Abstract
If dental pulp injury occurs prior to complete root formation and apical closure, normal root development is halted. This condition produces several complications. Firstly, the apical diameter of the canal is often larger than the coronal diameter, so debridement is difficult. Secondly, the lack of an apical stop makes the obturation in all dimensions virtually impossible. And finally, the thin walls of the root canal are prone to fracture, so that surgical treatment is generally not a viable option. There are a number protocols to manage non-vital open-apex teeth such as apexification, apical barrier technique (one-visit apexification), orthograde root filling using MTA, triple antibiotic paste, and tissue engineering concept. The aim of this paper is to review these treatment protocols.
Key words: Open apex, dental trauma, immature root development
During tooth development, the inner and outer dental epithelia fuse and form the cervical loop, which results in Hertwig’s epithelial root sheath, a structure responsible for root formation1. The presence of healthy pulp is essential for root development and apical closure. When the pulp is vital and the apex is not fully formed, it is imperative to maintain the pulp vitality for dentine formation2. Dental caries and trauma are the most common challenges to the integrity of a tooth as it matures. Both insults can render the pulp non-vital2. If this occurs prior to complete root formation and apical closure, normal root development is halted2. Clinically, there are several conditions associated with treating non-vital teeth that have a widened or open apical foramen. Firstly, the apical diameter of the canal is often larger than the coronal diameter, so debridement is difficult. In addition, the lack of an apical stop makes the obturation in all dimensions virtually impossible. Finally, the thin walls of the root canal are prone to fracture, so that surgical treatment is generally not a viable option3. To avoid these complications, apexification prior to root canal filling should be attempted.
HISTORICAL PERSPECTIVES
The use of calcium hydroxide for apical closure was first introduced in 1964 by Kaiser4, when he proposed that using it mixed with camphorated parachlorophenol (CMCP) would induce the formation of a calcified barrier across the apex. His procedure was popularised in 1966 by Frank5, who described a step-by-step technique and four types of apical closure. For the procedure to be effective, it has been suggested that that calcium hydroxide must contact vital tissue6. The larger the apical opening, the longer the time necessary to induce apical closure7.
Nygaard-Østby8 suggested that laceration of periapical tissues with a file results in the further development of the root apex. Zussman9 showed that the vascularity of the apical region facilitates and favours further root development by the root sheath once the tissues are in a healthy state. Ham et al.10 have not reported much success with the induced blood clot, and Citrome et al.11 showed that the blood clot maintained the initial inflammatory state and did not result in hard tissue bridging at the apex.
The mechanism of apical closure is still in question. Some researchers believe that the root sheath remain intact and resumes its function once the source of infection is eliminated. Torneck and Smith12 stated that remnants of the dental pulp remain vital and function in spite of inflammation. Klein and Levy13 believed that the cells of the dental sac around the apex retain their genetic code that predisposes them to form into cementoblasts. On the other hand, some other studies claimed that it was not possible to identify a root sheath in any section of teeth with either partial or complete root closure. West and Lieb14 reported a case in which the periapex of an incisor was curetted following conventional endodontic therapy. After 11 years, the tooth was then successfully treated by using Frank’s technique.
It has also been proposed that calcium hydroxide stimulates undifferentiated mesenchymal cells to differentiate into cementoblasts, which in turn, initiate cementogenesis at the apex13., 15.. Dylewski16 showed proliferating connective tissue in the apical region that differentiated into calcified material, which became continuous with the predentine at the apex. Schroder and Granath17 revealed that this calcification process occurs beneath a superficial layer of necrosis. In this procedure, the calcium hydroxide dressing is replaced every 3 months until a barrier is formed (usually 6 to 24 months)2.
Undoubtedly, this technique sometimes provides inconsistent results:
-
•
The apical foramen closes with a definite recession of the root canal. The apical aspect continues to develop with a seemingly obliterated apex1., 2., 7..
-
•
The obliterated apex develops without any change in the root canal space1., 2., 7..
-
•
A thin calcified bridge that is not radiographically discernable develops1., 2., 7..
-
•
Usually multiple visits and a long period are required to root end closure1., 2., 7..
-
•
Long-term dressing with calcium hydroxide makes the tooth mere sensitive to fracture18.
MTA consists of 50–75% (wt) calcium oxide and 15–25% silicon dioxide. These two components together comprise 70–95% of the cement19., 20.. When these raw materials are blended they produce tricalcium silicate, dicalcium silicate, tricalcium aluminate and tetracalcium aluminoferrite. On addition of water the cement hydrates to form silicate hydrate gel that solidifies into a hard structure during 3–4 hours21. The production of calcium hydroxide as a by-product of the hydration reaction of MTA has been reported22. The biological response to MTA had been likened to that of calcium hydroxide and it was postulated that their mechanisms of action were similar23. It has been reported that MTA releases calcium ions and promotes an alkaline pH23. The physicochemical basis for the biological properties of MTA has recently been attributed to the production of hydroxyapatite when the calcium ions released by the MTA came into contact with tissue fluid24.
MTA materials have been suggested to afford less microleakage than traditional materials in a majority of bacteria-based microleakage studies when used as an apical restoration25., 26., furcation repair27, and in the treatment of immature apices28., 29.. In both fluid filtration and bacterial leakage models, 3 mm of MTA material is suggested as the minimal amount for protection against microleakage while 5 mm is suggested in the treatment of immature apices19.
Regarding the biocompatibility of MTA materials, studies in general tend to support the biocompatibility of both grey-coloured MTA and white-coloured MTA, although cytotoxicity studies are inclined to suggest less response to the set material as compared to the freshly prepared material19.
There are two major solutions for the management of permanent non-vital teeth with open apices using MTA as follows.
Apical barrier technique
Induction of apical healing, regardless of the material used, takes at least 3–4 months and requires multiple appointments2., 7.. Patient compliance with this regimen may be poor and many fail to return for scheduled visits. The temporary seal may fail resulting in reinfection and prolongation or failure of treatment. The importance of the coronal seal in preventing endodontic failure is well established30. For these reasons one-visit apexification has been suggested. Morse et al.7 define one-visit apexification as the non-surgical condensation of a biocompatible material into the apical end of the root canal. The rationale is to establish an apical stop that would enable the root canal to be filled immediately. There is no attempt at root end closure. Rather an artificial apical stop is created. A number of materials have been proposed for this purpose including tricalcium phosphate, calcium hydroxide, freeze dried bone, and freeze dried dentine7. Steinig et al.31 stated that the importance of this technique lies in the expedient cleaning and shaping of the root canal system, followed by its apical seal with a material that favours regeneration. Furthermore, the potential for fractures of immature teeth with thin roots is reduced, as a bonded core can be placed immediately within the root canal. Furthermore, MTA provides scaffolding for the formation of hard tissue and the potential of a better biological seal31.
Clinical procedure
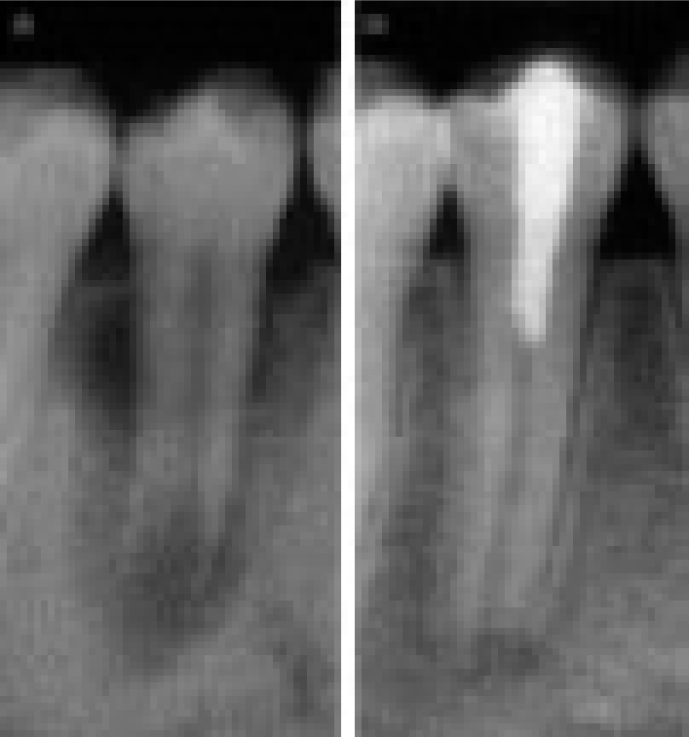
After obtaining anaesthesia, application of rubber dam, and preparing an adequate access preparation, the root canal system should be cleaned using intracanal instruments and sodium hypochlorite irrigation (NaOCl). To further disinfect the root canal system, a calcium hydroxide paste should be placed in the root canal for at least 1 week. Thereafter, calcium hydroxide paste is rinsed from the root canal with 5% NaOCl and 17% EDTA and the canal dried with sterile paper points. A thick application of MTA is prepared by mixing MTA powder with sterile water and is carried to the canal with a specific carrier. Then, MTA is condensed to the apical end of the canal with an adequate plugger and the back end of sterilised paper points. A 3–4 mm apical plug is created and its density, position and extension are checked radiographically (Figure 1). If creation of an ideal plug fails in the first attempt, MTA should be rinsed out with sterile water and the procedure should be repeated3. A moist cotton pellet is placed in the canal over MTA and the access cavity is closed with Cavit for at least 3–4 hours21.
Figure 1.
Apical MTA barrier.
ORTHOGRADE ROOT FILLING
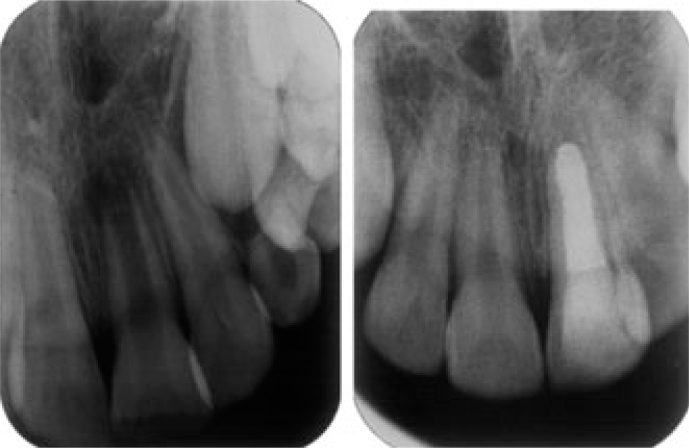
In selected cases such as situations where future nonsurgical retreatment is non-feasible or may not render better tooth prognosis, and in teeth with immature non-vital pulps and short roots (Figure 2), orthograde root canal filling with MTA should be considered as a valuable alternative for conventional apexification and even for one-visit apexification32.
Figure 2.
Complete root filling using MTA.
Al-Hezaimi et al.33 assessed the sealing ability of grey MTA and white MTA for a total period of 42 days in vitro and found that GMTA as well as WMTA had a better sealing ability than gutta-percha and Kerr Canal Sealer EWT. On the other hand, in another in vitro study Vizgirda et al.34 reported that the apical seal produced by laterally-condensed gutta-percha and sealer was superior to that produced by MTA.
Clinical support for the use of MTA as an obturating material, however, was presented in some case reports. O’Sullivan and Hartwell35 used MTA as the obturating material for the root canal system of a retained primary second molar. At the 4-month follow-up, the patient was asymptomatic, clinical findings were within normal limits, and there was evidence of radiographic healing. In another case report, Hayashi et al.36 used MTA for obturation of the root canal system of two mature mandibular central incisors with apical periodontitis. A 2-year follow-up radiographic examination demonstrated the dramatic regeneration of periradicular tissue.
TRIPLE ANTIBIOTIC PASTE
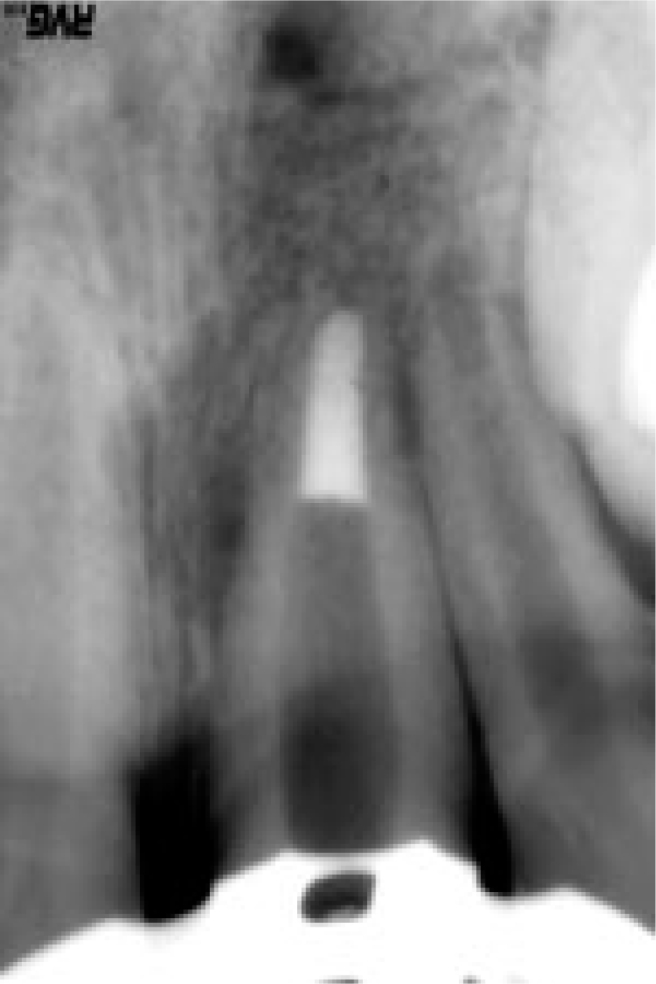
A newer approach to manage non-vital open-apex teeth is the use of a triple antibiotic paste (Figure 3). A combination of antibiotics may be needed to address the diverse root canal flora encountered and might also decrease the likelihood of the development of resistant bacterial strains. The combination that appears to be most promising consists of metronidazole, ciprofloxacin, and minocycline37. Sato et al.38 evaluated the potential of this mixture to kill bacteria in the deep layers of root canal dentine in situ. No bacteria were recovered from the infected dentine of the root canal wall 24 hours after application of the drug combination, except in one case in which a few bacteria were recovered. Hoshino et al.39 investigated the antibacterial effect of this same mixture, with and without the addition of rifampicin, on bacteria taken from the dentine of infected root canals. The efficacy was also determined against bacteria of carious dentine and infected pulps which may the precursory bacteria for an infected RCS. None of the individual drugs resulted in complete elimination of bacteria. However, in combination, these drugs were able to consistently disinfect all samples.
Figure 3.
Effect of triple antibiotic paste on the root-end closure.
Iwaya et al.40 presented a case report of an immature mandibular second premolar tooth with a pulpless, infected root canal with periapical involvement and a sinus tract. Instead of following the standard root canal treatment protocol and apexification, two antibiotics (metronidazole and ciprofloxacin) were placed in the canal, after which the canal was left empty. Radiographic examination showed the commencement of apical closure 5 months after the completion of the antimicrobial protocol. Thickening of the root dentine and complete apical closure was confirmed 30 months after the treatment, indicating the revascularisation potential of a young permanent tooth pulp into a bacteria-free root canal space.
Takushige et al.41 evaluated the efficacy of a poly-antibiotic paste consisting of ciprofloxacin, metronidazole and minocycline, on the clinical outcome of so-called ‘Lesion Sterilization and Tissue Repair’ (LSTR) therapy in primary teeth with periradicular lesions. They reported that the clinical symptoms (such as gingival swelling, sinus tracts, induced dull pain, spontaneous dull pain, and pain on biting) disappeared after treatment in all but four cases. The four cases that did not resolve after initial treatment had resolution of the clinical signs and symptoms after further treatment using the same procedures again. Thus, gingival abscesses and draining sinuses, if present, disappeared after a few days. Successor permanent teeth erupted without any disorders, or were found radiographically to be normal and in the process of eruption. All cases were evaluated as being successful. The mean functional time of the primary teeth was 680 days (range: 68–2,390 days), except for one case in which the successor permanent tooth was congenitally missing.
Windley et al.37 assessed the efficacy of a triple antibiotic paste in the disinfection of immature dog teeth with apical periodontitis. The canals were sampled before (S1) and after (S2) irrigation with 1.25% NaOCl, and after dressing with a triple antibiotic paste (S3) consisting of metronidazole, ciprofloxacin, and minocycline. At S1, 100% of the samples had a positive culture result for bacteria with a mean CFU count of 1.7 × 10. At S2, 10% of the samples were bacteria-free with a mean CFU count of 1.4 × 10, and at S3, 70% of the samples were bacteria-free with a mean CFU count of only 26. Reductions in mean CFU counts between S1 and S2 as well as between S2 and S3 were statistically significant.
TISSUE ENGINEERING CONCEPT
There are three major components of tissue engineering42, the first if which is a cell source42. Odontoblasts are of mesenchymal origin, and under appropriate conditions, cells from dental pulp, the apical papilla, and possibly other tissues can form odontoblast-like cells43., 44., 45., 46.. To date, the precise cell sources supporting the continued root development are unknown. However, it is possible that residual pulp cells might have remained vital in some cases, cells from the apical papilla underwent proliferation, or bleeding-induced angiogenesis might have recruited stem/progenitor cells from apical tissues including the apical papilla. The clinical challenge will be to find a reliable cell source capable of differentiating odontoblasts, convenient for harvesting, and autogenous to avoid tissue rejection or introduction of foreign pathogens47.
The second component is a physical scaffold42. Tissues are three dimensional structures and an appropriate scaffold is required to promote cell growth and differentiation. Extracellular matrix molecules control the differentiation of stem cells48., 49. and an appropriate scaffold might selectively bind and localise cells50, contain growth factors51, and undergo biodegradation over time52. Thus, a scaffold is far more than a lattice to contain cells. It has been suggested that platelet-rich plasma (PRP) satisfies many of these criteria. PRP is autogenous, fairly easy to prepare in a dental setting, rich in growth factors, degrades over time, and forms a three-dimensional fibrin matrix42.
The third component consists of signalling molecules42. Both growth factors and other compounds are capable of stimulating cellular proliferation and directing cellular differentiation. The observed radiographic thickening of the dentinal walls might be due to production of cementum, bone or dentine. It is likely that the cell source and the available signalling molecules play major roles in guiding the development of cells in the regenerating tissue. For example, the same cultures of human dental pulp cells can differentiate into cells resembling odontoblasts/osteoblasts, adipocytes, or chondrocytes, depending on the combination of signalling molecules such as dexamethasone43. Dentine or dentine extracts (rich in growth factors) will promote formation of an odontoblast phenotype. It has been shown that ethylenediaminetetraacetic acid (EDTA) is very effective in releasing growth factors from human dentine53. However, its effectiveness in promoting odontoblast differentiation and subsequently root development has not been yet evaluated.
CROWN RESTORATION OF IMMATURE TEETH
Because of the thin dentinal walls there is a high incidence of root fractures in teeth after apexification3. Restorative efforts should be directed towards strengthening the immature root. A number of studies have demonstrated that the use of the newer dentine bonding techniques can significantly increase the resistance to fracture of these teeth to levels close to that of intact teeth. Goldberg et al.54 have recently demonstrated the reinforcing effect of a resin glass ionomer in the restoration of immature roots. The risk of root fracture during apexification is a concern, but during this time it is essential that access to the apical portion of the canal is preserved. Katebzadeh et al.55 have described a technique in which the access is restored with a composite restoration. A clear curing post is inserted into the soft composite and cured. The post is then removed leaving a patent channel for calcium hydroxide replacement and subsequent obturation of the canal.
CONCLUSION
Endodontic management of pulpless permanent teeth with wide open apices has long presented a challenge to dentistry. A number of treatment modalities have been advocated for these teeth. Until recently, the most widely accepted technique has been cleaning and filling the canal with calcium hydroxide to stimulate the formation of a calcified tissue at the apex. The term apexification is used to describe this procedure. Apical barrier technique with MTA has now become the accepted treatment of choice. In nonvital open-apex teeth with short root lengths, the root can completely be filled with MTA. Intracanal medication with a triple antibiotic paste consisting of equal parts of metronidazole, minocycline and ciprofloxacin for 4 weeks is another effective approach to encourage continuing root development to manage nonvital open-apex teeth. Finally, it is likely that intracanal delivery of known signalling molecules or the solubilisation of endogenous signalling molecules will promote the formation of dentine.
REFERENCES
- 1.Hargreaves KM, Goodis HE. Quintessence Publishing Co, Inc; St Louis: 2002. Seltzer and Bender’s Dental Pulp; pp. 13–40. [Google Scholar]
- 2.Farhad A, Mohammadi Z. Calcium hydroxide: a review. Int Dent J. 2005;55:293–301. doi: 10.1111/j.1875-595x.2005.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S, Hargreaves KM. Mosby Inc.; St Louis: 2006. Pathways of the Pulp; pp. 610–649. [Google Scholar]
- 4.Kaiser HJ. Management of Wide Open Apex Canals With Calcium Hydroxide. Presented at the 21th Annual meeting of the American Association of Endodontists, April 17, 1964.
- 5.Frank AL. Therapy for the divergent pulpless tooth by continued apical formation. J Am Dent Assoc. 1966;72:87–93. doi: 10.14219/jada.archive.1966.0017. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell DF, Shankwalker GB. Osteogentic potential of calcium hydroxide and other materials in soft tissue and bone wounds. J Dent Res. 1958;37:1157–1163. doi: 10.1177/00220345580370061501. [DOI] [PubMed] [Google Scholar]
- 7.Morse DR, O’Larnic J, Yesilsoy C. Apexification: review of the literature. Quintessence Int. 1990;21:589–598. [PubMed] [Google Scholar]
- 8.Nygaard-Østby B. The role of blood clot in endodontic therapy. Acta Odontol Scand. 1961;19:323–346. [PubMed] [Google Scholar]
- 9.Zussman WV. The interaction of ameloblasts and odontoblasts in transplants. Oral Surg Oral Med Oral Pathol. 1966;21:388–396. doi: 10.1016/0030-4220(66)90078-8. [DOI] [PubMed] [Google Scholar]
- 10.Ham JW, Patterson SS, Mitchell DF. Induced apical closure of immature pulpless teeth in monkeys. Oral Surg Oral Med Oral Pathol. 1972;33:438–449. doi: 10.1016/0030-4220(72)90474-4. [DOI] [PubMed] [Google Scholar]
- 11.Citrome GP, Kaminski EJ, Heuer MA. A comparative study of tooth apexification in the dog. J Endod. 1979;5:290–297. doi: 10.1016/S0099-2399(79)80077-1. [DOI] [PubMed] [Google Scholar]
- 12.Torneck CD, Smith J. Biologic effects of endodontic procedures on developing incisor teeth. I. Effect of partial and total pulp removal. Oral Surg Oral Med Oral Pathol. 1970;30:258–266. doi: 10.1016/0030-4220(70)90371-3. [DOI] [PubMed] [Google Scholar]
- 13.Klein SH, Levy BA. Histologic evaluation of induced apical closure of a human pulpless tooth. Oral Surg Oral Med Oral Pathol. 1974;38:954–959. doi: 10.1016/0030-4220(74)90349-1. [DOI] [PubMed] [Google Scholar]
- 14.West NM, Lieb RJ. Biologic root-end closure on a traumatized and surgically resected maxillary central incisor: an alternative method of treatment. Endod Dent Traumatol. 1985;1:146–149. doi: 10.1111/j.1600-9657.1985.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 15.Steiner JC, Van Hassel HJ. Experimental root apexification in primates. Oral Surg Oral Med Oral Pathol. 1971;37:409–415. doi: 10.1016/0030-4220(71)90163-0. [DOI] [PubMed] [Google Scholar]
- 16.Dylewski JJ. Apical closure of nonvital teeth. Oral Surg Oral Med Oral Pathol. 1971;32:82–89. doi: 10.1016/0030-4220(71)90253-2. [DOI] [PubMed] [Google Scholar]
- 17.Schroder U, Granath LE. Early reaction of intact human teeth to calcium hydroxide following experimental pulpotomy and its significance to the development of hard tissue barrier. Odontol Revy. 1971;22:379–395. [PubMed] [Google Scholar]
- 18.Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134–137. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 19.Roberts HW, Toth JM, Berzins DW, et al. Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater. 2008;24:149–164. doi: 10.1016/j.dental.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri J, Pitt Ford TR. Mineral trioxide aggregate: a review of the constituents and biological properties of the material. Int Endod J. 2006;39:747–754. doi: 10.1111/j.1365-2591.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- 21.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 22.Fridland M, Rosado R. Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J Endod. 2003;29:814–817. doi: 10.1097/00004770-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Fridland M, Rosado R. MTA solubility: a long term study. J Endod. 2005;31:376–379. doi: 10.1097/01.don.0000140566.97319.3e. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar NK, Caicedo R, Rirwik P, et al. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 25.Fischer EJ, Arens DE, Miller CH. Bacterial leakage of mineral trioxide aggregate as compared with zinc-free amalgam, intermediate restorative material, and Super EBA as a root-end filling material. J Endod. 1998;24:176–179. doi: 10.1016/S0099-2399(98)80178-7. [DOI] [PubMed] [Google Scholar]
- 26.Tang HM, Torabinejad M, Kettering JD. Leakage evaluation of root end filling materials using endotoxin. J Endod. 2002;28:5–7. doi: 10.1097/00004770-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Ferris DM, Baumgartner JC. Perforation repair comparing two types of mineral trioxide aggregate. J Endod. 2004;30:422–424. doi: 10.1097/00004770-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 28.de Leimburg ML, Angeretti A, Ceruti P, et al. MTA obturation of pulpless teeth with open apices: bacterial leakage as detected by polymerase chain reaction assay. J Endod. 2004;30:883–886. doi: 10.1097/01.don.0000128749.50151.24. [DOI] [PubMed] [Google Scholar]
- 29.Al-Kahtani A, Shostad S, Schifferle R, et al. In vitro evaluation of microleakage of an orthograde apical plug of mineral trioxide aggregate in permanent teeth with simulated immature apices. J Endod. 2005;31:117–119. doi: 10.1097/01.don.0000136204.14140.81. [DOI] [PubMed] [Google Scholar]
- 30.Camps JH, Fuks AB. In: Pathways of the Pulp. Cohen S, Hargreaves KM, editors. Mosby Inc.; St Louis: 2006. Pediatric endodontics: endodontic treatment of the primary and young permanent dentition; pp. 822–882. [Google Scholar]
- 31.Steinig TH, Regan JD, Gutmann JL. The use and predictable placement of Mineral Trioxide Aggregate in one-visit apexification cases. Aust Endod J. 2003;29:34–42. doi: 10.1111/j.1747-4477.2003.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 32.Mohammadi Z. Orthograde root filling of an immature nonvital tooth using MTA. Dent Today. 2008;27:102. [PubMed] [Google Scholar]
- 33.Al-Hezaimi K, Naghshbandi J, Oglesby S, et al. Human saliva penetration of root canals obturated with two types of mineral trioxide aggregate cements. J Endod. 2005;31:453–456. doi: 10.1097/01.don.0000145429.04231.e2. [DOI] [PubMed] [Google Scholar]
- 34.Vizgirda PJ, Liewehr FR, Patton WR, et al. A comparison of laterally condensed gutta-percha, thermoplasticized gutta-percha, and mineral trioxide aggregate as root canal filling materials. J Endod. 2004;30:103–106. doi: 10.1097/00004770-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 35.O’Sullivan SM, Hartwell GR. Obturation of a retained primary mandibular second molar using mineral trioxide aggregate: a case report. J Endod. 2001;27:703–705. doi: 10.1097/00004770-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi M, Shimizu A, Ebisu S. MTA for obturation of mandibular central incisors with open apices: case report. J Endod. 2004;30:120–122. doi: 10.1097/00004770-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Windley W, Teixeira F, Levin L, et al. Disinfection of immature teeth with a triple antibiotic paste. J Endod. 2005;31:439–443. doi: 10.1097/01.don.0000148143.80283.ea. [DOI] [PubMed] [Google Scholar]
- 38.Sato I, Ando-Kurihara N, Kota K, et al. Sterilization of infected root-canal dentin by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29:118–124. doi: 10.1111/j.1365-2591.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 39.Hoshino E, Ando-Kurihara N, Sato I, et al. In vitro antibacterial susceptibility of bacteria taken from infected root dentin to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125–130. doi: 10.1111/j.1365-2591.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 40.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185–187. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 41.Takushige T, Cruz EV, Asgor Moral A, et al. Endodontic treatment of primary teeth using a combination of antibacterial drugs. Int Endod J. 2004;37:132–138. doi: 10.1111/j.0143-2885.2004.00771.x. [DOI] [PubMed] [Google Scholar]
- 42.Hargreaves KM, Giesler T, Henry M, et al. Regeneration potential of the young permanent tooth: what does the future hold? J Endod. 2008;34:S51–S56. doi: 10.1016/j.joen.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 43.Wei X, Ling J, Wu L, et al. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33:703–708. doi: 10.1016/j.joen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Huang GT, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–1073. doi: 10.1016/j.joen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Sonoyama W, Liu Y, Yamaza T, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentin regeneration and repair. Oral Dis. 2007;13:151–157. doi: 10.1111/j.1601-0825.2006.01346.x. [DOI] [PubMed] [Google Scholar]
- 47.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 49.Yamamura T. Differentiation of pulpal cells and inductive influences of various matrices with reference to pulpal wound healing. J Dent Res. 1985;64:530–540. doi: 10.1177/002203458506400406. [DOI] [PubMed] [Google Scholar]
- 50.Vacatello M, D’Auria G, Falcigno L, et al. Conformational analysis of heparin binding peptides. Biomaterials. 2005;26:3207–3214. doi: 10.1016/j.biomaterials.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Yamada Y, Ueda M, Naiki T, et al. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 2004;10:955–964. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 52.Young CS, Terada S, Vacanti JP, et al. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81:695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 53.Smith AJ. In: Seltzer and Bender’s Dental Pulp. Hargreaves KM, Goodis HE, editors. Quintessence Publishing Co, Inc.; St Louis: 2002. Dentin formation and repair; pp. 41–62. [Google Scholar]
- 54.Goldberg F, Kaplan A, Roitman M, et al. Reinforcing effect of a resin glass ionomer in the restoration of immature roots in vitro. Dent Traumatol. 2002;18:70–72. doi: 10.1034/j.1600-9657.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 55.Katebzadeh N, Dalton BC, Trope M. Strengthening immature teeth during and after apexification. J Endod. 1998;24:256–259. doi: 10.1016/s0099-2399(98)80108-8. [DOI] [PubMed] [Google Scholar]