Abstract
Carbon catabolite repression (CCR) of Bacillus subtilis catabolic genes is mediated by CcpA and in part by P-Ser–HPr. For certain operons, Crh, an HPr-like protein, is also implicated in CCR. In this study we demonstrated that in ptsH1 crh1 and hprK mutants, expression of the lev operon was completely relieved from CCR and that both P-Ser–HPr and P-Ser–Crh stimulated the binding of CcpA to the cre sequence of the lev operon.
The main function of histidine-containing protein (HPr) is to participate in the phosphotransferase system (PTS)-catalyzed transport and phosphorylation of carbohydrates. Being part of a phosphoenolpyruvate-dependent protein phosphorylation chain, HPr is phosphorylated by enzyme I (EI) at His-15 (8) and transfers the phosphoryl group to the sugar-specific EIIAs. In gram-positive bacteria, the phosphoryl carrier protein HPr is also phosphorylated at a regulatory serine (Ser-46) by ATP and the HPr kinase (HprK) (2, 7, 22). This ATP-dependent phosphorylation regulates the induction and carbon catabolite repression (CCR) of several catabolic genes (23). Replacement of Ser-46 with alanine (ptsH1 mutation) prevents the ATP-dependent phosphorylation of HPr and almost completely abolishes CCR of many operons (3). However, several operons such as the xyn, iol, and lev operons were not relieved or only partly relieved from CCR in the ptsH1 mutant (3, 6a, 18). Growth conditions were also found to influence CCR in ptsH1 mutants, and operons which were completely derepressed in minimal medium were only partly relieved from CCR in complex medium (3).
In addition to HPr, an HPr-like protein called Crh (for “catabolite repression HPr”), which was discovered during the Bacillus subtilis sequencing project and exhibits 45% sequence identity to HPr, was suggested to participate in CCR (6a). Since His-15 of HPr is replaced by a glutamine in Crh, no phosphoenolpyruvate-dependent, EI-catalyzed phosphorylation of Crh could be detected. However, Crh becomes phosphorylated by ATP and the metabolite-activated HprK at the conserved Ser-46 (6a, 7). If the crh gene of a ptsH1 mutant was disrupted, almost complete relief from CCR was observed for β-xylosidase, inositol dehydrogenase, and levanase, indicating that both HPr and Crh are implicated in CCR of the corresponding operons (6a). In addition, HPr and Crh participate in glucose-induced activation of ackA expression (24).
Catabolite control protein A (CcpA), a member of the LacI-GalR family of repressors, acts as a pleiotropic regulator of CCR in B. subtilis and binds to the cis-active operator sequences (cre, for “catabolite response element”) (11, 12, 25). An interaction of P-Ser–HPr with CcpA has been demonstrated in vitro (4, 13). The resulting complex binds specifically to the cre sequences of the gnt, xyl, and xyn operons and of the amyE gene (5, 6, 9, 14). P-Ser–Crh presumably also exerts its effect on CCR via CcpA, since those operons, which were only slightly relieved from CCR in a ptsH1 mutant, were similarly relieved from CCR in ccpA and ptsH1 crh double mutants (6a).
The levanase operon of B. subtilis (levDEFG-sacC) encodes a fructose-specific PTS (lev-PTS) and the extracellular levanase, which hydrolyzes fructose polymers and sucrose (17). Expression of this operon is induced by fructose and repressed by glucose (16). CCR of the B. subtilis levanase operon seems to involve at least two mechanisms: one mediated by phosphorylation of the transcriptional activator LevR by P-H15–HPr observed in a constitutive background (levR8) (19) and the other mediated by the repressor CcpA (18). HPr and Crh are also involved in the CcpA-dependent CCR mechanism operative for the levanase operon (6a, 18). A potential CcpA binding site, cre, was identified between the −12, −24 promoter and its upstream activating sequence, the target site for LevR, the specific activator of the operon (18).
In this study, we have confirmed the role of P-Ser–HPr and P-Ser–Crh in CCR of the levanase operon by constructing a ptsH1 crh1 double mutant and by testing the interaction of the CcpA/P-Ser–HPr and CcpA/P-Ser–Crh complexes with the cre sequence of the levanase operon. We have also tested the involvement of HPr kinase in the regulation of the levanase operon.
Ser-46 is the unique site of phosphorylation in Crh.
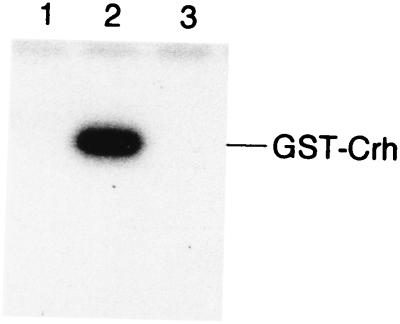
The site of phosphorylation in Crh had been determined by mass spectroscopy to be Ser-46 (6a). However, mass spectroscopy can fail to detect minor phosphorylation sites. We therefore wanted to make sure that Ser-46 represents the only site of phosphorylation in Crh. For this purpose, a 250-bp DNA fragment containing either the crh or crh1 allele was amplified by PCR with chromosomal DNA of the ptsGHI deletion strain GM273 (3) or plasmid pRC17 (6a) and appropriate primers containing an EcoRI site (3′ end) or a BamHI site (5′ end). The EcoRI-BamHI fragments were cloned into the expression vector pGEX-KT (10). Crh or CrhS46A fused to glutathione S-transferase (GST) was expressed from the resulting plasmids after transformation in E. coli NM522 and isopropyl-β-d-thiogalactopyranoside (IPTG) induction. Protein purification was carried out as described by Hakes and Dixon (10). Phosphorylation of GST-Crh and GST-CrhS46A was tested in the presence of HprK as described by Galinier et al. (6a) (Fig. 1). GST-Crh was phosphorylated by [γ-32P]ATP (Fig. 1, lane 2), while GST-CrhS46A was not (lane 3), confirming that Ser-46 is the only site of HprK-catalyzed phosphorylation in Crh.
FIG. 1.
ATP-dependent phosphorylation of GST-Crh and GST-CrhS46A. [γ-32P]ATP and HprK were incubated together with GST-Crh (lane 2) or GST-CrhS46A (lane 3). Lane 1 is the control without GST-Crh or GST-CrhS46A. The phosphorylation reaction was stopped by adding sample buffer to the assay mixtures before loading them onto a sodium dodecyl sulfate-polyacrylamide gel. After electrophoresis, the gel was treated for 5 min with boiling 16% trichloroacetic acid before being dried and exposed to autoradiography (Biomax MR; Kodak). Coomassie blue-stained gels on which the same samples had been loaded revealed a single band for HPr and Crh (data not shown).
P-Ser–Crh participates in CCR of the levanase operon.
Similar to the situation for a ccpA mutant, CCR of the levanase operon was abolished in a ptsH1 crh::aphA3 double mutant (6a). Crh was therefore assumed to carry out its function in CCR via interaction of phosphorylated Crh with CcpA. To test this hypothesis, a chromosomal crh1 mutant was constructed by using a pHT315 derivative (1) carrying the crh1 allele and part of the downstream yvcN gene (pRC23, Fig. 2). A kanamycin resistance cassette was introduced into yvcN, giving plasmid pRC33 (Fig. 2). This plasmid was linearized with PstI and used to transform QB5081 (levD′-′lacZ) and QB7148 (ptsH1 levD′-′lacZ). Cotransformation of the crh1 allele with the kanamycin resistance cassette allowed us to isolate Kmr and Ems clones containing the crh1 allele (QB7158) or both the ptsH1 and crh1 mutations (QB7159). The presence of the mutations was confirmed by sequencing appropriate PCR products of these strains. Expression of the levD′-′lacZ fusion in the wild-type QB5081 and the crh1 mutant QB7158 was decreased 13- and 10-fold, respectively, by the addition of glucose (Table 1). A 4.5-fold repression was observed in the ptsH1 mutant, whereas the ptsH1 crh1 double mutant was almost completely relieved from CCR (1.5-fold repression). These results suggest that CCR of the levanase operon is mediated via phosphorylation of HPr and Crh at Ser-46. However, under the experimental conditions used, HPr can completely replace Crh in CCR of the lev operon whereas Crh can only partly substitute for HPr, since a ptsH1 mutant is partially relieved from CCR.
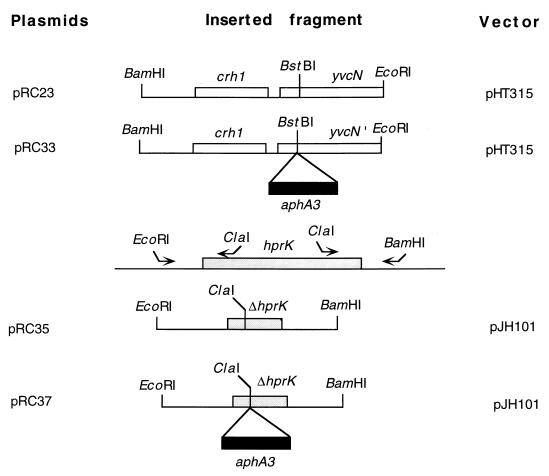
FIG. 2.
Construction and restriction map of plasmids used in this study. A 1-kb DNA fragment containing the crh1 allele and part of yvcN was cloned between the BamHI and EcoRI sites of pHT315 (1) to give plasmid pRC23. A 1.5-kb ClaI DNA fragment containing the kanamycin resistance gene aphA3 was inserted in yvcN at the unique BstBI restriction site, providing plasmid pRC33. Plasmid pRC35 was constructed as follows. An EcoRI-ClaI and a ClaI-BamHI fragment, corresponding to the 5′ and 3′ ends of hprK, respectively, were amplified by PCR. These two fragments were cloned into the integrative vector pJH101 digested with EcoRI and BamHI, thus reconstituting an hprK gene deleted from codons 26 to 232. pRC37 was obtained by insertion of the 1.5-kb kanamycin cassette into the newly created ClaI site of hprK.
TABLE 1.
Regulation of the expression of a levD′-′lacZ fusion by CcpA, P-Ser–HPr, and P-Ser–Crh
| Straina | Relevant genotype | β-Galactosidase activity (U/mg of protein)b in:
|
Repression factor | |
|---|---|---|---|---|
| CSK Fru | CSK Fru Glu | |||
| QB5081 | crh+ ptsH+ | 220 | 17 | 13 |
| QB5224 | ptsH1 | 405 | 87 | 4.5 |
| QB7158 | crh1 | 270 | 27 | 10 |
| QB7159 | ptsH1 crh1 | 390 | 220 | 1.5 |
| QB7160 | hprK::aphA3 | 370 | 282 | 1.3 |
All strains contain a levD′-′lacZ translational fusion integrated in the amyE gene and a trpC2 mutation.
Specific activities of β-galactosidase were determined in extracts prepared from exponentially growing cells (absorbance at 600 nm, 0.7 to 1). The mean values of at least three independent experiments are presented. Cells were grown in CSK medium, which is C minimal medium supplemented with potassium succinate (6 g/liter) and potassium glutamate (8 g/liter) (17), or on CSK medium containing 0.2% fructose or 0.2% fructose plus 1% glucose. The method of Miller was used for the determination of β-galactosidase activity (21).
Both HPr and Crh are phosphorylated by ATP and HprK (6a, 7). The B. subtilis hprK (former yvoB) gene has recently been identified (7, 22), and HprK was found to be bifunctional, also catalyzing the dephosphorylation of P-Ser–HPr (15). To confirm the importance of ATP-dependent HPr and Crh phosphorylation for CCR of the levanase operon, we constructed an hprK mutant by using plasmid pRC37 containing hprK carrying a deletion from codons 26 to 232 and a kanamycin resistance cassette inserted at the newly created ClaI restriction site (Fig. 2). Plasmid pRC37 was cut with ScaI and used to replace hprK in QB5081 (levD′-′lacZ) with the modified hprK. A Kmr Cms clone (QB7160) was isolated, and the presence of the kanamycin cassette in hprK was confirmed by PCR. Similar to the situation for the ptsH1 crh1 double mutant, expression of the levD′-′lacZ fusion in QB7160 was reduced only 1.3-fold by glucose (Table 1), confirming that ATP-dependent phosphorylation of HPr and Crh is part of the CCR signal transduction pathway operative for the levanase operon. However, we cannot exclude that in addition to the hprK mutation, alterations in the expression of the downstream genes due to the insertion of the kanamycin resistance cassette into hprK might influence CCR.
Binding of the CcpA/P-Ser–HPr and CcpA/P-Ser–Crh complexes to the lev cre sequence.
To completely understand the signal transduction pathway in CCR of the levanase operon, we wanted to investigate whether both P-Ser-HPr and P-Ser-Crh influence the binding of CcpA to the lev cre sequence. P-Ser–HPr has been demonstrated to interact in vitro with CcpA (4, 13), and the resulting protein complex binds specifically to the cre sequences of the gnt, xyn, and xyl operons and of the amyE gene (5, 6, 9, 14). To test whether P-Ser–Crh can also interact with CcpA and allow specific binding of CcpA to the lev cre sequence, we performed DNase I footprinting experiments. A 178-bp fragment containing the lev promoter region (from positions −148 to +30) was 3′-end labelled with [α-32P]dATP at the EcoRI site. The assay mixture contained 10 mM HEPES (pH 7.6), 1 mM MgCl2, 200 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, 50 μg of poly(dI-dC)-(dI-dC) per ml as the bulk carrier DNA, the radioactive DNA probe (100,000 cpm), 2.5 μM CcpA, and 10 μM HPr, P-Ser–HPr, Crh, or P-Ser–Crh. After a 15-min incubation at room temperature, 2 ng of bovine pancreatic DNase I (Worthington) was added to the assay mixture, which was incubated for a further 60 s at room temperature. DNase I digestion was terminated by adding stop solution (final concentration, 2.5 mM EDTA, 0.4 M sodium acetate, and 50 μg of calf thymus DNA) and subjecting the mixture to phenol extraction. All the proteins were purified on Ni-nitrilotriacetate-agarose columns, and HPr(His)6 and Crh(His)6 were phosphorylated by HprK(His)6 in the presence of ATP as described by Galinier et al. (6a, 7). Under these conditions, HPr and Crh were about 90% phosphorylated. After phosphorylation, HprK was inactivated by keeping the assay mixture for 10 min at 80°C.
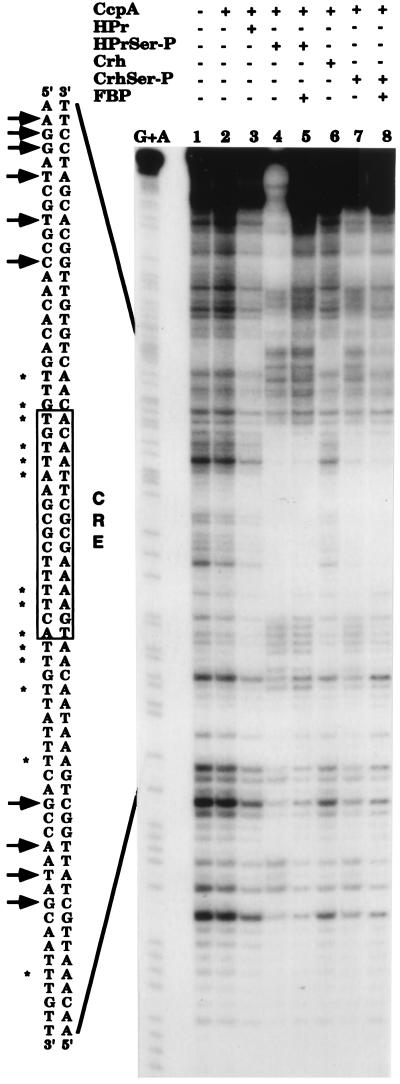
The results of the DNase I footprinting experiments are presented in Fig. 3. In the presence of either P-Ser–HPr or P-Ser–Crh, CcpA specifically recognized the lev cre sequence (Fig. 3, lanes 4 and 7, respectively). This interaction was not observed with HPr or Crh (data not shown) or when CcpA, CcpA and HPr, or CcpA and Crh were present in the assay mixture (lanes 2, 3, and 6, respectively). The slightly increased protection in the presence of CcpA and HPr or of CcpA and Crh appears to be nonspecific, since it affects the total DNA (lanes 3 and 6). By contrast, the region strongly protected in the presence of CcpA and P-Ser–HPr or of CcpA and P-Ser–Crh (AAATAACAACAATGAAAACGCTTAACACAA) (lanes 4 and 7) contains the presumed cre-like sequence (in bold letters) located between positions −50 and −36 upstream of the promoter of the levanase operon (18). Moreover, sites of hypersensitivity to DNase I digestion were observed only when CcpA and either P-Ser–HPr or P-Ser–Crh were present in the reaction mixture. The addition of 20 mM fructose-1,6-bisphosphate (FBP) did not modify the binding of the CcpA protein in the presence of P-Ser–HPr or P-Ser–Crh (lanes 5 and 8).
FIG. 3.
DNase I footprinting experiments with the lev cre sequence in the presence of CcpA, P-Ser–HPr, and P-Ser–Crh. A 178-bp EcoRI-PstI fragment (from positions −148 to +30) containing the lev promoter and the cre sequence (from position −50 to −36) was labeled at the 3′-end as described in the text. DNA was digested with DNase I in the absence of any protein (lane 1) or in the presence of 5 μM CcpA (lane 2); 2.5 μM CcpA and 10 μM HPr (lane 3), 2.5 μM CcpA and 10 μM P-Ser–HPr (lane 4), 2.5 μM CcpA, 10 μM P-Ser–HPr, and 20 mM FBP (lane 5), 2.5 μM CcpA and 10 μM Crh (lane 6), 2.5 μM CcpA and 10 μM P-Ser–Crh (lane 7), or 2.5 μM CcpA, 10 μM P-Ser–Crh, and 20 mM FBP (lane 8). The base-specific chemical cleavage reaction at guanine and adenine (20) is shown in lane G+A. The cre sequence is boxed, and the cre consensus sequence is indicated. The bases protected against digestion by DNase I are indicated by asterisks, while the sites of hyperdigestion by DNase I are indicated by arrows.
Compared to the consensus cre sequence, the lev cre sequence contains additional base pairs close to the 3′ end. In addition, most of the cre sequences were found to be located either within the coding sequence of the corresponding gene or close to the transcription start site (12). In the levanase operon, the cre sequence (−50 to −36) is situated upstream from the −12, −24 promoter. Binding of the CcpA/P-Ser–HPr or CcpA/P-Ser–Crh complexes to the cre sequence located between the LevR binding site (upstream activating sequence) and the −12, −24 promoter may influence the formation of the complex between LevR and the RNA polymerase-ς54 which is necessary for melting the DNA and activating transcription. In this context, it is interesting that binding of the CcpA/P-Ser–HPr or CcpA/P-Ser–Crh complexes also caused significant alterations in the pattern of DNase I digestion outside the cre sequence (Fig. 3), suggesting that binding of CcpA to the lev cre sequence might induce changes in the DNA structure.
CCR of the B. subtilis levanase operon seems to involve at least two mechanisms: one mediated by the transcriptional activator LevR (19) and the other mediated by the repressor CcpA. The first CCR mechanism is based on activation of LevR by P-His–HPr-dependent phosphorylation at His-585. In the presence of a PTS sugar, the phosphoryl group of P-His–HPr is thought to be preferentially used for sugar phosphorylation, leading to poor phosphorylation of LevR. The reduced phosphorylation of LevR lowers its transcriptional activator function and leads to slowed expression of the levanase operon (19). The second CCR mechanism is based on the interaction of P-Ser–HPr or P-Ser–Crh with CcpA. Under the reaction conditions used, we observed similar binding affinities with both complexes. In the case of the B. subtilis xyn operon, a more detailed study had revealed that the CcpA/P-Ser–HPr complex is more effective in protecting the xyn cre sequence (6). The increased production of glycolytic intermediates accompanying the rapid metabolism of a carbon source is thought to activate HprK, which catalyzes the ATP-dependent phosphorylation of HPr and Crh. P-Ser–HPr and P-Ser–Crh function as corepressors by interacting with CcpA, the global regulator of CCR. They enable CcpA to bind to the lev cre sequence located between the binding site of the transcriptional activator LevR and the −12, −24 promoter, thus preventing expression of the lev operon.
Acknowledgments
We are grateful to G. Rapoport, in whose laboratory part of this work was carried out, for continuous encouragement and critical reading of the manuscript and to A. Kolb for helpful discussions.
This research was supported by the Ministère de l’Education Nationale, de la Recherche et de la Technologie, the Centre National de la Recherche Scientifique, the Institut National de la Recherche Agronomique, the Institut Pasteur, the Université de Lyon, and the Université Paris 7.
REFERENCES
- 1.Arantes O, Lereclus D. Construction of cloning vectors for Bacillus thuringiensis. Gene. 1991;108:115–119. doi: 10.1016/0378-1119(91)90495-w. [DOI] [PubMed] [Google Scholar]
- 2.Deutscher J, Saier M H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci USA. 1983;80:6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deutscher J, Reizer J, Fischer C, Galinier A, Saier M H, Jr, Steinmetz M. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier of the phosphostransferase system, by mutation of the ptsH gene confers catabolite repression resistance to several catabolic genes of Bacillus subtilis. J Bacteriol. 1994;176:3336–3344. doi: 10.1128/jb.176.11.3336-3344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deutscher J, Küster E, Bergstedt U, Charrier V, Hillen W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in Gram-positive bacteria. Mol Microbiol. 1995;15:1049–1053. doi: 10.1111/j.1365-2958.1995.tb02280.x. [DOI] [PubMed] [Google Scholar]
- 5.Fujita Y, Miwa Y, Galinier A, Deutscher J. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol Microbiol. 1995;17:953–960. doi: 10.1111/j.1365-2958.1995.mmi_17050953.x. [DOI] [PubMed] [Google Scholar]
- 6.Galinier A, Deutscher J, Martin-Verstraete I. Phosphorylation of either Crh or HPr mediates binding of CcpA to the xyn cre and catabolite repression of the Bacillus subtilis xyn operon. J Mol Biol. 1999;286:307–314. doi: 10.1006/jmbi.1998.2492. [DOI] [PubMed] [Google Scholar]
- 6a.Galinier A, Haiech J, Kilhoffer M C, Jaquinod M, Stülke J, Deutscher J, Martin-Verstraete I. The Bacillus subtilis crh gene encodes a HPr-like protein involved in carbon catabolite repression. Proc Natl Acad Sci USA. 1997;94:8439–8444. doi: 10.1073/pnas.94.16.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galinier A, Kravanja M, Engelmann R, Hengstenberg W, Kilhoffer M C, Deutscher J, Haiech J. New protein kinase and protein phosphatase families mediate signal transduction in bacterial catabolite repression. Proc Natl Acad Sci USA. 1998;95:1823–1828. doi: 10.1073/pnas.95.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gassner M, Stehlik D, Schrecker O, Hengstenberg W, Maurer W, Rüterjans H. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. 2. 1H and 31P nuclear-magnetic-resonance studies on the phosphocarrier protein HPr, phosphohistidines and phosphorylated HPr. Eur J Biochem. 1977;75:287–296. doi: 10.1111/j.1432-1033.1977.tb11528.x. [DOI] [PubMed] [Google Scholar]
- 9.Gösseringer R, Küster E, Galinier A, Deutscher J, Hillen W. Cooperative and non-cooperative DNA binding modes of catabolite control protein CcpA from Bacillus megaterium result from sensing two different signals. J Mol Biol. 1997;266:665–676. doi: 10.1006/jmbi.1996.0820. [DOI] [PubMed] [Google Scholar]
- 10.Hakes D J, Dixon J E. New vectors for high level expression of recombinant proteins in bacteria. Anal Biochem. 1992;202:293–298. doi: 10.1016/0003-2697(92)90108-j. [DOI] [PubMed] [Google Scholar]
- 11.Henkin T M. The role of CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol Lett. 1996;135:9–15. doi: 10.1111/j.1574-6968.1996.tb07959.x. [DOI] [PubMed] [Google Scholar]
- 12.Hueck C J, Hillen W, Saier M H., Jr Analysis of a cis-active sequence mediating catabolite repression in Gram-positive bacteria. Res Microbiol. 1994;145:503–518. doi: 10.1016/0923-2508(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 13.Jones B E, Dossonnet V, Küster E, Hillen W, Deutscher J, Klevit R E. Binding of the catabolite repressor protein CcpA to its DNA target is regulated by phosphorylation of its corepressor HPr. J Biol Chem. 1997;272:26530–26535. doi: 10.1074/jbc.272.42.26530. [DOI] [PubMed] [Google Scholar]
- 14.Kim J-H, Voskuil M I, Chambliss G H. NADP, corepressor for the Bacillus catabolite control protein CcpA. Proc Natl Acad Sci USA. 1998;95:9590–9595. doi: 10.1073/pnas.95.16.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kravanja M, Engelmann R, Dossonnet V, Blüggel M, Meyer H E, Frank R, Galinier A, Deutscher J, Schnell N, Hengstenberg W. The hprK gene of Enterococcus faecalis encodes a novel bifunctional enzyme: the HPr kinase/phosphatase. Mol Microbiol. 1999;31:59–66. doi: 10.1046/j.1365-2958.1999.01146.x. [DOI] [PubMed] [Google Scholar]
- 16.Martin I, Débarbouillé M, Klier A, Rapoport G. Induction and metabolite regulation of levanase synthesis in Bacillus subtilis. J Bacteriol. 1989;171:1885–1892. doi: 10.1128/jb.171.4.1885-1892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Verstraete I, Débarbouillé M, Klier A, Rapoport G. Levanase operon of Bacillus subtilis includes a fructose-specific phosphotransferase system regulating the expression of the operon. J Mol Biol. 1990;214:657–671. doi: 10.1016/0022-2836(90)90284-S. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Verstraete I, Stülke J, Klier A, Rapoport G. Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon. J Bacteriol. 1995;177:6919–6927. doi: 10.1128/jb.177.23.6919-6927.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Verstraete I, Charrier V, Galinier A, Stülke J, Erni B, Rapoport G, Deutscher J. Antagonistic effects of dual PTS-catalysed phosphorylation on the Bacillus subtilis transcriptional activator LevR. Mol Microbiol. 1998;28:293–303. doi: 10.1046/j.1365-2958.1998.00781.x. [DOI] [PubMed] [Google Scholar]
- 20.Maxam A M, Gilbert W. Sequencing end-labelled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 22.Reizer J, Hoischen C, Titgemeyer F, Rivolta C, Rabus R, Stülke J, Karamata D, Saier M H, Jr, Hillen W. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol Microbiol. 1998;27:1157–1169. doi: 10.1046/j.1365-2958.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 23.Saier M H., Jr Cyclic AMP-independent catabolite repression in bacteria. FEMS Microbiol Lett. 1996;138:97–103. doi: 10.1111/j.1574-6968.1996.tb08141.x. [DOI] [PubMed] [Google Scholar]
- 24.Turinsky A J, Grundy F J, Kim J-H, Chambliss G H, Henkin T M. Transcriptional activation of the Bacillus subtilis ackA gene requires sequences upstream of the promoter. J Bacteriol. 1998;180:5961–5967. doi: 10.1128/jb.180.22.5961-5967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weickert M J, Chambliss G H. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]