Abstract
Objective: Casein phosphopeptide-amorphous calcium phosphate nanocomplexes (CPP-ACP) exhibit anticariogenic potential in laboratory, animal and human experiments. The remineralising potential of synthetic CPPs on early enamel caries was investigated. Design:In vivo study. Setting: University of Naples ‘Federico II’, School of Dentistry, Department of Paediatric Dentistry, 2010, Italy. Participants: 40 volunteers (age range 10–16 years) were recruited and divided in two groups of 20 (Group A and B). Interventions: In Group A subjects two demineralised enamel specimens were placed on the buccal surfaces of the first molars and subjects were instructed to apply a commercial product containing CPPs (GC Tooth Mousse) only on the right-sided specimen and a placebo mousse on the left, for 1 month. In Group B subjects two enamel specimens were similarly placed into the mouth and used as controls. Results: SEM analysis revealed a diffuse and homogeneous mineral coating, reducing the surface alterations only in the demineralised specimens treated with synthetic CPPs into the mouth. Conclusions: Results demonstrate that CPPs are able to promote remineralisation of early enamel lesions.
Key words: Bioactive peptides, caries, casein phosphopeptides, prevention, GC Tooth Mousse
INTRODUCTION
Dairy products have been recognised as a food group that is effective in preventing dental caries1. Using in vitro, animal and human in situ models, the anti-cariogenic activity of milk and milk products was attributed to the direct chemical effects of casein phosphopeptides, calcium and phosphate2.
Casein phosphopeptides (CPPs) are phosphorylated casein-derived peptides produced by proteolytic digestion of αs1-, αs2-, and ß-casein in vitro or in the digestive tract. CPPs, containing the sequence Ser(P)-Ser(P)-Ser-(P)-Glu-Glu, stabilize nanoclusters of amorphous calcium phosphate (ACP) in metastable solution3.
Casein phosphopeptides-amorphous calcium phosphate localise ACP in dental plaque, which buffers the free calcium and phosphate ion activities, helping to maintain a state of supersaturation with respect to tooth enamel, depressing demineralisation and enhancing remineralisation4.
In liquid milk the majority of the casein and calcium and phosphate ions are bound in micelles and then upon consumption would not necessarily be available to promote enamel remineralisation5.
In fact, the calcium in casein micelles is unlikely to be available to diffuse across a relatively intact enamel surface layer into a subsurface lesion and therefore milk may have limited ability to remineralise enamel lesions in situ. Therefore, since milk could not be used as a natural product against dental caries, because it needs the addition of 2.0–5.0 g CPP-ACP to substantially increases its ability to remineralise enamel subsurface lesions, a commercial paste containing CPP-ACP showing a remineralisation effect on early enamel lesions is investigated6.
The application of a CPPs toothpaste and sodium fluoride (Colgate Neutrafluor 9,000 ppm) (NaF) can provide significant additional prevention of enamel demineralisation when resin-modified glass ionomer cement (RMGIC) is used for bonding molar tubes for orthodontic patient as preventive actions7. An in vitro study to evaluate the remineralisation of incipient enamel lesions by the topical application of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) using laser fluorescence and scanning electron microscope showed high scores of remineralisation8.
Therefore, the aim of this study was to test in vivo the effectiveness of synthetic casein phosphopeptides (CPPs) present in a commercial product to promote remineralisation of early enamel caries.
MATERIALS AND METHODS
Subjects and study design
The study was carried out in the Department of Paediatric Dentistry of the University of Naples ‘Federico II’, Italy. For this study 100 subjects were recruited but only 40 healthy patients (18 males and 22 females) with an age range from 10 to 16 years were selected with the following criteria: good general health (ASA I-II) and agreement to comply with study procedures. An intra-oral examination confirmed that each member had no periodontal disease or other oral pathology.
Exclusion criteria were: milk protein allergies and conditions that interfered with the examination procedure (non-co-operating subjects).
Participation was voluntary. After parents and patients had been given verbal and written explanations of the experimental protocol and the study aims, written informed consent was signed by them prior to the start of the study. Permission was received from the appropriate authorities. The subjects were divided into two groups of 20 (Groups A and B).
Preparation of enamel specimens
Forty human teeth, extracted for orthodontic reasons or impaction, were obtained from the Department of Paediatric Dentistry of the University of Naples ‘Federico II’, Italy.
Teeth were cleaned with sterile gauze imbibed in distilled water and stored at 4 °C in sterile containers with 5% formalin solution. The roots were cut by mean of a diamond disk assembled on a laboratory handpiece and the crowns polished with pumice dust and non-fluoride toothpaste, using a circular brush with nylon bristles mounted on a dental handpiece and then rinsed in distilled water. From each crown four longitudinal specimens of enamel of about 3 × 2 × 2 mm were obtained by cutting with a diamond disk.
Experiment design-Group A
Eighty specimens were used for this group. Three specimens of the four obtained from each tooth were submitted to a demineralisation procedure by immersion for 96 hours (with one change of solution after 48 hours) in a demineralising solution containing: 50 ml of 0.1 M lactic acid, 0.02% carboxymethylcellulose (Akzo Nobel, Amsterdam, Netherlands) (pH 4.8, T° 37 °C)9. The other one specimen from each tooth was left intact and stored in distilled water (with one change of solution after 48 hours).
After 4 days, all specimens were rinsed in distilled water and dried with a jet of warm air for 3 seconds, packaged in individual paper autoclavable bags and autoclaved at 121 °C at 15 lbs psi for 30 minutes10. Two enamel specimens (one sound and one demineralised, respectively) of four obtained from each tooth were stored in distilled water (with one change of solution every 48 hours) and used as control outside the oral environment (Subgroup α1 and α2) (Table 1).
Table 1.
Group A: remineralisation protocol with GC Tooth Mousse
| 20 sound specimens | Subgroup α1 | Outside of oral environment |
| 20 demineralized specimens | Subgroup α2 | Outside of oral environment |
| 20 demineralized specimens | Subgroup α3 | Intra-oral environment and GC Tooth Mousse |
| 20 demineralized specimens | Subgroup α4 | Intra-oral environment and placebo |
In 20 subjects belonging to Group A the remaining two enamel specimens (both demineralised) were placed, with the dentinal face towards the tooth, through an adhesive procedure, on buccal surfaces of the right and the left first or second upper molars for the remineralisation protocol with synthetic casein phosphopeptide; therefore each subject was required to apply a commercial product for professional use containing 10% of casein phosphopeptide (Tooth Mousse GC Corp., Tokyo, Japan) only on the right-side specimen (Subgroup α3) (Table 1) and a glycerine based (without remineralising substances) placebo gel on the left-side specimen (Subgroup α4) (Table 1), on a daily basis, three times per day for a month at the following times 7.00, 14.00, 21.00 hours. In addition, subjects were asked to complete a diary documenting their Tooth Mousse application habits.
The GC Tooth Mousse was provided by GC industry as coded products and stored in a secured area at room temperature. No alterations were made to the subjects’ diet and oral hygiene procedures during the study.
Experiment design-Group B
Eighty specimens were used for this group. Two of the four specimens obtained from each tooth were submitted to a demineralisation procedure by immersion for 96 hours (with one change of solution after 48 hours) in a demineralsing solution containing: 50 ml of 0.1 M lactic acid, 0.02% carboxymethylcellulose (Akzo Nobel, Netherlands) (pH 4.8, T° 37 °C)9. The other two specimens from each tooth were left intact and stored in distilled water (with one change of solution after 48 hours). After 4 days, all specimens were rinsed in distilled water, dried with a jet of warm air for 3 seconds, packaged in individual paper autoclavable bags and autoclaved at 121 °C at 15 lbs psi for 30 minutes10.
Two enamel specimens (one sound and one demineralised, respectively) of four obtained from each tooth were stored in distilled water (with one change of solution every 48 hours) and used as control outside of the oral environment (Subgroup β1 and β2) (Table 2).
Table 2.
Group B: control group
| 20 sound specimens | Subgroup β1 | Outside of oral environment |
| 20 demineralised specimens | Subgroup β2 | Outside of oral environment |
| 20 sound specimens | Subgroup β3 | Intra-oral environment |
| 20 demineralised specimens | Subgroup β4 | Intra-oral environment |
To 20 subjects belonging to Group B the remaining two enamel specimens (one sound and one demineralised, respectively) (Subgroup β3 and β4) (Table 2) were placed, with the dentinal face towards the tooth, through adhesive cementing systems, on buccal surfaces of first or second upper molars for a month. No alterations were made to the subjects’ diet and oral hygiene procedures during the study.
SEM analysis
After completion of each treatment period, the enamel specimens in the mouth were removed by orthodontic pliers and were polished with pumice dust and non-fluoride toothpaste, using a circular brush with nylon bristles mounted on a dental handpiece. They were then rinsed in distilled water and compared with the control specimens to assess the morphological changes by SEM. For the ultra-structural examination of the tooth surfaces, the specimens were dehydrated in a graded series of ethanol, critical point dried (SPC-900/EX; The Bomar Co., Tacoma, Washington, USA), mounted in stubs, sputter-coated with gold (E 306; Edwards, Crawley, UK) and observed by SEM (Stereoscan 250 MK3, Cambridge, UK) at 50×, 100×, 600×, 3000×, 6000× magnifications.
RESULTS
Images that are representative for each group are shown.
Specimens from Group A
Subgroup α1
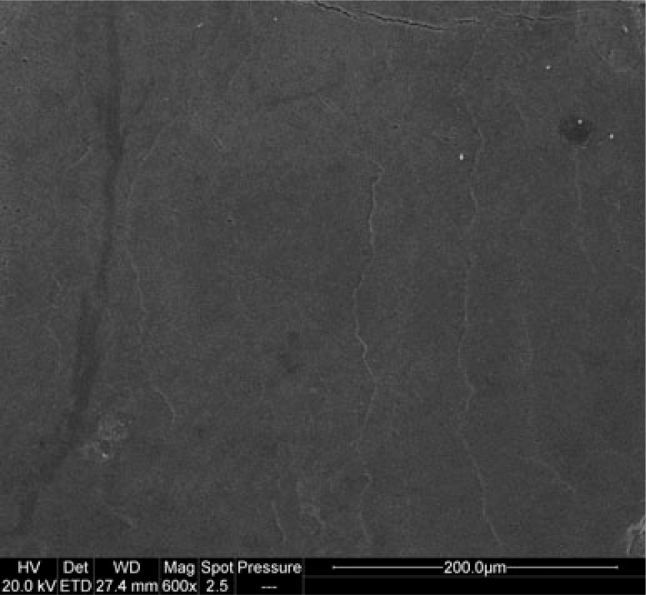
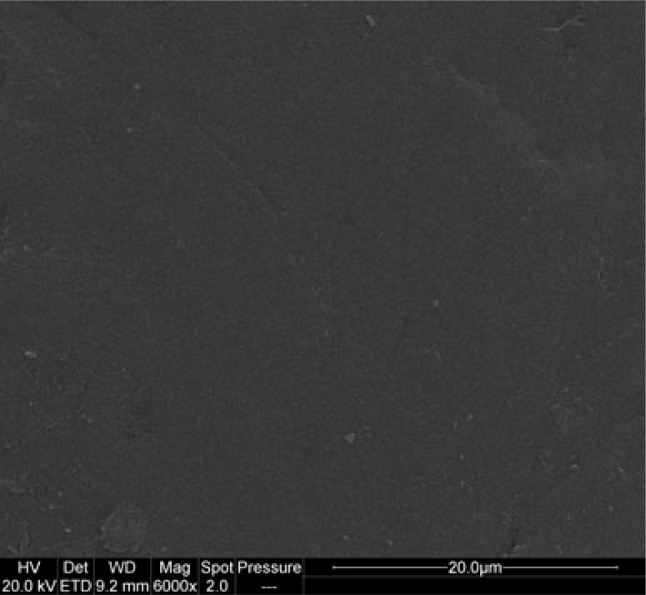
SEM images of the enamel specimens from subgroup α1 are suggestive of sound and smooth surface (Figures 1 and 2).
Figure 1.
Sound enamel surface subgroup α1 (3,000×).
Figure 2.
Sound enamel surface subgroup α1 (6,000×).
Subgroup α2
Characteristic etching patterns are found on the surfaces of the specimens from subgroup α2. Acid-etching of sound enamel creates microporosities from 4 to 20 μm in diameter (Figures 3 and 4).
Figure 3.
Demineralised enamel surface subgroup α2 (600×).
Figure 4.
Demineralised enamel surface subgroup α2 (6,000×).
Subgroup α3
The enamel specimens from subgroup α3 do not show any erosion or demineralisation of the enamel surface. A mineral sediment with a trend of wavy lines is clearly visible at low magnification (600×) (Figure 5). At higher magnification (6,000×) it can be clearly seen that the surfaces are covered with an amorphous deposit, which completely obscures the underlying prism structure. In all the specimens this mineral sediment entirely fills the previously created lesions (Figure 6).
Figure 5.
Remineralised enamel surface with GC Tooth Mousse subgroup α3 (600×).
Figure 6.
Remineralised enamel surface with GC Tooth Mousse subgroup α3 (6,000×).
Subgroup α4
The enamel specimens from subgroup α4 show strong superficial alterations, loss of enamel and pronounced holes from 5 to 25 μm in diameter, higher than the typical lesions created by the acid solution used for the demineralisation protocol. In particular, the surfaces appear disorganised, without any distinct etching pattern (Figures 7 and 8).
Figure 7.
Demineralised enamel surface with placebo subgroup α4 (600×).
Figure 8.
Demineralised enamel surface with placebo subgroup α4 (6,000×).
Specimens from Group B
Subgroup β1
SEM evaluation of the enamel specimens from subgroup β1 reveals that they are like a typical sound enamel surface at low and high magnification (Figures 9 and 10).
Figure 9.
Sound enamel surface subgroup β1 (600×).
Figure 10.
Sound enamel surface subgroup β1 (6,000×).
Subgroup β2
The specimens from subgroup β2 present roughening surfaces, in which tissue loss gives the enamel a porous appearance. The entire prism core is dissolved. In all the samples, the characteristic honeycomb structure of demineralised enamel is clearly evident with relatively deep, but tapered etched pits varied in size from 4 to 20 μm in diameter (Figure 11).
Figure 11.
Demineralised enamel surface subgroup β2 (3,000×).
Subgroup β3
The images of the subgroup β3 specimens are similar to the images of the sound specimens stored in distilled water outside of the oral environment (Figure 12).
Figure 12.
Sound enamel surface subgroup β3 (6,000×).
Subgroup β4
The enamel specimens from subgroup β4 show loss of enamel and evident holes from 5 to 25 μm in diameter, deeper than the typical damages caused by the acid solution used for the demineralisation protocol (Figure 13).
Figure 13.
Demineralised enamel surface subgroup β4 (6,000×).
DISCUSSION
SEM analysis provided useful information about structural changes occurring on the enamel surfaces during the test period. In particular, the effectiveness of synthetic CPPs was clearly visible from the images of the specimens demineralised for 4 days at pH 4.8 and then subjected to the treatment with CPPs in the mouth, in which the previously created microporosities were covered and filled by an amorphous mineral sediment. In fact, the inorganic components contained in high concentrations in CPP–ACP acted to enhance remineralisation of the enamel structure.
The increased enamel remineralisation found in vivo in this study by synthetic CPPs was consistent with previous studies showing the anti-cariogenic and remineralisation potential of CPP-ACP in solution and the proposed mechanism of the localisation of amorphous calcium phosphate on the tooth surface by the CPPs, depressing enamel demineralisation and enhancing remineralisation11., 12., 13., 14., 15., 16.. GC Tooth Mousse can be considered a valid product with anti-caries effect. Furthermore, the intent of this in vivo model was also to mimic what occurred in the natural caries process, and to give clinically relevant information about the remineralisation mechanism over a short period of time without causing irreversible tissue changes in the natural dentition.
It should be emphasised that this in vivo system could be considered, as suggested by Zero, as a bridge between the natural uncontrolled clinical situation and the highly controlled laboratory situation17. The main advantages of this model are: use of a ‘natural’ environment consisting of tooth substrate; formation or presence of dental plaque with cariogenic potential; carbohydrate challenge, provided by the subject’s normal diet, oral microflora, host factors such as saliva. Enamel specimens fixed in the mouth also had the advantage that subject compliance with wearing an appliance is not a factor, although model systems that use the subjects’ normally worn partial dentures ensure a level of certainty of compliance with the study requirements. Furthermore, the enamel specimens, mounted on the buccal sites, were well tolerated by subjects.
CONCLUSION
Casein phosphopeptides exhibit physiological functions such as minerals carrier activity that is one of the main mechanisms in dental caries prevention3. The remineralisation potential of CPP-ACP highlighted the importance of using milk-derived peptides against dental caries in daily oral care (delivered by commercial toothpaste).
Future studies should, then, be focused on the evaluation of the potential effects of synthetic CPPs, in order to create a new preventive strategy based on utilising these bio-active principles in personal hygiene products to reduce cariogenicity.
REFERENCES
- 1.Aimutis WR. Bioactive properties of milk proteins with particular focus on anticariogenesis. J Nutr. 2004;134:989S–995S. doi: 10.1093/jn/134.4.989S. [DOI] [PubMed] [Google Scholar]
- 2.McDougall WA. Effect of milk on enamel demineralization and remineralization in vitro. Caries Res. 1977;11:166–172. doi: 10.1159/000260263. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds EC. Anticariogenic complexes of amorphous calcium phosphate stabilized by casein phosphopeptides: a review. Spec Care Dentist. 1998;18:8–16. doi: 10.1111/j.1754-4505.1998.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 4.Cai F, Shen P, Morgan MV, et al. Remineralization of enamel subsurface lesions in situ by sugar-free lozenges containing casein phosphopeptide-amorphous calcium phosphate. Aust Dent J. 2003;48:240–243. doi: 10.1111/j.1834-7819.2003.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 5.Walker G, Cai F, Shen P, et al. Increased remineralization of tooth enamel by milk containing added casein phosphopeptide-amorphous calcium phosphate. J Dairy Res. 2006;73:74–78. doi: 10.1017/S0022029905001482. [DOI] [PubMed] [Google Scholar]
- 6.Kumar VL, Itthagarun A, King NM. The effect of casein phosphopeptide-amorphous calcium phosphate on remineralization of artificial caries-like lesions: an in vitro study. Aust Dent J. 2008;53:34–40. doi: 10.1111/j.1834-7819.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 7.Sudjalim TR, Woods MG, Manton DJ, et al. Prevention of demineralization around orthodontic brackets in vitro. Am J Orthod Dentofacial Orthop. 2007;131:1–9. doi: 10.1016/j.ajodo.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Pai D, Bhat SS, Taranath A, et al. Use of laser fluorescence and scanning electron microscope to evaluate remineralization of incipient enamel lesions remineralized by topical application of casein phosphopeptide amorphous calcium phosphate (CPP-ACP) containing cream. J Clin Pediatr Dent. 2008;32:201–206. doi: 10.17796/jcpd.32.3.d083470201h58m13. [DOI] [PubMed] [Google Scholar]
- 9.Iijima Y, Takagi O, Ruben J, et al. In vitro remineralization of in vivo and in vitro formed enamel lesions. Caries Res. 1999;33:206–213. doi: 10.1159/000016518. [DOI] [PubMed] [Google Scholar]
- 10.Kumar M, Sequeira PS, Peter S, et al. Sterilisation of extracted human teeth for educational use. Indian J Med Microbiol. 2005;23:256–258. [PubMed] [Google Scholar]
- 11.Reynolds EC, Cain CJ, Webber FL, et al. Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptides in the rat. J Dent Res. 1995;74:1272–1279. doi: 10.1177/00220345950740060601. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–1595. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 13.Iijima Y, Cai F, Shen P, et al. Acid resistance of enamel subsurface lesions remineralized by a sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. Caries Res. 2004;38:551–556. doi: 10.1159/000080585. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi K, Miyazaki M, Takamizawa T, et al. Effect of CPP-ACP paste on mechanical properties of bovine enamel as determined by an ultrasonic device. J Dent. 2006;34:230–236. doi: 10.1016/j.jdent.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Rahiotis C, Vougiouklakis G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine in vitro. J Dent. 2007;35:695–698. doi: 10.1016/j.jdent.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds EC. Calcium phosphate-based remineralization systems: scientific evidence? Aust Dent J. 2008;53:268–273. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 17.Zero DT. In situ caries models. Adv Dent Res. 1995;9:214–230. doi: 10.1177/08959374950090030501. [DOI] [PubMed] [Google Scholar]