Abstract
Brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1) promote the development and maintenance of neural circuits. Alterations in these factors might contribute to autism spectrum disorder (ASD). We asked whether serum BDNF, proBDNF, and IGF-1 levels are altered in an ASD population compared to controls. We measured serum BDNF, proBDNF, and IGF-1 immunoreactive protein in boys and girls aged 5–15 years old with mild to moderate ASD and non-autistic controls by ELISA. IGF-1 was increased in ASD serum compared to controls and was correlated with age and with CARS scores. Serum BDNF levels did not differ between groups, however, proBDNF serum levels were decreased in subjects with ASD compared to non-autistic controls. Medicated, but not unmedicated, ASD subjects exhibited lower serum proBDNF levels compared to controls, while neither IGF-1 nor BDNF levels differed between treatment groups. These data support the involvement of proBDNF and IGF-1 in the pathogenesis and treatment of autism.
Subject terms: Neuroscience, Diseases, Molecular medicine
Introduction
Autism spectrum disorder (ASD) is defined by persistent deficits in communication and social interaction accompanied by restricted, repetitive patterns of behavior, interests, or activities1. In addition to core symptoms, many individuals with ASD have co-occurring medical conditions, most frequently developmental or psychiatric conditions. The worldwide prevalence of ASD is approximately 1%, and more males than females are affected, although evidence suggests that autism in females may be under-diagnosed2. While synaptic dysfunction leading to impaired establishment and maintenance of functional neuronal networks occurs in ASD, the molecular mechanisms remain unclear3,4. Studies in humans with ASD and corresponding animal models have demonstrated that alterations of neurotrophic factor levels and their associated signaling pathways might contribute to the underlying disease pathophysiology5–9.
Neurotrophic factors are secreted proteins promoting the development, maintenance and function of neural circuits. They are essential regulators of neuronal maturation including synaptogenesis. The role of brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1) has been investigated in ASD. Altered BDNF signaling is implicated in ASD pathology, while IGF-1 is a potential candidate for treatment in this disorder8,10,11.
BDNF plays a pivotal role in the development and plasticity of the brain and is particularly important for the development of neural circuits and the regulation of glutamatergic and GABAergic synapses12–14. BDNF contributes to pre- and postnatal brain development and survival, has potent effects on synaptic and structural plasticity, strengthens excitatory (glutamatergic) synapses and weakens inhibitory (GABAergic) synapses15,16. BDNF binds to its receptor (TrkB) to activate canonical survival and neurite outgrowth signaling pathways such as the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI3K) serine-threonine-specific protein kinase (AKT1) pathways17,18, while its precursor, proBDNF, binds to the pan-neurotrophin receptor (p75NTR) to signal neurite retraction and apoptosis19–21. BDNF immunoreactivity measured by ELISA detects both mature BDNF and proBDNF, whereas proBDNF-specific ELISA or Western blotting distinguishes the isoforms. Higher levels of BDNF immunoreactivity have been found in post-mortem brain tissue of subjects with autism9,22, at least in part due to elevated proBDNF9. Elevated proBDNF may increase signaling through the p75NTR-RhoA pathway, which opposes the Rac pathway, and may contribute to aberrant connectivity in ASD5. In addition, TrkB receptor and signaling through the PI3K-AKT1 pathway are reduced in idiopathic autism compared to controls8. ProBDNF levels have not been measured in peripheral blood of subjects with autism, whereas BDNF immunoreactivity measured by ELISA in peripheral blood of children with autism compared to controls has resulted in mixed findings. However, systematic reviews and meta-analyses found increased BDNF blood levels (serum and plasma) in subjects with autism23–25.
The members of the insulin-like growth factor gene family, insulin-like growth factors 1 and 2 (IGF-1, IGF-2), play a key trophic role in the central nervous system (CNS). IGF-1 acts on its receptor (IGF1R) to activate the same signaling pathways as BDNF, the MAPK and PI3K-AKT1 pathways26,27, which play a crucial role in synaptic protein expression and inhibition of apoptosis. IGF-1 improves aberrant brain network connectivity, which is known to be an important neurobiological feature in ASD6,11, possibly by normalizing PI3K-AKT1 signaling. IGF-1 levels have been measured in brain parenchyma, CSF, plasma, serum and urine in autistic compared to control subjects, and the results are mixed28–34. CSF IGF-1 is significantly lower only in autistic children under 5 years old, whereas there is no IGF-1 difference in CSF or most brain areas in older children29,30,33. In contrast, serum and plasma levels of IGF-1 are reportedly higher in children with autism than controls32,34.
The development of effective pharmacological treatments for human psychiatric and neurological disorders, including ASD, represents one of the most challenging fields of neuroscience, due mainly to current limitations in our understanding of neurodevelopment, neurological function, and their links to psychiatry. Pharmacological treatment of ASD is focused on symptoms and not on progression of the disorder. Treatment is complicated by the heterogeneity of ASD and the presence of comorbid psychiatric illnesses or conditions in the majority of cases35. Blood BDNF levels have been highlighted as a potential biomarker of therapeutic efficacy36. Indeed, some of the drugs used to treat ASD and its accompanying comorbidities, such as depression, are known to affect BDNF levels37. However, there is still a gap in our understanding of the mechanisms underlying the differential changes in BDNF levels in the periphery and brain and their impact following pharmacological interventions in this disease.
The current study aimed to compare BDNF, proBDNF and IGF-1 serum levels between children aged 5–15 years old with mild to moderate autism and controls. Because pharmacological treatments can influence serum levels of these molecules, in addition to considering the groups as a whole, in this study we also separately analyzed BDNF, proBDNF and IGF-1 levels and the ratio of proBDNF/total BDNF in medication-naïve subjects versus those on medication. There are many gaps in our understanding of autism etiology, which limits its clinical management and treatment. These neurotrophic factors may not only provide blood biomarkers but may also offer clues to the pathophysiology of the disorder.
Materials and methods
Study subjects
The analysis included 22 children with a clinical diagnosis of ASD from the ambulatory services of the International Center for Neurological Restoration (CIREN), Borrás-Marfán Hospital and Las Católicas Hospital (Havana, Cuba) and 29 typically developing controls at similar ages. The control group included typically developing children gathered from the Surgery Service of both hospitals, which required blood for routine analysis prior to surgery. The age range of the subjects included in this study was 5–15 years old (Tables 1 and 2). The mean age of the control group was 8.68 ± 0.54 years and 9.45 ± 0.63 years for the autistic group. A 2-tailed unpaired t-test confirmed that there was no difference in age between the groups (p = 0.396). The control group contained 8 females and 21 males, the autistic group 5 females and 17 males (Tables 1 and 2). On average, 25% of the analyzed serum samples were from female subjects.
Table 1.
Characteristics of subjects with autism.
| ID# | Age years | Sex | BDNF ng/ml | ProBDNF ng/ml | ProBDNF/total BDNF | IGF-1 ng/ml | T/H | CGI-S | ABC-T | ADI-T | ATEC-T | CARS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 5 | M | 40.58 | 10.03 | 0.247 | 115.04 | CBZ | 4 | 99 | 44a | 70 | 28.5 |
| A16 | 5 | F | 29.11 | 11.35 | 0.390 | 113.36 | CBZ | 4 | 87 | 74 | 38 | 36 |
| A17 | 6 | M | 48.65 | 28.45a | 0.585 | 76.09 | No Tx | 3 | 98 | 63 | 46 | 26 |
| A2 | 7 | M | 39.97 | 10.52 | 0.263 | 98.19 | CBZ | 3 | 81 | 69 | 36 | 26 |
| A3 | 7 | M | 40.67 | 10.29 | 0.253 | 314.73c | No Tx | 4 | 89 | 65 | 36 | 29.5 |
| A18 | 7 | F | 19.71 | 10.38 | 0.526 | 93.01 | VPA | 4 | 121 | 69 | 62 | 33.5 |
| A19 | 7 | M | 43.7 | 10.36 | 0.237 | 114.21 | No Tx | 4 | 85 | 72 | 57 | 28 |
| A4 | 8 | F | 42.46 | 9.9 | 0.233 | 288.34 | CBZ | 4 | 121 | 64 | 70 | 32 |
| A5 | 8 | M | 30.49 | 9.93 | 0.326 | 72.46 | CBZ | 3 | 102 | 61 | 46 | 26 |
| A6 | 8 | F | 36.29 | 21.08 | 0.581 | 96.92 | No Tx | 3 | 110 | 70 | 58 | 27.5 |
| A7 | 9 | M | 29.56 | 18.79b | 0.636 | 134.91 | CBZ | 2 | 89 | 69 | 41 | 24 |
| A8 | 9 | M | 33.94 | 9.66 | 0.285 | 125.33 | CBZ | 3 | 103 | 65 | 20 | 29 |
| A9 | 10 | M | 35.85 | 6.01 | 0.168 | 105.47 | CBZ | 4 | 85 | 74 | 59 | 31.5 |
| A10 | 10 | F | 26.79 | 14.43 | 0.538 | 104.27 | MTPHE | 3 | 94 | 65 | 62 | 28.5 |
| A11 | 11 | M | 36.86 | 11.38 | 0.309 | 322.93 | HLP | 3 | 85 | 64 | 44 | 36 |
| A20 | 11 | M | 39.23 | 15.95 | 0.407 | 92.4 | No Tx | 3 | 89 | 63 | 41 | 27.5 |
| A12 | 13 | M | 42.3 | 7.5 | 0.177 | 228.1 | CBZ | 3 | 108 | 58 | 44 | 29.5 |
| A13 | 13 | M | 40.64 | 11.85 | 0.292 | 255.01 | CBZ | 4 | 82 | 74 | 36 | 36.5 |
| A14 | 13 | M | 28.79 | 7.77 | 0.27 | 143.17 | CBZ + R | 4 | 161a | 69 | 103a | 35 |
| A15 | 13 | M | 36.67 | 9.31 | 0.254 | 177.35 | No Tx | 4 | 95 | 58 | 39 | 35 |
| A21 | 13 | M | 35.95 | 9.4 | 0.262 | 164.83 | No Tx | 2 | 81 | 67 | 37 | 24 |
| A22 | 15 | M | 43.83 | 19.31 | 0.441 | 131.78 | No Tx | 4 | 102 | 62 | 45 | 30 |
| Mean | 9.45 | M = 17 | 36.46 | 12.44 | 0.349 | 153.09 | ||||||
| SEM ± | 0.63 | F = 5 | 1.45 | 1.13 | 0.030 | 16.56 | ||||||
| Median | 9.0 | 36.76 | 10.37 | 0.288 | 120.19 |
M male, F female, BDNF brain-derived neurotrophic factor, IGF-1 insulin-like growth factor 1, CBZ carbamazepine, R risperidone, MTPHE methylphenidate, HLP haloperidol, VPA valproic acid, No Tx no treatment, CGI-S Clinical Global Impression-Scale, ABC-T Autism Behavior Checklist total, ADI-T Autism Diagnostic Interview total, ATEC-T Autism Treatment Evaluation Checklist total, CARS Childhood Autism Rating Scale, a,b,coutliers detected by Grubb’s test, ain autism group, n = 22, bin medicated ASD, n = 14, cin unmedicated ASD, n = 8.
Table 2.
Characteristics of control subjects.
| ID# | Age years | Sex | BDNF ng/ml | ProBDNF ng/ml | IGF1 ng/ml |
|---|---|---|---|---|---|
| C1 | 5 | F | 34.74 | 8.70 | 103.39 |
| C2 | 5 | M | 38.07 | 14.56 | 71.43 |
| C3 | 5 | M | 40.37 | 13.82 | 106.83 |
| C4 | 5 | M | 43.35 | 10.94 | 84.8 |
| C5 | 5 | M | 47.36 | 12.37 | 96.39 |
| C6 | 6 | M | 7.14 | 13.94 | 65.71 |
| C8 | 6 | M | 43.33 | 13.43 | 157.61 |
| C11 | 6 | M | 42.96 | 11.50 | 95.24 |
| C12 | 7 | M | 45.47 | 10.65 | 124.53 |
| C13 | 7 | F | 38.1 | 8.23 | 108.7 |
| C14 | 7 | F | 42.27 | 17.48 | 123.03 |
| C15 | 7 | M | 27.95 | 17.48 | 113.51 |
| C16 | 8 | M | 9.38 | 17.01 | 58.53 |
| C17 | 9 | M | 35.56 | 14.90 | 89.44 |
| C18 | 9 | M | 35.37 | 15.52 | 85.62 |
| C19 | 9 | F | 34.04 | 13.95 | 107.17 |
| C20 | 9 | M | 41.17 | 30.56§ | 127.78 |
| C21 | 10 | M | 30.73 | 14.55 | 103.2 |
| C22 | 10 | M | 34.63 | 17.32 | 53.63 |
| C23 | 11 | M | 38.05 | 15.22 | 272.04 |
| C24 | 11 | M | 26.56 | 19.85 | 47.52 |
| C25 | 11 | M | 20.58 | nd | 46.63 |
| C26 | 11 | F | 27.01 | 19.36 | 50.32 |
| C27 | 11 | F | 21.35 | 16.28 | 230.54 |
| C28 | 11 | F | 36.47 | 17.01 | 115.41 |
| C29 | 12 | M | 25.1 | 16.53 | 141.43 |
| C30 | 13 | M | 26.41 | 17.40 | 144.11 |
| C32 | 14 | F | 43.08 | 11.09 | 100.22 |
| C33 | 14 | M | 35.5 | 16.31 | 250.52 |
| Mean | 8.68 | M = 21 | 33.99 | 15.21 | 115.31 |
| SEM ± | 0.54 | F = 8 | 1.87 | 0.80 | 10.53 |
| Median | 9.00 | 35.53 | 15.06 | 105.11 |
M male, F female, BDNF brain-derived neurotrophic factor, IGF-1 insulin-like growth factor 1, §outlier detected by Grubb’s test, nd not determined.
The diagnosis of autism followed DSM-V criteria1. Additionally, subjects were evaluated according to the Clinical Global Impression (CGI-S) Autism Treatment Evaluation Checklist (ATEC), the Autism Diagnostic Interview-Revised (ADI-R), the Autism Behavior Checklist (ABC) and the Childhood Autism Rating Scale (CARS). Autistic children with a history of seizure or psychotic disorders, severe head injury, or any other acute or chronic mental disease, inflammatory illness, or comorbidities were excluded. Control participants with a history of any immunological or infectious illness, metabolic, psychiatric, or neurological disorders were also excluded. All recruited subjects were of normal weight and free of any active treatment with immunomodulatory drugs. All subjects had IQ scores within the normal range. Fifteen of the subjects with autism (Table 1, subjects A1–A15) were included in previously published studies38,39.
Sample collection
Fasting peripheral blood samples were collected in the morning in 2 ml Vacutainer blood collection tubes and kept for 30 min at room temperature until the retraction of the clot. The blood was centrifuged for 10 min at 1000×g in a swinging bucket centrifuge pre-chilled to 4 °C. The serum was harvested, divided into aliquots, and stored at − 80 °C until the analysis. All experimental protocols were in accordance with the norms of the Advisory Scientific Committee and the Ethics Approval Board of the International Center for Neurological Restoration (CIREN).
Neurotrophic factor analysis
BDNF, proBDNF and IGF-1 ELISA: Serum BDNF, proBDNF and IGF-1 were measured40 using the Human BDNF DuoSet ELISA kit DY248, Human proBDNF DuoSet ELISA kit DY3175 and Human IGF-1 Quantikine ELISA kit DG100 (R&D Systems, Minneapolis, MN, US). The ancillary kit was used for BDNF and proBDNF. For BDNF measurements, serum samples were diluted 75 ×; the results are shown as the mean of four readings for each sample, each reading representing an independent dilution. For proBDNF measurements, serum samples were diluted 20 × and represent the mean of two independent plates. For IGF-1 quantification, serum samples were pre-treated according to the manufacturer’s instructions (R&D Systems); final dilution of the samples was 100 ×. Samples and standards for IGF-1 ELISA were analyzed in duplicate, and the final values were the average of two readings, each reading representing a separate dilution. Absorbances for BDNF, proBDNF and IGF-1 were read at 450 nm, with a reference reading at 540 nm, using a Multiskan GO plate reader and SKANIT 3.2 software (Thermo-Fisher Scientific, Waltham, MA, USA). BDNF, proBDNF and IGF-1 serum levels were expressed in ng/ml.
Data analysis
Statistical analyses were performed using SPSS 26 software (SPSS, Chicago, IL, USA) and GraphPad Prism software version 8.0.2 (GraphPad, La Jolla, CA, USA). The distribution of the data was examined by the Shapiro–Wilk normality test, and the presence of outliers was determined using the Grubbs' test. The significance of differences between groups was determined by 2-tailed unpaired t-test, Independent-Samples Mann–Whitney U test, Kruskal–Wallis test or 2-way ANOVA, according to the experimental design. Spearman’s correlation test was used to evaluate associations between variables. Statistical significance was set at p < 0.05.
Ethical standards
The Ethical Committee of the International Center approved the experimental protocols as part of the National Program of Neuroscience and Neurotechnology (Code PN305LH013-056) in accordance with the Helsinki norms and rights for human research. Informed consent from parents or caregivers was obtained for the study. All methods were carried out in accordance with relevant guidelines and regulations.
Results
Subject characteristics
Tables 1 and 2 show the demographic data and serum measurements for subjects with autism and controls. BDNF and IGF-1 measures from many of the subjects with autism were previously published as part of an analysis of non-invasive brain stimulation effectiveness in these patients39.
The Shapiro–Wilk normality test revealed that no variables except age, CARS scores and BDNF were normally distributed within the autism group (n = 22). The Shapiro–Wilk normality test on medicated subjects (n = 14) and unmedicated subjects (n = 8) revealed normal distribution for BDNF and proBDNF and the ratio of proBDNF/total BDNF. IGF-1 levels in medicated subjects only were not normally distributed. No other variables passed the Shapiro–Wilk normality test. Grubbs' test detected one outlier in the autism group for proBDNF levels and ABC-T, ADI-T and ATEC-T scores. In the control group, one outlier for proBDNF levels and two outliers for the ratio of proBDNF/total BDNF were detected (outliers marked in Tables 1 and 2). In the medicated ASD group, one outlier was detected for proBDNF levels and in the unmedicated ASD group, one outlier was detected for IGF-1 levels. The outliers were included in all analyses. For some analyses we also presented values when outliers were removed.
A two-way Univariate Analysis of Variance, group × sex, showed no significant effect of group or sex and no group × sex interaction for IGF-1, BDNF or proBDNF levels (IGF-1 group p = 0.133, sex p = 0.789, group × sex p = 0.589; total BDNF group p = 0.829, sex p = 0.326, group × sex p = 0.133; proBDNF group p = 0.195, sex p = 0.899, group × sex p = 0.351. Therefore, subsequent analyses combined male and female subjects.
Increased levels of serum IGF-1, no difference in serum BDNF and decreased serum proBDNF levels in subjects with autism compared to controls
Mean IGF-1 immunoreactivity was significantly increased by 36% in the serum of subjects with autism compared to controls (Fig. 1a, 2-tailed Mann–Whitney test, p = 0.037, n = 51). The higher points in Fig. 1a represent older subjects. Serum BDNF immunoreactivity was not significantly different between the autistic group and controls (Fig. 1b, 2-tailed Mann–Whitney test, p = 0.350, n = 51). Serum levels of proBDNF were significantly lower in subjects with autism compared to controls (Fig. 1c, 2-tailed Mann–Whitney U test, p = 0.005, n = 50). This was also true after removing outliers A17 and C20 (2-tailed Mann–Whitney U test, p = 0.002, n = 48). The ratio of proBDNF to total BDNF was also reduced in the ASD group (Fig. 1d, 2-tailed Mann–Whitney U test, p = 0.024, n = 50). After removing two outliers from the control group, C6 and C16, there was only a strong trend towards significance (2-tailed Mann–Whitney U test, p = 0.055, n = 48).
Figure 1.
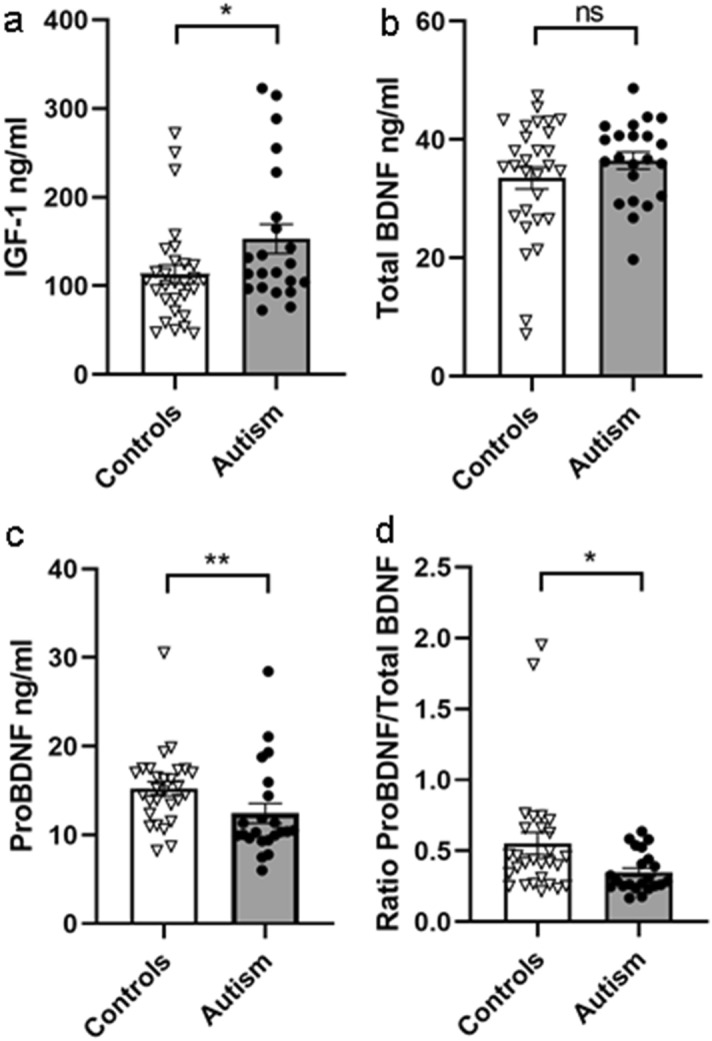
Serum protein levels measured by ELISA. (a) IGF-1, (b) total BDNF, (c) proBDNF and (d) the ratio of proBDNF/total BDNF immunoreactive protein levels in serum of controls (n = 29, or n = 28 where indicated) and subjects with autism (n = 22). 2-tailed Mann–Whitney test, IGF-1 *p = 0.037, total BDNF p = 0.350, ns not significant; proBDNF **p = 0.005, controls n = 28; proBDNF/total BDNF *p = 0.024, controls n = 28. Data are shown as mean ± SEM.
There was no correlation between total BDNF and IGF-1, proBDNF and IGF-1 or between proBDNF and total BDNF serum levels (Spearman’s correlation BDNF vs. IGF-1 rs = 0.236, p = 0.096, n = 51; proBDNF vs. IGF-1: rs = − 0.245, p = 0.087, n = 50; proBDNF vs. total BDNF: rs = − 0.176, p = 0.221, n = 50). However, there was a significant negative correlation between the ratio of proBDNF/total BDNF and IGF-1 levels when all samples were analyzed (Spearman’s correlation rs = − 0.284, p = 0.046, n = 50). This was maintained in the autism group when analyzed separately (Spearman’s correlation ASD: rs = − 0.468, p = 0.028, n = 22), but not in the control group (Spearman’s correlation controls: rs = − 0.121, p = 0.540, n = 28).
Different levels of serum proBDNF, but not BDNF or IGF-1, in medicated versus unmedicated subjects with autism
There was a trend towards increased levels of serum total BDNF immunoreactivity in unmedicated subjects with ASD compared to subjects with ASD taking medication and to non-autistic controls (Fig. 2a, Kruskal–Wallis test, p = 0.073, Dunn’s test unmedicated vs. medicated p = 0.122, unmedicated vs. controls p = 0.096; medicated vs. controls p > 0.999).
Figure 2.

Serum BDNF and IGF-1 protein levels in unmedicated and medicated subjects. BDNF and IGF-1 immunoreactive protein levels in serum of unmedicated (n = 8) and medicated (n = 14) subjects with autism and in non-autistic controls (n = 29 or n = 28 where indicated). Kruskal–Wallis test, (a) total BDNF, p = 0.073; (b) proBDNF **p = 0.004 followed by Dunn’s test unmedicated vs. medicated p = 0.187, unmedicated vs. controls p > 0.999, medicated vs. controls **p = 0.003, controls n = 28; (c) proBDNF/total BDNF p = 0.070, controls n = 28; (d) IGF-1 p = 0.108. Data are shown as mean ± SEM.
In contrast, while serum proBDNF levels did not differ between unmedicated ASD and non-autistic control subjects, the levels of serum proBDNF immunoreactivity in medicated subjects with ASD were significantly lower than those in non-autistic controls (Fig. 2b, Kruskal–Wallis test, p = 0.004; Dunn’s test medicated vs. controls p = 0.003; unmedicated vs. medicated p = 0.187, unmedicated vs. controls p > 0.999). Removing outliers A7 and C20 from the analysis did not alter this result (Kruskal–Wallis test, p = 0.001; Dunn’s test medicated vs. controls p < 0.001; unmedicated vs. medicated p = 0.081, unmedicated vs. controls p > 0.999). There was a trend toward significant differences between groups in the ratio of proBDNF to total BDNF. This trend was lost after removing outliers C6 and C16 from the control group (Fig. 2c, Kruskal–Wallis test, p = 0.070, n = 50; p = 0.136, n = 48).
There were no significant differences between groups when serum levels of IGF-1 immunoreactivity were analyzed in ASD subgroups taking medication and unmedicated and controls (Fig. 2d, Kruskal–Wallis test, p = 0.108, n = 51).
Correlations of IGF-1, BDNF and proBDNF serum levels with age
There was a significant positive association of serum IGF-1 immunoreactivity with age (Fig. 3a, Spearman’s correlation rs = 0.345, p = 0.013, n = 51). Although our study did not reveal a statistically significant correlation between IGF-1 and age in the control group analyzed separately (Spearman’s correlation rs = 0.207, p = 0.282, n = 29), the correlation with age was maintained in the autism group (Spearman’s correlation rs = 0.429, p = 0.046, n = 22, Fig. 3b). Serum total BDNF immunoreactivity did not correlate with age (Fig. 3c, Spearman’s correlation rs = − 0.233, p = 0.101, n = 51). When the groups were analyzed separately, in the control group (Fig. 3d), total BDNF was negatively correlated with age (rs = − 0.436, p = 0.018, n = 29) while proBDNF was positively correlated with age (Spearman’s correlation rs = 0.516, p = 0.005, n = 28). However, there was no correlation between total BDNF or proBDNF and age in the autism group (Fig. 3e, Spearman’s correlation BDNF rs = − 0.010, p = 0.966; proBDNF rs = − 0.205, p = 0.359, n = 22). There was a correlation in the control group between the ratio of proBDNF/BDNF and age (Fig. 3f, Spearman correlation rs = 0.469, p = 0.012, n = 28), which became stronger after removal of outliers C6 and C16 (Spearman correlation rs = 0.602, p = 0.001, n = 26), while there was no significant correlation between proBDNF/BDNF ratio and age in the ASD group (Spearman correlation rs = − 0.075, p = 0.739, n = 22). The correlation of the ratio of proBDNF/total BDNF with age when all subjects were analyzed together was not significant either without or with outliers (Spearman correlation rs = 0.247, p = 0.091, n = 48 and rs = 0.247, p = 0.196, n = 50 respectively).
Figure 3.
Correlations between serum IGF-1 and BDNF immunoreactivity and age. Spearman’s correlations: (a) IGF-1 correlation of all samples with age, rs = 0.345, p = 0.013, n = 51. (b) IGF-1 correlation of each group separately with age (control group rs = 0.207, p = 0.282, n = 29; autism group rs = 0.429, p = 0.046, n = 22). (c) Total BDNF correlation of all samples with age, rs = − 0.233, p = 0.101, n = 51. (d) Total BDNF and proBDNF correlations with age in the control group (total BDNF rs = − 0.436, p = 0.018, n = 29; proBDNF rs = 0.516, p = 0.005, n = 28). (e) Total BDNF and proBDNF correlations with age in the autism group (total BDNF rs = − 0.010, p = 0.966, n = 22; proBDNF rs = − 0.205, p = 0.359, n = 22. (f) Correlation of proBDNF/total BDNF in each group separately with age (control group rs = 0.469, p = 0.012, n = 28; autism group rs = − 0.075, p = 0.739, n = 22). Open triangles, controls; filled circles, autism; open diamonds, total BDNF; closed diamonds, proBDNF.
Correlations of autism diagnostic scores with serum IGF-1 and BDNF levels
There was a significant positive correlation between Childhood Autism Rating Scale (CARS) scores and IGF-1 immunoreactive levels (Fig. 4, Spearman’s correlation rs = 0.486, p = 0.022, n = 22). There was no correlation of CARS scores with total BDNF or with proBDNF immunoreactivity levels (Spearman’s correlation rs = − 0.069, p = 0.760 and rs = − 0.238, p = 0.287, n = 22, respectively), however, there was a trend toward a negative correlation between the proBDNF/BDNF ratio and CARS scores (Spearman’s correlation rs = − 0.407, p = 0.060, n = 22). We did not detect any relationship between CGI-S, ATEC, ADI-R or ABC scores with IGF-1, BDNF or proBDNF immunoreactivity.
Figure 4.
Correlations between Childhood Autism Rating Scale (CARS) scores and IGF-1 and proBDNF/BDNF. (a) IGF-1 immunoreactivity, Spearman’s correlation rs = 0.486, p = 0.022 and (b) ratio proBDNF/BDNF, Spearman’s correlation rs = − 0.407, p = 0.060. n = 22.
Discussion
Growth factors are a set of proteins that play a crucial role in cellular growth, proliferation, and differentiation in the CNS. Because of their role in CNS development, growth factors have been studied in neuroplasticity modulation and behavioral effects, with a particular emphasis on neurodevelopmental disorders6,11,41,42. Insulin-like growth factors (IGFs) are a family of proteins forming part of a complex signaling pathway including IGFs (i.e., IGF-1 and IGF-2), IGF receptors, binding proteins, and proteases. IGF-1 is neuroprotective and is involved in brain development44–45. Changes in IGF-1 signaling are implicated in the pathogenesis of neurological and psychiatric disorders including autism and major depressive disorder6,46,47. Both BDNF and IGF-1 are known to increase signaling through the PI3K-Akt-mTOR pathway26,48, a signaling pathway that is decreased in idiopathic ASD5,49–52. Because of its pharmacological properties, IGF-1 has been suggested as a treatment for autism53,54. In fact, IGF-1 has been useful in Rett syndrome (RTT) and in Phelan–McDermid Syndrome, both neurodevelopmental ASDs10,54–55. Trofinetide, an analog of the amino-terminal tripeptide of IGF-1, has also induced clinically relevant improvements in RTT56. IGF-1 may exert its effects in RTT by acting on the mTOR pathway, which is down-regulated in RTT57. Several clinical trials are currently testing the safety and efficacy of IGF-1 or IGF-1 analogues in RTT patients, and preliminary analysis demonstrates improvement in cardiorespiratory parameters, disease severity, and some behavioral and EEG measures of mood and anxiety10,53,54,56. Despite the promise of IGF-1 treatment for autism, CNS IGF-1 levels do not differ between subjects with autism and controls. In CSF of children with autism younger than 5 years old, IGF-1 levels are reportedly lower than in controls28,30. However, IGF-1 levels in CSF from children with autism at 5–15 years old were not different from controls at those ages30, consistent with a lack of difference in IGF-1 levels in post-mortem brain tissue29,33.
In contrast, several reports, including this one, document changes in IGF-1 levels in the periphery of children with autism30–32. The significantly increased peripheral blood levels of IGF-1 immunoreactivity observed in our subjects with autism compared to controls are in agreement with the results reported by Mills et al.32 and Şimşek et al.34, who showed IGF-1 increases in ASD plasma and serum, respectively. Mills et al.32 restricted their study sample to only boys between 4 and 8 years old, whereas Şimşek et al.34 found a significantly higher level of IGF-1 protein in a mixed population of boys and girls ages 4–12, consistent with the broader age range of 5–15 years and both sexes (25% females) in our study. Although IGF-1 may be a beneficial treatment for ASD, there appears to be no alteration in IGF-1 levels in autism CNS tissue or fluids compared to controls29,30,33. Increased blood levels of IGF-1 reported here, coupled with decreased levels of IGF-1 in urine of young subjects with autism31, instead suggest reduced excretion of peripheral IGF-1 in autism subjects compared to controls. Reduced excretion might be due to increased binding of IGF-1 by the IGF-1 binding protein, insulin-like growth factor binding protein 3 (IGFBP-3) that is increased in blood from autism patients32. Analysis of serum IGF-1 in children younger than 4–5 years old and of urine IGF-1 in older children would be of interest to clarify if reduced IGF-1 excretion is responsible for increased serum IGF-1 levels in autism.
In agreement with previous reports58–61, we found that serum IGF-1 immunoreactivity increases with prepubertal age. The IGF-1 levels of all measured samples were significantly correlated with the age of the subjects. Although our study did not show a statistically significant correlation between IGF-1 immunoreactivity and age in the control group analyzed separately (which may be attributable to our small sample size), the statistically significant correlation in the autism group and the parallel fitting lines in Fig. 3b suggest a relationship in each group. The parallel fitting lines demonstrate also, that across the tested range of 5–15 years of age, serum IGF-1 levels of children with autism were higher than levels of controls.
In this study, we found a significant positive correlation between Childhood Autism Rating Scale (CARS) scores and IGF-1 immunoreactivity. Why IGF-1 should correlate with CARS scores but not with other measures of autistic behavior is unclear.
Among the different families of growth factors, the neurotrophin family has received much attention for its role in neuropsychiatric conditions such as neurodegenerative disorders (e.g., Alzheimer’s disease), neurodevelopmental disorders (e.g., autism), and psychiatric disorders (e.g., depression, bipolar disorder)5,8,42,62–64. BDNF, in particular, is a neurotrophin that is abundantly expressed in the adult CNS, playing a prominent role in cognitive processes such as learning, memory, and behavioral consolidation16,65. BDNF and its precursor, proBDNF, are also widely expressed in the developing and postnatal brain. BDNF and proBDNF exhibit opposite activities on synapses, with BDNF signaling spine maturation, neurite outgrowth, survival and LTP while proBDNF signals spine weakening, neurite retraction, apoptosis and LTD20,21. BDNF and proBDNF regulate synaptogenesis and neuronal migration as well as influence synaptic plasticity and cortical circuitry development, all relevant for autism12,13,66.
In this study, we did not find statistically significant differences in serum BDNF-immunoreactive levels between subjects with autism and controls. However, proBDNF levels were lower in serum of subjects with ASD compared to controls. These results are in contrast to Bryn et al.67, who found increased BDNF, but not proBDNF levels, in plasma of children with ASD compared to controls. This is also inconsistent with our findings in post-mortem brain tissue, in which we found higher proBDNF levels in ASD fusiform gyrus than in controls9. These discrepancies may reflect poor efflux of proBDNF from brain compared to BDNF, differences in commercial proBDNF and BDNF ELISA assays and in Western blotting versus ELISA quantification of proBDNF, or altered proBDNF secretion from platelets into peripheral blood compared to BDNF68. Le Blanc et al.68 noted very different proBDNF/BDNF ratios in serum versus plasma, supporting the latter hypothesis.
When we stratified subjects with ASD according to medication/no medication, we found that subjects treated with mood stabilizers including carbamazepine, risperidone, haloperidol, methylphenidate and valproic acid exhibited lower serum proBDNF-immunoreactive levels than controls, suggesting that the inclusion of drug-treated subjects in the ASD group could be responsible for the reduced proBDNF levels when all ASD subjects were grouped together. Interestingly, neither BDNF nor IGF-1 serum levels differed between medicated and unmedicated subjects.
Previous findings support increased BDNF immunoreactivity in post-mortem ASD brain tissue compared to controls, in addition to decreases in the full-length isoform of the BDNF receptor, TrkB-FL, and its downstream PI3K-Akt-mTOR signaling pathway8,9,22. Likewise, in the periphery, most but not all groups report higher BDNF-immunoreactive levels in ASD compared to controls24,25. Western blots indicate that, in the brain, the increase in BDNF immunoreactivity in subjects with ASD is due to higher levels of proBDNF9. Since commercial BDNF ELISAs generally recognize both proBDNF and BDNF but with differing affinities, the contribution of proBDNF to changes in peripheral BDNF levels is difficult to discern without performing Western blots to distinguish BDNF isoforms. Nevertheless, in this study we used a commercial proBDNF ELISA to determine the effects of medication on the proBDNF isoform. Although the proBDNF ELISA failed to confirm higher amounts of serum proBDNF in subjects with ASD versus controls, normal or reduced levels of proBDNF may be the result of medications taken by the ASD group. Indeed, while proBDNF levels in drug naïve ASD subjects were comparable to control levels, proBDNF levels in medicated ASD subjects were significantly lower than in controls. This suggests that the normalization of BDNF levels by mood-stabilizing drugs in subjects with autism may include a reduction in proBDNF, which represents a portion of total BDNF levels. This possibility is supported by a report that tissue plasminogen activator (t-PA), an activator of plasmin that processes proBDNF to BDNF, is increased by valproate69. Unfortunately, previous reports of increased t-PA in children with autism70 do not mention the medication status of the children.
Current pharmacological treatment of ASDs includes antidepressants, anxiolytics and atypical antipsychotics36,71–73. Only two medications have been approved by the US FDA as useful for alleviating irritability, aggression and self-injury and stereotypy disorders, but not sociability defects, in autism: risperidone (Risperdal®), and aripiprazole (Abilify®)74,75. Normalization of BDNF levels following effective treatment with these antipsychotics has been reported76,77. Serum proBDNF levels following these treatments have not been investigated.
Methylphenidate (MTPHE) is the most commonly used psychostimulant in children with attention deficit hyperactivity disorder, as it significantly reduces hyperactivity and improves social skills in these children78,79. MTPHE affects predominantly dopamine and noradrenergic systems, with evidence of its role in neuroplasticity and behavior via dopamine D3 receptors and BDNF80,81. Although the literature is mixed, two reports demonstrate reductions of BDNF in ADHD children treated with MTPHE82,83 and two reports demonstrate increases84,85. ProBDNF levels have not been investigated in MTPHE-treated subjects with autism.
Carbamazepine (CBZ) and valproate (VPA) are two of the most common therapeutic drugs used to stabilize mood disorders and are also used frequently in autism. Both increase BDNF mRNA expression in individuals with temporal lobe epilepsy86. VPA is a histone deacetylase inhibitor that is known to increase BDNF expression in hippocampus, cortex and amygdala when given to adult animals87. However, most of these studies have assayed BDNF at the mRNA rather than the protein level, and none has attempted to distinguish BDNF from proBDNF. Counter-intuitively, the one subject in our study treated with VPA exhibited the lowest BDNF levels of any of the autism subjects but did not exhibit unusual proBDNF levels. Although proBDNF, not BDNF, levels are elevated in post-mortem brain tissue of humans with autism9, proBDNF levels have not been assayed following VPA administration in humans with autism.
Our report is one of the first to examine both BDNF and proBDNF in peripheral blood of autistic children. Another study67 assayed plasma rather than serum BDNF and proBDNF in a slightly older study population (average age 11 instead of our 9 years) and did not test the correlation of BDNF or proBDNF with age. We report here that serum levels of both BDNF and proBDNF were associated with age, but only in the control group. BDNF levels decreased with age, whereas proBDNF increased with increasing age. This suggests that processing of proBDNF to mature BDNF becomes progressively less efficient over time such that proBDNF represents a greater proportion of the total BDNF immunoreactivity in older subjects compared to younger. This is consistent with reports that both BDNF and proBDNF are crucial for synaptic remodeling during development14,88, and that BDNF gives way to proBDNF as circuits depend less on growth and more on fine tuning. The lack of correlation between these BDNF isoforms and age in the autism group provides further evidence that disruption in BDNF isoform balance during development is associated with defects in the establishment and maintenance of functional neuronal networks9.
A major limitation of this analysis is the small group size and high variability in the unmedicated ASD group. Further experiments are required to verify the isoform of BDNF that is elevated in serum. Notably, BDNF serum levels are affected by the Val66Met BDNF polymorphism89,90. The genotype of the study subjects is not known, and thus the degree to which the Val66Met BDNF polymorphism might have increased variability is unknown.
In summary, we found a significant increase in serum IGF-1 and a significant decrease in serum proBDNF in young boys and girls with mild to moderate autism compared to controls. The increase in serum IGF-1 levels may be due to decreased excretion in urine. IGF-1 levels were positively correlated with age and did not vary with medication status. Total BDNF levels in subjects with autism were similar to controls, regardless of medication status. However, proBDNF levels in subjects with autism, in particular, those being treated with medication, were lower than in controls, suggesting that the beneficial actions of these medications may include lowering proBDNF levels. The significant elevation in IGF-1 in the autism group and the reduced proBDNF immunoreactivity in medicated subjects with autism compared to controls reinforces the involvement of those two growth factors in the pathophysiology and treatment of autism. The role of neurotrophic factors in autism should be studied further, with necessary inclusion of both sexes, larger study populations (particularly medication-naïve subjects) and, in the case of IGF-1 serum levels, inclusion of subjects younger than 4 years old.
Acknowledgements
The authors gratefully acknowledge the participation of the children and their families in this study.
Author contributions
M.R.-A. designed the study, recruited subjects, collected serum samples, helped with sample analysis, and wrote the manuscript. B.M. analysed serum samples, performed statistical analyses, prepared the figures and wrote the manuscript. B.V.-M., L.R.H., and M.W.S. performed diagnosis and treatment. M.F. designed the study, wrote and reviewed the manuscript. All authors reviewed and approved the final manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Asken MJ, Grossman D, Christensen LW. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Baron-Cohen S, et al. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011;9:e1001081. doi: 10.1371/journal.pbio.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat. Rev. Neurosci. 2015;16:551–563. doi: 10.1038/nrn3992. [DOI] [PubMed] [Google Scholar]
- 4.Guang S, et al. Synaptopathology involved in autism spectrum disorder. Front. Cell Neurosci. 2018;12:470. doi: 10.3389/fncel.2018.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahnestock M, Nicolini C. Bridging the gap between genes and behaviour: Brain-derived neurotrophic factor and the mTOR pathway in idiopathic autism. Autism-Open Access. 2015;5:143. doi: 10.4172/2165-7890.1000143. [DOI] [Google Scholar]
- 6.Riikonen R. Insulin-like growth factors in the pathogenesis of neurological diseases in children. Int. J. Mol. Sci. 2017;18:2056. doi: 10.3390/ijms18102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohja K, et al. Neuroimmunologic and neurotrophic interactions in autism spectrum disorders: Relationship to neuroinflammation. Neuromol. Med. 2018;20:161–173. doi: 10.1007/s12017-018-8488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolini C, Ahn Y, Michalski B, Rho JM, Fahnestock M. Decreased mTOR signaling pathway in human idiopathic autism and in rats exposed to valproic acid. Acta Neuropathol. Commun. 2015;3:1–13. doi: 10.1186/s40478-015-0184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia KL, et al. Altered balance of proteolytic isoforms of pro-brain-derived neurotrophic factor in autism. J. Neuropathol. Exp. Neurol. 2012;71:289–297. doi: 10.1097/NEN.0b013e31824b27e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khwaja OS, et al. Safety, pharmacokinetics, and preliminary assessment of efficacy of mecasermin (recombinant human IGF-1) for the treatment of Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 2014;111:4596–4601. doi: 10.1073/pnas.1311141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costales J, Kolevzon A. The therapeutic potential of insulin-like growth factor-1 in central nervous system disorders. Neurosci. Biobehav. Rev. 2016;63:207–222. doi: 10.1016/j.neubiorev.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuman EM. Neurotrophin regulation of synaptic transmission. Curr. Opin. Neurobiol. 1999;9:105–109. doi: 10.1016/S0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 13.Luikart BW, Parada LF. Receptor tyrosine kinase B-mediated excitatory synaptogenesis. Prog. Brain Res. 2006;157:15–24. doi: 10.1016/S0079-6123(06)57002-5. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Wang KH, Nose A. Molecular mechanisms underlying neural circuit formation. Curr. Opin. Neurobiol. 2009;19:162–167. doi: 10.1016/j.conb.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 16.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–389. doi: 10.1016/S0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 18.Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron Glia Biol. 2001;32:767–770. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- 19.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 2002;67:203–233. doi: 10.1016/S0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 20.Koshimizu H, et al. Multiple functions of precursor BDNF to CNS neurons: Negative regulation of neurite growth, spine formation and cell survival. Mol. Brain. 2009;13:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leal G, Bramham CR, Duarte CB. BDNF and hippocampal synaptic plasticity. Vitam. Horm. 2017;104:153–195. doi: 10.1016/bs.vh.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Perry EK, et al. Cholinergic activity in autism: Abnormalities in the cerebral cortex and basal forebrain. Am. J. Psychiatry. 2001;158:1058–1066. doi: 10.1176/appi.ajp.158.7.1058. [DOI] [PubMed] [Google Scholar]
- 23.Qin XY, et al. Association of peripheral blood levels of brain-derived neurotrophic factor with autism spectrum disorder in children: A systematic review and meta-analysis. J. Am. Med. Assoc. Pediatr. 2016;170:1079–1086. doi: 10.1001/jamapediatrics.2016.1626. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z, et al. Peripheral brain-derived neurotrophic factor in autism spectrum disorder: A systematic review and meta-analysis. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep31241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saghazadeh A, Rezaei N. Brain-derived neurotrophic factor levels in autism: A systematic review and meta-analysis. J. Autism Dev. Disord. 2017;47:1018–1019. doi: 10.1007/s10803-016-3024-x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, et al. IGF-1 promotes Brn-4 expression and neuronal differentiation of neural stem cells via the PI3K/Akt pathway. PLoS One. 2014;9:e113801. doi: 10.1371/journal.pone.0113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z, Yu Y, Niu L, Fei A, Pan S. IGF-1 protects tubular epithelial cells during injury via activation of ERK/MAPK signaling pathway. Sci. Rep. 2016;6:28066. doi: 10.1038/srep28066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanhala R, Turpeinen U, Riikonen R. Low levels of insulin like growth factor-I in cerebrospinal fluid in children with autism. Dev. Med. Child Neurol. 2001;43:614–616. doi: 10.1017/S0012162201001116. [DOI] [PubMed] [Google Scholar]
- 29.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 30.Riikonen R, et al. Cerebrospinal fluid growth factors IGF-1 and IGF-2 in infantile autism. Dev. Med. Child Neurol. 2006;48:751–755. doi: 10.1017/S0012162206001605. [DOI] [PubMed] [Google Scholar]
- 31.Anlar B, et al. Urinary epidermal and insulin-like growth factor excretion in autistic children. Neuropediatrics. 2007;38:151–153. doi: 10.1055/s-2007-990282. [DOI] [PubMed] [Google Scholar]
- 32.Mills J, et al. Elevated levels of growth-related hormones in autism and autism spectrum disorder. Clin. Endocrinol. (Oxf) 2007;67:230–237. doi: 10.1111/j.1365-2265.2007.02868.x. [DOI] [PubMed] [Google Scholar]
- 33.Cioana M, Michalski B, Fahnestock M. Insulin-like growth factor and insulin-like growth factor receptor expression in human idiopathic autism fusiform gyrus tissue. Autism Res. 2020;13:897–907. doi: 10.1002/aur.2291. [DOI] [PubMed] [Google Scholar]
- 34.Şimşek F, Işık U, Aktepe E, Kılıç F, Şirin FB, Bozkurt M. Comparison of serum VEGF, IGF-1, and HIF-1α levels in children with autism spectrum disorder and healthy controls. J. Autism Dev. Disord. 2021;51:3564–3574. doi: 10.1007/s10803-020-04820-w. [DOI] [PubMed] [Google Scholar]
- 35.Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: Classification, diagnosis and therapy. Pharmacol. Ther. 2018;190:91–104. doi: 10.1016/j.pharmthera.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Farmer C, Thurm A, Grant P. Pharmacotherapy for the core symptoms in autistic disorder: Current status of the research. Drugs. 2013;73:303–314. doi: 10.1007/s40265-013-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Björkholm C, Monteggia LM. BDNF—A key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gómez L, et al. Non-invasive brain stimulation for children with autism spectrum disorders: A short-term outcome study. Behav. Sci. (Basel) 2017;7(3):63. doi: 10.3390/bs7030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson-Agramonte MA, et al. Effect of non-invasive brain stimulation on behavior and serum brain-derived neurotrophic factor and insulin-like growth factor-1 levels in autistic patients. Drug Dev. Res. 2021 doi: 10.1002/ddr.21808. [DOI] [PubMed] [Google Scholar]
- 40.Heisz JJ, et al. The effects of physical exercise and cognitive training on memory and neurotrophic factors. J. Cogn. Neurosci. 2017;29:1895–1907. doi: 10.1162/jocn_a_01164. [DOI] [PubMed] [Google Scholar]
- 41.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galvez-Contreras AY, Campos-Ordonez T, Gonzalez-Castaneda RE, Gonzalez-Perez O. Alterations of growth factors in autism and attention-deficit/hyperactivity disorder. Front. Psychiatry. 2017;8:126. doi: 10.3389/fpsyt.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werther GA, Russo V, Baker N, Butler G. The role of the insulin-like growth factor system in the developing brain. Horm. Res. 1998;49:37–40. doi: 10.1159/000053066. [DOI] [PubMed] [Google Scholar]
- 44.Russo VG, Gluckman PD, Feldman EL, Werther G. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr. Rev. 2005;26:919–943. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- 45.Dyer AH, Vahdatpour C, Sanfeliu A, Tropea D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience. 2016;325:89–99. doi: 10.1016/j.neuroscience.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 46.Tu KY, et al. Significantly higher peripheral insulin-like growth factor-1 levels in patients with major depressive disorder or bipolar disorder than in healthy controls: A meta-analysis and review under guideline of PRISMA. Medicine (Baltimore) 2016;95:e2411. doi: 10.1097/MD.0000000000002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zegarra-Valdivia JA. Insulin-like growth factor type 1 and its relation with neuropsychiatric disorders. Medwave. 2017;17:e7031. doi: 10.5867/medwave.2017.07.7031. [DOI] [PubMed] [Google Scholar]
- 48.Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 2012;4:a009886. doi: 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magdalon J, Sánchez-Sánchez SM, Griesi-Oliveira K, Sertié AL. Dysfunctional mTORC1 signaling: A convergent mechanism between syndromic and nonsyndromic forms of autism spectrum disorder? Int. J. Mol. Sci. 2017;18:659. doi: 10.3390/ijms18030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onore C, Yang H, Van de Water J, Ashwood P. Dynamic Akt/mTOR signaling in children with autism spectrum disorder. Front. Pediatr. 2017;5:43. doi: 10.3389/fped.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganesan H, et al. mTOR signalling pathway—A root cause for idiopathic autism? BMB Rep. 2019;52:424–433. doi: 10.5483/BMBRep.2019.52.7.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pini G, et al. IGF1 as a potential treatment for Rett syndrome: Safety assessment in six Rett patients. Autism Res. Treat. 2012;2012:679801. doi: 10.1155/2012/679801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vahdatpour C, Dyer AH, Tropea D. Insulin-like growth factor 1 and related compounds in the treatment of childhood-onset neurodevelopmental disorders. Front. Neurosci. 2016;10:450. doi: 10.3389/fnins.2016.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolevzon, et al. A pilot controlled trial of insulin-like growth factor-1 in children with Phelan–McDermid syndrome. Mol. Autism5, 54. 10.1186/2040-2392-5-54 (2014) [Erratum: Mol. Autism6, 31. 10.1186/s13229-015-0025-0 (2015)]. [DOI] [PMC free article] [PubMed]
- 56.Glaze D, et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology. 2019;92:e1912–e1925. doi: 10.1212/WNL.0000000000007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rangasamy S, Olfers S, Gerald B, Hilbert A, Svejda S, Narayanan V. Reduced neuronal size and mTOR pathway activity in the Mecp2 A140V Rett syndrome mouse model. F1000Res. 2016;5:2269. doi: 10.12688/f1000research.8156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juul A, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: Relation to age, sex, stage of puberty, testicular size, and body mass index. J. Clin. Endocrinol. Metab. 1994;78:744–752. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- 59.Fleisch AF, et al. Blood lead levels and serum insulin-like growth factor 1 concentrations in peripubertal boys. Environ. Health Perspect. 2013;121:854–858. doi: 10.1289/ehp.1206105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain N, et al. Serum IGF-1, IGFBP-3 and their ratio: Potential biochemical growth maturity indicators. Prog. Orthod. 2017;18:11. doi: 10.1186/s40510-017-0165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang HP, Yang SF, Wang SL, Su PH. Associations among IGF-1, IGF2, IGF-1R, IG-2R, IGFBP-3, insulin genetic polymorphisms and central precocious puberty in girls. BMC Endocr. Disord. 2018;18:66. doi: 10.1186/s12902-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fahnestock M. Brain-derived neurotrophic factor: The link between amyloid-β and memory loss. Future Neurol. 2011;6:627–639. doi: 10.2217/fnl.11.44. [DOI] [Google Scholar]
- 63.Fernandes BS, et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: A meta-analysis of 52 studies. BMC Med. 2015;13:995–1004. doi: 10.1186/s12916-015-0529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castrén E, Monteggia LM. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol. Psychiatry. 2021;90:128–136. doi: 10.1016/j.biopsych.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez MC, Radiske A, Cammarota M. On the involvement of BDNF signaling in memory reconsolidation. Front. Cell. Neurosci. 2019;13:383. doi: 10.3389/fncel.2019.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horch HW. Local effects of BDNF on dendritic growth. Rev. Neurosci. 2004;15:117–129. doi: 10.1515/REVNEURO.2004.15.2.117. [DOI] [PubMed] [Google Scholar]
- 67.Bryn V, et al. Brain derived neurotrophic factor (BDNF) and autism spectrum disorders (ASD) in childhood. Eur. J. Paediatr. Neurol. 2015;19:411–414. doi: 10.1016/j.ejpn.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Le Blanc J, et al. Platelets selectively regulate the release of BDNF, but not that of its precursor protein, proBDNF. Front. Immunol. 2020;25:575607. doi: 10.3389/fimmu.2020.575607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larsson P, et al. Role of histone acetylation in the stimulatory effect of valproic acid on vascular endothelial tissue-type plasminogen activator expression. PLoS One. 2012;7:e31573. doi: 10.1371/journal.pone.0031573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Şimşek S, Cetin I, Cim A, Kaya S. Elevated levels of tissue plasminogen activator and E-selectin in male children with autism spectrum disorder. Autism Res. 2016;9:1241–1247. doi: 10.1002/aur.1638. [DOI] [PubMed] [Google Scholar]
- 71.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeFilippis M, Wagner KD. Treatment of autism spectrum disorder in children and adolescents. Psychopharmacol. Bull. 2016;46:18–41. doi: 10.64719/pb.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eissa N, et al. Current enlightenment about etiology and pharmacological treatment of autism spectrum disorder. Front. Neurosci. 2018;12:304. doi: 10.3389/fnins.2018.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alsayouf HA, et al. Pharmacological intervention in children with autism spectrum disorder with standard supportive therapies significantly improves core signs and symptoms: A single-center, retrospective case series. Neuropsychiatr. Dis. Treat. 2020;16:2779–2794. doi: 10.2147/ndt.s277294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alsayouf HA, Talo H, Biddappa ML, De Los Reyes E. Risperidone or aripiprazole can resolve autism core signs and symptoms in young children: Case study. Children (Basel) 2021;8:318. doi: 10.3390/children8050318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rizos E, et al. A longitudinal study of alterations of hippocampal volumes and serum BDNF levels in association to atypical antipsychotics in a sample of first-episode patients with schizophrenia. PLoS One. 2014;9:e87997. doi: 10.1371/journal.pone.0087997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bakirhan A, Yalcin Sahiner S, Sahiner IV, Safak Y, Goka E. Association of serum brain derived neurotropic factor with duration of drug-naive period and positive-negative symptom scores in drug naive schizophrenia. PLoS One. 2017;12:e0189373. doi: 10.1371/journal.pone.0189373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sturman, N., Deckx, L. & van Driel, M. L. Methylphenidate for children and adolescents with autism spectrum disorder. Cochrane Database Syst. Rev.2017. 10.1002/14651858.CD011144.pub2 (2017). [DOI] [PMC free article] [PubMed]
- 79.Storebø, O. J. et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents—Assessment of adverse events in non‐randomised studies. Cochrane Database Syst. Rev.2018. 10.1002/14651858.CD012069.pub2 (2018). [DOI] [PMC free article] [PubMed]
- 80.Andersen SL, Sonntag KC. Juvenile methylphenidate reduces prefrontal cortex plasticity via D3 receptor and BDNF in adulthood. Front. Synaptic Neurosci. 2014;6:4. doi: 10.3389/fnsyn.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schrantee A, et al. Age-dependent effects of methylphenidate on the human dopaminergic system in young vs adult patients with attention-deficit/hyperactivity disorder: A randomized clinical trial. JAMA Psychiat. 2016;73:955–962. doi: 10.1001/jamapsychiatry.2016.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahin S, et al. Effect of methylphenidate treatment on appetite and levels of leptin, ghrelin, adiponectin, and brain-derived neurotrophic factor in children and adolescents with attention deficit and hyperactivity disorder. Int. J. Psychiatry Clin. Pract. 2014;18:280–287. doi: 10.3109/13651501.2014.940054. [DOI] [PubMed] [Google Scholar]
- 83.Cubero-Millán I, et al. BDNF concentrations and daily fluctuations differ among ADHD children and respond differently to methylphenidate with no relationship with depressive symptomatology. Psychopharmacology. 2017;234:267–279. doi: 10.1007/s00213-016-4460-1. [DOI] [PubMed] [Google Scholar]
- 84.Amiri A, et al. Changes in plasma brain-derived neurotrophic factor (BDNF) levels induced by methylphenidate in children with Attention deficit-hyperactivity disorder (ADHD) Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;47:20–24. doi: 10.1016/j.pnpbp.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 85.Akay AP, et al. Serum brain-derived neurotrophic factor levels in treatment-naive boys with attention-deficit/hyperactivity disorder treated with methylphenidate: An 8-week, observational pretest–posttest study. Eur. Child Adolesc. Psychiatry. 2018;27:127–135. doi: 10.1007/s00787-017-1022. [DOI] [PubMed] [Google Scholar]
- 86.Martínez-Levy GA, et al. Increased expression of brain-derived neurotrophic factor transcripts I and VI, cAMP response element binding, and glucocorticoid receptor in the cortex of patients with temporal lobe epilepsy. Mol. Neurobiol. 2018;55:3698–3708. doi: 10.1007/s12035-017-0597-0. [DOI] [PubMed] [Google Scholar]
- 87.Whittle N, Singewald N. HDAC inhibitors as cognitive enhancers in fear, anxiety and trauma therapy: Where do we stand? Biochem. Soc. Trans. 2014;42:569–581. doi: 10.1042/BST20130233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun W, et al. Requirements of postnatal proBDNF in the hippocampus for spatial memory consolidation and neural function. Front. Cell Dev. Biol. 2021;9:678182. doi: 10.3389/fcell.2021.678182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lang UE, Hellweg R, Sander T, Gallinat J. The Met allele of the BDNF Val66Met polymorphism is associated with increased BDNF serum concentrations. Mol. Psychiatry. 2009;14:120–122. doi: 10.1038/mp.2008.80. [DOI] [PubMed] [Google Scholar]
- 90.Meng WD, et al. Elevated serum brain-derived neurotrophic factor (BDNF) but not BDNF gene Val66Met polymorphism is associated with autism spectrum disorders. Mol. Neurobiol. 2017;54:1167–1172. doi: 10.1007/s12035-016-9721-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.




