Abstract
Piperic acid, a natural product-based derivative, has been used with polyvinyl alcohol for the first time to form polymer composite films for its suitable modification in physicochemical and antimicrobial properties. Initially, piperic acid was synthesized from piperine, a natural alkaloid extracted from black pepper (Piper nigrum). The solvent casting method was used for the synthesis of PVA–piperic acid composite films. The films were characterized by various spectral and microscopic techniques like UV–visible spectroscopy, FT-IR, SEM, XRD, and TGA. The antibacterial activity was shown by these polymer composites of piperic acid against Gram-positive Staphylococcus aureus (S. aureus-ATCC8738P) and Gram-negative Escherichia coli (E. coli—ATCC8739) was worthwhile. The antifungal activity of the composite films was evaluated by the food poisoning technique. Percentage mycelial growth inhibition was found maximum against Fusarium solani than Aspergillus and Penicillium. The water vapour and oxygen barrier properties are enhanced with the incorporation of increased content of piperic acid. Also, enhancement in the tensile strength of PVA/PA composite film was observed, while elongation at break shows decreased trend with the addition of piperic acid. The surface properties of polymer composite films were determined by contact angle measurements. Contact angle shows a considerable increase in these films when compared to virgin PVA film. It was increased by 56.1° in 15 mL composite film containing a higher concentration of piperic acid than virgin PVA.
Keywords: Piperic acid, Polyvinyl alcohol, Antimicrobial activity, Composite
Introduction
Polyvinyl alcohol (PVA), a synthetic, inexpensive, and biodegradable polymer, has been commonly used for food packaging (Yun et al. 2010). Its use in the food industry is increasing progressively over 40 years of packaging (Zainab et al. 2017). PVA, one of the most common unnatural polymers, is the prime subject of this research paper due to its good biodegradability, compatibility, non-toxicity, processability, and admissible mechanical and thermal properties. The phonological structure of PVA, methods of its preparation, its blends, and nanocomposites are extensively studied, together with certain descriptions of permeability design for resulting barrier properties of their composites. PVA is a semi-crystalline polymer with some degree of crystallinity and consists of 1, 3-diol units or 1, 2-diol units, depending on the degree of hydrolysis (Guimaraes et al. 2015; Tang et al. 2008). Methods of processing PVA like suspension, water, and melt via extrusion or injection molding are more cost-effective than other biodegradable polymers (Park et al. 1994). The high moisture sensitivity of PVA due to hydroxyl groups in its structure makes it hydrophilic. This hydrophilic character limits its use in packaging as pure polymer; hence, its resulting blends and composite materials have become common for packages (Zhou et al. 2009; Rahman et al. 2010; Luo et al. 2012). Polymer blends of PVA starch have been used to reduce such limitations (Chen et al. 2008; Jayasekara et al.2004; Lawton et al. 1996; Shogren et al.1998; Siddaramaiah et al. 2004; Yoon et al. 2006; Zhou et al.2006). Similarly, the incorporation of layered silicates like montmorillonite into the PVA polymer matrix has resulted in improved properties (Purwar et al. 2015; Saha et al. 2016). Composite materials of PVA and natural products have resulted in additional properties like antimicrobial and antioxidant properties (Annu et al. 2021; Abdelghany et al. 2019). These natural products provide bioactive characteristics to PVA polymer and decrease hydrophilicity by decreasing the availability of free OH groups via some interactions. Organic components, namely, dimethyl carbonate (Jihui et al.2017), formaldehyde (Pan et al. 2015), glutaraldehyde (Destaye et al. 2013), dicarboxylic acids (Siracusa et al. 2008), and inorganic reagents like boric acid (Chen et al. 2015), which can react with hydroxyl groups of PVA can improve its physicochemical properties. The hydrophilic character of PVA-based polymer films containing organic acids like malic acid, tartaric acid, and lactic acid decreases due to an increase in contact angle (Suganthi et al. 2020). PVA polymer has got film-forming property; hence, it can act as a carrier for natural products. For the preparation of antimicrobial films, two components are needed, a film-forming polymer, water, ethanol, and others which act as a solvent for dissolution of the polymer, and an antimicrobial agent (Pareda et al. 2011; Ramos et al. 2012; Sanchez-Gonzalez et al. 2013; Sivarooban et al. 2008). Sometimes glutaraldehyde acts as cross-linking agents, glycerol, sorbitol, and others as plasticizers, and pH adjusting agents (acid, sodium hydroxide, and others) are added when needed.
Incorporation of natural products with antimicrobial activity in polymer PVA results in the formation of antimicrobial films. Such antimicrobial films can be used to reduce, minimize, and stop the growth of microbes inside the food. The antimicrobial activity is imperative for food packaging (Appendini et al. 2002). The antimicrobial active packaging is different from traditional packaging in its capability to minimize or retard the microbial growth in the headspace of food (Soares et al. 2009). To get the antimicrobial characteristics of food packages many antimicrobial acids, such as sorbic acid, nisin, and pectin, have been successfully impregnated in them (Silveira et al. 2007; Chan et al. 2004; Linshu et al. 2009). In addition to this, natural extracts have also been incorporated. Different packaging systems like low-density polythene, nylon, chitosan, starch, etc. have been used for the incorporation of extracts like clove extract, horseradish seed extract, etc.
Piperine, an important natural bioactive compound, is used for the synthesis of piperic acid. Piperine is extracted from black pepper (Piper nigrum) using ethanol as a solvent (Dayanand et al. 2013). Black pepper is known for its antimicrobial and antioxidant activity. Its use in dairy products like cheese and paneer reduces the growth of psychrotrophic bacteria, yeasts, and molds (Makhal et al. 2003). Its antimicrobial activity was found higher than ginger used in soy milk and kunun Zaki at 0.4–1%; hence, it acts as a natural preservative to extend the shelf life of foods (Udensi et al. 2012). Black pepper essential oil inhibits the growth of a variety of organisms. Piperic acid, an acid derivative of piperine, belongs to the alkaloid class of organic compounds. It has been used against a wide spectrum of microorganisms for its antimicrobial and antioxidant activity. The results show its potential use in the food industry as a natural food preservative (Zied et al. 2013).
Keeping in view the above facts, this work describes the synthesis of PVA composite film containing piperic acid, a plant-derived organic acid by solution casting technique. The composite films were fully characterized by physical techniques. Further from an application point of view, their antimicrobial properties are evaluated. Also as for as our literature survey is concerned, we did not find any research article regarding piperic acid–PVA composite film.
Materials and methods
Synthesis of piperic acid
Piperic acid was acquired by alkaline hydrolysis of piperine. Piperine was extracted from the fruit part of Piper nigrum as per the procedure already given by Leila et al. (2017). The following reaction in Fig. 1 shows the scheme for preparation of piperic acid from piperine.
Fig. 1.
Scheme for synthesis of piperic acid
Preparation of PVA film
5% (w/v) Solution of pure Polyvinyl alcohol was heated at 75 °C for 3 h on a magnetic stirrer with constant stirring to get a transparent and clear solution of pure PVA (Solution-A) (Usman et al. 2016). One-fourth (25 mL) of the above solution was cast on a Petri plate and kept for drying at 40 0C inside oven for 48 h. The resultant films as shown in Fig. 2 were peeled out from the plates and placed inside desiccators for further studies.
Fig. 2.
a Pure PVA film. b PVA–piperic acid composite film (10 mL)
Preparation of PVA/PA composite films
To prepare PVA–piperic acid composite films (PVA/PA), 0.063 wt% solutions of pure piperic acid were prepared by the dissolution of 15 mg of piperic acid in 30 mL ethanol (Solution-B). Then, 5 mL, 10 mL, and 15 mL volumes of solution-B were mixed with 25 mL proportions of solution-A at 80 °C, with constant stirring for 2 h. The weight percent (wt%) of above solutions (A + B) are 0.011, 0.018, and 0.024, respectively. These solutions were placed in an ultrasonic bath for 15 min to remove air bubbles if any. The three solutions were transferred to Petri plates and kept for drying in an oven at 40 °C for 4 days (Feroz et al. 2016). Finally, the prepared composite films shown in Fig. 2 were removed from Petri plates and were packed in black paper sheets to avoid solar exposure and for further characterization.
Pure PVA film obtained was smooth and transparent. With the inclusion of piperic acid, the film appeared thick, rough, and less transparent. Also, with the addition of piperic acid molecules in PVA, the color became slightly milky and with an increase in the concentration of filler, the said coloration also increased. This decrease in transparency or transmission is also supported by UV–Vis absorption spectroscopy and will be discussed in the future section of this article.
XRD analysis
For crystal structural study of these samples, the XRD was carried out using Rigaku smart lab 9 kW rotating-anode X-ray diffractometer; Cu Kα radiation of wavelength (λ) = 1.54050 Å).
FT-IR spectral studies
The prepared samples were characterized by the FT-IR spectrophotometry using a Perkin-Elmer spectrum 2 spectrometer in the range of 500–4000 cm−1.
Field emission scanning electron microscopy
The surface morphological studies were done using the field emission scanning electron microscopy (FESEM) ZEISS Gemini SEM 500 operated at 15 kV. Further imaging of the polymer composite samples was taken as per their standard procedures.
UV–visible spectroscopy
The optical property of the prepared films was measured using Shimadzu UV-1601 spectrophotometer in the wavelength range of 200–800 nm.
Thermo-gravimetric analysis
The thermo-gravimetric analysis was done using Mettler Toledo DSC/TGA instrument. For TGA measurements, the heating rate was kept at 10 °C/min under nitrogen flow.
Contact angle measurements
The surface properties (hydrophobicity and hydrophilicity) of the polymer composite films were obtained by contact angle (ϴ) measurements. Microscope MicroView (USB Digital Microscope) coupled with an image analysis software (220*2.0 MP) was employed. A distilled water (5μL) bubble was dropped on the surface of each film. The angle formed by the intersection of the liquid–solid interface (a drop of water surface of the film) and the liquid–vapor interface (tangent on the boundary of the drop) was measured (Karbowiak et al. 2006).
Moisture content
The drying oven method was used for getting moisture content. Initially, 2 × 2 cm.2 film pieces were cut and weighed (W1). Then, at 105 °C for 24 h in a hot air oven, films were dried and dry weight (W2) was recorded (Orsuwan et al. 2016). The experiment was done in triplicates. The moisture content (MC) in % was calculated from the weight loss using Eq. (1)
| 1 |
Water solubility
The film solubility was determined according to the methodology given in Orsuwan et al. (2016). After immersion of films in water, the percentage of dissolved dry matter was calculated. For this, film samples of 2 cm × 2 cm size were cut and dried at 60 °C for 24 h and the initial dry weight was determined (Mi). 30 mL De-ionized water was used for dipping film samples followed by mild shaking for 24 h. Then, the samples were removed and dried at 100 °C for 24 h to determine the un-dissolved final dry weight (Mf). The % film solubility (FS) was calculated using Eq. (2)
| 2 |
Water vapor transmission rate
The water vapor transmission rate (WVTR) of films was determined by the standard method of ASTM E96-95 with modification (Gennadios et al. 1994). 18 mL of distilled water was filled into WVP measuring cup. Then, cup was covered with (7.5 cm × 7.5 cm) film sample on the top and sealed tightly to prevent the water vapor loss. The assembled WVP cup was weighed and subsequently placed in a controlled environmental chamber set at 25 °C and 50% RH. Weight change of the cup was determined every1 h for 8 h. The water vapor transmission rate (WVTR; g/m2 s) of the film was calculated using the slope of the steady-state (linear) portion of the weight loss versus the time plot.
Oxygen transmission rate
Oxygen transmission rate was determined using OTR measuring system (Labthink; C230) according to ASTM D3985—17 standard at 23 ◦C and relative humidity of 0%. Two parallel samples were tested for each packaging film.
Film thickness
Calibrated digital micrometer Vernier caliper was used for film thickness measurements at three different places of the film. Then, an average of the three measurements was taken.
Mechanical properties
According to ASTM D, 882 standard mechanical properties of composite films such as tensile strength and elongation at break % were measured using Tinius Olsen H50 KT universal testing by applying 5 kg (50 N) load cell at 5 mm/min of crosshead speed. Film samples of dimensions 5 × 1cm2 were placed between two gripping units of tensometer, with a 3 cm gauge length left for mechanical loading. From the results of five tests, average values are observed and expressed as mean ± standard deviation (SD).
Antibacterial activity
For the evaluation of the antibacterial activity of the above-mentioned polymer composites against two bacterial strains E. coli and S. aureus, the liquid test culture method was used (Teixeira et al. 2014). In this method, circular composite films of 20 mm diameter were ripped along, sterilized by UV radiations for 20 min, and immersed in Tryptic Soy Broth (TSB) medium placed in four tubes. Then, 100 µl of bacterial inoculum (~ 108 CFU/ml) was mixed with the TSB medium in tubes in which films were offhand placed. These four test tubes were kept in a shaker incubator (200 rpm) at 37 °C for 24 h. After that 1 ml of the sample was separated from all the four test tubes and separately tenfold diluted with maximum recovery diluent media. These tenfold dilutions were spread on Trypticase Soy Agar (TSA) medium by spread plate method. These plates were kept in an incubator at 37 °C for 24 h to get colonial forming units per ml (CFU/ml) to count.
Antifungal activity
Polymer composite films of piperic acid were evaluated for their efficacy on mycelial growth of pathogenic fungi Fusarium solani, Penicillium, Aspergillus species by food poisoning technique (Zhiqi et al. 2008). These 20 mm circular films were dipped in potato dextrose agar (PDA) medium and about 15 mL mixtures were transferred into sterile Petri plates and allowed to solidify in a laminar airflow chamber. After solidification, inoculation of 2 mm mycelial discs of the above fungus was done at the center of each plate. The discs were obtained from the actively growing colonies. The whole procedure was done thrice to get accurate results. The Petri plates were incubated at 24° ± 2 °C. Colony diameter was measured and recorded after 3 days. The PDA plate without polymer-piperic acid composite film served as a negative control. The percentage inhibition of fungal growth by these composite films can be obtained by Vincent formulae
| 3 |
where I is the percent inhibition, C is the colony diameter in control, and T is the colony diameter in treatment.
Results and discussion
XRD analysis
The XRD pattern of pure PVA and piperic acid composite film is shown in Fig. 3. The PVA film showed an intense peak at 2θ = 19.68° (Tripathi et al. 2013).
Fig. 3.

XRD of pure PVA and PVA/PA composite film at different concentrations of piperic acid. Inset show XRD pattern of piperic acid
This intense peak observed in PVA inferring its semi-crystalline nature arises from [101] crystal plane due to hydrogen bonding between polymeric chains (Mathew et al. 2018; Zainab et al. 2017). The individual piperic acid peaks are not present in the graph. However, increasing the addition of piperic acid decreases the relative intensity of the characteristic peak of PVA, therefore indicating the loss of the semi-crystalline nature of PVA. This supports the existence of interaction between the two (Jie et al. 2015). Besides broadening of the characteristic peak could be due to polymer complex formation which in turn results in the isolation of polymer chains followed by different rearrangements in its structure. The presence of hydrogen and oxygen atoms in our dopant molecule could show interactions via Vander Waals and weak interactions with polymer hydrogen atoms (Feroz et al. 2016. This interaction is seen in the crystalline region of the host molecule, which shows a significant decrease in crystallinity. Another reason for semi-crystalline nature transforming into amorphous may be due to the changes induced by heat (˃70 °C) during sample preparation (Tripathi et al. 2013). Furthermore, the supplied heat causes movement of the lattice points like phonons and results in the breakage of existing bonds and the formation of new bonds present in the lattice systems (Tripathi et al. 2013). Thereof, this non-covalent interaction between piperic acid and PVA was also supported by FT-IR data as aforementioned.
FT-IR analysis
Figure 4 shows the FT-IR spectrum of PVA, piperic acid, and their composite films. The FT-IR spectrum of piperic acid shows a typical high intense peak at 1674 cm−1 which corresponds to the C=O carboxylic group. The bands observed between 2700 and 3000 cm−1 correspond to aliphatic & aromatic C-H bonds. The high-intensity peak observed at around 1490–1600 cm−1 is because of the aromatic moiety. Another absorption peak at 1255 cm−1 corresponds to C–O–Ar methylenedioxy symmetric stretch (Bahri et al. 2019). The pure PVA spectrum showed a strong and broadband at 3263 cm−1, which is due to the characteristic stretching vibrations of O–H groups involved in inter- and intramolecular H-bonding between polymer chains. However, with piperic acid incorporation in the polymer matrix, this band starts broadening, which could be due to weak forces like electrostatic interactions between aromatic C–H stretches of piperic acid and PVA–OH electric dipoles (Feroz et al.2016; Abdelghany et al. 2018). Again, a blue shift of the band which corresponds to asymmetric –C–H stretches from 2926 to 2922 cm−1is observed. This peak broadening and shifting in the above two regions may be due to the hydrogen-bonding type of interaction between the reactive functional groups of two molecules (Feroz et al. 2016).
Fig. 4.

FT-IR analysis of piperic acid, PVA, and PVA/PA composite film
The band at 1428 cm−1 corresponds to C–H bends of methylene group vibrations of PVA (Rahman et al. 2014). The decreased intensity at this band in the composites due to decoupling supports the interaction between the two (Usman et al. 2016). Again, merging of the peaks at 1710 cm−1 of pure PVA and 1674 cm−1 of piperic acid is an indicative of hydrogen bonding between the two molecules (Feroz et al. 2016). Again the appearance of a peak corresponding to C–O–Ar methylenedioxy stretch of pure piperic acid in the composites confirms its presence strongly and thereafter shifting of the same peak to 1246 cm−1supports interactions between the two. Precisely, the change in PVA structure with piperic acid incorporation is correlating well with the XRD data of these samples.
Scanning electron microscopy
Figure 5 shows the SEM images of PVA and its piperic acid composite. The surface morphology of PVA is changed when piperic acid is incorporated. The micrograph of PVA film is less rough; however, with piperic acid incorporation, the roughness of PVA films shows an increase in roughness. Usually, more roughness means more surface area. The increased roughness could increase the hydrophobicity of films, which in turn could increase the applicability of such hydrophobic materials for food packaging purposes (Erbil et al. 2003). This is well supported by contact angle measurements as mentioned below. A similar observation was well supported by several studies (Mishra et al. 2014; Ismail et al. 2011; Kokila et al. 2015).
Fig. 5.
SEM micrographs of PVA/PA film composites indicate PVA with increasing concentrations of piperic acid
Optical absorption studies
Figure 6 shows the UV spectrum of piperic acid, PVA, and PVA/PA composite films with different piperic acid concentrations. For pure piperic acid molecules, the absorption peaks are observed at 330, 298, and 199 nm, and these peaks are due to π–π* electronic transitions. The electronic transitions in UV and near-visible regions in the current and other kinds of organic molecules are due to σ → σ*, n → π*, and π → π* transitions (Mir et al. 2015).
Fig. 6.

UV–Vis absorbance spectrum of pure PVA and PVA/PA composite at different concentrations of Piperic acid
In the case of pure PVA, the UV absorption spectrum does not show any distinguishing peak in the visible region. It offers a characteristic absorption peak at 197 nm in the UV region. However, after doping with piperic acid, the absorption peaks of the filler molecule are shifted to 311, 294, and 215 nm and intensified (Bahri et al.2019). The same observation was obtained by high polyphenolic compounds containing natural extracts and essential oils (Davis et al. 1960). This shifting of the peaks towards a lower wave number supports the interaction between the polymer and natural product (Feroz et al.2016). It correlates well with its XRD and FT-IR data.
TGA studies
Polymeric materials used in industrial processes like food packaging are preferred to have high thermal stability to persist high heat and moisture retort treatments. TGA test is performed to determine the thermal and oxidative stability, chemical structure and composition, and water activity of films. It is a useful technique to verify the incorporation of bioactive components in polymer films by determining the increase or decrease in thermal degradation peak. In PVA molecule inter-chain and intra-chain interactions arise due to H-bonding in between hydroxyl (–OH) groups. These interactions between polymer chains are changed due to hydroxyl groups (OH) present in piperic acid in said composite, thus modifying the physical structure and crystalline behavior. The TGA curves of pure PVA and PVA/PA composites are shown in Fig. 7 which shows three weight loss stages. Initial weight loss can be due to the evaporation of small molecules like water (Guiping et al. 2009). The second stage of weight loss gives degradation or decomposition temperature of PVA. It can be due to the fission of building (monomer) units and bond cleavage in the PVA backbone. In the final stage, weight loss can be due to the cleavage of the macromolecular structures of both PVA and piperic acid (Deshmukh et al. 2014; Zhi-Qiang et al. 2013). The TGA curves show that the thermal stability of piperic acid PVA composite films has improved as compared to neat PVA.
Fig. 7.

Relative loss of weight with temperature
The thermal stability is expected to increase due to the hydrogen-bonding type of interactions between PVA and piperic acid, as both the molecules contain reactive hydroxyl(-OH) groups. Furthermore, the hydrogen bonding between PVA and PA could have decreased PVA chain movements which resulted in the repression of chain transfer reaction, and hence slackening of the degradation process (Ahmad et al. 2014; Morimune-Moriya et al. 2014). Pour et al. found increased thermal stability of starch PVA composite film containing citric acid and suggested cross-linking interaction of critic acid molecules with starch and PVA chains (Sekhavat et al. 2015). The incorporation of piperic acid in the polymer films enhanced their thermal stability by increasing the thermal degradation temperature from 251 to 275 °C. The enhanced thermal stability can be very useful during the production of films via extrusion/compression molding. Furthermore, it can be concluded that natural products enhance the functionality of edible films for food applications. Further increase in thermal stability exhibits the higher cross-linking, density, and lower porosity of films with good oxidative stability. Moisture content can be obtained from the initial stage of weight loss. Moisture content has decreased from 8.73 to 1.82% with increasing content of piperic acid in composite films.
Contact angle
The relationship between hydrophobicity and surface roughness of a material is reported in literature (Erbil et al. 2003). The larger the surface roughness, the greater is its hydrophobicity and vice versa. Similarly, authors have reported the relationship between contact angle, hydrophobicity, and surface roughness of a material, implying that greater roughness surfaces exhibit more contact angle and hence will be more hydrophobic (Carolina et al. 2015; Jiang et al. 2009; Seungil et al. 2014). Materials with uneven top leave the surface for air fraction to get into it and subsequently increase the water contact angle (Kim et al. 2014; Zisman et al. 1964). The hydrophobicity of PVA composite films was determined by contact angle measurements, as shown in Fig. 8. The contact angle of pure PVA is 43°. With the incorporation of piperic acid, it is increased to 57.4° in 5 ml composite, 72.6° in 10 mL composite, and 99.1° in 15 mL composite. The terms “hydrophobic” and “hydrophilic” are defined, respectively, for ϴ > 65o and ϴ < 65o (Volger et al. 1998); thus, the incorporation of piperic acid results in hydrophobic film surfaces. Another reason for the increase in contact angle could be increased roughness as clear from SEM images.
Fig. 8.
Image of a drop of water on the surface of a PVA, b 5 mL, c 10 mL, and d 15 mL Films
Moisture content
The films with hydrophobic surfaces (10 mL, 15 mL composite films) exhibited lower moisture content as in Table1. For 15 mL PVA/PA composite film, a reduction of moisture content was observed when compared with the neat PVA. This could probably be due to non-covalent interactions between the polymer network and the piperic acid molecule, and hence controls the accessibility of hydroxyl groups for hydrogen bonding with water, subsequently leading to a decrease in the affinity of the films towards water (Siripatrawan et al. 2010).
Table 1.
Thickness, moisture content, and film solubility of films
| Samples | Thickness (mm) | Moisture content (MC) (%) | Film solubility (FS) (%) |
|---|---|---|---|
| Pure PVA | 0.11 ± 0.002 | 10.23 | 98.32 |
| 5 ml PP | 0.16 ± 0.003 | 8.06 | 89.24 |
| 10 ml PP | 0.18 ± 0.001 | 7.24 | 9.32 |
| 15 ml PP | 0.20 ± 0.004 | 5.82 | 8.97 |
*Values are expressed as mean ± Standard Deviation (SD) in the thickness column
Film solubility
To ensure the retention of sensory qualities of food products and to improve the integrity, water insolubility of film is considered significant (Tongdeesoontorn et al. 2011). Film solubility indicates the water resistance of film. Pure PVA films are almost entirely soluble in distilled water. With the addition of different concentrations of piperic acid in PVA films, the solubility of the films was considerably reduced. For example, in 15 mL PVA–piperic acid composite film, solubility was found reduced compared to pure PVA film. The results in Table 1 show more water resistance of those PVA films with more piperic acid concentration. This could probably be due to an increase in the hydrophobic character of the film. The tenfold decrease in film solubility of PVA/PA composite films incorporated with different concentrations of piperic acid can be explained based on the very low solubility of piperic acid molecule in water than PVA which is completely soluble (Annu et al. 2021). Another reason for solubility reduction may be due to the interaction between reactive hydroxyl groups (–OH) in PVA and PA molecule, which reduce the degree of hydrophilicity in the films and as a result shows a reduction in solubility (Ghelejlu et al. 2016). Finally, a significant reduction in water solubility could be correlated well with an increase in contact angle from 43° (PVA) to 72.6° in 10 mL composite film and to 99.1° in 15 mL composite film. This makes the composite films highly hydrophobic (ϴ > 90°) in nature compared to PVA film which is hydrophilic (ϴ < 90°). Abdullah et al. (2019) found that in the case of PVA film containing starch, glycerol, and halloysite nanotube increase in contact angle shows a correlation with a significant reduction in water solubility of nanocomposite film with increasing wt% of halloysite nanotubes.
Water vapor transmission rate and oxygen transmission rate
Materials employed for packaging purposes should possess better oxygen and water barrier properties to ensure the integrity and maintenance of food products. Packaging materials with both good oxygen and water barrier properties are non-existent. Generally, olefin polymers having non-polar functional groups in the repeating units have excellent moisture barrier properties for example polythene and polypropylene. Meanwhile, PVA is having good oxygen barrier properties due to its crystalline nature and intermolecular attractions between hydroxyl groups in its repeating monomeric units. The barrier properties of PVA/PA films were observed as a function of increasing volumes of piperic acid molecule or wt% of piperic acid, as shown in Table 2.
Table 2.
OTR, WVTR, TS, and % EB of all film samples
| Film samples | OTR (cc/m2/day) | WVTR (gm/m2/day) | Tensile strength (TS) | %Elongation at break (EB) |
|---|---|---|---|---|
| PVA | 8.69 ± 0.026 | 920.33 ± 1.247 | 18.73 ± 0.81 | 165 ± 1.87 |
| 5 ml | 6.28 ± 0.029 | 836.00 ± 1.633 | 21.11 ± 0.65 | 145 ± 1.63 |
| 10 ml | 5.39 ± 0.134 | 749.67 ± 1.699 | 23.24 ± 0.73 | 137 ± 1.27 |
| 15 ml | 4.97 ± 0.045 | 688.67 ± 2.054 | 29.87 ± 0.88 | 121 ± 1.73 |
*Values are expressed as mean (±) Standard deviation (SD) in TS and %EB column
The oxygen transmission rate (OTR) value for pure PVA is 8.7 cc/m2/day. The OTR value for PVA/PA films varies from 6.3 to 4.7 cc/m2/day. This indicates that OTR of virgin PVA films was reduced by the incorporation of piperic acid. A similar result of a reduction in OTR values has been observed with PVA-based PVA boric acid hybrid films (Limet et al. 2015).
Water vapor permeability is strongly based on chemical structure, film morphology, nature of the permeate, and measurement conditions, such as temperature and water vapor pressure gradient (Park et al. 2002). Usually, the water vapor transmission rate (WVTR) of packaging films should be below the range of 1000 g/m2/day. The water vapor transmission rate of a packaging material below 1000 g/m2/day ensures the protection of food from moisture and subsequent microbial growth there off. The WVTR of pure PVA film is 920 gm/m2/day as given in Table 2. The value varies in descending order from 920 to 690 gm/m2/day with an increasing concentration of piperic acid. The lower value of WVTR of 15 mL composite film compared to pure PVA might be attributed to the hydrophobic nature of the film. The lower OTR and WVTR of the PVA films incorporated with piperic acid could be because of hydrogen-bonding interactions between hydroxyl groups of PVA and piperic acid. This interaction could decrease the availability of free hydroxyl groups of polymer for hydrogen bonding with water; hence, it could enhance its barrier properties. Similar findings were observed by Siripatrawan et al. (2010) by incorporating green tea extract into chitosan-based films. Hydrogen and covalent type interactions between chitosan and polyphenolic compounds of green tea extract resulted in the reduction of water vapor permeability. Similarly, Gómezguillin et al. (2007) observed a reduction in the number of hydroxyl groups available for hydrogen bonding with water due to cross-linking reaction between antioxidant extracts from murta leaves and tuna-fish gelatin.
Thickness and mechanical properties
The thicknesses of the composite films as shown in Table 1 vary from 0.11 to 0.20 mm. Pure PVA film has the least thickness. Due to PA incorporation, film thickness augments.
Mechanical properties such as Tensile strength (TS) and Elongation at break (EB) can have determining effects on the quality of food packaging materials. The mechanical properties of different PVA/PA films were obtained using a universal testing machine. As shown in Fig. 9, the introduction of PA into virgin PVA improved the mechanical properties of the pure PVA film.
Fig. 9.
Tensile strength and % elongation at break of film samples
Given in Table 2 tensile strength of virgin PVA is enhanced with increasing wt% of piperic acid into PVA, whereas the % elongation at break was reduced. The tensile strength of pure PVA is 18.73 ± 0.81 Mpa, and the PVA/PA composite films exhibited an increasing trend from 21.11 ± 0.65 Mpa to 29.87 ± 0.88 Mpa. Reduction in EB is preferable in food packaging, as this property is directly related to the biodegradability of the films (Voon et al. 2012). The elongation of the PVA/PA hybrid films decreased with increasing PA content from 165% ± 1.87 to 121% ± 1.73. Increment in TS is an essential criterion in food packaging applications, because high TS is required to bear common stresses encountered during handling, food storage, delivery, and transportation. The polymeric chains in pure PVA can show sliding movements easily by the application of external force; hence, it has low tensile strength and high elongation at break. However, after the incorporation of piperic acid into PVA polymer, hydrogen-bonding interactions between the polymer and piperic acid hydroxyl groups could have restricted the movement of polymer chains by making the polymer chains tight and rigid. Therefore, tensile strength shows an increase and elongation at break shows a decrease (Lim et al. 2015). Similarly, improvement in the mechanical property of PVA was observed when boric acid was added to it as a cross-linking agent (Chen et al. 2015).
Biological evaluation
Antimicrobial activity
Antimicrobial activity is desirable for food packaging to reduce the growth of microbes in food. The antibacterial properties of polymer composite films against S. aureus and E. coli were evaluated by the colony count method. The results obtained from the method are shown in Fig. 10 against S. aureus and E. coli, respectively. Results were obtained by counting the number of viable cells, i.e., colonial forming units per mL.
Fig. 10.
Antimicrobial activity of PVA/PA composites against S. aureus & E. coli, respectively
From the graph in Fig. 11, which shows log CFU/mL against all-composite film samples, it is clear that 15 mL composite film has shown antimicrobial activity against both tested pathogens and 10 mL composite film has demonstrated antimicrobial activity against S. aureus. This significant activity by 15 mL composite against both shows its provision in active packaging antimicrobial films.
Fig. 11.
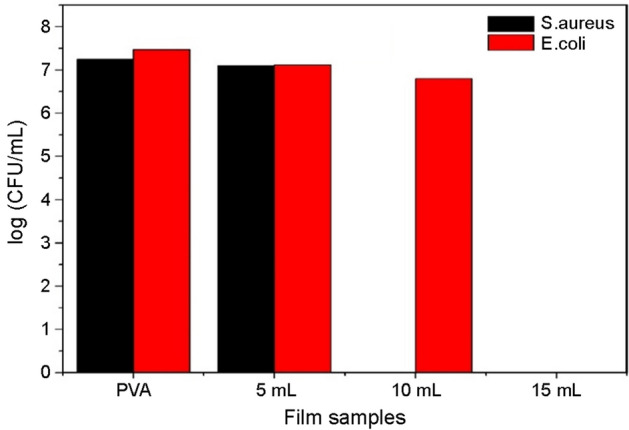
Log CFU/ml of films against S. aureus and E. coli
Antifungal activity
Composite films of piperic acid were evaluated for their efficacy on mycelial growth of pathogenic fungi Fusarium solani, penicillium species, and Aspergillus by food poisoning technique. As indicated in Fig. 12a and Table 3, we can see that the radial growth of Fusarium solani is inhibited by increasing the concentration of piperic acid in composite films.
Fig. 12.
Antifungal activity of films against a Fusarium solani, b Penicillium, and c Aspergillus
Table 3.
Concentration of piperic acid in composite films vs the mean diameter of radial growth and % inhibition against Fusarium solani
| Diameter (cm) | Conc. (µg/ml) | % Inhibition |
|---|---|---|
| 8.87 ± 0.15 | 0 | 0% |
| 3.53 ± 0.20 | 83 | 61% |
| 1.07 ± 0.25 | 143 | 89% |
| 0.53 ± 0.15 | 187 | 94% |
In control, the mean diameter of mycelial growth is maximum and has covered almost the whole plate. There has been a drastic decrease in the diameter of mycelial growth with an increase in the concentration of piperic acid in polymer films. Table 3 shows the same results where the mean diameter of mycelial growth is compared with the concentration (µg/mL) and percentage inhibition of piperic acid in the polymer composite. Similarly in Fig. 12b, c, and Tables 4, 5, radial growth of Penicillium and Aspergillus was inhibited by increasing the concentration of piperic acid. As per Vincent formulae, the percentage of mycelial radial growth inhibition at 72 h after inoculation was highest in the case of composite having a higher concentration of piperic acid against all the tested pathogens. Hence, 15 mL composite was found more effective against all three tested pathogens. Its percentage inhibition is maximum against Fusarium solani than Penicillium and Aspergillus.
Table 4.
Concentration of piperic acid in composite films vs the mean diameter of radial growth and % inhibition against Penicillium
| Diameter (cm) | Conc. (µg/ml) | % Inhibition |
|---|---|---|
| 8.03 ± 0.25 | 0 | 0% |
| 3.97 ± 0.15 | 83 | 50% |
| 3.17 ± 0.15 | 143 | 60% |
| 2.02 ± 0.07 | 187 | 75% |
Table 5.
Concentration of piperic acid in composite films vs the mean diameter of radial growth and % inhibition against Aspergillus
| Diameter (cm) | Conc. (µg/ml) | % Inhibition |
|---|---|---|
| 8.77 ± 0.21 | 0 | 0% |
| 6.97 ± 0.25 | 83 | 22% |
| 5.50 ± 0.10 | 143 | 39% |
| 5.05 ± 0.11 | 187 | 44% |
Conclusion
To improve thermal properties and to increase the hydrophobicity of PVA-based packaging films, PVA impregnated piperic acid composite films were prepared. These composite films could find suitability in food packaging applications due to their excellent light transmittance, moisture content, film solubility, barrier properties, and antimicrobial properties. Characterization of the composite films was done by SEM, XRD, FT-IR, and UV–Vis spectroscopic analysis. FT-IR analysis revealed the involvement of hydroxyl (OH) group interactions between the polymer and natural product. SEM micrographs also indicated healthy dispersion of natural molecules at higher concentrations. 15 mL composite films exhibited good antibacterial activity against food-borne pathogens tested in this study. Also, the antifungal activity shown by these composites is worthwhile. Hence, the results suggest that composite films of piperic acid could be used for food packaging film to ensure the maintenance of sensory properties of food and increased shelf life.
Acknowledgements
One of the authors IG would like to thank the Ministry of Human Resource Development (MHRD) New Delhi for Fellowship. JAB would like to thank JK Science Technology & Innovation Council for research grants (No. JKST&IC/SRE/1014-16). FAM would like to thank the JK Department of Science and Technology for research grants and UGC India for the start-up project (No.F.30-59/2019 (BSR).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The data generated during the current study are available with the corresponding author.
Declarations
Conflict of interest
We do not have any conflict of interest related to this paper. The authors have no relevant financial or nonfinancial interests to disclose.
Compliance with ethical standards
The present study does not involve any human or animal subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelghany AM, Meikhail MS, Abdelraheem GEA, Badr SI, Elsheshtawy N. Lepidium sativum natural seed plant extract in the structural and physical characteristics of polyvinyl alcohol. Int J Environ Stud. 2018;75:965–977. doi: 10.1080/00207233.2018.1479564. [DOI] [Google Scholar]
- Abdelghany AM, Menazea AA, Ismail AM. Synthesis, characterization and anti-microbial activity of chitosan/polyvinyl alcohol blend doped with Hibiscus sabdariffa L. extract. J Mol Struct. 2019;5:603–609. doi: 10.1016/j.molstruc.2019.07.089. [DOI] [Google Scholar]
- Abdullah ZW, Dong Y, Han N, Liu S. Water and gas barrier properties of polyvinyl alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) bionanocomposite films: experimental characterization and modeling approach. Compos B Eng. 2019;174:107033. doi: 10.1016/j.compositesb.2019.107033. [DOI] [Google Scholar]
- Ahmad J, Deshmukh K, Habib M, Hägg MB. Influence of TiO2nanoparticles on the morphological, thermal and solution properties of PVA/TiO2nanocomposite membranes. Arab J Sci Eng. 2014;39:6805–6814. doi: 10.1007/s13369-014-1287-0. [DOI] [Google Scholar]
- Annu AA, Shakeel A. Eco-friendly natural extract loaded anti-oxidative chitosan/polyvinyl alcohol-based active films for food packaging. Heliyon. 2021;7:3. doi: 10.1016/j.heliyon.2021.e06550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appendini P, Hotchkiss JH. Review of antimicrobial food packaging. Innov Food Sci Emerg Technol. 2002;3:113–126. doi: 10.1016/S1466-8564(02)00012-7. [DOI] [Google Scholar]
- Bahri S, Ambarwati Y, Iqbal M, Baihaqy AA. Synthesis 4-piperoilmorpholine from piperine. J Phys Conf Ser. 2019;1338:9–11. doi: 10.1088/1742-6596/1338/1/012010. [DOI] [Google Scholar]
- Carolina MJ, Paula GS, Silvia G, Celina B, Lucia F. Biofilms based on cassava starch-containing starch of yerba mate as antioxidant and plasticizer. Starke. 2015;67:780–789. doi: 10.1002/star.201500033. [DOI] [Google Scholar]
- Yoon SD, Chough SH, Park HR. Effects of additives with different functional groups on the physical properties of starch/PVA blend film. J Appl Polym Sci. 2006;100:3733–3740. doi: 10.1002/app.23303. [DOI] [Google Scholar]
- Chan HL, Duck SA, Seung CL, Hyun JP, Dong SL. A coating for use as an antimicrobial and anti-oxidative packaging material incorporating nisin and α-tocopherol. J Food Eng. 2004;62:323–329. doi: 10.1016/S0260-8774(03)00246-2. [DOI] [Google Scholar]
- Chen Y, Cao X, Chang PR, Huneault MA. A Comparative study on the films of Poly(vinyl alcohol)/pea starch nanocrystals and poly(vinyl alcohol)/native pea starch. Carbohydr Polym. 2008;73:8–17. doi: 10.1016/j.carbpol.2007.10.015. [DOI] [Google Scholar]
- Chen J, Li Y, Zhang Y, Zhu Y. Preparation and characterization of graphene oxide reinforced PVA film with boric acid as cross-linker. J Appl Polm Sci. 2015;132:42000. [Google Scholar]
- Davis PS, Shalliday TS. Some optical properties of cadmium telluride. Phys Rev. 1960;118:1020. doi: 10.1103/PhysRev.118.1020. [DOI] [Google Scholar]
- Dayanand K, Shingate PN, Prerana D. New method development for extraction and isolation of piperine from black pepper. Int J Pharm Sci. 2013;4:3165–3170. [Google Scholar]
- Deshmukh K, Jamil A, May-Britt H. Fabrication and characterization of polymer blends consisting of cationic poly-allyl-amine and anionic polyvinyl alcohol. Ionics. 2014;20:957–967. doi: 10.1007/s11581-013-1062-3. [DOI] [Google Scholar]
- Destaye AG, Lin C-K, Lee C-K. Glutaraldehyde vapor crosslinked nanofibrous PVA Mat with in situ formed silver nanoparticles. ACS Appl Mater. 2013;5:4745–4752. doi: 10.1021/am401730x. [DOI] [PubMed] [Google Scholar]
- Erbil HY, Demirel AL, Avcı Y, Mert O. Transformation of a simple plastic into a superhydrophobic surface. Science. 2003;299:1377–1380. doi: 10.1126/science.1078365. [DOI] [PubMed] [Google Scholar]
- Feroz AM, Adil G, Asokan K. Gamma irradiation studies of composite thin films of poly vinyl alcohol and coumarin. RSC Adv. 2016;6:1554–1561. doi: 10.1039/C5RA15633E. [DOI] [Google Scholar]
- Gennadios A, Weller CL, Gooding CH. Measurement errors in water vapor permeability of highly permeable, hydrophilic edible films. J Food Eng. 1994;21:395–401. doi: 10.1016/0260-8774(94)90062-0. [DOI] [Google Scholar]
- Ghelejlu SB, Esmaiili M, Almasi H. Characterization of chitosan–nano clay bionanocomposite active films containing milk thistle extract. Int J Biol Macromol. 2016;86:613–621. doi: 10.1016/j.ijbiomac.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Gómez Guillén MC, Ihl M, Bifani V, Silva A, Montero P. Edible films made from tuna fish gelatin with antioxidant extracts of two different murta ecotypes leaves (Ugni molinae Turcz) Food Hydrocoll. 2007;21:1133–1143. doi: 10.1016/j.foodhyd.2006.08.006. [DOI] [Google Scholar]
- Guimarães M, Botaro VR, Novack KM, Teixeira FG, Tonoli GH. Starch/PVA-based nanocomposites reinforced with bamboo nanofibrils. Ind Crops Prod. 2015;70:72–83. doi: 10.1016/j.indcrop.2015.03.014. [DOI] [Google Scholar]
- Guiping M, Dongzhi Y, Dandan S, Xueyan M, John FK, Jun N. Preparation and properties of water-soluble chitosan and polyvinyl alcohol blend films as potential bone tissue engineering matrix. Polym Adv Technol. 2009;21:189–195. doi: 10.1002/pat.1415. [DOI] [Google Scholar]
- Ismail H, Zaaba NF. Effect of additives on properties of polyvinyl alcohol (PVA) tapioca starch biodegradable films. Polym Plast Technol Eng. 2011;50:1214–1219. doi: 10.1080/03602559.2011.566241. [DOI] [Google Scholar]
- Jayasekara R, Harding I, Bowater I, Christie G, Lonergan G. Preparation, surface modification, and characterization of solution cast starch PVA blended films. Polym Test. 2004;23:17–27. doi: 10.1016/S0142-9418(03)00049-7. [DOI] [Google Scholar]
- Jiang Z, Yunhai M, Lili R, Jin T, Ziqin L, Liang X. Preparation and characterization of surface crosslinked TPS/PVA blend films. Carbohydr Polym. 2009;76:632–638. doi: 10.1016/j.carbpol.2008.11.028. [DOI] [Google Scholar]
- Jie C, Li Y, Yin Z, Yangguang Z. Preparation and characterization of graphene oxide reinforced PVA film with boric acid as cross linker. J Appl Polym Sci. 2015;132:42000. doi: 10.1002/app.42000. [DOI] [Google Scholar]
- Jihui L, Yongshen L, Shuai N, Jie L, Lizhen W. Synthesis of a new “green” sponge via transesterifcation of dimethyl carbonate with polyvinyl alcohol and foaming approach. J Porous Mater. 2017;24:1595–1604. doi: 10.1007/s10934-017-0399-9. [DOI] [Google Scholar]
- Karbowiak T, Debeaufort F, Champion D, Voilley A. Wetting properties atthe surface of iota-carrageenan-based edible films. J Colloid Interface Sci. 2006;294:400–410. doi: 10.1016/j.jcis.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Kim SI, Lee BR, Lim JI, Mun CH, JungY KJH, Kim SH. Preparation of topographically modified poly (L-lactic acid)-b-poly (ɛ-caprolactone)-b-poly (L-lactic acid) tri-block copolymer film surfaces and their blood compatibility. Macromol Res. 2014;22:1229–1237. doi: 10.1007/s13233-014-2168-9. [DOI] [Google Scholar]
- Kokila T, Ramesh PS, Geetha D. Biosynthesis of silver nanoparticles from Cavendish banana peel extract and its antibacterial and free radical scavenging assay: a novel biological approach. Appl Nanosci. 2015;5:911–920. doi: 10.1007/s13204-015-0401-2. [DOI] [Google Scholar]
- Lawton JW. Effect of starch type on the properties of starch-containing films. Carbohydr Polym. 1996;29:203–208. doi: 10.1016/0144-8617(96)00028-8. [DOI] [Google Scholar]
- Leila G, Maedeh M, Ghasem DN, Maryam N. Piperine-the bioactive compound of black pepper: from isolation to medicinal formulations. Compr Rev Food Sci Food Saf. 2017;16:124–140. doi: 10.1111/1541-4337.12246. [DOI] [PubMed] [Google Scholar]
- Lim M, Kwon H, Kim D, Seo J, Han H, Khan SB. Highly-enhanced water-resistant and oxygen barrier properties of cross-linked poly (vinyl alcohol) hybrid films for packaging applications. Prog Org Coat. 2015;85:68–75. doi: 10.1016/j.porgcoat.2015.03.005. [DOI] [Google Scholar]
- Linshu L, Jin T, Finkenstadt V, Cheng-Kung L, Cooke P, Coffin D, Hicks KB, Samer C, Liu L, Jin T, Finkenstadt V, Liu C-K. Antimicrobial packaging materials from poly(lactic acid)incorporated with pectin-Nisaplin ® microparticles. Chem Technol. 2009;3:221–230. [Google Scholar]
- Luo X, Li J, Lin X. Effect of gelatinization and additives on morphology and thermal behavior of corn starch/PVA blend films. Carbohydr Polym. 2012;90:1595–1600. doi: 10.1016/j.carbpol.2012.07.036. [DOI] [PubMed] [Google Scholar]
- Makhal S, Mandal S, Kanawjia SK. Micro GARDT Min food preservation: a shifted towards natural preservatives. J Dairy Bio Sci. 2003;14:70–78. [Google Scholar]
- Mathew S, Snigdha S, Mathew J, Radhakrishnan EK. Montmorillonite: boiled rice water (starch) blend film reinforced with silver nanoparticles; characterization and antibacterial properties. Appl Clay Sci. 2018;16:464–473. doi: 10.1016/j.clay.2018.05.009. [DOI] [Google Scholar]
- Mir FA. Spectrophotometric and electrical properties of imperatorin: an organic molecule. Appl Phys A. 2015;120:1659–1663. doi: 10.1007/s00339-015-9384-9. [DOI] [Google Scholar]
- Mishra RK, Ramasamy K, Lim SM, Ismail MF, Majeed ABA. Antimicrobial and in vitro wound healing properties of novel clay-based bio-nanocomposite films. J Mater Sci Mater Med. 2014;25:1925–1939. doi: 10.1007/s10856-014-5228-y. [DOI] [PubMed] [Google Scholar]
- Morimune-Moriya S, Kotera M, Nishino T, Goto T. Uniaxial drawing of poly (vinyl alcohol)/graphene oxide nanocomposites. Carbon. 2014;70:38–45. doi: 10.1016/j.carbon.2013.12.055. [DOI] [Google Scholar]
- Orsuwan A, Shankar S, Wang LF, Sothornvit R, Rhim J-W. Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocoll. 2016;60:476–485. doi: 10.1016/j.foodhyd.2016.04.017. [DOI] [Google Scholar]
- Pan Y, Shi K, Liu Z, Wang W, Peng C, Xiangling J. Synthesis of a new kind of macroporous polyvinyl-alcohol formaldehyde-based sponge and its water super absorption performance. RSC Adv. 2015;5:78780–78789. doi: 10.1039/C5RA11958H. [DOI] [Google Scholar]
- Pareda M, Ponce AG, Marcovich NE, Ruseckaite RA, Martucci JF. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011;25:1372–1381. doi: 10.1016/j.foodhyd.2011.01.001. [DOI] [Google Scholar]
- Park EH, George ER, Muldoon MA, Flammino A. Thermoplastic starch blends with poly (vinyl alcohol): processability, physical properties, and bio-degradability. Polym News. 1994;19:230–238. [Google Scholar]
- Park SY, Marsh KS, Rhim JW. Characteristics of different molecular weight chitosan films affected by the type of organic solvents. J Food Sci. 2002;67:194–197. doi: 10.1111/j.1365-2621.2002.tb11382.x. [DOI] [Google Scholar]
- Purwar R, Sharma S, Sahoo P, Srivastava CM. Flexible sericin/polyvinyl alcohol/clay blend films. Fibers Polym. 2015;16:761–768. doi: 10.1007/s12221-015-0761-y. [DOI] [Google Scholar]
- Rahman W, Sin LT, Rahmat A, Samad A. Thermal behavior and interactions of cassava starch filled with glycerol plasticized polyvinyl alcohol blends. Carbohydr Polym. 2010;81:805–810. doi: 10.1016/j.carbpol.2010.03.052. [DOI] [Google Scholar]
- Rahman MM, Afrin S, Haque P. Characterization of crystalline cellulose of jute reinforced poly (vinyl alcohol) (PVA) bio composite film for potential biomedical applications. Prog Biomater. 2014;3:23. doi: 10.1007/s40204-014-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos OL, Silva SI, Soares JC, Fernandes JC, Poças MF, Pintado ME, Malcata FX. Features and performance of edible films, obtained from whey protein isolate is formulated with antimicrobial compounds. Food Res Int. 2012;45:351–361. doi: 10.1016/j.foodres.2011.09.016. [DOI] [Google Scholar]
- Saha NR, Sarkar G, Roy I, Rana D, Bhattacharyya A, Adhikari A, Mukhopadhyay A, Chattopadhyay D. Studies on methylcellulose/pectin/montmorillonite nanocomposite films and their application possibilities. Carbohydr Polym. 2016;136:1218–1227. doi: 10.1016/j.carbpol.2015.10.046. [DOI] [PubMed] [Google Scholar]
- Sánchez-González L, Saavedra JIQ, Chiralt A. Physical properties and antilisterial activity of bioactive edible films containing Lactobacillus plantarum. Food Hydrocoll. 2013;33:92–98. doi: 10.1016/j.foodhyd.2013.02.011. [DOI] [Google Scholar]
- Sekhavat PZ, Makvandi P, Ghaemy M. Performance properties and antibacterial activity of crosslinked films of quaternary ammonium modified starch and poly(vinyl alcohol) Int J Biol Macromol. 2015;80:596–604. doi: 10.1016/j.ijbiomac.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Seungil K, Boram L, Lim J, Mun C, Youngmee J, Kim JH, Kim SH. Preparation of topographically modified poly (L-lactic acid)-b-Poly(b-caprolactone)-b- Poly (L-lactic acid) tri-block copolymer film surfaces and their blood compatibility. Macromol Res. 2014;22:1229–1237. doi: 10.1007/s13233-014-2168-9. [DOI] [Google Scholar]
- Shogren RL, Lawton JW, Tiefenbacher KF, Chen L. Starch Poly (vinyl-alcohol) foamed articles are prepared by a baking process. J Appl Polym Sci. 1998;68:2129–2140. doi: 10.1002/(SICI)1097-4628(19980627)68:13<2129::AID-APP9>3.0.CO;2-E. [DOI] [Google Scholar]
- Siddaramaiah S, Raj B, Somashekar R. Structure-property relation in polyvinyl alcohol/starch composites. J Appl Polym Sci. 2004;91:630–635. doi: 10.1002/app.13194. [DOI] [Google Scholar]
- Silveira MFA, Soares NFF, Geraldine RM, Andrade NJ, Gonçalves MPJ. Antimicrobial efficiency and sorbic acid migration from active films into a pastry dough. Package Technol Sci. 2007;20:287–292. doi: 10.1002/pts.757. [DOI] [Google Scholar]
- Siracusa V, Rocculi P, Romani S, Rosa MD. Biodegradable polymers for food packaging: a review. Trends Food Sci Technol. 2008;19:634–643. doi: 10.1016/j.tifs.2008.07.003. [DOI] [Google Scholar]
- Siripatrawan B, Bruce RH. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010;24:770–775. doi: 10.1016/j.foodhyd.2010.04.003. [DOI] [Google Scholar]
- Sivarooban T, Hettiarachchy NS, Johnson MG. Physical and antimicrobial of grape seed extract, nisin, and EDTA incorporated soy protein edible films. Food Res Int. 2008;41:781–785. doi: 10.1016/j.foodres.2008.04.007. [DOI] [Google Scholar]
- Soares NFF, Pires ACS, Camilloto GP, Santiagosilva P, Espitia PJP, Silva WA. Recent patents on active packaging for food application. Recent Pat Food, Nutr Agric. 2009;1:171–178. doi: 10.2174/2212798410901020171. [DOI] [PubMed] [Google Scholar]
- Suganthi S, Vignesh S, Kalyana Sundar J, Raj V. Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications. Appl Water Sci. 2020;10:100. doi: 10.1007/s13201-020-1162-y. [DOI] [Google Scholar]
- Tang S, Zou P, Xiong H, Tang H. Effect of nano-SiO2 on the performance of starch/polyvinyl alcohol blend films. Carbohydr Polym. 2008;72:521–526. doi: 10.1016/j.carbpol.2007.09.019. [DOI] [Google Scholar]
- Teixeira B, Marques A, Pires C, Ramos C, Batista I, Saraiva JA, Nunes ML. Characterization of fish protein films incorporated with essential oils of clove, garlic, and origanum: physical, antioxidant, and antibacterial properties. LWT-Food Sci Technol. 2014;59:533–539. doi: 10.1016/j.lwt.2014.04.024. [DOI] [Google Scholar]
- Tongdeesoontorn W, Mauer LJ, Wongruong S, Sriburi P, Rachtanapun P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem Cent J. 2011;5(1):6. doi: 10.1186/1752-153X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi J, Tripathi S, Keller JM, Das K, Shripathi T. Degradation study on structural and optical properties of annealed Rhodamine B doped poly(vinyl) alcohol films. J Environ Polym Degrad. 2013;98:12–21. doi: 10.1016/j.polymdegradstab.2012.11.003. [DOI] [Google Scholar]
- Udensi EA, Odom TC, Dike CO. Comparative studies of ginger (Zingiber officinale) and West African black pepper (Piper guineense) extracts at different concentrations on the microbial quality of soymilk and kunun-Zaki. Niger Food J. 2012;30:38–43. doi: 10.1016/S0189-7241(15)30033-3. [DOI] [Google Scholar]
- Usman A, Hussain Z, Riaz A, Ahmad NK. Enhanced mechanical, thermal and antimicrobial properties of poly(vinyl alcohol)/graphene oxide/starch/silver nanocomposites films. Carbohydr Polym. 2016;153:592–599. doi: 10.1016/j.carbpol.2016.08.026. [DOI] [PubMed] [Google Scholar]
- Vogler EA. Structure and reactivity of water at biomaterial surfaces. Adv Colloid Interface Sci. 1998;74:69–117. doi: 10.1016/s0001-8686(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Voon HC, Bhat R, Easa AM, Liong MT, Karim AA. Effect of addition of halloysite nanoclay and SiO2 nanoparticles on the barrier and mechanical properties of bovine gelatin films. Food Bioprocess Technol. 2012;5:1766–1774. doi: 10.1007/s11947-010-0461-y. [DOI] [Google Scholar]
- Yun YH, Yoon SD. Effect of amylose contents of starches on physical Properties and biodegradability of starch/PVA-blended films. Polym Bull. 2010;64:553–568. doi: 10.1007/s00289-009-0158-4. [DOI] [Google Scholar]
- Zainab WA, Yu D, Ian JD, Salim B. PVA, PVA blends, and their nanocomposites for biodegradable packaging applications. Polym Plast Techn Eng. 2017;56:1307–1344. doi: 10.1080/03602559.2016.1275684. [DOI] [Google Scholar]
- Zhiqi S, Shougou S, Wei Z, Fei W, Yongjian F. Fusarium Graminearum growth inhibition due to glucose starvation caused by osthol. Int J Mol Sci. 2008;9:371–382. doi: 10.3390/ijms9030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi-Qiang D, Yi-Tao L, Xu-Ming X, Xiong-Ying Y. A simple and green route to transparent boron nitride/PVA nanocomposites with significantly improved mechanical and thermal properties. Chin Chem Lett. 2013;24:17–19. doi: 10.1016/j.cclet.2012.12.014. [DOI] [Google Scholar]
- Zhou G, Liu Y, Fang C, Zhang M, Zhou C, Chen Z. Water resistance, mechanical properties and biodegradability of methylated-cornstarch/poly(vinyl alcohol) blend film. Polym Degrad Stab. 2006;91:703–711. doi: 10.1016/j.polymdegradstab.2005.06.008. [DOI] [Google Scholar]
- Zhou J, Ma Y, Ren L, Tong J, Liu Z, Xie L. Preparation and characterization of surface cross-linked TPS/PVA blend films. Carbohydr Polym. 2009;76:632–638. doi: 10.1016/j.carbpol.2008.11.028. [DOI] [Google Scholar]
- Zied Z, Emna B, Nadia BS, Youssef G, Adel S. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT Food Sci Technol. 2013;50:634–641. doi: 10.1016/j.lwt.2012.07.036. [DOI] [Google Scholar]
- Zisman WA. Contact Angle, Wettability, And Adhesion. Washington, DC: RF Gould American Chemical Society; 1964. pp. 1–51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated during the current study are available with the corresponding author.









