Abstract
How the brain represents gender identity is largely unknown, but some neural differences have recently been discovered. We used an intrinsic ignition framework to investigate whether there are gender differences in the propagation of neural activity across the whole‐brain and within resting‐state networks. Studying 29 trans men and 17 trans women with gender incongruence, 22 cis women, and 19 cis men, we computed the capability of a given brain area in space to propagate activity to other areas (mean‐ignition), and the variability across time for each brain area (node‐metastability). We found that both measurements differentiated all groups across the whole brain. At the network level, we found that compared to the other groups, cis men showed higher mean‐ignition of the dorsal attention network and node‐metastability of the dorsal and ventral attention, executive control, and temporal parietal networks. We also found higher mean‐ignition values in cis men than in cis women within the executive control network, but higher mean‐ignition in cis women than cis men and trans men for the default mode. Node‐metastability was higher in cis men than cis women in the somatomotor network, while both mean‐ignition and node‐metastability were higher for cis men than trans men in the limbic network. Finally, we computed correlations between these measurements and a body image satisfaction score. Trans men's dissatisfaction as well as cis men's and cis women's satisfaction toward their own body image were distinctively associated with specific networks in each group. Overall, the study of the whole‐brain network dynamical complexity discriminates gender identity groups, functional dynamic approaches could help disentangle the complex nature of the gender dimension in the brain.
Keywords: cisgender, dynamical complexity, ignition, sex/gender differences, transgender, whole‐brain dynamics
1. INTRODUCTION
A significant number of studies have explored sex‐related differences in brain connectivity (Biswal et al., 2010; de Lacy et al., 2019; Eliot et al., 2021; Ritchie et al., 2018). However, the proposed sexual dimorphism, as observed in the reproductive organs, has been rejected in terms of sex/gender differences in the brain (Eliot et al., 2021; Hyde et al., 2019) and a meta‐analysis described probable excessive significance in the reports, that is, the existence of a positive reporting bias (David et al., 2018). Furthermore, when investigating the brain differences between females and males (i.e., in reference to one's sex assigned at birth), it is also important to consider gender‐variant minority groups such as transgender, which have been traditionally overlooked or investigated from a pathologized point of view. Gender identity can be defined as a complex multidimensional construct resulting from one's feelings and thoughts about the gender category and membership experience in such a category (Carver et al., 2003; Egan & Perry, 2001). Gender identity may or may not be binary and may correspond either to one's sex assigned at birth, that is, cisgender, or be incongruent with the sex assigned at birth, that is, transgender (Polderman et al., 2018).
Understanding gender incongruence in transgender people has been a growing focus of interest, especially for transgender people who have not undergone gender‐affirmative hormone treatment (GAHT) and/or surgery (Selvaggi & Bellringer, 2011). Studies investigating intrinsic brain functional connectivity in specific networks (e.g., intra‐network group differences in the default mode, executive control, attentional network, or sensorimotor networks) have reported gender differences (Clemens et al., 2020; Nota et al., 2017; Uribe et al., 2020b). However, as these brain networks are constantly interacting (Chen et al., 2013; Menon, 2011), the underlying whole‐brain dynamics is something worth exploring in terms of gender differences (Uribe et al., 2020b). In our initial work (Uribe et al., 2020b), we studied the intra‐ and inter‐network connectivity of four known networks (the default mode, sensorimotor, salience, and executive control networks) through stationary approaches: an independent component analysis, threshold‐free node‐based statistics (Baggio et al., 2018), and graph theory analysis. Briefly, we found that trans men, trans women, and cis women had decreased connectivity with respect to cis men in superior parietal regions, and more importantly, trans men displayed weaker connectivity than cis men between intra‐salience network regions and decreased inter‐network connectivity among nodes of the salience, default mode, executive control, and sensorimotor networks. On the other hand, trans women displayed a lower small worldness, modularity, and clustering coefficient than cis men.
However, stationary measures may be too simplistic an approach to capture the full extent of resting brain activity (Preti et al., 2017). The study of brain network interactions is enriched by investigating the cerebral spatiotemporal fluctuations in response to internal and external stimuli. Differences in whole‐brain dynamics between cis men and cis women have been described using a sliding window approach (de Lacy et al., 2019), and more recently, within a small dataset of trans men (Uribe et al., 2021). A sliding window approach makes it possible to obtain metrics with a dynamic view of the coupling between resting‐state network activity, and it is based on windowed correlations between temporally coherent networks captured with multivariate approaches (Allen et al., 2014; Calhoun et al., 2014). In our study (Uribe et al., 2021), we investigated the connectivity dynamics of 30 independent components grouped into 10 functional networks and obtained 4 brain states by applying a k‐means clustering. Briefly, there were three states with sparse overall connections, but two presented specific positive couplings within the sensorimotor and salience networks and a fourth showed couplings involving components of the salience, default, and executive control networks. Temporally, we mainly differentiated cis men from trans men and cis women, while the latter groups had statistically equivalent fluidity and range dynamism values (Uribe et al., 2021). On the other hand, the brain dynamics of trans women remain elusive. Although differences in the interactions among large‐scale networks have been described between cis‐ and transgender groups (Uribe et al., 2020b), it remains unclear how such networks cooperate in different gender identity groups.
In recent years, a growing number of data‐driven approaches have been proposed to describe spatiotemporal brain dynamics (Allen et al., 2014; Deco, Kringelbach, et al., 2017; Hansen et al., 2015). Among them, the novel intrinsic ignition framework has been recently developed to investigate the propagation over time of activity across the whole brain (Deco & Kringelbach, 2017). This data‐driven method was conceived to capture the influence of local activity on the global brain computation by describing the broadness of communication (Deco & Kringelbach, 2017). In particular, the concept of intrinsic ignition reflects a degree of global integration induced by the capability of a given brain area to propagate neural activity across the whole‐brain network.
In this work, we explore for the first time gender‐related differences in whole‐brain dynamics by leveraging the intrinsic ignition framework to study information transmission across the whole‐brain network as well as at the network level. The gender dimension was explored including gender‐variant groups (transgender) in addition to cisgender groups, and we employed the same dataset analyzed in the two previous studies described above (Uribe et al., 2020b; Uribe et al., 2021). Specifically, we investigated the dynamical complexity across the whole‐brain network of four gender groups (trans men and trans women with gender incongruence, cis men, and cis women) by looking at the effects of spontaneously occurring local activation (i.e., events) on global integration (Deco et al., 2015) through the intrinsic ignition framework (Deco & Kringelbach, 2017; Deco, Tagliazucchi, et al., 2017). Furthermore, based on our previous findings of brain network interactions with intra‐ and inter‐network connectivity differences (Uribe et al., 2020b; Uribe et al., 2021), we also explored the underlying dynamics for each resting‐state network. As a secondary objective to test the relationship between the intrinsic ignition measurements and clinical outcomes, we were also interested in exploring the associations of the functional connectivity dynamics with the degree of satisfaction toward body parts for both trans‐ and cis‐groups. The two groups of transgender participants assessed in the present study did not go through a GAHT and thus reported gender nonconformity toward the sex assigned at birth. We hypothesized that trans men and women included in this study would present a significantly greater dissatisfaction toward body parts than the cisgender groups and this dissatisfaction would be linked to specific brain networks.
2. MATERIALS AND METHODS
2.1. Participants and instruments
Twenty‐nine trans men participants who had not begun GAHT (age: mean(SD) = 24.7(6.2), range = 17–39; education: mean(SD) = 11.7(1.7), range = 9–15), 17 trans women with no GAHT (age: mean(SD) = 21.4(3.9), range = 18–34, education: mean(SD) = 13.1(1.8), range = 10–16), 19 cis men (age: mean(SD) = 22.2(4.4), range = 18–32, education mean(SD) = 14.4(3.0), range = 10–20), and 22 cis women (age: mean(SD) = 19.6(2.4), range = 18–27, education: mean(SD) = 13.3(1.6), range = 12–17) were enrolled. All participants explicitly stated they had a binary identity, so trans men and cis men identified themselves as a man, and trans women and cis women as a woman. Detailed demographic information, such as age and education, and information of the assessment and recruitment protocol can be found in the data article (Uribe et al., 2020a) and our previous work (Uribe et al., 2020b). All trans men and trans women met diagnostic criteria for gender identity disorder according to the DSM‐IV‐TR and ICD‐10 when recruited. Nonetheless, the diagnosis was relabeled to gender incongruence as per the change to ICD‐11 and recommended by EPATH and WPATH v7 (Bouman et al., 2017).
Participants answered the Body Image scale (Lindgren & Pauly, 1975), this test has been previously used to assess the body image satisfaction of transgender cohorts (e.g., [Shirdel‐Havar et al., 2019]). This auto‐administered questionnaire has a total averaged score that includes 30 body parts: the nose, shoulders, chin, calves, hands, Adam's apple, eyebrows, face, feet, height, hips, figure, waist, arms, buttocks, biceps, appearance, stature, muscles, weight, thighs, breasts, chest, body hair, facial hair, hair, voice, penis/vagina, scrotum/clitoris, and testicles/uterus. Participants scored each body part on a Likert scale ranging from 1 to 5, 1—very satisfied, 2—satisfied, 3—neutral, 4—dissatisfied, and 5—very dissatisfied. The total score is obtained by averaging the sum of the 30 responses to each body part, thus higher scores indicate higher dissatisfaction. Subscores can also be computed to obtain the satisfaction toward “neutral,” “primary,” and “secondary” sex body characteristics. However, to reduce the number of multiple comparison correction, we only used the global total score.
Written informed consent was obtained from all participants after a full explanation of the procedures. The study was approved by the ethics committee of the Hospital Clinic of Barcelona.
2.2. MRI acquisition and preprocessing
Raw and processed imaging data are available online (Uribe et al., 2020a). MRI data were acquired with a 3T scanner (MAGNETOM Trio, Siemens, Germany). Briefly, T1‐weighted images were acquired in the sagittal plane, TR = 2300 ms, TE = 2.98 ms, TI = 900 ms, 240 slices, FOV = 256 mm, matrix size = 256 × 256; 1 mm isotropic voxel. A total of 240 resting‐state T2*‐weighted echo planar images (scan duration of 10 min) were acquired with a TR = 2500 ms s, TE = 28 ms, flip angle = 80°, slice thickness = 3 mm, FOV = 240 mm, matrix size = 256 × 256, 40 slices, and bandwidth = 2404 Hz/pixel. Participants were instructed not to fall asleep and not to focus on any specific thought, keeping their eyes closed. Basic preprocessing was conducted with AFNI using an in‐house shell script. ICA‐AROMA was applied for the automatic removal of motion‐related artifacts. No motion parameter differed between groups (Uribe et al., 2020a).
We extracted the time series of the 1000 nodes parcellation in Schaefer et al. (2018) from Yeo's resting‐state networks (Thomas Yeo et al., 2011) with the fslmeants tool from FSL v5.0.10 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). The 17‐network Schaefer parcellation was used to define networks by grouping, for example, the A, B, and C components of the default mode or the executive control networks as one. We chose the 17th partition as there was a unique temporal parietal network, otherwise subdivided by several other networks in the 7‐network partition, namely the default mode, ventral attention, and somatomotor networks. For a comprehensive description of the method, we refer readers to Schaefer et al. (2018) and Thomas Yeo et al. (2011).
2.3. Intrinsic ignition framework
We applied the intrinsic ignition framework to characterize gender‐related differences in the spatiotemporal transmission of information across the whole brain over time (Deco & Kringelbach, 2017; Deco, Tagliazucchi, et al., 2017). This framework has been used to successfully discriminate between different brain states, such as sleep (Deco, Tagliazucchi, et al., 2017) and meditation (Escrichs et al., 2019), as well as to explore differences in the healthy elderly brain (Escrichs et al., 2020), depression (Alonso Martínez et al., 2020; Mayneris‐Perxachs et al., 2021), and even in preterm children (Padilla et al., 2020). It allows us to compute the effect of spontaneous local activation on the whole‐brain integration using the phase space of the signals. First, we filtered the blood oxygen level–dependent (BOLD) time series parcellated in 1000 brain regions within the narrowband 0.04–0.07 Hz to avoid artifacts (Glerean et al., 2012) (Figure 1a‐A). We then calculated the instantaneous phase of the BOLD signals by computing the Hilbert transform of the filtered time series (Figure 1a‐B).
FIGURE 1.
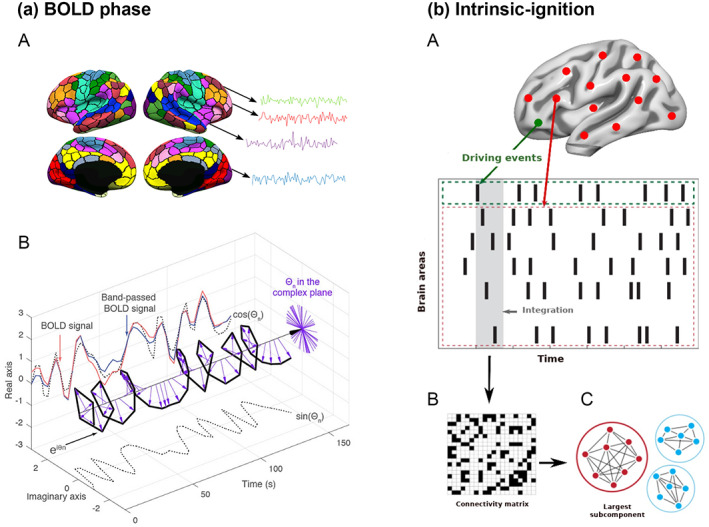
Intrinsic ignition framework. (a) We extracted the BOLD time series for each of the 1000 brain areas and computed the phase space of the BOLD signal. (a‐A) We obtained the time series for each parcellation using the resting‐state Schaefer atlas (Schaefer et al., 2018). (a‐B) Then, we measured the phase space of the BOLD signal through the Hilbert transform for each region. The BOLD signal (red) was band‐pass filtered between 0.04 and 0.07 Hz (blue) and using the Hilbert transform. The phase dynamics can be represented in the complex plane as eiφ (black bold line), the real part as cos φ, and the imaginary part as sin φ (black dotted lines). The purple arrows represent the Hilbert phases at each TR (2.5 s). (b) Intrinsic ignition measurements. (b‐A) Events were captured by applying a threshold method (Tagliazucchi et al., 2012 see green node). For each event elicited, the activity in the rest of the network (see red stippled region) was measured in the time window of 4‐TR (4 × 2.5 s) (gray area). (b‐B) A binarized phase lock matrix was obtained from the time window. (b‐C) From this phase lock matrix, we obtained the integration measurement by computing the largest subcomponent, that is, by applying the global integration measurement (Deco et al., 2015; Deco, Tagliazucchi, et al., 2017). Repeating the procedure for each driving event, we obtained the mean‐ignition and node‐metastability of the intrinsic‐driven integration for each brain region across the whole‐brain network. Figure adapted from Deco et al. (2019); Deco and Kringelbach (2017), and Escrichs et al. (2020).
Figure 1b gives a graphic representation of the algorithm used to compute the ignition value for each brain area evoked for an event within a fixed time window of 4‐TR (TR = 2.5 s). In brief, a binary event was defined by transforming the time series into z‐scores, z i (t), and by fixing a threshold θ (Deco, Tagliazucchi, et al., 2017; Tagliazucchi et al., 2012). Then, the phase lock matrix P jk (t) was calculated, representing the state of pair‐wise phase synchronization at each time point t between regions j and k, as given by:
| (1) |
where φ j (t) and φ k (t) represent the phase obtained at time t for the regions j and k. Given the fixed threshold θ, the symmetric phase lock matrix P jk (t) was binarized (Figure 1b‐B) so that σ(t) = 1 if zi(t) > θ and otherwise as 0. We computed the integration value as the length of the connected component (i.e., the largest subcomponent) considered as an adjacent graph (Figure 1b‐C). Finally, we obtained the average integration value (i.e., mean‐ignition) by averaging across all events and we obtained the variability (i.e., node‐metastability) by calculating the standard deviation, reflecting the spatial diversity across the whole‐brain network and the level of variability over time for each brain region, respectively. The framework was applied to the whole‐brain network parcellated into 1000 brain areas and to each resting‐state network separately: the dorsal and ventral attentional, executive control, default mode, somatomotor, limbic, visual, and temporal parietal networks (Schaefer et al., 2018; Thomas Yeo et al., 2011).
2.4. Statistical analyses
A general linear model and Monte Carlo permutation test (1000 iterations) to control for the family‐wise error (FWE) rate were applied to perform group comparisons. Cohen's d effect sizes were also calculated. Age and education were entered as covariates when comparing trans men and cis women, and the education variable alone when comparing trans men and cis men. Spearman correlations and 95% confidence interval were computed between the quantitative functional dynamic metrics and the total body image scale score for each group. We did not include the visual network in the correlations as there were no group differences within this network in the mean‐ignition or node‐metastability results.
3. RESULTS
3.1. Intrinsic ignition across nodes
There were significant differences (FWE corrected p < .004) between all gender groups when the intrinsic ignition framework was computed across the whole‐brain functional network in both mean‐ignition and node‐metastability measurements (Figure 2a and Table 1). There was a gradual progression in the mean‐ignition, that is, cis men > cis women > trans men > trans women. On the other hand, the average node‐metastability was the highest in the cis woman group, and the trans man group showed the lowest average.
FIGURE 2.
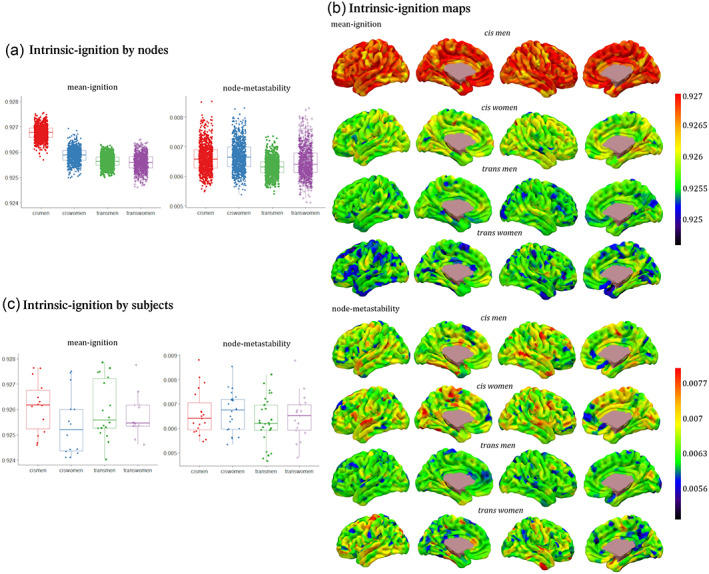
Whole‐brain mean‐ignition and node‐metastability measurements for each of the 1000 brain regions by each group. (a) The boxes in the plots indicate the second and third quartiles (IQR), and middle lines are medians. Each dot represents a brain region. Means and standard deviations can be found in Table 1. There were significant differences between all groups' contrasts with Monte Carlo 1000 permutations and the Bonferroni correction p < .0004. (b) Brain renderings show the distribution of mean‐ignition and node‐metastability values per each brain region by group. Red warm regions had the highest mean‐ignition and node‐metastability values, and dark blue ones the lowest. (c) Mean‐ignition and node‐metastability measurements with averaged regions for each participant by groups. Each dot represents a participant. No group comparison reached the significance threshold after the Bonferroni correction. Metadata can be downloaded at https://doi.org/10.6084/m9.figshare.14622564.
TABLE 1.
Statistics from the whole‐brain intrinsic ignition framework by nodes
| Contrast | Mean‐ignition—Mean (SD) | t statistic | Uncorr‐p | FWE‐p | Node‐metastability—Mean (SD) | t statistic | Uncorr‐p | FWE‐p |
|---|---|---|---|---|---|---|---|---|
| cis men vs. cis women |
cis men: 0.9268 (0.0003) cis women: 0.9259 (0.0003) trans men: 0.9259 (0.0002) trans women: 0.9256 (0.0003) |
72.459 | .001 | <.004 |
cis men: 0.0066 (0.0005) cis women: 0.0067 (0.0005) trans men: 0.0063 (0.0003) trans women: 0.0065 (0.0005) |
−3.194 | .002 | <.004 |
| cis men vs. trans men | 91.394 | .001 | <.004 | 15.656 | .001 | <.004 | ||
| cis men vs. trans women | 95.284 | .001 | <.004 | 6.912 | .001 | <.004 | ||
| cis women vs. trans men | 18.936 | .001 | <.004 | 18.850 | .001 | <.004 | ||
| cis women vs. trans women | 22.826 | .001 | <.004 | 10.106 | .001 | <.004 | ||
| trans men vs. trans women | 3.890 | .001 | <.004 | −8.744 | .001 | <.004 |
Note: Data are means and standard deviations. There were significant differences between all contrast groups with Monte Carlo 1000 permutations and family‐wise error (FWE) correction.
Figure 2b shows brain renderings, where the hot colors represent those regions with the highest mean‐ignition and node‐metastability per group, while cold and dark tonalities represent the lowest values. The regional distribution of the highest mean‐ignition and node‐metastability measurements included regions across the whole‐brain from all networks. There was no hemisphere predominance among the 100 areas with the highest mean‐ignition in any group (left/right: cis men 54/46—out of the 100 regions—, cis women 43/57, trans men 51/49, and trans women 38/62, χ 2 = 6.472; p = 0.091). Nodes in the right hemisphere were more frequent for the node‐metastability values except in the trans woman group (left/right: cis men 41/59, cis women 47/53, trans men 48/52, and trans women 61/39, χ 2 = 8.512; p = 0.037).
3.2. Intrinsic ignition across participants
Whole brain. There were no group differences that survived FWE correction (Figure 2c). When computing the intrinsic ignition framework by networks, group differences were present in all networks except for the visual network (Table 2, Figures 3 and 4). Attentional network. In the dorsal attention network, the mean‐ignition (Figure 3a‐C, b‐C) and node‐metastability (Figure 4a‐B,b‐B) measurements were higher in the cis man group with respect to cis women, trans men, and trans women. Regarding the ventral subdivision of the attentional network, only the node‐metastability mean of cis men was higher than those of the other three gender groups, namely cis women, trans men, and trans women (Figure 4a‐C,b‐C). Executive control network. Cis men's mean‐ignition values were significantly higher than cis women (Figure 3a‐A, b‐A). Cis men also had higher node‐metastability than cis women, trans men, and trans women (Figure 4a‐A, b‐A). Default mode network. Cis women had higher mean‐ignition than cis men and trans men (Figure 3a‐B,b‐B). Limbic network. Both mean‐ignition and node‐metastability measurements were higher within the cis man group with respect to trans men (Figures 3a‐D, b‐D and 4a‐D,b‐D). Somatomotor. Cis men showed higher node‐metastability than cis women (Figure 4a‐E,b‐E). Temporal parietal. Cis men had greater mean‐ignition than cis women, trans men, and trans women (Figure 3a‐E,b‐E).
TABLE 2.
Intrinsic ignition by networks of averaged nodes across groups
| Measure | Contrast | t statistic | Uncorr‐p | Corrected‐p | Cohen's d |
|---|---|---|---|---|---|
| Dorsal attention network | |||||
| Mean‐ignition | |||||
| cis men vs. cis women | 3.463 | .002 | .002 | 1.1 | |
| cis men vs. trans men (education as covariate) |
2.784 no‐cov: 3.368 |
.005 no‐cov: .001 |
.016 no‐cov: .007 |
.94 | |
| cis men vs. trans women | 2.666 | .008 | .016 | .79 | |
| Node‐metastability | |||||
| cis men vs. cis women | 2.515 | .006 | .015 | .8 | |
| cis men vs. trans men (education as covariate) |
2.476 no‐cov: 2.681 |
.006 no‐cov: .008 |
.025 no‐cov: .023 |
.71 | |
| cis men vs. trans women | 2.407 | .007 | .030 | .86 | |
| Ventral attention network | |||||
| Node‐metastability | |||||
| cis men vs. cis women | 2.269 | .010 | .024 | .74 | |
| cis men vs. trans men (education as covariate) |
2.455 no‐cov: 2.252 |
.010 no‐cov: .022 |
.026 no‐cov: .048 |
.66 | |
| cis men vs. trans women | 2.190 | .016 | .044 | .82 | |
| Control executive network | |||||
| Mean‐ignition | |||||
| cis men vs. cis women | 2.379 | .008 | .021 | .71 | |
| Node‐metastability | |||||
| cis men vs. cis women | 2.100 | .015 | .037 | .61 | |
| cis men vs. trans men (education as covariate) |
2.875 no‐cov: 2.895 |
.004 no‐cov: .008 |
.012 no‐cov: .018 |
.8 | |
| cis men vs. trans women | 2.092 | .019 | .055 | .68 | |
| Default mode network | |||||
| Mean‐ignition | |||||
| cis men vs. cis women | −2.246 | .019 | .042 | −.67 | |
| cis women vs. trans men (age & education as covs) |
2.050 no‐covs: 2.863 |
.020 no‐covs: .003 |
.055 no‐covs: .009 |
.91 | |
| somatomotor network | |||||
| Node‐metastability | |||||
| cis men vs. cis women | 2.442 | .007 | .019 | .72 | |
| limbic network | |||||
| Mean‐ignition | |||||
| cis men vs. trans men (education as covariate) |
2.869 no‐cov: 2.551 |
.003 no‐cov: .014 |
.012 no‐cov: .032 |
.75 | |
| Node‐metastability | |||||
| cis men vs. trans men (education as covariate) |
2.257 no‐cov: 1.75 |
.016 no‐cov: .05 |
.039 no‐cov: .105 |
.54 | |
| temporal parietal network | |||||
| Mean‐ignition | |||||
| cis men vs. cis women | 2.625 | .007 | .013 | .74 | |
| cis men vs. trans men (education as covariate) |
3.479 no‐cov: 2.896 |
.001 no‐cov: .007 |
.002 no‐cov: .018 |
.94 | |
| cis men vs. trans women | 2.954 | .002 | .005 | .91 | |
Note: General linear model with 1000 permutations and family‐wise error (FWE) correction were applied. Cohen's d effect sizes were computed. The cis men versus trans men contrast was tested with and without (no‐cov) education as covariate, and the comparison between cis women and trans men with and without (no‐covs) age and years of education as covariates.
FIGURE 3.
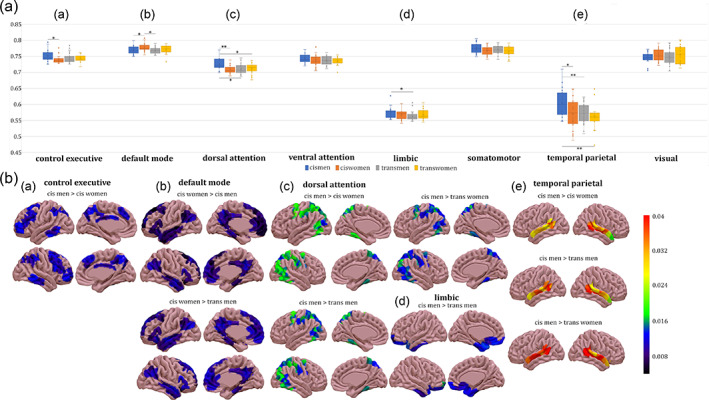
Mean‐ignition measurements by network. (a) Group ignition values by network. The boxes in the plots indicate the second and third quartiles (IQR), middle lines are mean‐ignition medians, and X are mean‐ignition means. Legend: *p ≤ .05; **p < .01. (b) Brain renderings represent the differences in mean‐ignition between groups and were plotted with the SurfIce software. There were group differences in the (A) executive control, (B) default mode, (C) dorsal attentional, (D) limbic, and (E) temporal parietal networks. Metadata can be downloaded at https://doi.org/10.6084/m9.figshare.14622564.
FIGURE 4.
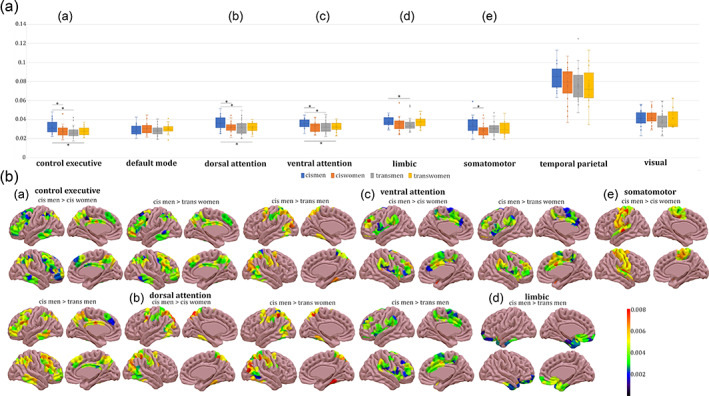
Node‐metastability measurements by network. (a) Group node‐metastability values by network. The boxes in the plots indicate the second and third quartiles (IQR), middle lines are node‐metastability medians, and X are node‐metastability means. Legend: *p ≤ .05; **p < .01. (b) Rendered brains depict the node‐metastability difference between groups and were plotted with the SurfIce software. There were group differences in the (A) executive control, (B) dorsal attention, (C) ventral attention, (D) limbic, and (E) somatomotor networks. Metadata can be downloaded at https://doi.org/10.6084/m9.figshare.14622564.
3.3. Body image satisfaction correlations
The degree of dissatisfaction toward body parts was significantly higher in the two transgender groups in comparison to either cis group. On the other hand, satisfaction in the two cisgender groups was not statistically different (Figure 5a). The satisfaction/dissatisfaction with the body image within each gender group was distinctively associated with intrinsic ignition measurements in specific networks. Cis men were the group with the highest overall mean satisfaction with their body, and this correlated negatively with mean‐ignition in the executive control network and positively with node‐metastability in the limbic network (Figure 5b). On the other hand, in cis women, body image satisfaction correlated with mean‐ignition in the default mode network, which was reported to be higher in the cis woman group comparisons, and the temporal parietal's node‐metastability (Figure 5c). Ventral attentional mean‐ignition was positively associated with the global score of the body image scale in trans men (Figure 5d).
FIGURE 5.
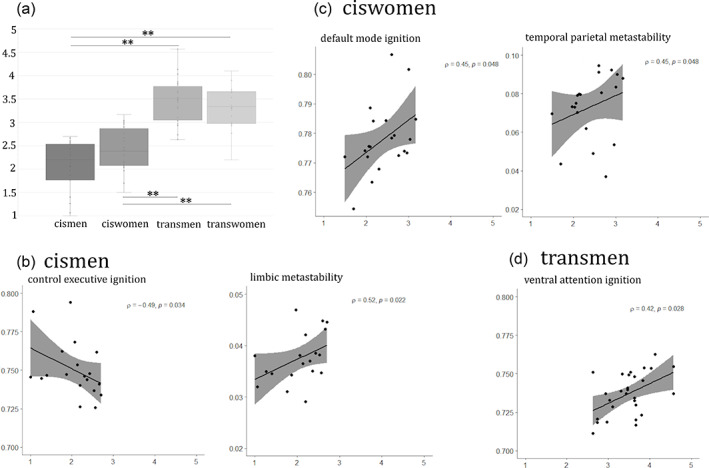
Correlations between network‐based mean‐ignition and node‐metastability measurements and body image satisfaction scores. (a) Group comparisons of the body image scale scores. A general linear model with 1000 permutation testing and Bonferroni correction was applied. The boxes in the plots indicate the second and third quartiles (IQR), middle lines are total body image scores medians, and X are the means. Legend: * p ≤ .05; ** p < .01. Correlations within (b) the cis men group, (c) the cis women, and (d) the trans men. There were no significant correlations within the trans women group. Correlations are Spearman's ρ and shadowed areas are 95% confidence interval.
Data on mean‐ignition and node‐metastability matrices per gender group and group comparison statistics are publicly available (https://doi.org/10.6084/m9.figshare.14622564).
4. DISCUSSION
For the first time, we characterize the spatiotemporal whole‐brain dynamics of cisgender and transgender binary groups. Our findings corroborate the existence of four brain phenotypes (Guillamon et al., 2016; Uribe et al., 2020b) beyond the classic, lately questioned, conception that the human brain can be split into two configurations, the male and the female (Legato, 2018). To characterize the propagation of information and measure the degree of integration of spontaneously occurring events while at rest, we applied the intrinsic ignition framework (Deco & Kringelbach, 2017; Deco, Tagliazucchi, et al., 2017). This framework was very sensitive in detecting functional dynamics differences between young adults grouped by gender. Some of these group differences had been elusive when using stationary functional connectivity measurements (Uribe et al., 2020b), or sliding windows approach to study brain connectivity states (Uribe et al., 2021). In addition, spatial and temporal brain dynamics measurements were specifically related with the satisfaction toward body parts for cis men, cis women, and trans men.
The main novelty here is that we provide the first description of the spatiotemporal dynamics underlying the gender dimension in the brain, and more importantly, characterize the regional contribution to the whole‐brain dynamics. Mean‐ignition is an informative measurement of the spatial diversity and broadness of communication across the brain. On the other hand, node‐metastability captures the variability over time across the whole brain. Both the spatial and temporal variability that defined each gender group were widespread across the whole brain, with nodes from all functional networks. Likewise, when using a support vector machine algorithm inputting stationary group independent component maps and clinical data as features, four gender groups were obtained based on the different patterns of brain connectivity (Clemens et al., 2020). In addition, our results stress the importance of using fine‐grained dynamic measurements to study spatiotemporal oscillations over grand averaged functional connectivity measurements; these latter enabling a more narrowed investigation of differences that would be accountable for gender, and the incongruence felt in the transgender community.
Group differences in the two subdivisions of the attentional networks and in executive control were in line with previous findings of functional connectivity differences, both stationary (Uribe et al., 2020a) and dynamic (Uribe et al., 2021). The particular group differences in the dorsal and the ventral subdivisions of the attentional network underline the need to study them separately. More relevantly, the spatial broadness of communication of nodes in the default mode network was higher in cis women with respect to cis men and trans men. Higher functional connectivity in default mode regions has been reported in cis women in contrast to cis men (Biswal et al., 2010; de Lacy et al., 2019; Ritchie et al., 2018). Also, in the transgender literature, weaker connectivity strength has been reported in these network regions in the trans men group in contrast to cis men (Feusner et al., 2017; Uribe et al., 2020a) and cis women (Feusner et al., 2017), but this finding is not generalized as other studies had negative reports (Clemens et al., 2017; Nota et al., 2017).
On the other hand, the reported pattern of activation in cis men relies on sensory–motor regions (Ritchie et al., 2018). The somatomotor network in the Schaefer parcellation included areas of motor action and sensory inputs from the external world, making it the network with the most direct interaction with our environment. Despite the previous relevance given to this functional network in understanding the own body perception and subsequently explaining the incongruence in transgender people (Burke et al., 2019; Manzouri et al., 2017), the intrinsic ignition framework only differentiated between cisgender groups in terms of temporal variability. Indeed, our previous work on functional connectivity dynamics identified a sensorimotor state, although no differences between trans‐ and cisgender groups were noted (Uribe et al., 2021). The spatial and temporal dynamism of the limbic network was greater in the cis man group than in trans men. On the other hand, increased limbic connectivity in transgender individuals has been reported when viewing “ambiguous, androgynous images of themselves morphed toward their gender identity” (Majid et al., 2020). Such findings should be further explored. Different functional MRI measurements do not permit further discussion, and greater integration, broadness of communication, and temporal variability do not necessarily translate to increased averaged connectivity.
The superior parietal cortex has been previously linked to gender differences when comparing cis men with cis women and transgender groups, structurally (Zubiaurre‐Elorza et al., 2013) and functionally (Uribe et al., 2020b). The choice of the Schaefer parcellation (Schaefer et al., 2018) allowed a high representation of the temporal parietal network in terms of brain dynamics in agreement with temporoparietal junction findings in trans men with respect to cisgender groups (Manzouri et al., 2017). The spatial diversity and broadness of communication of temporal parietal regions were greater in cis men than in the other three gender groups, namely cis women, trans men, and trans women.
The fact that cis men present higher brain dynamism than other gender groups, especially cis women and trans men, would be in line with previous brain states occupancy where cis men occupied more combinations of connectivity patterns over time than cis women (Yaesoubi et al., 2015). Nonetheless, these results have not been consistently replicated, as has occurred with other brain flexibility measurements through brain states using sliding windows that reported differential regional brain dynamism for both cis men and cis women (Mao et al., 2017). In addition, the increased spatial and temporal variability of brain oscillations in cis men was not homogeneous across all networks, for instance, in the default mode network.
Trans women presented a lateralized predominance in the regions with the highest node‐metastability in the left hemisphere. The discussion of these findings is hampered by the scarcity of the literature investigating gender differences in brain dynamism. To the best of our knowledge, previous reports of the gender effects in the lateralization of brain connectivity patterns found these were mostly comparable between a large sample of cis men and women, with two marginal findings that did not survive false discovery rate correction and were considered a trend‐level effect (Agcaoglu et al., 2015; Eliot et al., 2021), although there was a marginal leftwards lateralization in cis women only in the inferior frontal cortex (Tomasi & Volkow, 2012). Given these and the small sample of individuals investigated, especially in the trans woman group, our results should be taken carefully.
Finally, the (dis)satisfaction toward one's own body parts is not simply associated with a specific network, but differently according to the group, which suggests a different way of understanding and accepting the body depending on gender. The trans men group image (dissatisfaction) relied on the ventral attentional, that is, salience network. If one key element in the construction of gender is the perception of our own body (Burke et al., 2019; Peelen & Downing, 2007), the salience network has been highly related to trans‐ and cisgender differences that may explain the gender incongruence (Uribe et al., 2020b; Uribe et al., 2021). However, such a landmark is not helpful for the functional correlates of cisgender groups. These differences in the network correlates could be driven by the fact that the trans men group scores were within the range of the unconformity toward the body parts—4–5 points in the Likert scale of Lindgren and Pauly (1975)—, while cisgender groups would range mainly within the neutral satisfaction scores (1– points). Another potential explanation is that trans‐ and cisgender individuals' ratings may not be comparable between groups as the reported dissatisfaction may underlie different reasons for transgender people in contrast to what it would mean for cisgender groups. The cis man group's satisfaction and/or neutrality was positively associated with the limbic network and negatively with executive control. On the other hand, the network with higher spatial dynamical complexity, that is, the default mode, was also associated with body parts satisfaction in cis women. These networks have been largely associated with gender groups' differences described in previous works (Clemens et al., 2017; Manzouri et al., 2017; Uribe et al., 2020b; Uribe et al., 2021). However, our work provide evidence that gender group differences depict interplay by a whole‐brain network in terms of spatial and temporal variability that exceeds the rather specific correlates of the degree of satisfaction toward the own body.
Some shortcomings should be addressed in future works. First, the need to increase the sample size that would add more power to the findings. Currently ongoing collaborative initiatives like the ENIGMA initiative on transgender health are trying to overcome this persistent limitation in the neuroimaging field (Mueller et al., 2021). However, this initiative lacks standard acquisition protocols to reduce the variability among sites that may hamper group discrimination. Second, the menstrual cycle of cis women and trans men was not accounted as a variable of interest, while there is growing evidence of functional dynamic differences between the phases of the menstrual cycle (De Filippi et al., 2021). Likewise, the sexual orientation of all participants was not systematically assessed as a variable of interest (Frigerio et al., 2021; Guillamon et al., 2016; Skorska et al., 2021). Including minority gender groups when investigating the gender phenomenon in the brain is imperative to understand the complexity of the gender experience. Nonetheless, future studies should include other gender groups, such as nonbinary or other genderqueer identities. Our exploratory work could potentially impact awareness, the development of healthcare guidelines, societal and political evidence‐based changes accounting for this heterogeneity, and improve the quality of life while raising visibility that can help fight stigma (Janssen & Voss, 2021). Finally, it is important to note that the sample characteristics and analytical approach employed here prevent us from discriminating actual gender identity differences from other phenomena such as experiences of stigma that transgender people may have undergone. Future experimental designs should address such issues.
5. CONCLUSION
First, we propose a gendered brain perspective of spatiotemporal whole‐brain communications across networks that characterize four binary gender groups, namely cis and trans men and women. Second, we propose the study of the brain as a complex whole system beyond the localizationist paradigm when investigating complex phenomena such as the gender dimension. Third, taking advantage of novel techniques to study brain dynamics and understand network cooperation and the brain's dynamical complexity, we confirm and expand previous findings on the interplay of the attentional, default mode, executive control, limbic, somatomotor, and temporal parietal networks associated with differences in information propagation between cis‐ and transgender identities. Finally, novel cutting‐edge frameworks in studying brain dynamics are needed to untangle the complex and very intimate experience of a gendered self.
AUTHOR CONTRIBUTIONS
Carme Uribe: Designed research, Analyzed data, Investigation, and Writing–Original draft preparation. Anira Escrichs: Designed research, Analyzed data, Software, and Writing–Original draft preparation. Eleonora de Filippi: Visualization and Writing–Original draft preparation. Yonatan Sanz‐Perl: Software and Writing–Reviewing and Editing. Carme Junque: Supervision and Writing–Reviewing and Editing. Esther Gomez‐Gil: Investigation and Writing–Reviewing and Editing. Morten Kringelbach: Methodology, Software, and Writing–Reviewing and Editing. Antonio Guillamon: Funding acquisition and Writing–Reviewing and Editing. Gustavo Deco: Methodology, Software, and Writing–Reviewing and Editing.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
CU was supported by the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska‐Curie grant agreement 888692. AG was supported by the Spanish Ministry of Science and Innovation grant number PGC2018‐094919‐B‐C21. AE was supported by the European Union Horizon 2020 FET Flagship Human Brain Project (grant agreement number 945539, HBP SGA3). GD was supported by the Spanish Research Project (ref. PID2019‐105772GB‐I00 AEI FEDER EU), funded by the Spanish Ministry of Science, Innovation and Universities (MCIU), State Research Agency (AEI), and European Regional Development Funds (FEDER); and HBP SGA3 Human Brain Project Specific Grant Agreement 3 (grant agreement no. 945539), funded by the EU H2020 FET Flagship program and SGR Research Support Group support (ref. 2017 SGR 1545), funded by the Catalan Agency for Management of University and Research Grants (AGAUR). EDF was supported by the Doctorate Scholarship FI‐2020 from the Catalan Agency for Management of University and Research Grants (AGAUR). The authors are thankful to the participants for their time, without them this work would not be a reality. We are also indebted to the Magnetic Resonance Imaging core facility of the IDIBAPS for technical support, especially to Cesar Garrido and Gema Lasso; and we acknowledge the CERCA Programme/Generalitat de Catalunya. Finally, we also acknowledge Carol Fox Warren for her editorial help.
Uribe, C. , Escrichs, A. , de Filippi, E. , Sanz‐Perl, Y. , Junque, C. , Gomez‐Gil, E. , Kringelbach, M. L. , Guillamon, A. , & Deco, G. (2022). Whole‐brain dynamics differentiate among cisgender and transgender individuals. Human Brain Mapping, 43(13), 4103–4115. 10.1002/hbm.25905
Carme Uribe and Anira Escrichs contributed equally to this study.
Funding information H2020 Excellent Science, Grant/Award Number: 945539; H2020 Marie Skłodowska‐Curie Actions, Grant/Award Number: 888692; Ministerio de Ciencia e Innovación, Grant/Award Numbers: PGC2018‐094919‐B‐C21, PID2019‐105772GB
Contributor Information
Carme Uribe, Email: carme.uribe@ub.edu.
Antonio Guillamon, Email: aguillamon@psi.uned.es.
DATA AVAILABILITY STATEMENT
Imaging raw and processed data used for this study and demographical variables are publicly available in Data Mendeley repositories [Uribe et al., 2020a]: https://doi.org/10.17632/hjmfrv6vmg.2, https://doi.org/10.17632/rw2yhtpj96.3, https://doi.org/10.17632/bgyzz94mz9.3. The data that support the findings of this study are openly available in https://doi.org/10.6084/m9.figshare.14622564.
REFERENCES
- Agcaoglu, O. , Miller, R. , Mayer, A. R. , Hugdahl, K. , & Calhoun, V. D. (2015). Lateralization of resting state networks and relationship to age and gender. NeuroImage, 104, 310–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, E. A. , Damaraju, E. , Plis, S. M. , Erhardt, E. B. , Eichele, T. , & Calhoun, V. D. (2014). Tracking whole‐brain connectivity dynamics in the resting state. Cerebral Cortex, 24, 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso Martínez, S. , Marsman, J. B. C. , Kringelbach, M. L. , Deco, G. , & ter Horst, G. J. (2020). Reduced spatiotemporal brain dynamics are associated with increased depressive symptoms after a relationship breakup. NeuroImage Clinical, 27, 102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio, H. C. , Abos, A. , Segura, B. , Campabadal, A. , Garcia‐Diaz, A. , Uribe, C. , Compta, Y. , Marti, M. J. , Valldeoriola, F. , & Junque, C. (2018). Statistical inference in brain graphs using threshold‐free network‐based statistics. Human Brain Mapping, 39, 2289–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. B. , Mennes, M. , Zuo, X.‐N. , Gohel, S. , Kelly, C. , Smith, S. M. , Beckmann, C. F. , Adelstein, J. S. , Buckner, R. L. , Colcombe, S. , Dogonowski, A.‐M. , Ernst, M. , Fair, D. , Hampson, M. , Hoptman, M. J. , Hyde, J. S. , Kiviniemi, V. J. , Kotter, R. , Li, S.‐J. , … Milham, M. P. (2010). Toward discovery science of human brain function. Proceedings of the National Academy of Sciences, 107, 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman, W. P. , Schwend, A. S. , Motmans, J. , Smiley, A. , Safer, J. D. , Deutsch, M. B. , Adams, N. J. , & Winter, S. (2017). Language and trans health. International Journal of Transgenderism, 18, 1–6. [Google Scholar]
- Burke, S. M. , Majid, D. S. A. , Manzouri, A. H. , Moody, T. , Feusner, J. D. , & Savic, I. (2019). Sex differences in own and other body perception. Human Brain Mapping, 40, 474–488. 10.1002/hbm.24388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun, V. D. , Miller, R. , Pearlson, G. , & Adali, T. (2014). The Chronnectome: time‐varying connectivity networks as the next frontier in fMRI data discovery. Neuron, 84, 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver, P. R. , Yunger, J. L. , Perry, D. G. (2003). Gender identity and adjustment in middle childhood. Sex Roles, 49, 95–109. 10.1023/A:1024423012063 [DOI] [Google Scholar]
- Chen, A. C. , Oathes, D. J. , Chang, C. , Bradley, T. , Zhou, Z. W. , Williamsa, L. M. , Glover, G. H. , Deisseroth, K. , & Etkin, A. (2013). Causal interactions between fronto‐parietal central executive and default‐mode networks in humans. Proceedings of the National Academy of Sciences of the United States of America, 110, 19944–19949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, B. , Derntl, B. , Smith, E. , Junger, J. , Neulen, J. , Mingoia, G. , Schneider, F. , Abel, T. , Bzdok, D. , & Habel, U. (2020). Predictive pattern classification can distinguish gender identity subtypes from behavior and brain imaging. Cerebral Cortex, 30, 2755–2765. [DOI] [PubMed] [Google Scholar]
- Clemens, B. , Junger, J. , Pauly, K. , Neulen, J. , Neuschaefer‐Rube, C. , Frölich, D. , Mingoia, G. , Derntl, B. , & Habel, U. (2017). Male‐to‐female gender dysphoria: gender‐specific differences in resting‐state networks. Brain and Behavior: A Cognitive Neuroscience Perspective, 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, S. P. , Naudet, F. , Laude, J. , Radua, J. , Fusar‐Poli, P. , Chu, I. , Stefanick, M. L. , & Ioannidis, J. P. A. (2018). Potential reporting bias in neuroimaging studies of sex differences. Scientific Reports, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippi, E. , Uribe, C. , Avila‐varela, D. S. , Martínez‐molina, N. , Gashaj, V. , Pritschet, L. , Santander, T. , Jacobs, E. G. , Kringelbach, M. L. , Sanz Perl, Y. , Deco, G. , Escrichs, A. , De, F. E. , Uribe, C. , Avila‐varela, D. S. , & Martínez‐molina, N. (2021). The menstrual cycle modulates whole‐brain turbulent dynamics. Frontiers in Neuroscience, 15, 1–11. 10.3389/fnins.2021.753820/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lacy, N. , McCauley, E. , Kutz, J. N. , & Calhoun, V. D. (2019). Sex‐related differences in intrinsic brain dynamism and their neurocognitive correlates. NeuroImage, 202, 116116. 10.1016/j.neuroimage.2019.116116 [DOI] [PubMed] [Google Scholar]
- Deco, G. , Cruzat, J. , Cabral, J. , Tagliazucchi, E. , Laufs, H. , Logothetis, N. K. , & Kringelbach, M. L. (2019). Awakening: predicting external stimulation to force transitions between different brain states. Proceedings of the National Academy of Sciences of the United States of America, 116, 18088–18097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco, G. , & Kringelbach, M. L. (2017). Hierarchy of information processing in the brain: a novel ‘intrinsic ignition’ framework. Neuron, 94, 961–968. 10.1016/j.neuron.2017.03.028 [DOI] [PubMed] [Google Scholar]
- Deco, G. , Kringelbach, M. L. , Jirsa, V. K. , & Ritter, P. (2017). The dynamics of resting fluctuations in the brain: Metastability and its dynamical cortical core. Scientific Reports, 7, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco, G. , Tagliazucchi, E. , Laufs, H. , Sanjuán, A. , & Kringelbach, M. L. (2017). Novel intrinsic ignition method measuring local‐global integration characterizes wakefulness and deep sleep. eNeuro, 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco, G. , Tononi, G. , Boly, M. , & Kringelbach, M. L. (2015). Rethinking segregation and integration: contributions of whole‐brain modelling. Nature Reviews. Neuroscience, 16, 430–439. https://pubmed.ncbi.nlm.nih.gov/26081790/ [DOI] [PubMed] [Google Scholar]
- Egan, S. K. , & Perry, D. G. (2001). Gender identity: a multidimensional analysis with implications for psychosocial adjustment. Developmental Psychology, 37, 451–463 /record/2001‐01084‐001. [DOI] [PubMed] [Google Scholar]
- Eliot, L. , Ahmed, A. , Khan, H. , & Patel, J. (2021). Dump the “dimorphism”: comprehensive synthesis of human brain studies reveals few male‐female differences beyond size. Neuroscience and Biobehavioral Reviews, 125, 667–697. 10.1016/j.neubiorev.2021.02.026 [DOI] [PubMed] [Google Scholar]
- Escrichs, A. , Biarnes, C. , Garre‐Olmo, J. , Fernández‐Real, J. M. , Ramos, R. , Pamplona, R. , Brugada, R. , Serena, J. , Ramió‐Torrentà, L. , Coll‐De‐Tuero, G. , Gallart, L. , Barretina, J. , Vilanova, J. C. , Mayneris‐Perxachs, J. , Essig, M. , Figley, C. R. , Pedraza, S. , Puig, J. , & Deco, G. (2020). Whole‐brain dynamics in aging: Disruptions in functional connectivity and the role of the Rich Club. Cereb Cortex, 31, 2466–2481. [DOI] [PubMed] [Google Scholar]
- Escrichs, A. , Sanjuán, A. , Atasoy, S. , López‐González, A. , Garrido, C. , Càmara, E. , & Deco, G. (2019). Characterizing the dynamical complexity underlying meditation. Frontiers in Systems Neuroscience, 13, 2015–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner, J. D. , Lidström, A. , Moody, T. D. , Dhejne, C. , Bookheimer, S. Y. , & Savic, I. (2017). Intrinsic network connectivity and own body perception in gender dysphoria. Brain Imaging and Behavior, 11, 964–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, A. , Ballerini, L. , & Valdés Hernández, M. (2021). Structural, functional, and metabolic brain differences as a function of gender identity or sexual orientation: a systematic review of the human neuroimaging literature. Archives of Sexual Behavior, 50, 3329–3352. 10.1007/s10508-021-02005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glerean, E. , Salmi, J. , Lahnakoski, J. M. , Jääskeläinen, I. P. , & Sams, M. (2012). Functional magnetic resonance imaging phase synchronization as a measure of dynamic functional connectivity. Brain Connectivity, 2, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamon, A. , Junque, C. , & Gómez‐Gil, E. (2016). A review of the status of brain structure research in transsexualism. Archives of Sexual Behavior, 45, 1615–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, E. C. A. , Battaglia, D. , Spiegler, A. , Deco, G. , & Jirsa, V. K. (2015). Functional connectivity dynamics: Modeling the switching behavior of the resting state. NeuroImage, 105, 525–535. 10.1016/j.neuroimage.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Hyde, J. S. , Bigler, R. S. , Joel, D. , Tate, C. C. , & van Anders, S. M. (2019). The future of sex and gender in psychology: five challenges to the gender binary. The American Psychologist, 74, 171–193. [DOI] [PubMed] [Google Scholar]
- Janssen, A. , & Voss, R. (2021). Policies sanctioning discrimination against transgender patients flout scientific evidence and threaten health and safety. Transgender Health, 6, 61–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legato, M. J. (2018). Untangling the Gordian knot of human sexuality. Gender and the Genome, 2, 62–67. [Google Scholar]
- Lindgren, T. W. , & Pauly, I. B. (1975). A body image scale for evaluating transsexuals. Archives of Sexual Behavior, 4, 639–656. 10.1007/BF01544272 [DOI] [PubMed] [Google Scholar]
- Majid, D. S. A. , Burke, S. M. , Manzouri, A. , Moody, T. D. , Dhejne, C. , Feusner, J. D. , & Savic, I. (2020). Neural systems for own‐body processing align with gender identity rather than birth‐assigned sex. Cerebral Cortex, 30, 2897–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzouri, A. , Kosidou, K. , & Savic, I. (2017). Anatomical and functional findings in female‐to‐male transsexuals: testing a new hypothesis. Cerebral Cortex, 27, 998–1010. 10.1093/cercor/bhv278 [DOI] [PubMed] [Google Scholar]
- Mao, N. , Zheng, H. , Long, Z. , Yao, L. , & Wu, X. (2017). Gender differences in dynamic functional connectivity based on resting‐state fMRI. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS (Vol. 2017, pp. 2940–2943). Institute of Electrical and Electronics Engineers Inc. [DOI] [PubMed] [Google Scholar]
- Mayneris‐Perxachs, J.,Castells‐Nobau, A., Arnoriaga‐Rodríguez, M., Martin, M., de la Vega‐Correa, L.,Zapata, C., Burokas, A., Blasco, G., Coll, C., Escrichs, A., Biarnés, C.,Moreno‐Navarrete, J. M., Puig, J., Garre‐Olmo, J., Ramos, R., Pedraza, S.,Brugada, R., Vilanova, J. C., Serena, J., … Fernández‐Real, J. M. (2022). Microbiota alterations in proline metabolism impact on depression. Cell Metabolism, 34(5), 681–701.e10. 10.1016/J.CMET.2022.04.001. [DOI] [PubMed]
- Menon, V. (2011). Large‐scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences, 15, 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Mueller, S. C. , Guillamon, A. , Zubiaurre‐Elorza, L. , Junque, C. , Gomez‐Gil, E. , Uribe, C. , Khorashad, B. S. , Khazai, B. , Talaei, A. , Habel, U. , Votinov, M. , Derntl, B. , Lanzenberger, R. , Seiger, R. , Kranz, G. S. , Kreukels, B. P. C. , Kettenis, P. T. C. , Burke, S. M. , Lambalk, N. B. , … Luders, E. (2021). The neuroanatomy of transgender identity: mega‐analytic findings from the ENIGMA transgender persons working group. The Journal of Sexual Medicine, 18, 1122–1129. [DOI] [PubMed] [Google Scholar]
- Nota, N. M. , Burke, S. M. , den Heijer, M. , Soleman, R. S. , Lambalk, C. B. , Cohen‐Kettenis, P. T. , Veltman, D. J. , & Kreukels, B. P. (2017). Brain sexual differentiation and effects of cross‐sex hormone therapy in transpeople: a resting‐state functional magnetic resonance study. Neurophysiologie Clinique, 47, 361–370. 10.1016/j.neucli.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Padilla, N. , Saenger, V. M. , Van Hartevelt, T. J. , Fernandes, H. M. , Lennartsson, F. , Andersson, J. L. R. , Kringelbach, M. , Deco, G. , & Åden, U. (2020). Breakdown of whole‐brain dynamics in preterm‐born children. Cerebral Cortex, 30, 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen, M. V. , & Downing, P. E. (2007). The neural basis of visual body perception. Nature Reviews neuroscience, 88(8), 636–648. [DOI] [PubMed] [Google Scholar]
- Polderman, T. J. C. , Kreukels, B. P. C. , Irwig, M. S. , Beach, L. , Chan, Y. M. , Derks, E. M. , Esteva, I. , Ehrenfeld, J. , Den, H. M. , Posthuma, D. , Raynor, L. , Tishelman, A. , & Davis, L. K. (2018). The biological contributions to gender identity and gender diversity: bringing data to the table. Behavior Genetics, 48, 95–108. [DOI] [PubMed] [Google Scholar]
- Preti, M. G. , Bolton, T. A. , & Van De Ville, D. (2017). The dynamic functional connectome: state‐of‐the‐art and perspectives. NeuroImage, 160, 41–54. [DOI] [PubMed] [Google Scholar]
- Ritchie, S. J. , Cox, S. R. , Shen, X. , Lombardo, M. V. , Reus, L. M. , Alloza, C. , Harris, M. A. , Alderson, H. L. , Hunter, S. , Neilson, E. , Liewald, D. C. M. , Auyeung, B. , Whalley, H. C. , Lawrie, S. M. , Gale, C. R. , Bastin, M. E. , Mcintosh, A. M. , & Deary, I. J. (2018). Sex differences in the adult human brain: evidence from 5216 UKbiobank participants. Cerebral Cortex, 28, 2959–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, A. , Kong, R. , Gordon, E. M. , Laumann, T. O. , Zuo, X.‐N. , Holmes, A. J. , Eickhoff, S. B. , & Yeo, B. T. T. (2018). Local‐global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cerebral Cortex, 28, 3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaggi, G. , & Bellringer, J. (2011). Gender reassignment surgery: an overview. nature reviews. Urology, 8, 274–282. [DOI] [PubMed] [Google Scholar]
- Shirdel‐Havar, E. , Steensma, T. D. , Cohen‐Kettenis, P. T. , & Kreukels, B. P. C. (2019). Psychological symptoms and body image in individuals with gender dysphoria: a comparison between Iranian and Dutch clinics. International Journal of Transgender Health, 20, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorska, M. N. , Chavez, S. , Devenyi, G. A. , Patel, R. , Thurston, L. T. , Lai, M. C. , Zucker, K. J. , Chakravarty, M. M. , Lobaugh, N. J. , & Vanderlaan, D. P. (2021). A multi‐modal MRI analysis of cortical structure in relation to gender dysphoria, sexual orientation, and age in adolescents. Journal of Clinical Medicine, 10, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi, E. , Balenzuela, P. , Fraiman, D. , & Chialvo, D. R. (2012). Criticality in large‐scale brain fmri dynamics unveiled by a novel point process analysis. Frontiers in Physiology, 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Yeo, B. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , Roffman, J. L. , Smoller, J. W. , Zöllei, L. , Polimeni, J. R. , Fisch, B. , Liu, H. , & Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2012). Laterality patterns of brain functional connectivity: gender effects. Cerebral Cortex, 22, 1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe, C. , Junque, C. , Gómez‐Gil, E. , Abos, A. , Mueller, S. C. , & Guillamon, A. (2020a). Data for functional MRI connectivity in transgender people with gender incongruence and cisgender individuals. Data Brief, 31, 105691. 10.1016/j.dib.2020.105691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe, C. , Junque, C. , Gómez‐Gil, E. , Abos, A. , Mueller, S. C. , & Guillamon, A. (2020b). Brain network interactions in transgender individuals with gender incongruence. NeuroImage, 211, 116613. [DOI] [PubMed] [Google Scholar]
- Uribe, C. , Junque, C. , Gómez‐Gil, E. , Díez‐Cirarda, M. , & Guillamon, A. (2021). Brain connectivity dynamics in cisgender and transmen people with gender incongruence before gender affirmative hormone treatment. Scientific Reports, 11, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaesoubi, M. , Miller, R. L. , & Calhoun, V. D. (2015). Mutually temporally independent connectivity patterns: A new framework to study the dynamics of brain connectivity at rest with application to explain group difference based on gender. NeuroImage, 107, 85–94. 10.1016/j.neuroimage.2014.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubiaurre‐Elorza, L. , Junque, C. , Gómez‐Gil, E. , Segovia, S. , Carrillo, B. , Rametti, G. , & Guillamon, A. (2013). Cortical thickness in untreated transsexuals. Cerebral Cortex, 23, 2855–2862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Imaging raw and processed data used for this study and demographical variables are publicly available in Data Mendeley repositories [Uribe et al., 2020a]: https://doi.org/10.17632/hjmfrv6vmg.2, https://doi.org/10.17632/rw2yhtpj96.3, https://doi.org/10.17632/bgyzz94mz9.3. The data that support the findings of this study are openly available in https://doi.org/10.6084/m9.figshare.14622564.


