Abstract
Introduction
The visual-well aerated lung (V-WAL) is a score for the visual quantification of the well aerated lung on CT scan in COVID-19 patients and its value at admission seems to predict future COVID-19 severity. The aim of the present study was to analyze the association between V-WAL and risk factors for severe COVID-19 evolution in people with multiple sclerosis.
Materials and methods
This is an observational retrospective study, including people with multiple sclerosis and concomitant COVID-19, who were investigated with a lung CT scan at Hospital admission. The association of V-WAL with age, sex, EDSS, comorbidities, recent steroid use, and treatment (anti-CD20 vs other) was assessed by a multivariate linear regression model.
Results
In this observational retrospective study, the only factor that was significantly associated to a lower V-WAL at multivariable analysis was an increasing level of the EDSS (R2 = 0.41, p = 0.001), with an average decrease of 8% of V-WAL for each additional EDSS point.
Discussion and conclusion
This analysis shows that a high EDSS level is the main factor associated to the severity of lung involvement in a group of people with multiple sclerosis who were hospitalized for Covid-19.
Keywords: Multiple sclerosis, DMTs, Disease modifying treatment, COVID-19, Visual well aerated lung, V-WAL
Introduction
Several risk factors additional to those of the general population (age, sex, comorbidities) expose people with multiple sclerosis (pwMS) to the risk of severe COVID-19 evolution, i.e., progressive disease, significant disability, and anti-CD20 therapies [1, 2]
The visual well aerated lung score (V-WAL) depicts the degree of lung involvement in COVID-19 pneumonia (a greater V-WAL indicate a smaller lung involvement) and V-WAL at admission seems to predict COVID-19 severity [3].
V-WAL has been associated with stroke severity in patients with concomitant stroke and COVID-19 [4]. V-WAL at admission has never been described in patients with MS and the aim of the present study was to analyze the association of the V-WAL to MS characteristics in a group of pwMS with COVID-19.
Materials and methods
This is an observational retrospective study, including a subgroup of pwMS with COVID-19 from the MUSC-19 study [1], who were investigated with a lung CT scan at hospital admission, in two Italian MS centers (Piacenza and Montichiari).
The same radiologist reviewed lung CT scans and estimated V-WAL, according to the methods described by Colombi et al. [3]
Demographics, MS disease course, EDSS, MS treatment and COVID-19 severity were collected.
The association of V-WAL with age, sex, EDSS, comorbidities, recent steroid use, and treatment (anti-CD20 vs other) was assessed by a multivariate linear regression model.
The study was approved by the regional ethics committee of Liguria (University of Genoa; n 130/2020–DB id 10,433) and at a national level by the Italian Medicines Agency.
Results
Twenty-four pwMS with COVID-19 were included, mean age was 47.7 years (Standard Deviation (SD) = 15.69), 62.5% were female, 70.8% had relapsing–remitting MS, 20.8% secondary progressive MS and 8.3% primary progressive MS. Median EDSS was 3.5 (range 1.5–6.3), mean MS duration was 12 (SD = 8.95) years and 29.2% had at least one comorbidity. Anti-CD20 was the current therapy in 45.8%, injectables in 25% and orals in 29.2%.
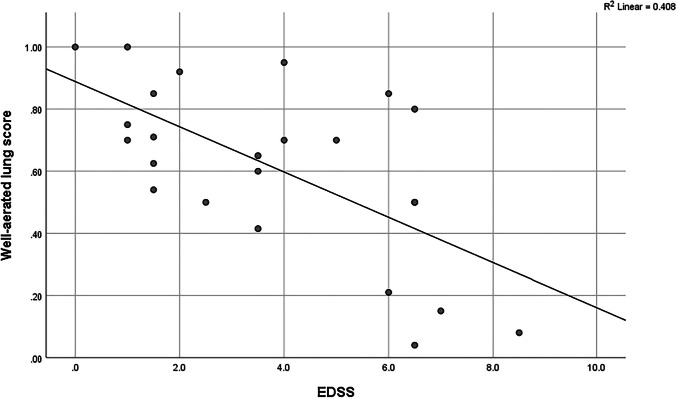
Nineteen pwMS had mild/moderate COVID-19 requiring hospitalization, seven were admitted to Intensive Care Unit and 2 of them died. Mean V-WAL in the entire cohort was 0.6 (standard error (SE) = 0.28). The only factor that was significantly associated to a lower V-WAL at multivariable analysis (Table 1) was an increasing level of the EDSS (R2 = 0.41, p = 0.001) (Fig. 1), with an average decrease of 8% of V-WAL for each additional EDSS point (Table 1).
Table 1.
Multivariable analysis assessing factors associated to high values of V-WAL score
| Parameter | Beta coefficients (SE) | p |
|---|---|---|
| Age | 0.002 (0.004) | 0.57 |
| Sex (males vs female) | − 0.049 (0.097) | 0.61 |
| Comorbidities (yes vs no) | − 0.090 (0.118) | 0.45 |
| Therapy (anti-CD20 vs others) | − 0.094 (0.090) | 0.29 |
| Recent use of methylprednisolone (yes vs no) | − 0.224 (0.236) | 0.34 |
| EDSS (1 point) | − 0.076 (0.022) | 0.001 |
Dependent Variable: Well-aerated lung score
Model: (Intercept), Age, Sex, Comorbidity, Therapy, Recent use of methylprednisolone, EDSS
Fig. 1.
V-WAL and EDSS correlation
Discussion
Previous studies have shown that in pwMS older age, male gender, higher EDSS, cardiac comorbidities, obesity, progressive MS course, administration of high doses of methylprednisolone in the month before infection and anti-CD20 therapy are risk factors for a severe COVID-19 evolution [1, 2].
Acute Respiratory Distress Syndrome is the hall-mark for severe evolution of acute respiratory failure in SARS-CoV-2 infection [5], and the extent of lung involvement at hospital admission has been associated with a severe COVID-19 evolution [3].
This analysis shows that a high EDSS level is the main factor associated to the severity of lung involvement in a group of pwMS who were hospitalized for Covid-19, after adjusting for all the other variables. For each EDSS additional point, there was an 8% decrease in well aerated lung: we suggest to consider lung CT scan in the diagnostic work-up of disabled pwMS with admitted to the emergency causality with SARS-CoV-2 infection.
Conclusion
This analysis better characterizes the “higher severity” of Covid-19 in patients with high EDSS.
Declarations
Ethical approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Patients signed informed consent regarding publishing their data.
Research involving human participants and/or animals
Not applicable.
Conflict of interest
P. Immovilli has received consulting fees from Roche, Biogen, Merck, Novartis and Sanofi. M.P. Sormani has received consulting fees from Biogen, Boehringer Ingelheim, GeNeuro, GSK, Immunic, Merck, Novartis, Roche, and Sanofi. C. Cordioli has received consulting fees from Biogen, Merck Serono, Novartis, Bristol Myers Squibb, Roche, Almirall. I. Schiavetti has received consulting fees from Associazione Commissione Difesa Vista, Eye Pharma, Hippocrates Research, and D.M.G Italia outside the submitted work. P. De Mitri, S. Grazioli, D Guidetti have nothing to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sormani MP, Salvetti M, Labauge P, et al. DMTs and COVID-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol. 2021;8(8):1738–1744. doi: 10.1002/acn3.51408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombi D, Bodini FC, Petrini M, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296(2):E86–E96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Immovilli P, Terracciano C, Zaino D, et al. Stroke in COVID-19 patients: a case-series from Italy. Int J Stroke. 2020;15(6):701–702. doi: 10.1177/1747493020938294. [DOI] [PubMed] [Google Scholar]
- 5.Berlin DA, Gulick RM, Martinez FJ. Severe COVID-19. NEJM. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]