Abstract
It has been widely demonstrated that time processing is altered in patients with schizophrenia. This perspective review delves into such temporal deficit and highlights its link to low-level sensory alterations, which are often overlooked in rehabilitation protocols for psychosis. However, if temporal impairment at the sensory level is inherent to the disease, new interventions should focus on this dimension. Beyond more traditional types of intervention, here we review the most recent digital technologies for rehabilitation and the most promising ones for sensory training. The overall aim is to synthesise existing literature on time in schizophrenia linking psychopathology, psychophysics, and technology to help future developments.
Subject terms: Schizophrenia, Scientific community, Neuroscience
Introduction
From the moment of their birth, newborns are immersed in time, experiencing a continuously mutating reality and creating temporal representations that naturally improve and become more sophisticated through childhood into adolescence (e.g. ref. [1–5]). Time is a fundamental element of human awareness and efficiently coding temporal proprieties of the environment is necessary to be connected with the outer world, coherently experience events, and produce adaptive behaviours.
During the last decade, research has suggested that time disturbance plays a role in the pathophysiology of schizophrenia [6–8]. However, since time is not a single, unitary dimension, literature about time representations in schizophrenia is heterogeneous, involves different terminology based on researchers’ background and does not support an easy overview and comparison between studies. The present perspective review aims at providing an overall picture of temporal representations in schizophrenia, showing that the deficit embraces time at different levels and encompasses low-level sensory alterations that must be considered when thinking of schizophrenia, its early diagnosis and its rehabilitation strategies.
The complexity of the concept time
Time is so intrinsic in human beings that sometimes we forget how wide the concept of time is [9–16]. From a phenomenological perspective, humans are time-producing organisms, whose awareness of being is strictly interconnected with the sense of a lived duration of experience [17]. From a perceptual perspective, temporal coding is a heterogeneous process, which intervenes every time an estimation of a time duration is made but also every time sensorimotor information takes place.
To grasp the concept of time, many definitions and models have been proposed. The models developed to describe timing functions in humans can be mostly grouped into dedicated or intrinsic models [18]. On the one hand, according to dedicated models, temporal coding relies on specialized neural functions of a specific timing network. On the other hand, intrinsic models consider the timing functions as an emergent property of neural activity [19]. Thus, prototypical dedicated models of time hypothesize the existence of a centralized internal clock: [11–13] a pacemaker emits pulses, these are stored into an accumulator, then transferred to working memory, and a final decision making stage compares the pulses accumulated in working memory to those already stored in a reference memory module and identifies an appropriate outcome. Prototypical intrinsic models instead, such as the striatal beat frequency model [14–16], suggest that timing does not rely on dedicated circuits but it consists of a general computation of most neural networks [20, 21].
Specific processing circuits have been investigated for at least three time scales: sub-second, near-second, tens-of-seconds scales. Based on the main brain areas involved, it has been suggested that sub-second intervals activate an automatic timing processing (expression of the right cerebellum activity, left sensorimotor cortex and bilateral supplemental motor areas [22–24]), whereas supra-second intervals involve a cognitive timing processing (expression of the activity of right dorsolateral-prefrontal cortex and right posterior-parietal cortex [23, 25]). Although the evidence of the recruitment of different circuits between short sub-second and long supra-second intervals, the mechanisms underlying processing of near-second intervals and the neural basis for the intrinsic sense of time are still not clear [9, 10]. Moreover, time scales do not represent the only parameter to classify and investigate time processing. The next section introduces two important concepts to have in mind when investigating time.
Distinction between perception of time and timing of perception
Traditionally, a distinction has been made between explicit and implicit level of processing of time [26, 27]. Explicit processing involves explicit judgements about external stimuli’s temporal properties, such as their duration, order or simultaneity [6]. On the contrary, implicit processing is automatically engaged whenever sensorimotor information is temporally structured, even without the specific instruction to focus on time [26, 28]. For example, it can happen that, although no overt estimates of stimulus or action duration are required, to make perceptual judgements about stimulus features or perform a specific motor act any temporal structure intrinsic in stimulus presentation or motor execution implicitly recalls timing mechanisms. Implicit and explicit processing have been dissociated with imaging methods [27]. Explicit timing engages specifically the basal ganglia, with co-activation of supplementary motor area, inferior frontal cortex and cerebellum; implicit timing activates most consistently cortical action circuits, such as left-lateralized premotor and inferior-parietal cortices.
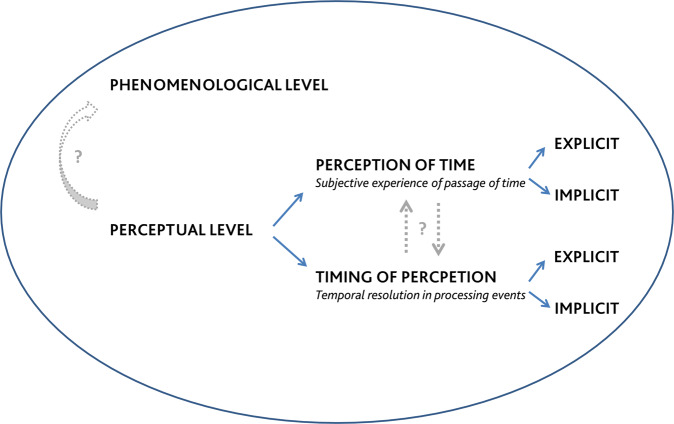
Stated the existing difference between explicit and implicit timing, we think that, in order to shed light on the literature about time perception in schizophrenia, it is important to introduce a new distinction between two concepts, which are often confused: perception of time and timing of perception. Perception of time refers to the subjective experience of the passage of time and of events duration. Instead, timing of perception refers to the temporal resolution in processing events. Indeed, every sensory experience is embedded in time and there is, unavoidably, a time in which perceived events take place. From a phenomenological perspective, the distinction between these two concepts overlaps with the distinction between structure and content. However, it is important to clarify that the distinction between perception of time and timing perception does not necessarily coincide with explicit and implicit temporal processing respectively. As illustrated in Fig. 1, both perception of time and timing of perception can be investigated explicitly and implicitly. Some examples are presented to illustrate possible experimental paradigms that outline the difference between implicit and explicit perception of time and timing of perception:
Explicit investigation of perception of time occurs for instance in duration discrimination tasks, where two time intervals are presented in sequence and the participant is asked to decide which interval is longer (e.g. refs. [29, 30]);
Implicit investigation of perception of time occurs for instance in tasks that require to evaluate whether moving stimuli will collide (e.g. ref. [27]). Participants implicitly use temporal information inherent to the speed of moving sensory stimuli to predict their eventual locations.
Explicit investigation of timing of perception occurs for instance in the simultaneity judgement task [31], which consists of evaluating the simultaneity vs. asynchrony of two stimuli. The specific instruction (“Are these stimuli simultaneous?”) makes it explicit, the possibility to estimate the timeframe within which multiple stimuli are highly likely to be perceived as one makes it a paradigm to explore timing of perception.
Implicit investigation of timing of perception occurs for instance in the double-flash illusion task [32], in which participants are asked how many flashes they perceive, and the illusion occurs when one flash simultaneously accompanied by two beeps is erroneously perceived as two flashes. The instructions are not about time, but the number of perceived flashes indirectly gives information about the temporal resolution in processing the sensory events.
Fig. 1. The concept of time.
Time involves both a phenomenological and a perceptual level. At perceptual level, it is possible to distinguish perception of time from timing of perception, which can both be investigated trough implicit or explicit experimental paradigms.
In our opinion, a categorization to better unfold the mechanisms underlying the concept of time and their role in schizophrenia would discern between perception of time and timing of perception regardless of the paradigm type, either explicit or implicit (Fig. 1).
Time representations in schizophrenia
Schizophrenia is characterized by positive symptoms (such as disorganized thinking, speech, delusions, hallucinations), negative symptoms (such as diminished emotional expression, social withdrawal, anhedonia) and cognitive dysfunction in several domains (such as attention, working memory and executive functioning) [33]. Since Stransky and Bleuler’s classical psychopathology up to Andreasen’s Cognitive Dysmetria model, the pathogenesis of schizophrenia has been hypothesized to depend on uncoordinated activity between different brain areas [34]. The model of Cognitive Dysmetria, in particular, suggests that impaired temporal information processing may represent one core deficit that triggers a detrimental cascade of effects [8, 34–36] contributing to the positive and negative symptoms of the disease.
Phenomenological evidence of time disturbance in schizophrenia
At phenomenological level, patients with schizophrenia report loss of continuity in the sense of time and temporal fragmentation of self-experience [37–39]. For example, they do not know the time of day, day of the week, month, or even year [40], and sometimes they feel time running slower or faster [41]. Abnormal temporal experience has been considered one key aspect of the basic self-disorder, a core trait-phenomenological feature of schizophrenia that implies structural instability of the first person-perspective [42–44]. Time, indeed, is a dynamic component of consciousness and phenomenological philosophy has interpreted the concept of time as a basic structure of the human self [45]. Fuchs and Pallagorsi [46] deeply addressed the phenomenological perspective of time in schizophrenia and concluded that “key schizophrenic symptoms such as thought disorder, thought insertion, auditory hallucinations, and passivity experiences may be described as disturbances of transcendental constitution of inner time consciousness or of the microstructure of temporality”.
Beyond the phenomenological evidence of time disturbance in schizophrenia, experimental psychology provided quantitative evidence of this temporal deficit showing that it also involves the perceptual level (see review of ref. [6, 47]). These aspects, albeit different, are likely to be interconnected (Fig. 1). Clarifying this link is important to understand the disease deeper and investigate how tailoring treatments on one ability can affect the other.
Overview of perception of time and timing of perception in schizophrenia
Experimental psychology demonstrated that perceptual temporal processing is compromised in schizophrenia. Several reviews have extensively and successfully addressed this topic [6, 44, 47]. Here, we argue that the well-documented temporal deficit in perception encompasses both perception of time and timing of perception.
Concerning the perception of time, literature is rich of examples (for a review see ref. [6, 47]). To briefly sum up, temporal precision (i.e. the consistency in perception) is clearly impaired in patients with schizophrenia, suggesting that the patients’ explicit and implicit judgements on time are significantly more variable compared to healthy controls. Patients with schizophrenia have deficits in explicitly detecting rhythm irregularities and in estimating durations ranging from milliseconds to several minutes, in a wide range of tasks, involving both verbal and motor responses, and affecting different sensory modalities. As stated above, while the higher time-scale processing is more related to high cognitive functions, such as attention and memory, the lower time-scale processing is thought to take place automatically and at low-sensory level [6]. Selective deficits in perception of time have also been observed with implicit paradigms. For instance, schizophrenic patients have problems in using temporal cues implicitly to anticipate target occurrence during an attention orienting task (e.g. ref. [48]).
Concerning the timing of perception, low-level processing of stimuli’s temporal features has often been reported altered in schizophrenia too. For example, tasks investigating the ability to judge the simultaneity of two events have widely demonstrated an enlarged temporal binding window in patients with schizophrenia (for a review [49]). The temporal binding window is a timeframe within which multiple stimuli are highly likely to be perceived as one [50]. Extended temporal binding window indicates imprecise temporal coding of sensory stimuli. Most studies on schizophrenia reported that the temporal binding window is tens of milliseconds wider in patients with schizophrenia compared to typical adults for unisensory modalities [51–53], and it widens to hundreds of milliseconds for audiovisual [53] and audiotactile [54] stimuli. A larger audiovisual temporal binding window encompasses both semantic [55] and pure non-semantic audiovisual stimuli [56]. In addition, the patients’ difficulties in the timing of perception go beyond explicit judgements and deficits have been reported with different implicit tasks too [55–59]. For instance, Giersch et al. [28] revealed that schizophrenic patients struggle to anticipate and follow information over time through a Stimulus-Response Compatibility paradigm [60], used for the implicit evaluation of sensory information processing over time. The latter branch of studies suggests an underlying difficulty exists in how information itself is processed in time and, consequently, in processing the flow of events [44].
Sensory processing underlying time representations at the core of schizophrenia
The excursus on time representations in schizophrenia highlights a strong deficit at the perceptual level that encompasses both the subjective experience of the passage of time (i.e. perception of time) and the ability to process temporal information intrinsic to any perceptual events (i.e. timing of perception). As a consequence, one might hypothesize that low-level sensory abnormalities underlying temporal processing are, at least partially, responsible for the overall temporal impairment and contribute to the other symptoms of schizophrenia.
To date, a growing body of literature supports such hypothesis. Indeed, overestimation of temporal intervals of both visual and auditory stimuli have been related to positive symptoms of schizophrenia (for a review see ref. [61],) and similar sensory alterations in time estimation have been observed in people without a diagnosis of schizophrenia but prone to visual hallucinations [62], individuals with schizotypal features [63] and subjects at high genetic risk for developing schizophrenia [64], suggesting that the deficit in perception of time may be an endophenotype of schizophrenia [6]. At the same time, anomalies in what we refer to as timing of perception have been proposed as a core deficit in the prodromal phase of schizophrenia [51]. For example, a larger temporal binding window is associated with disorganisation symptoms [52] and hallucination severity [53], and it matches higher levels of schizotypy in subclinical populations [54, 65, 66].
Moreover, timing of perception is crucial for multisensory integration, that is, the human ability to merge different sensory information from the same perceptual event into a unitary mental representation. The possibility and strength of multisensory integration depend on the low-level physical stimuli characteristics such as their timing (e.g. a pair of stimuli is likely to be integrated if they are closer in time) [50, 67, 68]. Research demonstrated that schizophrenia is characterized by different alterations in multisensory processing (for a review see ref. [49]). The ability to integrate sensory information has been associated with the emergence of a sense of self [69, 70] and, even more interestingly, there is a link between multisensory disintegration and self-disorders [71]. For example, integrating sensory information typically perceived as independent can cause sensory overload, alterations in sensory filters and ambiguous perceptual identity, causing a feeling of living in an incoherent world [72]. This symptomatology recalls what is observed at phenomenological level in patients with schizophrenia. In this regard, Martin et al. [44] have recently proposed that deficits in what we refer to as timing of perception could be associated with minimal self-disturbances in schizophrenic individuals. They argued that difficulties in integrating sensory information in time may affect the self because of their impact upon our ability to create single and stable representations, which would cause an uninterrupted flow of sensory inputs. In addition, Martin et al. [44] added that the impairment in timing of perception may also be responsible for the altered sense of time continuity also described at the phenomenological level in schizophrenia. Indeed, the representation of ourselves is stabilized within temporal windows and is experienced as being continuous in time. Instead, they demonstrated that, despite their enlarged temporal binding window, patients implicitly distinguish stimuli in time as controls do; however, differently from controls, they process stimuli individually rather than in sequence [57, 73, 74]. They interpreted their result as evidence of disturbed predictive coding in schizophrenia, suggesting that the ability to anticipate new events, while the focus is still on current ones, is compromised. This would lead to fragmentation in information processing, which impacts the sense of time continuity. This theory clearly supports a link between deficits in timing of perception, the temporal deficit reported at phenomenological level, and some main symptoms characterizing schizophrenia [75]. It is as if the sense of self required that sensory information were experienced as continuous in time, and in turn the sense of temporal continuity relied on the possibility to retain the latest past information and predict the imminent future events.
Therefore, research on both perception of time and timing of perception suggests that an impairment in the temporal aspects of early perceptual processing may be a pivotal substrate of psychotic functioning [76]. Starting from this perspective, major symptoms of schizophrenia, as those observed at phenomenological level, may be regarded as displays of globally disturbed time processing [46]. A recent study explored the relationship between perception of time and timing of perception and suggested that a higher precision in timing of perception implies a more fine-grained sense of the passage of time. Thus, there may be a common mechanism underlying these two perceptual dimensions [77] and its alteration may contribute to the pathophysiology of patients with schizophrenia. However, findings on the relationship between these dimensions of timing processing are inconsistent and do not allow any specific conclusion. The link between perception of time and timing of perception still needs further investigation, as well as the understanding of the interconnections between different aspects of the time deficit and psychosis. Yet, results presented in this paragraph are encouraging and, in our opinion, call clinicians and rehabilitators to consider the impairment in temporal representations at sensory level within the clinical rehabilitative framework.
New insight for rehabilitation of schizophrenia based on sensory processing of time representations
Despite the introduction of novel pharmacological agents and psychosocial interventions, schizophrenia remains one of the most severe and debilitating mental disorders [78], affecting 1% of the population [79, 80]. Although we have seen that a perceptual impairment in time processing has been frequently described, it is still neglected at both clinical and rehabilitative level. Indeed, existing treatments address neither the overall temporal deficit nor the related sensory alterations in perception. Beyond pharmacological treatments, interventions for schizophrenic patients are mostly focused on rehabilitation of cognitive and psychosocial skills. Some examples are the Cognitive Remediation Therapy (CRT) [81], cognitive-behavioural informed psychological interventions [82], or psychosocial lifestyle interventions [83]. However, if alterations in time representation at the perceptual level are at the core of psychosis, as hypothesized in previous sections, then they should be considered during both the assessment and rehabilitation phases of schizophrenia. In addition, since there is some evidence that they characterize the prodromal phase of the disease e.g. refs. [62–64], they could become a useful tool for screening or early intervention.
Notwithstanding that further research is necessary to clarify the role of the time deficit, all kinds of task presented above could potentially complement standard screening procedures to discriminate between healthy people and patients, to assess the level of severity of the disease, or, for example, to plan targeted interventions. For rehabilitation, sensory training addressing the specific impaired temporal skills could be planned. For example, previous research demonstrated that the most reliable sense to represent time is hearing [84], and hearing calibrates, regarding the perception of time, the other sensory modalities during development [85]. Hence, intervention strategies to improve the overall impairment in perception of time in schizophrenia could target hearing. Previous research demonstrated the potential of training auditory temporal skills and showed that the learned improvements on the temporal task transferred from the auditory modality to the visual one [86]. Similarly, other studies showed the benefits of perceptual learning to improve temporal judgements (e.g. refs. [87, 88]). Concerning the timing of perception, previous studies demonstrated high plasticity of multisensory integration in time, suggesting it is sensitive to experience and it can be successfully modified through perceptual training [89, 90] and temporal recalibration [91]. Perceptual training consists of, for instance, training procedures that aim to narrow the temporal binding window width through sensory exposure [92, 93]. Instead, temporal recalibration involves the exposure to temporally asynchronous pairs of stimuli to modify the temporal weight ascribed to each sense. For example, healthy individuals immersed in a visual-leading-auditory environment for several minutes develop a higher tolerance for visual-leading-auditory asynchrony [94, 95] and this aftereffect of asynchrony exposure is maintained until new discrepant sensory information is presented [96]. In addition, musical training could be considered since musicians show a much smaller temporal binding window compared to non-musicians [97].
The above trainings are only few examples of how the temporal deficit of schizophrenia could be addressed. Sensory and perceptual trainings, temporal recalibration and musical training could be exploited as clinical treatments for temporal distortions. Clearly, further research is needed to investigate the generalizability of the benefits of sensory training on time. Specifically, we might expect that other aspects of the disease associated with impaired temporal processing [98], such as incoherent perception, language and communication dysfunctions, impaired social cognition and self-disturbance, could improve following temporal trainings at perceptual level. The next section briefly illustrates the technology developed to assess and treat schizophrenia, discussing the digital revolution’s potential for delivering perceptual training on time.
Technology as tool for sensory intervention on time representations in schizophrenia
Nowadays, the world of e-mental Health is defined as “mental health services and information delivered or enhanced through the Internet and related technologies” [99]. This new category of technology-based assessment and intervention techniques can improve accessibility, reduce costs, ensures flexibility in terms of standardization and personalization, interactivity, and consumer engagement [100]. The potential benefits are such that various digital tools for both assessment and therapy of schizophrenia have been developed already (for reviews, see refs. [100–103]). Indeed, the disease itself does not set any specific limit to the development and use of e-mental Health-based solutions. People with schizophrenia who own digital devices do not differ from the typical population, and they have shown interest in using digital technologies for their mental health [103]. On the therapeutic side, the most popular digital interventions for schizophrenic patients include chatbots, which are software applications for monitoring signs of relapse in on-line chats, and computer-based cognitive training programs targeting different cognitive domains (e.g. CogPack) [104] or social cognition (e.g. the “Mind Reading: Interactive Guide to Emotions” [105]). These are only a few examples of technology-based interventions for psychosis; while some of them are at the very beginning, others are ready and available for clinical trials.
As the reader may have noticed, most of the efforts have been directed towards the creation of new technological ways to deliver classical therapies. However, the constant improvement of computational power and speed of digital devices [106] makes the new technologies valid tools to flexibly investigate earlier stages of sensory information processing. The study of perception requires stimuli presentation to be accurate in terms of intensity, onset, duration, and reproducibility across participants’ sessions. These characteristics are nowadays available even in smartphones, which are widely used, cheaper than standard experimental setups, and highly portable. Moreover, smartphones can deliver multimodal stimuli (i.e. visual, auditory and tactile) easily, as they can display images, animations or videos, reproduce sounds and vibrate. Thus, sensory processing underlying time representations, which requires multimodal stimulations at the millisecond scale, has become a research domain that today’s consumer electronics can probe. For instance, as described above, the deficit in the timing of perception of schizophrenic patients results in a temporal binding window around 10 to 500 ms larger than that of the typical population, depending on the task [49]. Consequently, typical paradigms on unisensory stimulation and multisensory integration require auditory, visual and/or haptic stimulations with a temporal delay among each other sometimes <20 ms e.g. ref. [107]. A few years ago, only computers with a strong GPU and a dedicated monitor with a refresh rate >100 Hz could reach such a degree of timing precision. Nowadays, as Inuggi et al. have demonstrated, consumer-level Android smartphones can deliver stimuli with the requested temporal accuracy [108, 109]. Based on their findings, Inuggi et al. developed Psysuite [108, 109], an Android app aimed to perform various psychophysics tasks that can probe, amongst others, the temporal correlates of perception. Specifically, two tasks have been validated with Psysuite: the double-flash illusion [108] and the temporal interval discrimination task [109]. The double-flash illusion task, mentioned above to illustrate the implicit investigation of timing of perception, requires inter-stimuli interval to be sometimes below few tens of milliseconds [32]. Users tested with Psysuite were subjected to this illusion. Albeit resistant to feedback training [110], the smartphone-based double-flash illusion could be used for remote assessment of temporal processing. The temporal interval discrimination task, instead, is one of the most common paradigms for explicit perception of time [111]. Participants trained in performing this task with Psysuite improved their performance after 4 days of unsupervised home training. These were just two practical examples of temporal tasks performed on smartphones, but all the above-mentioned sensory trainings on perception of time and timing of perception could be potentially delivered through new dedicated technologies. A remarkable body of literature is investigating the boundaries within which perceptual learning is obtained and generalizes to untrained stimuli [108, 109]. If temporal trainings at the perceptual level really work, they could be easily administrated during hospitalization or in the comfort of the patient’s house.
Another strength of modern digital devices yet to be fully exploited in regard to schizophrenia is immersive virtual reality. That is, the possibility to “immerse” users in virtual environments by fulfilling their senses with computer-generated visual, acoustic and haptic stimuli that mimic the perceptual features of physical stimuli and adapt in real-time to the users’ movements [112]. The power of immersive virtual reality resides in its ability to manipulate the users’ sense of embodiment [113], defined as the result of the sense of presence (i.e. self-location [114]), the sense of agency [115], and the sense of body-ownership [116]. In other words, immersive virtual reality offers a tool to investigate in a controlled and safe context the users’ sense of self, which is at the core of schizophrenia and, as hypothesized here, is likely interconnected with temporal processing. The implementation of real-time interactions with users allows to manipulate the temporal relationships between human predictions, actions, and sensory feedbacks, thus changing the sensorimotor contingencies and investigating the link between time and sense of self. Using virtual reality, it becomes possible, for instance, to control temporal delays of sensory feedback (e.g. delaying the incoming visual information), to create new sensory consequences for specific motor actions (e.g. bouncing a virtual ball by pressing a button), or to manipulate the temporal delay of sensory consequences (e.g. delaying the bounce of a virtual ball after pressing a button) within an ecological environment. All these possible experimental manipulations would allow to investigate sensory-motor temporal predictions and recalibration [117]. A link between the sense of agency in schizophrenia and imprecise motor predictions has already been identified thanks to virtual reality’s ability to on-line change the relationship between movements and corresponding sensory consequences [118–121]. Furthermore, since the human sensory-motor system seems able to adapt to temporal manipulations of the sensory-motor contingencies (see ref. [122] for a review), immersive virtual reality may offer a way to train the perception of time or the timing of perception and simultaneously assess the training effect on the sense of self [123, 124]. That said, despite the potential of such technology, to date virtual reality has been employed mainly to enhance and extend psychosocial and cognitive-behavioural treatments [125].
To sum up, the emergence of the e-mental Health has extensively expanded clinical possibilities by providing digital tools to modernise standard techniques. Digital technologies represent the most promising field of application for perceptual trainings. Considering results about sensory processing underlying time representations in schizophrenia, the scientific community should exploit the potentials of technology to create new science-based assessment and rehabilitation procedures working at sensory and sensory-motor levels. It is not about reinventing wheels; it is about using wings.
Conclusion
In this perspective review we provided a brief overview of the existing literature showing impaired ability to process temporal proprieties of the environment in people affected by schizophrenia. By distinguishing the phenomenological from the perceptual level and, within the latter, perception of time from timing of perception, we highlighted that the temporal deficit is multifaceted and encompasses low-level sensory alterations. However, these are not considered when thinking of rehabilitation strategies and technological devices. Although various reviews have stressed out the potential of digital tools on both assessment and therapy of schizophrenia, we think that the growth of the e-mental Health services must be expanded to the range of the possible assessment, early intervention and rehabilitation techniques based on scientific evidence. Digital technologies offer powerful resources for evaluating the soft signs of the disease, giving clinicians the possibility to dig into the lower levels of sensory processing with unprecedented ease. These potentials should be considered to develop new solutions that emerge from an interdisciplinary approach, involving experts on psychopathology, psychophysics and technology.
Acknowledgements
This work was developed within the framework of the DINOGMI Department of Excellence of MIUR 2018–2022 (law 232; 2016), and the joint-lab ANTARES based on the collaboration between the Unit for Visually Impaired People of the Italian Institute of Technology and the DINOGMI.
Author contributions
All authors contributed to conception of the manuscript. MBA, DE and AE wrote the first draft of the manuscript. MBA, DE, AE, CC, and AI contributed to manuscript revision. All authors read and approved the final version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allman MJ, Pelphrey KA, Meck WH. Developmental neuroscience of time and number: implications for autism and other neurodevelopmental disabilities. Front Integr Neurosci. 2012;6:7. doi: 10.3389/fnint.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Droit-Volet S. Time perception in children: a neurodevelopmental approach. Neuropsychologia. 2013;51:220–34. doi: 10.1016/j.neuropsychologia.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Pouthas V, Droit S, Jacquet A-Y. Temporal experiences and time knowledge in infancy and early childhood. Time Soc. 1993;2:199–218. doi: 10.1177/0961463X93002002004. [DOI] [Google Scholar]
- 4.Brackbill Y, Fitzgerald HE. Stereotype temporal conditioning in infants. Psychophysiology. 1972;9:569–77. doi: 10.1111/j.1469-8986.1972.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 5.Brannon EM, Roussel LW, Meck WH, Woldorff M. Timing in the baby brain. Brain Res Cogn Brain Res. 2004;21:227–33. doi: 10.1016/j.cogbrainres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Ciullo V, Spalletta G, Caltagirone C, Jorge RE, Piras F. Explicit time deficit in Schizophrenia: systematic review and meta-analysis indicate it is primary and not domain specific. Schizophr Bull. 2016;42:505–18. doi: 10.1093/schbul/sbv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnot O, de Montalembert M, Kermarrec S, Botbol M, Walter M, Coulon N. Are impairments of time perception in schizophrenia a neglected phenomenon. J Physiol Paris. 2011;105:164–9. doi: 10.1016/j.jphysparis.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–20. doi: 10.1016/S0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- 9.Wittmann M. The inner sense of time: how the brain creates a representation of duration. Nat Rev Neurosci. 2013;14:217–23. doi: 10.1038/nrn3452. [DOI] [PubMed] [Google Scholar]
- 10.Wittmann M, van Wassenhove V. Neural mechanisms and the interplay of emotion, cognition and embodiment. London: The Royal Society London; 2009. [DOI] [PMC free article] [PubMed]
- 11.Creelman CD. Human discrimination of auditory duration. J Acoustical Soc Am. 1962;34:582–93. doi: 10.1121/1.1918172. [DOI] [Google Scholar]
- 12.Treisman M. Temporal discrimination and the indifference interval. Implications for a model of the “internal clock”. Psychol Monogr. 1963;77:1–31. doi: 10.1037/h0093864. [DOI] [PubMed] [Google Scholar]
- 13.Gibbon J, Church RM, Meck WH. Scalar timing in memory. Ann N. Y Acad Sci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- 14.Buonomano DV, Mauk MD. Neural network model of the cerebellum: temporal discrimination and the timing of motor responses. Neural Comput. 1994;6:38–55. doi: 10.1162/neco.1994.6.1.38. [DOI] [Google Scholar]
- 15.Buonomano DV. Decoding temporal information: a model based on short-term synaptic plasticity. J Neurosci. 2000;20:1129–41. doi: 10.1523/JNEUROSCI.20-03-01129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci. 2004;27:307–40. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- 17.Biondi M, Pasquini M, Picardi A. Dimensional psychopathology. Springer; 2018.
- 18.Ivry RB, Schlerf JE. Dedicated and intrinsic models of time perception. Trends Cogn Sci. 2008;12:273–80. doi: 10.1016/j.tics.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paton JJ, Buonomano DV. The neural basis of timing: distributed mechanisms for diverse functions. Neuron. 2018;98:687–705. doi: 10.1016/j.neuron.2018.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:250–5. doi: 10.1016/S0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 21.Ivry RB. The representation of temporal information in perception and motor control. Curr Opin Neurobiol. 1996;6:851–7. doi: 10.1016/S0959-4388(96)80037-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee KH, Egleston PN, Brown WH, Gregory AN, Barker AT, Woodruff PW. The role of the cerebellum in subsecond time perception: evidence from repetitive transcranial magnetic stimulation. J Cogn Neurosci. 2007;19:147–57. doi: 10.1162/jocn.2007.19.1.147. [DOI] [PubMed] [Google Scholar]
- 23.Lewis PA, Miall RC. Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia. 2003;41:1583–92. doi: 10.1016/S0028-3932(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 24.Tregellas JR, Davalos DB, Rojas DC. Effect of task difficulty on the functional anatomy of temporal processing. Neuroimage. 2006;32:307–15. doi: 10.1016/j.neuroimage.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 25.Jones CR, Rosenkranz K, Rothwell JC, Jahanshahi M. The right dorsolateral prefrontal cortex is essential in time reproduction: an investigation with repetitive transcranial magnetic stimulation. Exp Brain Res. 2004;158:366–72. doi: 10.1007/s00221-004-1912-3. [DOI] [PubMed] [Google Scholar]
- 26.Piras F, Coull JT. Implicit, predictive timing draws upon the same scalar representation of time as explicit timing. PLoS One. 2011;6:e18203. doi: 10.1371/journal.pone.0018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coull J, Nobre A. Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol. 2008;18:137–44. doi: 10.1016/j.conb.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Giersch A, Lalanne L, Isope P. Implicit timing as the missing link between neurobiological and self disorders in Schizophrenia. Front Hum Neurosci. 2016;10:303. doi: 10.3389/fnhum.2016.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson VR, Feinberg I. Individual variations in time judgment and the concept of an internal clock. J Exp Psychol. 1968;77:631–40. doi: 10.1037/h0026048. [DOI] [PubMed] [Google Scholar]
- 30.Clausen J. An evaluation of experimental methods of time judgment. J Exp Psychol. 1950;40:756–61. doi: 10.1037/h0056354. [DOI] [PubMed] [Google Scholar]
- 31.van Eijk RL, Kohlrausch A, Juola JF, van de Par S. Audiovisual synchrony and temporal order judgments: effects of experimental method and stimulus type. Percept Psychophys. 2008;70:955–68. doi: 10.3758/PP.70.6.955. [DOI] [PubMed] [Google Scholar]
- 32.Shams L, Kamitani Y, Shimojo S. Visual illusion induced by sound. Brain Res Cogn Brain Res. 2002;14:147–52. doi: 10.1016/S0926-6410(02)00069-1. [DOI] [PubMed] [Google Scholar]
- 33.Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®). USA: American Psychiatric Pub; 2013. [DOI] [PubMed]
- 34.Escelsior A, Belvederi Murri M, Calcagno P, Cervetti A, Caruso R, Croce E, et al. Effectiveness of cerebellar circuitry modulation in Schizophrenia: a systematic review. J Nerv Ment Dis. 2019;207:977–86. doi: 10.1097/NMD.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 35.Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60:229–42. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- 36.Elvevag B, Brown GD, McCormack T, Vousden JI, Goldberg TE. Identification of tone duration, line length, and letter position: an experimental approach to timing and working memory deficits in schizophrenia. J Abnorm Psychol. 2004;113:509–21. doi: 10.1037/0021-843X.113.4.509. [DOI] [PubMed] [Google Scholar]
- 37.Stanghellini G, Ballerini M, Presenza S, Mancini M, Raballo A, Blasi S, et al. Psychopathology of lived time: abnormal time experience in persons with Schizophrenia. Schizophr Bull. 2016;42:45–55. doi: 10.1093/schbul/sbv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Northoff G, Stanghellini G. How to link brain and experience? Spatiotemporal psychopathology of the lived body. Front Hum Neurosci. 2016;10:76. doi: 10.3389/fnhum.2016.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuchs T. The temporal structure of intentionality and its disturbance in schizophrenia. Psychopathology. 2007;40:229–35. doi: 10.1159/000101365. [DOI] [PubMed] [Google Scholar]
- 40.Lewis A. The experience of time in mental disorder. Proc R Soc Med. 1932;25:611–20. [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman BJ. The subjective experience of perceptual and cognitive disturbances in schizophrenia. A review of autobiographical accounts. Arch Gen Psychiatry. 1974;30:333–40. doi: 10.1001/archpsyc.1974.01760090047008. [DOI] [PubMed] [Google Scholar]
- 42.Parnas J, Henriksen MG. Disordered self in the schizophrenia spectrum: a clinical and research perspective. Harv Rev Psychiatry. 2014;22:251–65. doi: 10.1097/HRP.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parnas J. The core Gestalt of schizophrenia. World Psychiatry. 2012;11:67–69. doi: 10.1016/j.wpsyc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin B, Wittmann M, Franck N, Cermolacce M, Berna F, Giersch A. Temporal structure of consciousness and minimal self in schizophrenia. Front Psychol. 2014;5:1175. doi: 10.3389/fpsyg.2014.01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zahavi D. Self and other: exploring subjectivity, empathy, and shame USA: Oxford University Press; 2014.
- 46.Fuchs T, Pallagrosi M. Dimensional psychopathology. Springer; 2018. 287–300.
- 47.Thoenes S, Oberfeld D. Meta-analysis of time perception and temporal processing in schizophrenia: differential effects on precision and accuracy. Clin Psychol Rev. 2017;54:44–64. doi: 10.1016/j.cpr.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Ciullo V, Piras F, Vecchio D, Banaj N, Coull JT, Spalletta G. Predictive timing disturbance is a precise marker of schizophrenia. Schizophrenia Res: Cognition. 2018;12:42–49. doi: 10.1016/j.scog.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou HY, Cai XL, Weigl M, Bang P, Cheung E, Chan R. Multisensory temporal binding window in autism spectrum disorders and schizophrenia spectrum disorders: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;86:66–76. doi: 10.1016/j.neubiorev.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Dixon NF, Spitz L. The detection of auditory visual desynchrony. Perception. 1980;9:719–21. doi: 10.1068/p090719. [DOI] [PubMed] [Google Scholar]
- 51.Di Cosmo G, Costantini M, Ambrosini E, Salone A, Martinotti G, Corbo M, et al. Body-environment integration: Temporal processing of tactile and auditory inputs along the schizophrenia continuum. J Psychiatr Res. 2021;134:208–14. doi: 10.1016/j.jpsychires.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 52.Foucher JR, Lacambre M, Pham BT, Giersch A, Elliott MA. Low time resolution in schizophrenia Lengthened windows of simultaneity for visual, auditory and bimodal stimuli. Schizophr Res. 2007;97:118–27. doi: 10.1016/j.schres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Stevenson RA, Park S, Cochran C, McIntosh LG, Noel JP, Barense MD, et al. The associations between multisensory temporal processing and symptoms of schizophrenia. Schizophr Res. 2017;179:97–103. doi: 10.1016/j.schres.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Cosmo G, Costantini M, Ambrosini E, Salone A, Martinotti G, Corbo M, et al. Body-Environment integration: temporal processing of tactile and auditory inputs along the schizophrenia continuum. J Psychiatr Res. 2020;134:208–14. doi: 10.1016/j.jpsychires.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 55.Martin B, Giersch A, Huron C, van Wassenhove V. Temporal event structure and timing in schizophrenia: preserved binding in a longer “now”. Neuropsychologia. 2013;51:358–71. doi: 10.1016/j.neuropsychologia.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Haß K, Sinke C, Reese T, Roy M, Wiswede D, Dillo W, et al. Enlarged temporal integration window in schizophrenia indicated by the double-flash illusion. Cogn Neuropsychiatry. 2017;22:145–58. doi: 10.1080/13546805.2017.1287693. [DOI] [PubMed] [Google Scholar]
- 57.Lalanne L, Van Assche M, Wang W, Giersch A. Looking forward: an impaired ability in patients with schizophrenia. Neuropsychologia. 2012;50:2736–44. doi: 10.1016/j.neuropsychologia.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 58.Exner C, Weniger G, Schmidt-Samoa C, Irle E. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophr Res. 2006;84:386–96. doi: 10.1016/j.schres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Posada A, Franck N. Use and automation of a rule in schizophrenia. Psychiatry Res. 2002;109:289–96. doi: 10.1016/S0165-1781(02)00027-6. [DOI] [PubMed] [Google Scholar]
- 60.Simon JR. Reactions toward the source of stimulation. J Exp Psychol. 1969;81:174–6. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- 61.Ueda N, Maruo K, Sumiyoshi T. Positive symptoms and time perception in schizophrenia: a meta-analysis. Schizophr Res Cogn. 2018;13:3–6. doi: 10.1016/j.scog.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coy AL, Hutton SB. The influence of hallucination proneness and social threat on time perception. Cogn Neuropsychiatry. 2013;18:463–76. doi: 10.1080/13546805.2012.730994. [DOI] [PubMed] [Google Scholar]
- 63.Lee K-H, Dixon JK, Spence SA, Woodruff PW. Time perception dysfunction in psychometric schizotypy. Personal Individ Differ. 2006;40:1363–73. doi: 10.1016/j.paid.2005.10.021. [DOI] [Google Scholar]
- 64.Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmeyer-Kimling L. Interval-timing deficits in individuals at high risk for schizophrenia. Brain Cogn. 2005;58:109–18. doi: 10.1016/j.bandc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 65.Ferri F, Venskus A, Fotia F, Cooke J, Romei V. Higher proneness to multisensory illusions is driven by reduced temporal sensitivity in people with high schizotypal traits. Conscious Cogn. 2018;65:263–70. doi: 10.1016/j.concog.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Ferri F, Nikolova YS, Perrucci MG, Costantini M, Ferretti A, Gatta V, et al. A neural “tuning curve” for multisensory experience and cognitive-perceptual Schizotypy. Schizophr Bull. 2017;43:801–13. doi: 10.1093/schbul/sbw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meredith MA, Stein BE. Spatial factors determine the activity of multisensory neurons in cat superior colliculus. Brain Res. 1986;365:350–4. doi: 10.1016/0006-8993(86)91648-3. [DOI] [PubMed] [Google Scholar]
- 68.Meredith MA, Nemitz JW, Stein BE. Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J Neurosci. 1987;7:3215–29. doi: 10.1523/JNEUROSCI.07-10-03215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rochat P. The self as phenotype. Conscious Cogn. 2011;20:109–19. doi: 10.1016/j.concog.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Bremner AJ, Lewkowicz DJ, Spence C. Multisensory development. Oxford: Oxford University Press; 2012.
- 71.Postmes L, Sno HN, Goedhart S, van der Stel J, Heering HD, de Haan L. Schizophrenia as a self-disorder due to perceptual incoherence. Schizophr Res. 2014;152:41–50. doi: 10.1016/j.schres.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 72.Borda JP, Sass LA. Phenomenology and neurobiology of self disorder in schizophrenia: primary factors. Schizophr Res. 2015;169:464–73. doi: 10.1016/j.schres.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 73.Lalanne L, van Assche M, Giersch A. When predictive mechanisms go wrong: disordered visual synchrony thresholds in schizophrenia. Schizophr Bull. 2012;38:506–13. doi: 10.1093/schbul/sbq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giersch A, Poncelet PE, Capa RL, Martin B, Duval CZ, Curzietti M, et al. Disruption of information processing in schizophrenia: The time perspective. Schizophr Res Cogn. 2015;2:78–83. doi: 10.1016/j.scog.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giersch A. The illusions of time. Springer; 2019.
- 76.Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–7. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 77.Fenner B, Cooper N, Romei V, Hughes G. Individual differences in sensory integration predict differences in time perception and individual levels of schizotypy. Conscious Cogn. 2020;84:102979.. doi: 10.1016/j.concog.2020.102979. [DOI] [PubMed] [Google Scholar]
- 78.Owen M, Sawa A. Mortensen pb. Schizophrenia Lancet. 2016;388:86–97. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bitanihirwe BK, Mauney SA, Woo TU. Weaving a net of neurobiological mechanisms in Schizophrenia and unraveling the underlying pathophysiology. Biol Psychiatry. 2016;80:589–98. doi: 10.1016/j.biopsych.2016.03.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18:727–40. doi: 10.1038/nrn.2017.125. [DOI] [PubMed] [Google Scholar]
- 81.Bowie CR, Bell MD, Fiszdon JM, Johannesen JK, Lindenmayer JP, McGurk SR, et al. Cognitive remediation for schizophrenia: An expert working group white paper on core techniques. Schizophr Res. 2020;215:49–53. doi: 10.1016/j.schres.2019.10.047. [DOI] [PubMed] [Google Scholar]
- 82.Wood L, Williams C, Billings J, Johnson S. A systematic review and meta-analysis of cognitive behavioural informed psychological interventions for psychiatric inpatients with psychosis. Schizophr Res. 2020;222:133–44. doi: 10.1016/j.schres.2020.03.041. [DOI] [PubMed] [Google Scholar]
- 83.Barber S, Thornicroft G. Reducing the mortality gap in people with severe mental disorders: the role of lifestyle psychosocial interventions. Front Psychiatry. 2018;9:463.. doi: 10.3389/fpsyt.2018.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burr D, Banks MS, Morrone MC. Auditory dominance over vision in the perception of interval duration. Exp Brain Res. 2009;198:49–57. doi: 10.1007/s00221-009-1933-z. [DOI] [PubMed] [Google Scholar]
- 85.Gori M, Sandini G, Burr D. Development of visuo-auditory integration in space and time. Front Integr Neurosci. 2012;6:77. doi: 10.3389/fnint.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McGovern DP, Astle AT, Clavin SL, Newell FN. Task-specific transfer of perceptual learning across sensory modalities. Curr Biol. 2016;26:R20–R21. doi: 10.1016/j.cub.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 87.Bratzke D, Seifried T, Ulrich R. Perceptual learning in temporal discrimination: asymmetric cross-modal transfer from audition to vision. Exp Brain Res. 2012;221:205–10. doi: 10.1007/s00221-012-3162-0. [DOI] [PubMed] [Google Scholar]
- 88.Nagarajan SS, Blake DT, Wright BA, Byl N, Merzenich MM. Practice-related improvements in somatosensory interval discrimination are temporally specific but generalize across skin location, hemisphere, and modality. J Neurosci. 1998;18:1559–70. doi: 10.1523/JNEUROSCI.18-04-01559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bidelman GM. Musicians have enhanced audiovisual multisensory binding: experience-dependent effects in the double-flash illusion. Exp Brain Res. 2016;234:3037–47. doi: 10.1007/s00221-016-4705-6. [DOI] [PubMed] [Google Scholar]
- 90.Powers AR, 3rd, Hillock AR, Wallace MT. Perceptual training narrows the temporal window of multisensory binding. J Neurosci. 2009;29:12265–74. doi: 10.1523/JNEUROSCI.3501-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van der Burg E, Goodbourn PT. Rapid, generalized adaptation to asynchronous audiovisual speech. Proc Biol Sci. 2015;282:20143083. doi: 10.1098/rspb.2014.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stevenson RA, Wilson MM, Powers AR, Wallace MT. The effects of visual training on multisensory temporal processing. Exp Brain Res. 2013;225:479–89. doi: 10.1007/s00221-012-3387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Powers AR, 3rd, Hevey MA, Wallace MT. Neural correlates of multisensory perceptual learning. J Neurosci. 2012;32:6263–74. doi: 10.1523/JNEUROSCI.6138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vroomen J, Keetels M, de Gelder B, Bertelson P. Recalibration of temporal order perception by exposure to audio-visual asynchrony. Brain Res Cogn Brain Res. 2004;22:32–35. doi: 10.1016/j.cogbrainres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 95.Roseboom W, Linares D, Nishida S. Adaptation reveals mechanisms for enhanced representation of common and novel temporal relationships. Procedia Soc Behav Sci. 2014;126:71. doi: 10.1016/j.sbspro.2014.02.320. [DOI] [Google Scholar]
- 96.Machulla TK, Di Luca M, Froehlich E, Ernst MO. Multisensory simultaneity recalibration: storage of the aftereffect in the absence of counterevidence. Exp Brain Res. 2012;217:89–97. doi: 10.1007/s00221-011-2976-5. [DOI] [PubMed] [Google Scholar]
- 97.Noppeney U, Lee HL. Causal inference and temporal predictions in audiovisual perception of speech and music. Ann N. Y Acad Sci. 2018. 10.1111/nyas.13615. [DOI] [PubMed]
- 98.Zhou HY, Cheung EFC, Chan RCK. Audiovisual temporal integration: cognitive processing, neural mechanisms, developmental trajectory and potential interventions. Neuropsychologia. 2020;140:107396. doi: 10.1016/j.neuropsychologia.2020.107396. [DOI] [PubMed] [Google Scholar]
- 99.Christensen H, Griffiths K, Evans K. e-Mental health in Australia: implications of the Internet and related technologies for policy. Commonwealth Department of Health and Ageing Canberra; 2002.
- 100.Lal S, Adair CE. E-mental health: a rapid review of the literature. Psychiatr Serv. 2014;65:24–32. doi: 10.1176/appi.ps.201300009. [DOI] [PubMed] [Google Scholar]
- 101.Ben-Zeev D, Buck B, Kopelovich S, Meller S. A technology-assisted life of recovery from psychosis. NPJ Schizophr. 2019;5:15.. doi: 10.1038/s41537-019-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mohr DC, Burns MN, Schueller SM, Clarke G, Klinkman M. Behavioral intervention technologies: evidence review and recommendations for future research in mental health. Gen Hosp Psychiatry. 2013;35:332–8. doi: 10.1016/j.genhosppsych.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bell IH, Alvarez-Jimenez M. Digital technology to enhance clinical care of early psychosis. Curr Treat Options Psychiatry. 2019;6:256–70. doi: 10.1007/s40501-019-00182-y. [DOI] [Google Scholar]
- 104.Moritz S, Mahlke CI, Westermann S, Ruppelt F, Lysaker PH, Bock T, et al. Embracing psychosis: a cognitive insight intervention improves personal narratives and meaning-making in patients with Schizophrenia. Schizophr Bull. 2018;44:307–16. doi: 10.1093/schbul/sbx072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lindenmayer JP, McGurk SR, Khan A, Kaushik S, Thanju A, Hoffman L, et al. Improving social cognition in schizophrenia: a pilot intervention combining computerized social cognition training with cognitive remediation. Schizophr Bull. 2013;39:507–17. doi: 10.1093/schbul/sbs120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leiserson CE, Thompson NC, Emer JS, Kuszmaul BC, Lampson BW, Sanchez D, et al. There’s plenty of room at the top: What will drive computer performance after Moore’s law? Science. 2020 10.1126/science.aam9744. [DOI] [PubMed]
- 107.Stevenson RA, Wallace MT. Multisensory temporal integration: task and stimulus dependencies. Exp Brain Res. 2013;227:249–61. doi: 10.1007/s00221-013-3507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inuggi A, Tonelli A, Gori M. 2021 IEEE international symposium on medical measurements and applications (MeMeA). IEEE; 2021. 10.1109/MeMeA52024.2021.
- 109.Domenici N, Inuggi A, Tonelli A, Gori M. 2021 43rd annual international conference of the IEEE engineering in medicine & biology society (EMBC). IEEE. 2021. 10.1109/EMBC46164.2021. [DOI] [PubMed]
- 110.Rosenthal O, Shimojo S, Shams L. Sound-induced flash illusion is resistant to feedback training. Brain Topogr. 2009;21:185–92. doi: 10.1007/s10548-009-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matthews WJ, Meck WH. Time perception: the bad news and the good. Wiley Interdiscip Rev Cogn Sci. 2014;5:429–46. doi: 10.1002/wcs.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Slater M, Sanchez-vives MV. Enhancing our lives with immersive virtual rality. Front Robot AI. 2016. 10.3389/frobt.2016.00074.
- 113.Kilteni K, Groten R, Slater M. The sense of embodiment in virtual reality. Presence: Teleoperators Virtual Environ. 2012;21:373–87. doi: 10.1162/PRES_a_00124. [DOI] [Google Scholar]
- 114.Servotte J-C, Goosse M, Campbell SH, Dardenne N, Pilote B, Simoneau IL, et al. Virtual reality experience: Immersion, sense of presence, and cybersickness. Clin Simul Nurs. 2020;38:35–43. doi: 10.1016/j.ecns.2019.09.006. [DOI] [Google Scholar]
- 115.Haggard P. Sense of agency in the human brain. Nat Rev Neurosci. 2017;18:196–207. doi: 10.1038/nrn.2017.14. [DOI] [PubMed] [Google Scholar]
- 116.Slater M, Perez-Marcos D, Ehrsson HH, Sanchez-Vives MV. Inducing illusory ownership of a virtual body. Front Neurosci. 2009;3:214–20. doi: 10.3389/neuro.01.029.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stetson C, Cui X, Montague PR, Eagleman DM. Motor-sensory recalibration leads to an illusory reversal of action and sensation. Neuron. 2006;51:651–9. doi: 10.1016/j.neuron.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 118.Synofzik M, Thier P, Leube DT, Schlotterbeck P, Lindner A. Misattributions of agency in schizophrenia are based on imprecise predictions about the sensory consequences of one’s actions. Brain. 2009;133:262–71. doi: 10.1093/brain/awp291. [DOI] [PubMed] [Google Scholar]
- 119.Rossetti I, Romano D, Florio V, Doria S, Nisticò V, Conca A, et al. Defective embodiment of alien hand uncovers altered sensorimotor integration in Schizophrenia. Schizophr Bull. 2020;46:294–302. doi: 10.1093/schbul/sbz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Werner JD, Trapp K, Wustenberg T, Voss M. Self-attribution bias during continuous action-effect monitoring in patients with schizophrenia. Schizophr Res. 2014;152:33–40. doi: 10.1016/j.schres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 121.Landgraf S, Krebs MO, Olié JP, Committeri G, van der Meer E, Berthoz A, et al. Real world referencing and schizophrenia: are we experiencing the same reality? Neuropsychologia. 2010;48:2922–30. doi: 10.1016/j.neuropsychologia.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 122.Rohde M, Ernst MO. Time, agency, and sensory feedback delays during action. Curr Opin Behav Sci. 2016;8:193–9. doi: 10.1016/j.cobeha.2016.02.029. [DOI] [Google Scholar]
- 123.Lallart E, Lallart X, Jouvent R. Agency, the sense of presence, and schizophrenia. Cyberpsychol Behav. 2009;12:139–45. doi: 10.1089/cpb.2008.0070. [DOI] [PubMed] [Google Scholar]
- 124.Davidson L. Recovering a sense of self in schizophrenia. J Pers. 2020;88:122–32. doi: 10.1111/jopy.12471. [DOI] [PubMed] [Google Scholar]
- 125.Bisso E, Signorelli MS, Milazzo M, Maglia M, Polosa R, Aguglia E, et al. Immersive virtual reality applications in Schizophrenia spectrum therapy: a systematic review. Int J Environ Res Public Health. 2020. 10.3390/ijerph17176111. [DOI] [PMC free article] [PubMed]