules and metabolites between cells by means of surface membrane channels (Goodenough et al., 1996). These channels are composed of gap junction proteins, called connexins (Kumar and Gilula, 1992). Connexin 43 (Cx43) is one of the most abundantly distributed members of the connexin protein family of which, in the human, there are twenty others that have been identified (Goodenough et al., 1996). Although gap junction-mediated communication has been suggested to play a critical role in regulating cell proliferation (Kanemitsu et al., 1998; Lampe et al., 1998; Lampe and Lau, 2000; Wynn et al., 2002), several different lines of evidence point to a loss of cell-cell communication during mitosis (Wilson et al., 2000; Goodall and Maro, 1987; Kanemitsu et al., 1997; Doble et al., 2004, Solan et al., 2003; Vincken et al., 2006). In dye communication studies, for example, gap junction-mediated communication was dramatically reduced during mitosis (Stein et al., 1992; Xie et al., 1997; Doble, et al., 2004, Solan et al., 2003; Vincken M. et al., 2006; Laird, 2005). Consistent with these findings, a decrease in the number of gap junction plaques was detected in dividing cells, with immunofluorescence and ultrastructural techniques (Boassa et al., 2010; Murray et al., 1981; Stein et al., 1992; Vinken et al., 2006). It has been previously reported that once the mitotic process is completed, gap junction communication is rapidly resumed without the need for new Cx43 protein synthesis (Xie et al., 1997). Along these same lines of evidence, Boassa and colleagues demonstrated that gap junction plaques were re-established at the cell surface from an “older” Cx43 pool, after the completion of mitosis, rather than from de novo Cx43 synthesis of gap junction protein (Boassa, et al., 2010). In that study, the site of the older connexins that contributed to the formation of new gap junction plaques was not identified nor was the method of gap junction plaque loss demonstrated. The removal process and fate of gap junction plaque channels once removed from the surface is an important consideration. The loss of gap junction plaques could reflect either the disaggregation of gap junction plaques by lateral movement of channels in the membrane or the recovery of channels from an intracellular site, both events independent of Cx43 synthesis. It has been well established however that portions or entire gap junction plaques can be removed from the cell surface (termed “endoexocytosis”) to form spherical, cytoplasmic, double-membraned annular gap junction vesicles in one of the two contacting cells (Jordan et al., 2001; Larsen & Hai, 1978; Larsen et al., 1979; Nickel et al., 2008; Nickel et al., 2013; Murray et al., 2004). We suggest that the removal of gap junctions from the cell surface during mitosis also occurs by an endoexocytotic process that results in the formation of annular gap junctions.
Here we investigate the Cx43 gap junction changes during mitosis with the human SW-13 adrenal cortical cell line as a model system in order to elucidate the mechanism of gap junction plaques loss from the surface of mitotic cells. We report the rapid removal of the gap junction from the cell surface and the formation of annular gap junction vesicles during mitosis. Further, we suggest the possibility that the annular gap junctions may contribute to the re-establishment of gap junction plaques at the end of the mitotic process.
2. MATERIALS AND METHODS
2.2. Cell Line
The cell line used in this study was a SW-13 human adrenocortical tumor cell line obtained from ATCC (Manassas, Virginia). These cells form numerous large gap junctions that are spontaneously internalized to form annular gap junction vesicles (Murray et al., 1981). As previously described, the cells were grown at 37°C under 5% CO2 in Leibovitz’s L15-medium (Gibco, Grand Island, NY) containing 200 U/ml penicillin, 200 μg/ml streptomycin, 5mg/ml fungizone (Amphotericin B), and 10% fetal calf serum (GIBCO, Grand Island, NY) (Defranco et al., 2008).
2.3. Transfection with cDNA
To visualize gap junction structures in living cells, cells were transfected with cDNA encoding for a fluorescent Cx43-GFP or GFP control vector (provided by Dr. M. Falk, Lehigh University). The Cx43-GFP was constructed by linking the GFP fluorescent reporter protein to the C-terminus of the rat Cx43 cDNA. This Cx43-GFP fusion protein has been well characterized and found to assemble into gap junction plaques similar to wild type Cx43 (Falk 2000, Nickel et al., 2008 and 2013). Lipofectamine 2000 (Invitrogen, Carlsbad, CA) transfection reagent was used to establish cell populations that transiently express fluorescently tagged Cx43, empty vector, or GFP. Specifically, cell populations expressing Cx43-GFP were established by treating cells grown to 70-80% confluency in 35 mm culture dishes, with 2 ml of Opti-MEM medium (GIBCO, Carlsbad, CA) which contained 10 μl Lipofectamine 2000 transfection reagent and 4 μg of plasmid DNA (Cx43-GFP DNA or GFP DNA) for six hours at 37°C in an atmosphere of 5% CO2. This transfection medium was removed by gentle aspiration and the cells were washed with phosphate buffered saline (PBS). Fresh L-15 complete cell growth medium was added to the dishes, and the cells were incubated at 5% CO2 for 24 hours before being processed for imaging.
2.3. Immunofluorescence
Immunofluorescence of gap junction proteins was performed as described previously (Oyoyo et al. 1997). Briefly, cells were grown to confluency on sterile coverslips. The coverslips were then fixed in 4% paraformaldehyde for 20 minutes at room temperature, permeabilized with cold acetone for 7 minutes, and washed several times with phosphate buffered saline (PBS). Primary antibody, rabbit polyclonal anti-connexin 43 (Zymed, Carlsbad, CA), was diluted (1:100) in a blocking solution (3% BSA), and then applied to the cells for 1 hour at 37°C in a humidity chamber. After washing 5 times in PBS, the coverslips were incubated with a secondary anti-rabbit antibody [Alexa-488 or Alexa-594 (Molecular Probes, Carlsbad, CA)] diluted (1: 1000) in 3% BSA blocking buffer for 1 hour at 37°C. After five washes in PBS, Hoechst 33342 nuclear stain (0.5 mg/ml) was applied for two minutes at room temperature. The coverslips were mounted in Fluoromont G (Southern Biotechnology Association Birmingham, AL) and imaged with an Olympus IMT2 or Nikon Microphot-FXA fluorescent microscope with a 60x oil objective (numerical aperture 1.4) and Metamorph Software (Universal Imaging). In some studies, cell cortical actin was stained with phalloidin Alexa-488 (Life Technologies, Carlsbad, CA) to assist in distinguishing the cell borders.
2.4. Analysis of Mitotic Stages
The mitotic stage of cells was determined with Hoechst nuclear staining. Cells at the stages of prophase, metaphase, anaphase, telophase and those undergoing cytokinesis were imaged.
2.5. Imaging of Gap Junction Plaques and Annular Vesicles
Gap junction plaque and cytoplasmic annular gap junction packet number, size and distribution were characterized with Olympus IMT2 or Nikon Microphot FXA fluorescence phase microscopes interfaced to an Optimas image analysis program.
2.6. Imaging of Cx43-GFP in Living Cells
Coverslips with cells expressing Cx43-GFP or GFP were placed into a temperature controlled FCS2 Bioptechs chamber (Butler, PA) and maintained at 37°C on a Zeiss Axovert 135 or the Nikon A1 microscopes. The culture media was supplemented with 10 mM HEPES at pH 7.2 during live cell imaging. Both differential interference contrast (DIC) and fluorescent microscopic images, obtained with standard Fluorescein isothiocyanate (FITC) filters (Chroma) with a 40x (numerical aperture 0.75) or 63x oil objective (numerical aperture 1.4), were collected at various intervals with an Olympus IX81 microscope equipped with a Hamamatsu ORCA ER camera. Metamorph software (Universal Imaging) was used to collect images. Focus was maintained by algorithms which established maximum contrast on the DIC image prior to each exposure. The focal plane thickness (Z-ordinate) was limited to 456 nm.
2.7. Transmission Electron Microscopy
Cell monolayers were fixed with 2.5% glutaraldehyde buffered with 0.05 M cacodylate followed by 1% osmium in 0.05 M cacodylate for 20 minutes, and then subsequently dehydrated in ethanol and embedded in Araldite (Cedar Grove, NJ) (Murray et al. 1981). Ultra thin sections were cut, mounted on grids, and imaged with a JEOL 1210 electron microscope.
2.8. Quantum Dot Immuno-electron Microscopy
Cells were fixed with 2% paraformaldehyde and 1% gluteraldehyde and permeabilized with 0.1% Triton X-100. Quantum dots (Q-dots) were then used to specifically label Cx43 gap junction protein with a modified procedure described by Murray and Shakespeare, 2015. Cells were incubated sequentially in Cx43 primary antibody, biotinylated secondary antibody and then in Streptavidin conjugated Q-dots [655 (red)] and then processed for fluorescent microscopy and TEM analysis (Murray and Shakespeare, 2015; Nickel et al., 2013; Giepmans et al., 2005).
3. RESULTS
3.1. Characterization of the SW-13 Cells
To assess the suitability of the cell population for studying gap junctions in mitotic cells, the behavior of the SW-13 cell population was characterized. Cells attached to the substrate and formed an epithelial-like monolayer of flattened cells within two hours of being seeded at subconfluent densities. Figure 1A (scanning electron microscopy) and 1B (phase contrast microscopy) show that a rounded SW-13 cell can remain attached to a layer of flattened cells. The same cell population demonstrated with phase contrast microscopy (1B) is shown with anti-Cx43 immunofluorescence in Figure 1C. There are more cytoplasmic puncta and fewer gap junction plaques in rounded cells than in cells of the monolayer.
Figure 1.
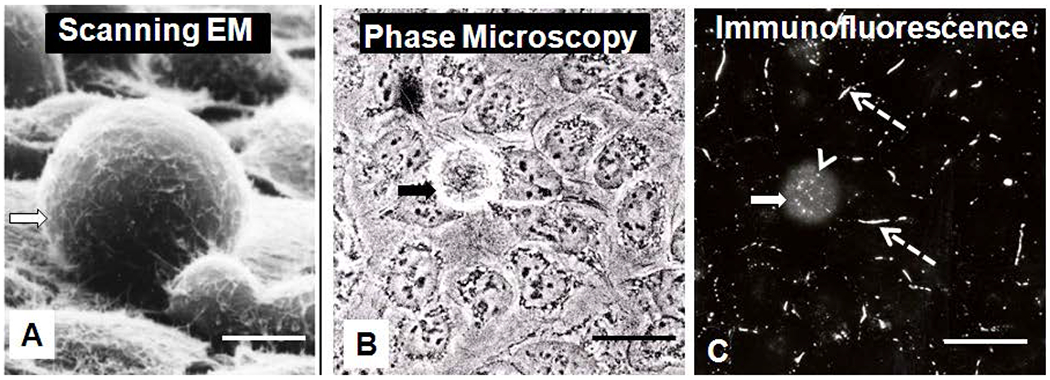
Characterization of SW-13 cell populations with scanning electron microscopy (SEM) (A), phase contrast (B), and Cx43 immunofluorescence (C). The figures B and C are images of the same cell populations, and the solid arrow points to a rounded cell which is attached to flattened cells within the monolayer. Note, the rounded cell has more cytoplasmic puncta (arrowhead) than flattened cells within the monolayer, while the flattened cells have more surface gap junction plaques (dotted arrows). Bars: 50μm A; 20μm B and C.
With time lapse imaging, the cells were seen to move around on the substrate and change positions within the monolayer, even after confluency was obtained (data not shown). The cells of the monolayer were observed to detach and re-attach to one another and, in some cases, to move under other cells in the population. Single cells within the monolayer were observed to round, detach into the medium, divide and either cluster with other rounded cells above the monolayer or, if space was available, they re-flattened to become part of the monolayer once again. As seen in figure 1, rounded cells could be easily distinguished from the flattened cells, with a number of different microscopic techniques. If the culture flask was agitated, rounded cells could be collected with the medium (Figure 2), while flattened cells remained attached to the substrate. The rounded cells were demonstrated with trypan blue dye exclusion technique to be 95% viable and most (86%) of the cells that rounded were observed with time-lapse microscopy to divide after rounding (results not shown). Only 14% of the rounded cells did not divide when viewed with time lapse imaging for as long as 42 hours. These non-dividing, rounded cells either remained in the medium or intermittently rounded and re-flattened without dividing. For the cells that divided, the two daughter cells flattened and became part of the monolayer once again. Once the monolayer became confluent, the rounded cells remained rounded and clustered with one another above the monolayer. The mitotic chromosome distribution could be detected in both the rounded cells that remained with the monolayer and in cells collected from the medium (results not shown).
Figure 2.
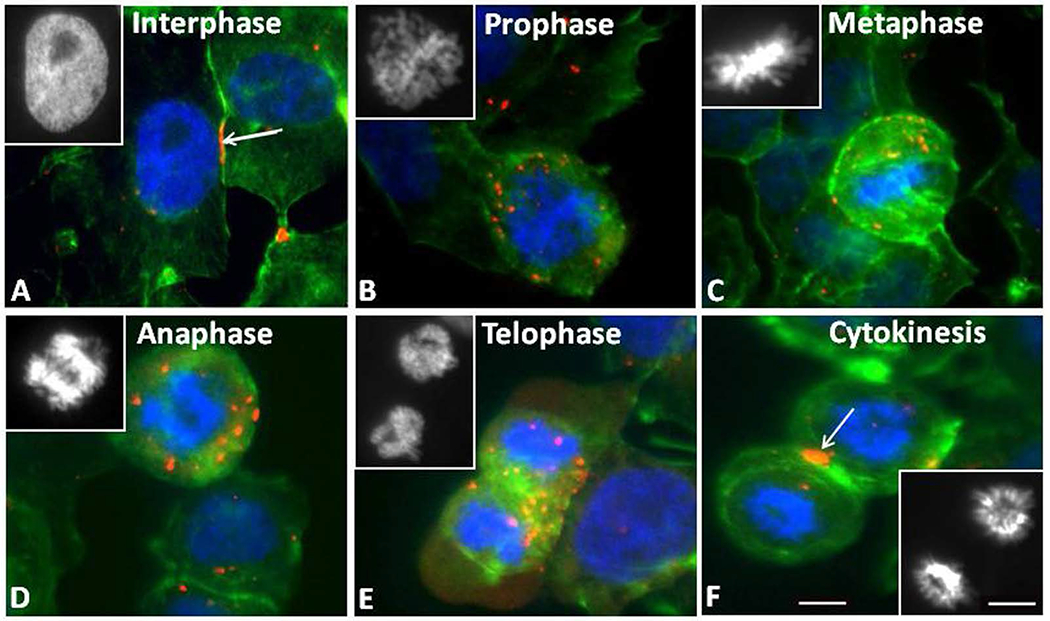
Cx43 immunofluorescence staining of SW-13 Cx43 gap junction protein (red) and nuclear chromatin with Hoechst Dye (blue) at different stages of mitosis. Gap junction plaques (arrows) and annular gap junction puncta (red) are seen in cells in interphase (A). The number of annular gap junctions was increased during prophase (B), metaphase (C), anaphase (D), and telophase (E). During cytokinesis, the punctate staining was reduced and large gap junction plaques were formed at the furrow between the two daughter cells. The cell peripheries are stained with FITC-actin phalloidin (green). The corresponding images of the chromatin can be seen in the inserts. Bars: 10μm A-F and inserts.
3.2. Characterization of Gap Junction Structures in SW-13 Cell Populations:
SW-13 cells expressed Cx43 protein that localized to areas of cell-cell contact in gap junction plaques or within cytoplasmic Cx43-GFP puncta (Figures 1C and 2A). By labeling the peripheral actin with FITC-phalloidin (green), Cx43 gap junction protein staining (red) at the cell surface could be distinguished from cytoplasmic staining (Figure 2A). It was confirmed that the majority of Cx43 were located in gap junction plaques found at sites of contact between cells, while smaller, punctate structures were seen within the cytoplasm (Figures 1C and 2A). Results from confocal microscopy observations are consistent with most of the punctate structures being within the cytoplasm and all of the long, linear staining representing gap junction plaques at the cell surface.
3.3. Changes in Localization of Cx43 During Mitosis
The rounded cells had few, if any, gap junction plaques between them and the surrounding non-dividing cells, however they did contain relatively large numbers of puncta, thought to be annular gap junction vesicles. In contrast, the cells of the monolayer have large gap junction plaques at sites of cell-cell contact and fewer annular gap junctions than the rounded cells (Figure 1C).
We analyzed the changes in the distribution of Cx43 in endogenous Cx43-expressing SW-13 cells during mitosis by immunofluorescence (Figures 2A–F). Mitotic stages were identified through staining of nuclei with Hoechst 33342 (blue). Cell borders were revealed by cortical actin staining with fluorescent phalloidin (green), and the Cx43 antibody was labeled with a fluorophore (red) (Figure Figures 2A–F). Endogenous Cx43 was localized primarily on the plasma membrane as large gap junction plaques at the beginning stages of mitosis, but became intracellular in the form of annular gap junctions as mitosis proceeded (Figure 2). By telophase, abundant intracellular structures that contained Cx43 were evident and some were located between the two nuclei (Figure 2E). At the stage of cytokinesis, gap junction plaques appear between the two connected cells (Figure 2F). In contrast, distribution of Cx43 in non-mitotic cells displayed the typical localization of gap junction plaques between cell-cell contacts (Figure 2A).
Similar to the findings for endogenous Cx43, when the distribution of tagged Cx43, Cx43-GFP, was studied, large surface gap junction plaques were observed during interphase, while only a few annular gap junction vesicles were found within the cytoplasm of cells expressing Cx43-GFP (Figure 3). The mitotic stages were detected distinguished with Hoechst staining (Figure 3A–E). During prophase, small gap junction plaques between the dividing cell and the non-mitotic cells were present but the number of cytoplasmic annular gap junctions continued to be greater than the number in the non-mitotic cell (Figure 3G). As in prophase, during metaphase there was a sparsity of gap junction plaques between contacting cells while most of the Cx43-GFP was located intracellularly (Figure 3H). At telophase, annular gap junctions were present as well as diffuse intracellular staining and there were few or no surface gap junction plaques (Figure 3I). At the end of cell division, large surface gap junction plaques again existed between apposed cells and only a few annular gap junction vesicles were present (Figure 3J). Since the Cx43-GFP behaved the same as the endogenous Cx43, cells expressing the tagged Cx43 were used to further investigate the gap junction plaques and the origin of the cytoplasmic Cx43 punctate stained structures with live cell imaging techniques.
Figure 3.
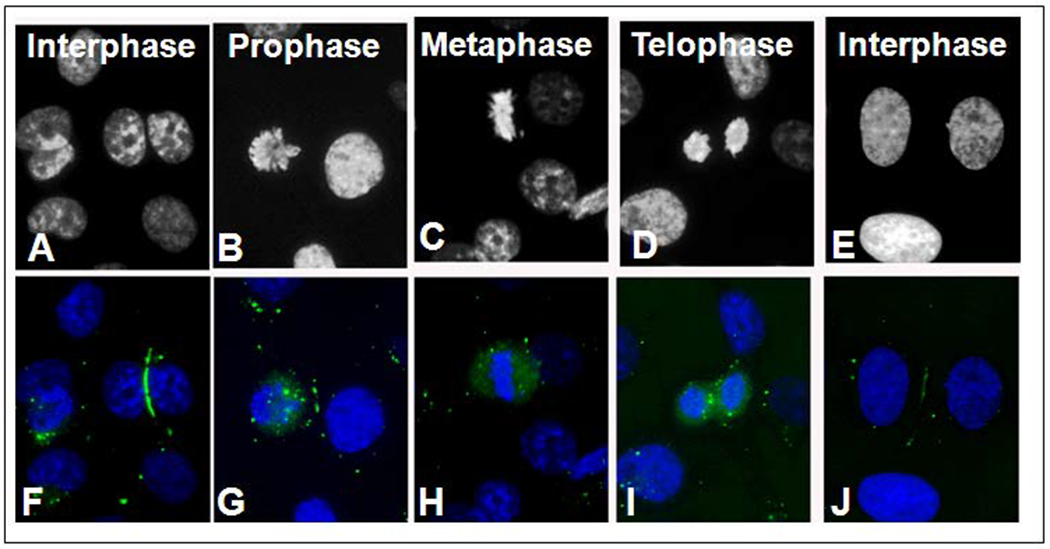
Chromatin and Cx43-GFP distribution during mitosis. The mitotic stages can be determined from the chromatin distribution seen with Hoechst staining (A-E). In the corresponding color images, Cx43-GFP gap junction plaques (green) decreased starting with prophase, while the number of cytoplasmic puncta increased (F-J). The number of puncta seen in the images for each mitotic stage are as follows: Interphase (1), Prophase (19), Metaphase (12), and Telophase (10). Bar: 10μm A-J.
3.4. Characterization of Gap Junction Structure Dynamics: Live Cell Imaging Microscopy
The Cx43-GFP localized to areas of cell-cell contact as gap junction plaques and within the cytoplasmic Cx43-GFP vesicles with a similar distribution pattern as for endogenous Cx43 protein (Figures 2 and 3). With time lapse microscopy, gap junction plaques or portions of gap junction plaques were observed to be internalized and to form cytoplasmic Cx43-GFP gap junction vesicles (Figure 4). The size and location of Cx43 cytoplasmic vesicles correlated with that of annular gap junctions observed with transmission electron microscopy (Figure 5) (Murray et al., 1981: Larsen et al., 1979; Nickel et al., 2008, Nickel et al., 2013). The morphology of the cytoplasmic puncta seen with time lapse and immunofluorescence microscopy were further characterized with standard transmission and Q-dot immuno-electron microscopic techniques.
Figure 4.
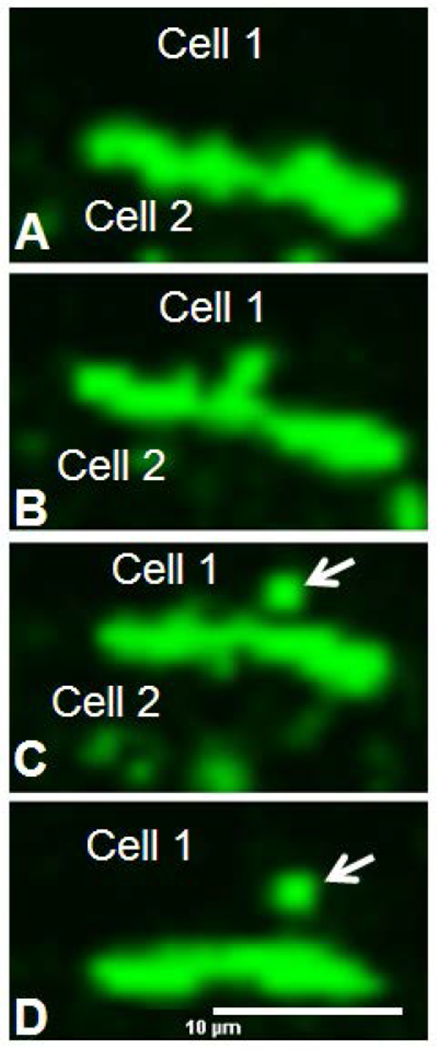
Time lapse image montage of cells expressing Cx43-GFP. Note the release of the annular gap junction vesicle (arrows) into the cytoplasm of one of the two contacting cells. Bar: 10μm A-D.
Figure 5.
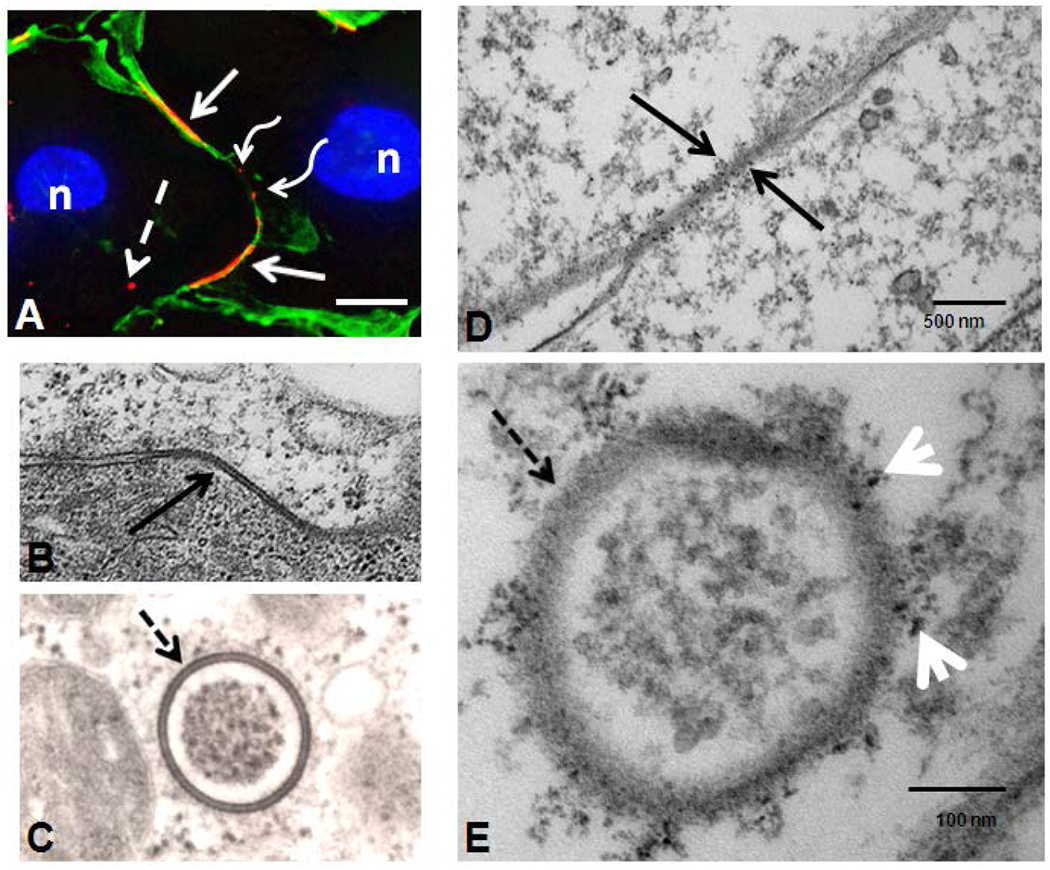
Gap junction plaques (solid arrows) and annular gap junction (dashed arrows) seen in immunofluorescence (A), standard transmission electron microscopy (B,C) and quantum dot immuno-electron microscopy (D, E). Both large (solid arrow) and small gap junction plaques (waved arrows) can be seen on the cell surface (A). Cortical actin was stained with phalloidin Alexa-488 (green) to help define the cell borders and distinguish the cytoplasmic punctate staining (dashed arrow) from small surface gap junction plaques (waved arrow)(A). Note the typical pentalaminar membrane of the gap junction plaque (solid arrow in B) and annular gap junction vesicle (dashed arrow in C). The association of phosphorylated Cx43 with a gap junction plaque (solid arrow in D) and an annular gap junction vesicle (dashed arrow in E) has been demonstrated with Q-dot immuno-electron microscopy labeling. In E arrowheads are pointing to Cx43 Q-dots. Bars: 10μm A; 100nm B; and 100nm C; 500nm D; and 100nm E.
3.5. Characterization of Gap Junction Structures with Ultrastructural Imaging Techniques
The typical pentalamellar, double membrane of the gap junction plaque was apparent with transmission electron microscopy (Figure 5) and correlated with the location of surface gap junction plaques detected with immunofluorescence (Figures 1C, 2A and 5A). Within the cytoplasm, annular gap junctions were found with the characteristic pentalamellar, double membrane which surrounded a central lumen area (Figure 5 C). These vesicles varied in size and could be found with various internal contents (data not shown). The morphology of the annular gap junctions were the same in the rounded cells collected above the media as those of the monolayer. Specifically, the characteristic pentalamellar membrane was separated by a 2-4 nm gap. This same width of the gap found separating the membranes of the gap junction plaque was found surrounding the central lumen in annular gap junctions.
To investigate and correlate the Cx43 structures seen with immunofluorescence with the morphological positively identified organelles, cells were examined at the ultrastructural level of resolution with Q-dot immuno-electron microscopic techniques. This technique used antibodies against Cx43 and phosphorylated Cx43 protein (phosphorylated at serine 368). As shown in figure 5D, Q-dots, indicative of Cx43, can be seen labeling gap junction plaques at the cell surface. Annular gap junction vesicles within the cytoplasm could also be seen decorated with Q-dots (Figure 5E). Annular gap junction vesicle membrane remnants fused with lysosomes were also occasionally seen presumably in the process of degradation (data not shown). However, such findings were rare. Importantly, single membrane vesicles were not found to be decorated with the Q-dots. Thus, it does not appear that gap junction plaques were internalized by splitting along the gap junction plaque horizontal plane. Such an event would have resulted in subsequent internalization of a single membrane into the cytoplasm of both cells or the remnant of one of the planes remaining on the cell surface. No examples of one side of the membrane between contacting cells being decorated while the other side remained undecorated with Cx43 Q-dots were observed. This localization pattern would be consistent with removal of gap junctions from the cell surface by endocytosis of the double membrane plaque to form a double membrane annular gap junction vesicle.
We thus suggest that the punctuate staining seen at the light microscopic level represents, for the most part, annular gap junction vesicles. Furthermore, other structures, such as clathrin coated pits and vesicles were not found to be decorated with the Q-dots, which would suggest that the staining for Cx43 is specific. More importantly, Cx43 was not on the surface of other structures within the cytoplasm that could have been identified at the fluorescent light microscopic level as annular gap junctions.
4. DISCUSSION
The SW-13 cell population is a valuable model system for studying changes and dynamics in Cx43 gap junction structures during mitosis. We have shown that cells are capable of forming and internalizing gap junctions both in non-dividing and dividing cells. Furthermore, this cell line has previously been demonstrated to express only Cx43 gap junction protein (Oyoyo et al., 1997). It is possible therefore to study and analyze gap junction plaque changes without the need to consider two or more other gap junction protein types. In this cell population, mitotic cells became rounded and could be easily collected from the medium or imaged while still attached to the substrate (Murray et al., 1981). The SW-13 cell line therefore provides a simple model for analysis of gap junction plaque dynamics during division.
In this study, gap junction plaques decreased between neighboring cells during cell mitosis, starting from prophase. Moreover, punctuate Cx43 staining observed in the cytoplasm of the non-dividing cells increased in the cells undergoing mitosis. Similar observations were made in a detailed study by Boassa and colleagues (2010), however, they did not identify the morphological structure of these puncta. Here we have demonstrated that the cytoplasmic Cx43 punctate staining observed with immunofluorescence correlated in size, with the double membrane annular gap junction vesicles detected with Q-dot Cx43 immunoelectron microscopic techniques, and not with single membrane vesicles such as endosomes or secretory vesicles. We and others have demonstrated with time lapse microscopy that annular gap junction vesicles result from the internalization of gap junction plaques into one of two contacting cells (Murray et al., 1981; Nickel et al., 2013; Jordan et al., 2001; Jordan et al., 1999; Lauf et al., 2002). In this study, the annular gap junction vesicle membranes were labelled with Cx43 Q-dots. Q-dot labelling however was not found on single membrane vesicles within the cytoplasm. These results are consistent with the gap junction plaques being removed as annular gap junctions, and the puncta, seen with immunofluorescence and live cell imaging, being annular gap junction vesicles. Further, there were no examples of Cx43 Q-dot localization on only one side of the gap junction plaque membrane, which would happen if only one half of the gap junction plaque was internalized to form a single membrane vesicle.
It was noted with Q-dot immuno-electron microscopy, that there were occasionally non-annular gap junction vesicle membrane “remnants” seen fused with lysosomes. Lysosomal degradation of gap junction has been documented and finding such remnants is consistent with some annular gap junctions being degraded once internalized (Caretta et al., 2015). However, most of the Cx43-labeled structures were annular gap junctions. The size, number and position of the annular gap junction vesicles observed at the electron microscopic level of resolution correlated with the puncta seen at the light level of resolution. It was therefore concluded that the puncta represent the annular gap junction vesicles. Further, loss of surface gap junction plaques correlates well to the increased appearance of annular gap junction vesicles during the division process and would be consistent with annular gap junction plaque internalization.
The loss of gap junction plaques observed with immunocytochemical methods is supported by our earlier work with freeze fracture techniques. In these studies, the fractional area covered by gap junction plaques was measured in round cells collected from above the monolayer in the media and in monolayer cell populations attached to the dish (Murray et al., 1981). In these early studies we found that the fractional area covered by gap junction plaques was significantly smaller in the dividing cell population (0.01 ± 0.01 μm2) than in the non-dividing, flattened cells of the monolayer (4.10 ± 2.19 μm2). The differences in fractional area covered by gap junctions reflects the difference in the number of gap junction plaques formed between contacting cells of the monolayer (20.7 ± 11.13) compared with the number counted on rounded cells (0.28 ± 0.3) (Murray et al., 1981). Our findings are consistent with the increase in gap junction removal from the cell surface during mitosis and thus would explain the observations of a loss of cell communication in dividing cells (Kanemitsu et al., 1997; Wilson et al., 2000; Solan et al., 2003).
It is well known that cells in culture round during mitosis (Lamp et al., 1998, Cardart et al., 2014, Hoijman et al., 2015, Maddox and Burridge 2003, Kunda et al., 2008). It is suggested that there are changes in the round cell not present in the flattened cell that signal initiation of gap junction plaque internalization. The changes may determine into which cell internalization will occur (in this study into the rounded cell). Further study is needed to elucidate these signals.
It is not clear why there would be a need for removal of gap junction plaques and thus a decrease in direct cell-cell communication during the division process. The loss of gap junction plaques is seen as early as prophase. It would therefore appear that mitosis may have been initiated before increased gap junction internalization was triggered. It seems less likely, that removal of gap junction plaques initiated division. However, only additional experimentation will elucidate which comes first, the signal for internalization and thus mitosis or the signal for mitosis and thus gap junction plaque internalization. The present study would facilitate additional investigations of the signals that trigger internalization during mitosis, which at this point, can only be speculated.
While very little is known about the molecular signals that initiate gap junction internalization, it has been shown that clathrin, acting via its adaptor proteins, facilitates gap junction plaque internalization (Nickel et al., 2008; Nickel et al., 2013; Fong et al., 2012; Girao et al., 2009; Piehl et al., 2007). It has been suggested that connexin modifications, as a result of kinase signaling, could influence gap junction internalization. Specifically, two different kinases, MAPK and PKC mediate phosphorylation of Cx43 on serines 252, 262, 279/282, and 368. These kinases have been suggested to promote clathrin recruitment to gap junction plaques and subsequent plaque internalization (Johnson et al., 2013; Schmitt et al., 2014; Fong et al., 2014). In addition, protein kinase Akt has been suggested to be an important mediator of signals for gap junction plaque turnover (Dunn, et al., 2012). How kinase-mediated phosphorylation/de-phosphorylation events might be linked to clathrin recruitment, gap junction internalization, and mitosis remains to be elucidated. It is tempting however to suggest a direct relationship between changes in specific kinases and gap junction internalization during mitosis. Such a possibility requires further study.
In this study, we report gap junction plaque re-formation at the cell surface between the newly forming daughter cells during cytokinesis. Boassa and colleagues (2010) similarly reported the rapid reformation of surface gap junction plaques. These researchers suggested that some of the reformation resulted from a recycling of connexin back to the membrane. Furthermore, Carette and colleagues (2015), based on time-lapse imaging data, suggested that an entire annular gap junction vesicle may return to the plasma membrane to form a new gap junction plaque. We suggest, based on our findings, that annular gap junctions appear to be the source of Cx43 that serves to re-establish gap junction plaques at the end of mitosis. This is an intriguing proposition that is suggestive of mechanisms for the specific localization of Cx43 to a particular region of the cell surface. It is plausible that the loss of the annular and gain of the surface gap junctions that we report are consistent with a recycling of the connexin, associated with annular gap junction vesicle, back to the cell surface.
5. CONCLUSION
In conclusion, Cx43 gap junction plaques are internalized to form annular gap junctions in the SW-13 cell population during mitosis. Cx43 cell surface gap junction plaques begin to reappear at the stage of cytokinesis and are located between the two newly forming cells. The rapid return and size of the newly formed surface gap junction plaques is suggestive of connexin recycling back to the cell surface after the division process. It is proposed that the recycling process utilizes Cx43 gap junction protein from annular gap junction vesicles. The possibility that gap junction proteins are not only degraded following gap junction plaque internalization but that they may recycle back to the surface is a intriguing hypothesis to further be tested.
6. ACKNOWLEDGEMENTS
The work was supported by National Science Foundation grants [# MCB-0444398 and #MCB-1408986] to S. Murray and National Institute of Health [# 5T36GM008622] to American Society for Cell Biology Visiting Professor Award to O.A. Vanderpuye. We greatly acknowledge the scientific and editing assistance of Beth Nickel and Margaret E. Bisher and the technical support of Dr. Simon Watkins and the Cell Biology Imaging Center.
Abbreviations:
- Akt
Protein kinase Akt (serine/threonine-specific protein kinase/protein kinase B)
- Cx43
Gap junction protein connexin 43
- Cx43-GFP
Green fluorescent protein-tagged Cx43
- DIC
Differential interference contrast
- FITC
Fluorescein isothiocyanate
- GFP
Green fluorescent protein
- MAPK
Mitogen-activated protein kinase
- PBS
Phosphate buffered saline (PBS)
- PKC
Protein kinase C
- Scanning EM (SEM)
Scanning electron microscopy
- SW-13
Scott White human adrenal cortical tumor cell
- Q-dot
Quantum dot
- TEM
Transmission electron microscopy
7. References
- Boassa D, Solan JL, Papas A, Thornton P, Lampe PD, Sosinsky GE. (2010). Trafficking and recycling of the connexin43 gap junction protein during mitosis. Traffic;11:1471–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta D, Gilleron J, Denizot JP, Grant K, Pointis G, Segretain D. (2015). New cellular mechanisms of gap junction degradation and recycling. Biol Cell;107:218–31. [DOI] [PubMed] [Google Scholar]
- Cadart C, Zlotek-Zlotkiewicz E, Le Berre M, Piel M, Matthews HK. (2014). Exploring the function of cell shape and size during mitosis. Developmental Cell; 29(2):159–169. [DOI] [PubMed] [Google Scholar]
- Defranco BH, Nickel BM, Baty CJ, Martinez JS, Gay VL, Sandulache VC, Hackam DJ, Murray SA. (2008). Migrating cells retain gap junction plaque structure and function. Cell Commun Adhes;15:273–88. [DOI] [PubMed] [Google Scholar]
- Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, Cattini PA, Kardami E. (2004). Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocyte. J Cell Sci;117:507–14. [DOI] [PubMed] [Google Scholar]
- Dunn CA, Su V, Lau AF and Lampe PD. (2012). Activation of Akt, not connexin 43 protein ubiquitination regulates gap junction stability. J Biol Chem;287:2600–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MM. (2000). Connexin-specific distribution within gap junctions revealed in living cells. J Cell Sci;113:4109–20. [DOI] [PubMed] [Google Scholar]
- Fong J, Kells R, Gumpert A, Marzillier J, Davidson M, Falk M. (2012). Internalized gap junctions are degraded by Autophagy. Autophagy;8:794–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong JT, Nimlamool W, Falk MM. (2014). EGF induces efficient Cx43 gap junction endocytosis in mouse embryonic stem cell colonies via phosphorylation of Ser262, Ser279/282, and Ser368. FEBS Letters;588(5):836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girao H, Catarino S, Pereira P. (2009). Eps15 interacts with ubiquitinated Cx43 and mediates its internalization. Exp Cell Res;315(20):3587–97. [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Deerinck T, Smarr BJY, Ellisman M. (2005). Correlated light and electron microscopic imaging of multiple endogenous proteins using quantum dots. Nature Methods ;2:743–49. [DOI] [PubMed] [Google Scholar]
- Goodall H, Maro B. (1986). Major loss of junctional coupling during mitosis in early mouse embryos. J Cell Biol;102:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL. (1996). Connexins, Connexons, and intercellular communication (Review). Ann Rev Biochem;65:475–502. [DOI] [PubMed] [Google Scholar]
- Hoijman E, Rubbini D, Colombelli J, Alsina B. (2015). Mitotic cell rounding and epithelial thinning regulate lumen growth and shape. Nature Communications; 6:1–12. [DOI] [PubMed] [Google Scholar]
- Johnson KE, Mitra S, Katoch P, Kelsey LS, Johnson KR, Mehta PP. (2013). Phosphorylation on Ser-279 and Ser-282 of connexin43 regulates endocytosis and gap junction assembly in pancreatic cancer cells. Mol Biol Cell;24(6):715–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan KR, Chodock AR, Hand A, Laird DW. (2001). The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci;114:763–73. [DOI] [PubMed] [Google Scholar]
- Jordan K, Solan JL, Dominguez M, Sia M, Hand A, Lampe PD, Laird DW. (1999). Trafficking, assembly, and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol Biol Cell;10:2033–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu MY, Jiang W, Eckhart W. (1998). Cdc2-mediated phosphorylation of the gap junction protein, connexin43 during mitosis. Cell Growth Differ;9:13–21. [PubMed] [Google Scholar]
- Kanemitsu MY, Loo LW, Simon S, Lau AF, Eckhart W. (1997). Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J Biological Chemistry;272:22824–31. [DOI] [PubMed] [Google Scholar]
- Kunda P, Pelling AE, Liu T, Baum B. (2008). Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Current Biology; 18:91–101. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB (1992) Molecular biology and genetics of gap junction channels. Seminars in Cell Biology 3:3–16. [DOI] [PubMed] [Google Scholar]
- Laird DW. (2005). Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta;1711:172–82. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Kurata WE, Warn-Kramer BJ, Lau AF. (1998). Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent on p34cdc2 kinase. J Cell Sci;111:833–41. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. (2000). Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys;384:205–15. [DOI] [PubMed] [Google Scholar]
- Larsen WJ, Hai N. (1978). Origin and fate of cytoplasmic gap junctional vesicles in rabbit granulosa cells. Tissue & Cell;10:585–98 [DOI] [PubMed] [Google Scholar]
- Larsen WJ, Tung HN, Murray SA, Swenson CA. (1979). Evidence for the participation of actin microfilaments and bristle coats in the internalization of gap junction membrane. J Cell Biol;83:576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. (2002). Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A;99:10446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox AS, Burridge K. (2003). RhoA is required for cortical retraction and rigidity during mitotic cell rounding. Journal of Cell Biology; 160:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SA, Larsen WJ, Trout J, Donta ST. (1981). Gap junction assembly and endocytosis correlated with patterns of growth in a cultured adrenocortical tumor cell line (SW-13). Cancer Res;41:4063–74. [PubMed] [Google Scholar]
- Murray SA, Nickel BM, Gay VL. (2004). Endocytosis of connexin protein in adrenal cells.Endocr Res;30:647–654. [DOI] [PubMed] [Google Scholar]
- Murray SA, and Shakespeare TI. (2015). Immunofluorescence: Applications for analysis of connexin a distribution and trafficking. Gap Junction and Pannexin Channels; CRC Methods in Signal AaaTransduction Series (Bai D and Saez JC, eds); in press. [Google Scholar]
- Nickel BM, DeFranco BH, Gay VL, Murray SA. (2008). Clathrin and Cx43 gap junction plaque endoexocytosis. Biochem Biophys Res Commun;374:679–82. [DOI] [PubMed] [Google Scholar]
- Nickel B, Boller M, Schneider K, Shakespeare T, Gay V, Murray SA. (2013). Visualizing the effects of dynamin inhibition on annular gap vesicles formation and fission. J Cell Sci;126: 2607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoyo UA, Shah US, Murray SA. (1997). The role of α1 (Connexin-43) gap junction expression in adrenal cortical cell function. Endocrinology;138:5385–97. [DOI] [PubMed] [Google Scholar]
- Piehl M, Lehmann C, Gumpert A, Denizol J, Segretain D, Falk MM. (2007). Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell;18:337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M, Leykauf K, Reinz E, Cheng H, Alonso A, Schenkel J. (2014). Mutation of Human Connexin43 Amino Acids S279/S282 Increases Protein Stability Upon Treatment with Epidermal Growth Factor. Cell Biochem Biophys; 69:379–84. [DOI] [PubMed] [Google Scholar]
- Stein LS,J Boonstra J, Burghardt RC. (1992). Reduced cell-cell communication between mitotic cells and non-mitotic coupled cells. Experimental Cell Research;198:1–7. [DOI] [PubMed] [Google Scholar]
- Solan JL, Fry MD, TenBroek EM, Lampe PD. (2003). Connexin43 phosphorylation is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci;116:2203–11. [DOI] [PubMed] [Google Scholar]
- Vinken M, Vanhaecke T, Papeleu P, Snykers S, Henkens T, Rogiers V. (2006). Connexins and their channels in cell growth and cell death. Cell Signal;18:592–600. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Close TW, Trosko JE (2000) Cell population dynamics (apoptosis, mitosis, and cell-cell communication) during disruption of homeostasis. Exp Cell Res 254:257–268 [DOI] [PubMed] [Google Scholar]
- Wynn J, Shah U, Murray SA (2002) Redistribution of connexin 43 by cAMP: a mechanism for growth control in adrenal cells. Endocr Res;28:663–68. [DOI] [PubMed] [Google Scholar]
- Xie H, Laird DW, Chang TH, Hu VW. (1997). A mitosis-specific phosphorylation of the gap junction protein connexin43 in human vascular cells: biochemical characterization and localization. J Cell Biol;137:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


